Abstract
The effects of hormonal and dietary stimuli on hepatic glucose and lipid homeostasis include regulation of gene expression. Berberine, an effective compound in certain Chinese medicinal herbs, has been reported to lower plasma glucose and lipid levels in diabetic and hypercholesterolemic patients. We hypothesized that it may affect the expression of hepatic genes involved in glucose and lipid metabolism. The effects of berberine hydrochloride on viability, gene expression, and activation of AMP activated protein kinase (AMPK) in primary hepatocytes from Sprague-Dawley (SD), Zucker lean (ZL) or fatty (ZF) rats were examined with MTT assay, real-time PCR, and western blotting, respectively. Berberine hydochloride at 50 µM or higher caused cytotoxic effects on hepatocytes. In SD and ZL hepatocytes, it induced Gck and suppressed G6pc expression at 10 and 25 µM, but not as potent as 1 nM insulin. Its effects on Pck1, and insulin-regulated Gck and G6pc expression depended on the hepatocyte sources and the dosage used. In ZF hepatocytes, it increased Gck, and suppressed Pck1 and G6pc expression without insulin. Its effects on Gck and G6pc, but not Pck1 expression, were additive with insulin. Berberine hydrochloride at 25 µM attenuated insulin-suppressed Pck1 (ZL/ZF cells), and insulin-induced Srebp-1c expression (SD/ZL/ZF cells), suggesting modulation of insulin action. Berberine hydrochloride did not alter these genes' mRNA stability. Its treatment caused a dose-dependent increase of phosphorylation of AMPKα, and its substrate, acetyl-CoA carboxylase, in primary hepatocytes. We conclude that berberine hydrochloride regulated the transcription of hepatic genes involved in glucose and fatty acid metabolism.
Keywords: Berberine, insulin, primary hepatocytes, Zucker fatty rats, gene expression
Introduction
The increased rate of metabolic diseases, such as obesity, diabetes and cardiovascular disease, has become a major public health concern [1]. The common characteristic of human obesity and type 2 diabetes is insulin resistance [2]. It has been shown that tight blood glucose control is helpful to reduce the risk of cardiovascular disease, a leading cause of death for patients with diabetes [3]. Liver plays a critical role in mediating glucose and lipid homeostasis regulated by hormones and nutrients. In liver and hepatocytes, insulin stimulates or inhibits the expression of a variety of genes responsible for glycolysis, glycogenesis and lipogenesis, and inhibits gluconeogenesis [4]. This insulin-regulated hepatic gene expression, at least in part, is responsible for the control of glucose and lipid homeostasis [5,6].
For hepatic glucose metabolism, insulin increases the expression of glucokinase gene (Gck) [7,8], the enzyme responsible for the first step of hepatic glucose utilization. It suppresses the expression of the cytosolic form of phosphoenolpyruvate carboxykinase (Pck1) [9] and glucose 6-phosphatase catalytic subunit (G6pc) [4], the first and last steps of gluconeogenesis, respectively. For hepatic lipid metabolism, insulin increases the expression levels of sterol regulatory element binding protein 1c gene (Srebp-1c) [10]. Sterol regulatory element-binding proteins (SREBPs) are critical transcription activators for hepatic cholesterol and fatty acid biosynthesis, and their homeostasis [11]. Therefore, any nutritional or pharmacological factor that alters the expression of these insulin-regulated genes will have the potential to modulate or regulate the hepatic glucose and lipid homeostasis.
Berberine is an isoquinoline alkaloid of the protoberberine type, which is found in many plant species such as Coptis Chinensis Franch used in traditional Chinese medicine. Because of its antimicrobial activity, it has been used conventionally for treatment of diarrhea [12]. Recently, it has been shown that berberine has anti-diabetic effects in experimental animals and clinical diabetic patients [13-16]. It lowered plasma glucose as effectively as metformin [17], a widely prescribed medicine for the treatment of type 2 diabetes. Multiple mechanisms of berberine actions have been proposed such as increase of glycolysis [18], insulin secretion [14], and insulin receptor expression level [17], etc. Recently, it has been shown that berberine activates AMP-activated protein kinase (AMPK) [13,19], a pathway activated by metformin as well [20]. In addition, berberine has been shown to reduce low density lipoprotein cholesterol by up-regulating LDL receptor mRNA expression [21]. This hypolipidemic effect of berberine has been attributed to its ability to activate AMPK [22]. Recently, it has been shown that berberine treatment inhibited hepatic gluconeogenesis in rats of an insulin-insufficient type 2 diabetic model. This has been attributed to berberine-mediated inhibition of Pck1 and G6pc expression in an insulin-independent pathway [23]. We hypothesize that the beneficiary effects of berberine on glucose and lipid metabolism may derive from its direct effects on the expression of hepatic genes involved. Since insulin also regulates the expression levels of many of them, we envision that berberine may have the potential to affect insulin action as well.
Herein, we examined the effects of berberine hydrochloride on the expression of insulin-regulated representative genes, Gck, Pck1, G6pc and Srebp-1c in primary hepatocytes from normal Sprague-Dawley (SD), Zucker lean (ZL) or Zucker fatty (ZF) rats. Both ZL and ZF rats have been widely used as rat models for the development of obesity, insulin resistance and other aspects of metabolic diseases [24,25]. We also examined the effects of berberine hydrochloride on AMPK activation in primary hepatocytes.
Materials and methods
Reagent: Berberine hydrochloride was purchased from National Institutes for Food and Drug Control (> 97.9 %, Beijing, China). Methylthiazolyldiphenyltetrazolium bromide (MTT) was obtained from Sigma (Saint Louis, MO). Antibodies to phospho-AMPKα (Thr172), total AMPKα, phospho-Akt (Ser473), total Akt, phospho-acetyl-CoA carboxylase (Ser79), total acetyl-CoA carboxylase (ACC), and β-actin, were obtained from Cell Signaling Technologies (Danvers, MA). Other reagents have been described previously [26].
Animal and hepatocytes: Male Sprague-Dawley or Zucker rats were purchased from Harlan Breeders (Indianapolis, IN) or bred in the Department of Nutrition at the University of Tennessee at Knoxville. All experimental procedures were approved by the Institutional Animal Care and Use Committee. All the guidelines for the use and care of laboratory animals were followed.
Primary hepatocytes were isolated from non-fasted male SD (10 to 14 weeks), ZL or ZF (9 to 11 weeks of ages) rats, and seeded as described [26]. After attachment, hepatocytes were washed once with 3 ml of PBS, and incubated in medium A (Medium 199 containing 100 nM dexamethasone, 100 nM T3, 100 U/ml sodium penicillin and 100 μg/ml streptomycin sulfate) with 1 nM insulin for overnight at 37°C and in 5% CO2. The hepatocytes were treated as indicated in the figure legends before the total RNA or protein were extracted for real-time PCR or Immunoblot analysis, respectively.
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) based Cell viability assay: Primary hepatocytes from ZL rats were washed once with 3 ml PBS and incubated in medium A without or with increasing concentrations of berberine hydrochloride in the absence or presence of 1 nM insulin for 4 h. After which, 20 μl MTT (5 mg/ml) was added to each well, and the cells were incubated for additional 4h. After removal of the medium, formazan crystal formed inside the cells was dissolved in 150 μl DMSO. The absorbance at 490 nm was measured using a Packard Spectra Count plate reader (Model#: 851).
Total RNA isolation, cDNA synthesis and quantitative real time PCR: Total RNA was isolated using the RNA STAT-60TM reagent (TEL-TEST, Inc, Friendswood, TX) according to the protocol. The procedures for cDNA synthesis and real time PCR have been described previously [26]. The relative amounts of mRNAs except for Fig. 3 were calculated using the comparative CT method as described [26] with 36B4 as the invariant control gene.
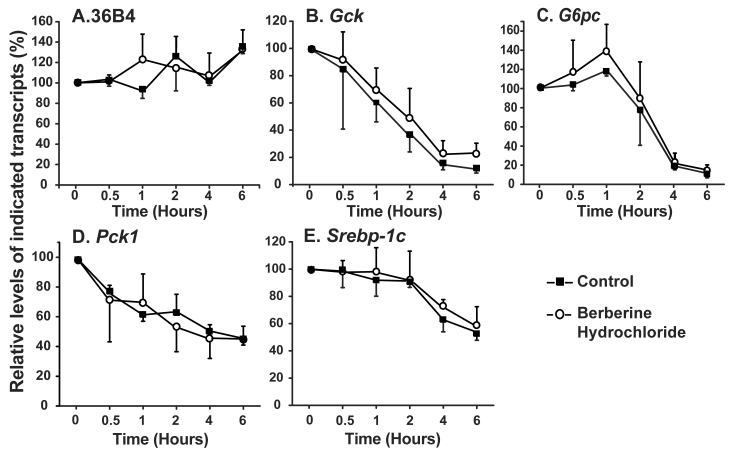
Fig 3.
The effects of berberine hydrochloride on the stabilities of 36B4 (A), Gck (B), G6pc (C), Pck1 (D) and Srebp-1c (E) transcripts. ZL rat primary hepatocytes were treated with medium A containing 3 μM α-amanitin in the absence or present of 25 µM berberine hydrochloride. Total RNAs were extracted at 0.5, 1, 2, 4 and 6 h after treatment, and subjected to real-time PCR analysis. The amount of indicated gene transcripts at time 0 was arbitrarily assigned as 100%. Results represented means ± SD of three independent experiments.
RNA stability assay: For RNA stability assay, primary hepatocytes were washed once with 3 ml PBS and incubated in medium A without or with 3 μM α-amanitin in the absence or presence of 25 μM berberine hydrochloride. Total RNAs were isolated at 0.5, 1, 2, 4, and 6 h, and subjected to real-time PCR analysis. The relative amounts of indicated transcripts were calculated using the method as described [26]. The level of each transcript at time 0 were assigned as 100%.
Immunoblot analysis. After indicated treatments in the figure legends, primary hepatocytes in a 60 mm dish were washed once with 3 ml PBS and scrapped from the dish in 400 µl of whole-cell lysis buffer (1% Triton X-100, 10% glycerol, 1.0% IGEPAL CA-630, 50 mM Hepes, 100 mM NaF, 10 mM EDTA, 5 mM Sodium orthovanadate, 1.9 mg/ml aprotinin, 5 µg/ml leupeptin, 1 mM Benzamide, 2.5 mM DMSF, pH 8.0). The lysates were allowed to sit on ice for at least 20 minutes before subjected to 20,000×g centrifugation for 20 minutes. The protein content in the supernatant was determined with PIERCE BCA protein assay kit (Rockford, IL). Proteins (30 µg/lane) in whole cell lysates were separated on SDS/PAGE, transferred to BIO-RAD Immuno-Blot PVDF membrane (Hercules, CA), and detected with primary antibodies according to the protocols provided by the manufacturers. Bound primary antibodies were visualized by chemiluminescence (ECL Western Blotting Substrate; Thermo Scientific) using a 1:5,000 dilution of goat anti-rabbit IgG (Upstate) conjugated to horseradish peroxidase. Filters were exposed to X-ray films (Phenix Research Products, Candler, NC) for protein band detection.
Statistics. Data are presented as means ± S.D. The number of experiments represents the independent experiments using hepatocytes isolated from different animals on different days. Levene's test was used to determined homogeneity of variance among groups using SPSS 19.0 statistical software and where necessary natural log transformation was performed before analysis. An independent-samples t-test was used to compare two conditions. Multiple comparisons were analyzed by one-way ANOVA using least significant different (LSD) when equal variance was assumed, and Games-Howell test was used when equal variance was not assumed. Differences were considered statistically significant at P < 0.05.
Results
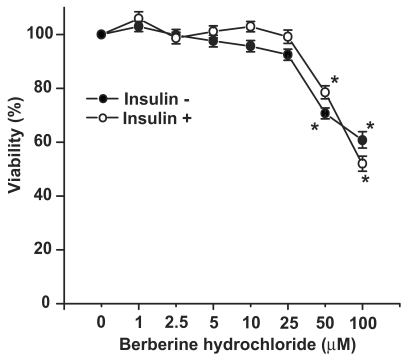
The effects of berberine hydrochloride on viability of primary hepatocytes from SD rats. To determine any cytotoxic effect, the viabilities of primary hepatocytes treated with increasing concentrations of berberine hydrochloride was measured with MTT assay as shown in Fig. 1. The viabilities of the hepatocytes treated with 1 to 25 μM of berberine hydrochloride were not significantly different from that of the control group without or with insulin. However, berberine hydrochloride at 50 or 100 µM reduced the viabilities to 70.7% or 60.7% (without insulin), and 78.5% or 52.0% (with insulin), respectively. These results demonstrated that berberine hydrochloride up to 25 µM can be used to treat rat primary hepatocytes without the concern of cytotoxic effects in the current experimental settings.
Fig 1.
The effects of berberine hydrochloride on viability of primary hepatocytes from SD rats. The primary hepatocytes from SD rats were first incubated in medium A without or with berberine hydrochloride (1 to 100 μM) for 4 hours in the absence or present of 1 nM insulin. After this pre-incubation, MTT was added for additional 4 hours before hepatocyte viabilities were determined as described in the Materials and Methods section. The OD value in control group without insulin was arbitrarily assigned as 100%. Results represented means ± SD of six experiments (* P < 0.01, for comparing the values of control with the values of the indicated treatments).
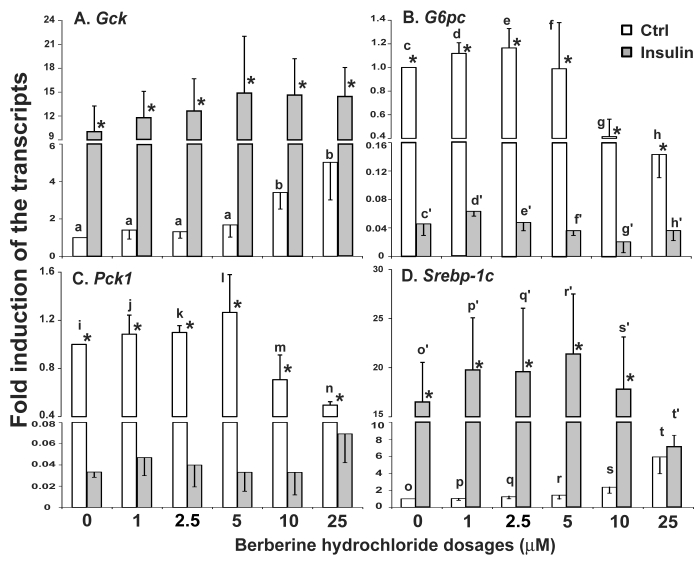
The effects of berberine hydrochloride on the expression of Gck, G6pc, Pck1 and Srebp-1c in hepatocytes from SD rats. Fig. 2A-D showed the relative expression levels of Gck, G6pc, Pck1 and Srebp-1c transcripts in SD primary hepatocytes treated with vehicle control or increasing concentrations of berberine hydrochloride in the absence or presence of insulin. Berberine hydrochloride alone at 10 and 25 μM significantly induced the expression of Gck by 3.4 ± 1.1-fold and 5.0 ± 2.5 -fold, respectively (Fig. 2A). Insulin induced Gck expression at all concentrations of berberine hydrochloride as expected. However, no additive effect was observed between them. Fig. 2B showed that 10 or 25 μM berberine hydrochloride alone significantly reduced G6pc mRNA to 0.41 ± 0.18 or 0.14 ± 0.04 -fold of the control value, respectively. Fig. 2C showed that 25 μM berberine hydrochloride reduced the Pck1 expression level to 0.50 ± 0.03 -fold of its value of the control group in the absence of insulin (P<0.01). Insulin significantly reduced the expression levels of G6pc and Pck1 as expected. However, berberine hydrochloride could not further reduce insulin-suppressed G6pc or Pck1 expression. Fig. 2D showed that 25 μM berberine hydrochloride induced the expression of Srebp-1c by 5.9 ± 2.0-fold (P<0.05) in the absence of insulin. Surprisingly, this concentration of berberine hydrochloride attenuated insulin-induced Srebp-1c expression, which was not affected by other concentrations of berberine hydrochloride tested.
Fig 2.
The effects of berberine hydrochloride on the levels of Gck (A), G6pc (B), Pck1 (C) and Srebp-1c (D) transcripts in hepatocytes from SD rat. Primary hepatocytes were treated with either vehicle or berberine hydrochloride (1 to 25 μM) for 6h in the absence or present of 1 nM insulin. The expression levels of indicated transcripts were analyzed using real-time PCR. The expression level in control group without insulin was arbitrarily assigned as 1. Results represented means ± SD of three independent experiments (* for comparing the fold induction of the indicated transcript at the berberine hydrochloride concentration in the absence with that in the presence of insulin; for Gck, b>a; for G6pc, d/e>g, c/d/e>h, d'>f'/g'/h', and e'>g'; for Pck1, n>i/j/k; for Srebp1c, t>s>o/p/q, t>r, and t'>o'/p'/q'/r'/s'; all P<0.05).
The effects of berberine hydrochloride on the stabilities of Gck, G6pc, Pck1 and Srebp-1c in primary hepatocytes from ZL rats. We next determined whether the change of the gene expression levels was caused by alteration of transcription or stability of the transcripts. The stabilities of the Gck, G6pc, Pck1 and Srebp-1c mRNA were measured in the presence of α-amanitin, a specific inhibitor of RNA polymerase II complex [27]. As shown in Fig. 3A, the contents of 36B4 transcripts exhibited no difference between the vehicle control and 25 µM berberine hydrochloride groups. Fig. 3B-E respectively showed the decay of Gck, G6pc, Pck1 and Srebp-1c transcripts in the absence or presence of berberine hydrochloride. At 0.5, 1, 2, 4, and 6 h after α-amanitin treatment, the contents of indicated transcripts in the berberine hydrochloride group were not significant different from those in the control groups of the corresponding genes, indicating no effect of berberine hydrochloride on the decay rates of those transcripts. The estimated half life of Gck, G6pc, Pck1 or Srebp-1c transcripts was about 1.5h, 3h, 4h or 6h for either control or berberine hydrochloride group, respectively. It indicated that berberine hydrochloride regulated the transcription of Gck, G6pc, Pck1 and Srebp-1c in primary rat hepatocytes.
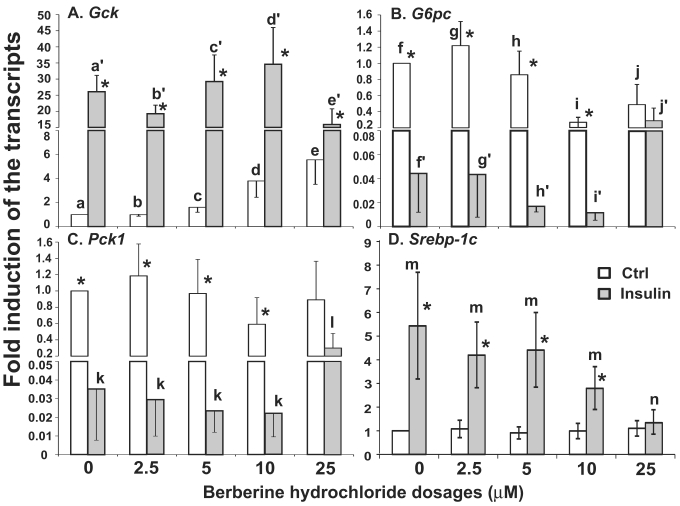
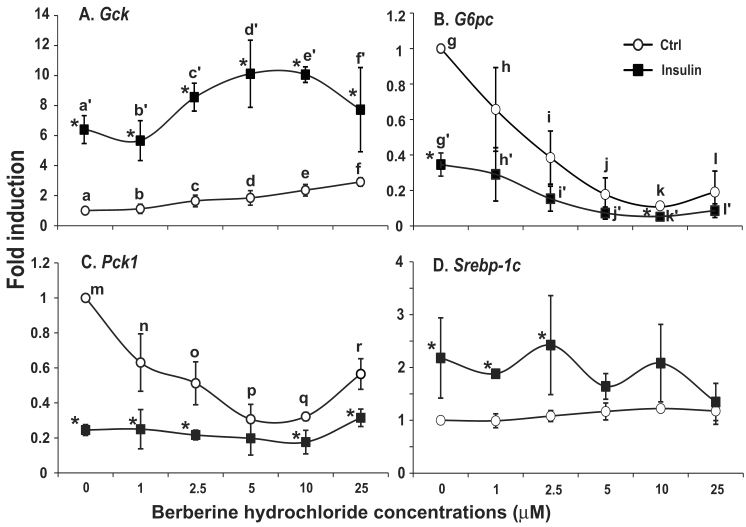
The effects of berberine hydrochlorides on the expression levels of Gck, G6pc, Pck1 and Srebp-1c in primary hepatocytes from ZL rats. To determine the berberine hydrochloride effects on gene expression in hepatocytes derived from a difference strain of rats, hepatocytes were isolated from ZL rats, which are insulin sensitive and have been used as controls for insulin resistant ZF rats [24,25]. The expression levels of Gck, G6pc, Pck1 and Srebp-1c in ZL hepatocytes treated with increasing concentrations of berberine hydrochloride (0 to 25 µM) in the absence or presence of insulin were analyzed as shown in Fig. 4. Berberine hydrochloride started to significantly induce Gck expression by 1.6 ± 0.4-fold at 5 μM in the absence of insulin (Fig. 4A). Insulin significantly induced Gck expression at all berberine hydrochloride concentrations tested. However, there was an attenuation of insulin-induced Gck expression when berberine hydrochloride was at 25 µM, a phenomenon not observed in SD hepatocytes. For G6pc, 10 or 25 μM berberine hydrochloride alone respectively reduced G6pc mRNA to 0.3 ± 0.1 or 0.5 ± 0.3 -fold of the control value in the absence of insulin (Fig. 4B). Interestingly, 10 µM or 25 µM berberine hydrochloride respectively potentiated or attenuated insulin-suppressed G6pc expression, a difference with SD hepatocytes. Fig. 4C showed that 25 μM berberine hydrochloride significantly attenuated insulin-suppressed Pck1 expression, but had no effects on its expression in the absence of insulin, a difference with SD hepatocytes. The expression levels of G6pc and Pck1 mRNA in control group was not significantly different from that of insulin group when berberine hydrochloride was at 25 µM, suggesting attenuation of insulin-mediated suppression of these two genes. Fig. 4D showed that in ZL hepatocytes, berberine hydrochloride up to 25 μM did not affect the expression of Srebp-1c without insulin, a difference with SD hepatocytes. Insulin no longer induced Srebp-1c when berberine hydrochloride concentration was 25 µM, a similar phenomenon in SD hepatocytes.
Fig 4.
The effects of berberine hydrochloride on the levels of Gck (A), G6pc (B), Pck1 (C) and Srebp-1c (D) transcripts in hepatocytes from Zucker lean rat. Primary hepatocytes from ZL rats were treated with either vehicle or berberine hydrochloride (2.5 to 25 μM) for 6h in the absence or present of 1 nM insulin. Total RNAs were extracted and subjected to real-time PCR analysis. The gene expression level in control group without insulin was arbitrarily assigned as 1. Results represented means ± SD of four independent experiments (* for comparing the fold induction of the indicated transcript at the berberine hydrochloride concentration in the absence with that in the presence of insulin; for Gck, e>c>a, d/e>a/c, a'>c'/d'>e'; for G6pc, f/g/h>i/j, and j'>f'/g'/h'>i'; for Pck1, l>k; for Srebp1c, m>n; all P<0.05).
The effects of berberine hydrochlorides on the expression levels of Gck, G6pc, Pck1 and Srebp-1c in primary hepatocytes from ZF rats. To determine the berberine effects on gene expression in hepatocytes from insulin resistant animals, we used primary hepatocytes from obese and insulin resistant ZF rats [24,25]. Fig. 5A-D respectively showed the relative expression levels of Gck, G6pc, Pck1 and Srebp-1c transcripts in ZF primary hepatocytes treated with vehicle control or increasing concentrations of berberine hydrochloride in the absence or presence of 1 nM insulin. In the absence of insulin, berberine hydrochloride at 5 μM started to induce Gck expression by 1.9 ± 0.5-fold (Fig. 5A). Insulin induced the level of Gck transcript to 6.5 ± 1.0- fold of the control value in the absence of berberine hydrochloride. Berberine hydrochloride at 5 and 10 μM significantly potentiated insulin-induced expression of Gck, suggesting an additive effect between insulin and these two concentrations of berberine on Gck expression in ZF hepatocytes. Fig. 5B and 5C showed that berberine hydrochloride alone at 2.5 and 1 µM started to significantly suppress G6pc and Pck1 expression in ZF hepatocytes, respectively. The levels of G6pc and Pck1 transcripts in 10 µM berberine hydrochloride treatment group reached 0.11 ± 0.01- and 0.32 ± 0.02-fold of the control value, respectively. Interestingly, the Pck1 expression levels in 25 µM treatment group were higher than those in 5 and 10 µM berberine hydrochloride groups (Fig. 5C). Insulin respectively reduced the levels of G6pc and Pck1 transcript to 0.35 ± 0.07- and 0.25 ± 0.03-fold of the control value. In the presence of 1 nM insulin, berberine hydrochloride at 2.5 to 25 μM further reduced the expression levels of G6pc transcript, but not Pck1, in ZF hepatocytes. The effects of berberine hydrochloride on the expression of Srebp-1c in hepatocytes from ZF rats were weak at the most. Fig. 5D showed that berberine hydrochloride did not significantly affect Srebp-1c expression in the absence of insulin. Insulin only slightly induced Srebp-1c expression in fatty hepatocytes. This weak induction no longer existed when berberine hydrochloride was at 5 µM or higher, suggesting attenuation of insulin-induced Srebp-1c expression by berberine treatment in ZF hepatocytes.
Fig 5.
The effects of berberine hydrochloride on the levels of Gck (A), G6pc (B), Pck1 (C) and Srebp-1c (D) in hepatocytes from Zucker fatty rat. Primary hepatocytes from ZF rats were treated with either vehicle or berberine hydrochloride (1 to 25 μM) for 6h in the absence or present of 1 nM insulin. Total RNAs were extracted and subjected to real-time PCR analysis. The gene expression level in control group without insulin was arbitrarily assigned as 1. Results represented means ± SD of three independent experiments (* for comparing the fold induction of the indicated transcript at the berberine hydrochloride concentration in the absence with that in the presence of insulin; for Gck, f>d>a, e>a, e/f>b, d'/e'>a'/b'; for G6pc, g>i>k, g>j/l, h>j/k/l, and g'/h'>i'/k'/l'; for Pck1, m>n/r>p/q, and m>o; all P<0.05).
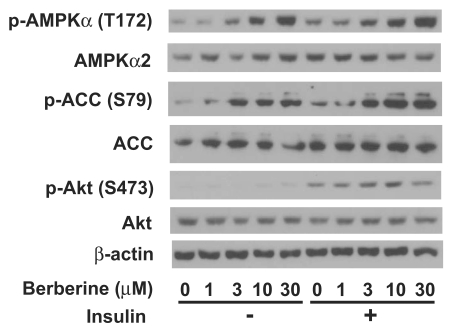
Berberine hydrochloride activated AMPK in primary hepatocytes. Fig. 6 showed that berberine hydrochloride treatment started to cause phosphorylation of AMPKα subunits at threonine 172 as low as 3 µM in ZL hepatocytes. This phosphorylation of AMPK correlated with an induction of phosphorylation of its substrate, ACC, at serine 79 in the absence or presence of 1 nM insulin. It demonstrated an increase of AMPK activity after berberine hydrochloride treatment. Insulin treatment resulted in phosphorylation of its downstream signaling target, Akt at serine 473, demonstrating the activation of insulin signaling transduction pathway. There are no significant changes of total AMPKα, ACC, Akt, and β-actin in the same hepatocytes treated without and with berberine hydrochloride in the absence or presence of insulin.
Fig 6.
Immunoblot analysis of phosphor-AMPKα, total AMPKα, phosphor-ACC, total ACC, phosphor-Akt, total Akt, and β-actin in primary hepatocyes treated with berberine hydrochloride in the absence or presence of insulin. After overnight pretreatment, primary hepatocytes from Zucker lean rats were incubated in medium A without or with increasing concentrations of berberine hydrochloride (1 to 30 µM) in the absence or presence of 1 nM insulin for 20 minutes. Total protein (30 µg/lane) was separated on SDS/PAGE, detected by specific primary antibodies as indicated, and visualized by chemiluminescence. Graph was the representative of three independent experiments with similar results using hepatocytes isolated from three different ZL rats in three different days.
Discussion
In this study, we reported the effects of berberine hydrochloride on the expression of hepatic genes involved in glucose and lipid metabolism. It has been reported that berberine administrated orally was well tolerated in rodents (100 to 300 mg/kg) and human (500 mg two to three times per day/person) [13-16]. The dosages used here were similar to the concentrations used (5 to 15 µg/ml which are equivalent to 15 to 45 µM) in previous reports showing activation of signal transduction pathways [13] and regulation of gene expression [21]. Our results demonstrated that 25 µM berberine hydrochloride was well tolerated by primary hepatocytes in the current experimental settings (Fig. 1). The observed cytotoxic effect of 50 or 100 µM berberine hydrochloride was reasonable as berberine and its derivatives inhibited activity of mitochondrial complex I of electron transport chain [28]. The tolerance of berberine in the in vivo studies may be attributed to its poor bioavailability due to the P-glycoprotein on small intestine, which actively pumps berberine back into the intestinal lumen [29]. This might have prevented higher concentrations of berberine in plasma and exerted toxic effects on liver. Recently, some attempts have been taken to modify the structure of berberine and identify its analogues for the improvement of its bioavailability and efficacy [28,30-33]. Whether any of those derivatives and analogues has the same effects on viability of primary hepatocytes remains to be seen. In addition, how berberine and its derivatives are transported and catabolized in primary hepatocytes also deserves to be investigated. Nevertheless, the results shown here have provided a reference for future berberine studies in primary hepatocytes.
We initiated our studies using hepatocytes from SD rats and observed the effects of berberine hydrochloride on gene expression in them (Fig. 2). Subsequently, we decided to examine berberine effects on hepatocytes in insulin resistant Zucker fatty rats. Therefore, we conducted similar dosage experiments in hepatocytes (Fig. 4) from ZL rats which are insulin sensitive and have been used as the controls for obese and insulin resistant ZF rats [24,25]. In hepatocytes from both SD and ZL rats, berberine hydrochloride at 10 and 25 µM increased Gck, and suppressed G6pc expression in the absence of insulin. However, the magnitude of the induction or suppression was not the same as that mediated by insulin (Fig. 2 and 4). In hepatocytes from either SD or ZL rats, berberine hydrochloride at 25 µM attenuated insulin-induced Srebp-1c expression. Nevertheless, the hepatocytes from these two strains of rats showed some differences regarding the responses of these genes to berberine hydrochloride treatments. For Gck, berberine hydrochloride at 25 µM attenuated insulin-induced its expression in ZL, but not SD hepatocytes. The same treatment reversed berberine hydrochloride- and insulin-suppressed G6pc expression to a certain extent in ZL, but not in SD hepatocytes. For Pck1, berberine hydrochloride at 25 µM reduced its expression in SD, but not in ZL hepaotcytes in the absence of insulin. In the presence of insulin, it only attenuated insulin-suppressed Pck1 expression in ZL, but not in SD hepatocytes. Berberine hydrochloride started to induce Srebp-1c expression at 10 µM in SD, but not ZL in hepatocytes. All these results demonstrated that berberine hydrochloride had the ability to regulate the expression of these four insulin-regulated genes in primary hepatocytes. The regulation can be masked by insulin treatment (such as Gck and G6pc). On the other hand, berberine hydrochloride can modify insulin's action on the expression of its downstream target gene (such as Srebp-1c). In addition, some of the effects on a particular gene expression (such as Pck1) may depend on the strain of rats from which the hepatocytes were obtained.
The fact that berberine hydrochloride did not change the mRNA stabilities of these four genes (Fig. 3) indicated that it regulated their transcription in primary hepatocytes, which is also regulated by insulin. In the process of submitting our current manuscript, a report came out and demonstrated that berberine inhibited hepatic Pck1 and G6pc expression in an insulin-independent pathway [23]. Our results showed that berberine directly regulated these two genes' transcription, which is in agreement with their observations. In addition, we also demonstrated that berberine can induce the expression of Gck, suggesting glucose usage as another target of berberine action in liver. It has been shown the metformin, another reagent that activates AMPK [20], and insulin suppressed hepatic Pck1 and G6pc expression through phosphorylation of CREB binding protein [34]. Both metformin and insulin treatments caused activation of PKCι/λ via activation of AMPK and phosphoinositide-dependent protein kinase 1 (PDK1), respectively. However, only insulin activates Akt/PKB, and metformin activates AMPK [34]. It indicated that activation metformin and insulin shared a common signal transduction pathway. Given the effects of berberine hydrochloride on Gck, Pck1 and G6pc expression, it seems that insulin and berberine activated a common signaling pathway that leads to regulation of these genes' transcription in primary rat hepatocytes. However, given the effects of berberine hydrochloride on Srebp-1c expression in the presence of insulin, it seems that berberine antagonized part of insulin-regulated gene expression. It has been shown that insulin-mediated induction of Srebp-1c expression and suppression of Pck1 expression bifurcated at mTOR step, which is downstream of PDK1 activation [35]. Berberine hydrochloride treatment might have activated part of insulin-mediated pathways involved in glucose metabolism and antagonized insulin-induced Srebp-1c expression. Whether the berberine signaling in hepatocytes shares part of insulin pathways or initiates a novel pathway deserves further investigation.
The beneficial effects of berberine on type 2 diabetes and insulin resistance subjects [13-16] indicated that it may have effects on hepatocytes isolated from insulin resistant fatty rats. In a different manuscript, we have reported that the insulin-regulated gene expression in ZF hepatocytes was impaired or diminished when compared to that in ZL hepatocytes. Our results shown here also supported this claim when the fold inductions of these four genes in ZL and ZF hepatocytes were compared (Fig. 4 and Fig. 5). We showed here that berberine hydrochloride increased Gck expression in fatty hepatocytes as it did in SD and ZL hepatocytes (Fig. 4 and Fig. 5). The difference here is that berberine and insulin had additive effects on the induction of Gck expression in fatty hepatocytes, but not in SD or ZL cells. For G6pc expression, berberine suppressed its expression in ZF hepatocytes as low as 2.5 µM, a concentration that could not affect G6pc expression in SD and ZL hepatocytes. In addition, berberine potentiated insulin-suppressed G6pc expression at concentration as low as 2.5 µM. For Pck1 expression in ZF hepatocytes, berberine suppressed its expression levels in the absence of insulin at concentration as low as 1 µM, which was not observed in SD or ZL hepatocytes. Unlike its effects on G6pc expression, berberine did not potentiated insulin-suppressed Pck1 expression. For Srebp-1c, berberine hydrochloride had almost no effect on its expression in fatty hepatocytes without or with insulin. The unresponsiveness of Srebp-1c to berberine hydrochloride treatment in fatty hepatocytes could be caused by the elevated expression of Srebp-1c [36] or alteration of the mechanisms of its regulation in fatty hepatocytes. These results indicated that the expression levels of Gck, G6pc and Pck1 were more sensitive to berberine treatment in ZF hepatocytes than in SD or ZL hepatocytes. In addition, insulin-regulated Gck and G6Pc can be modified in the presence of berberine hydrochloride (5 and 10 µM), suggesting improvement of insulin action. Whether the pre-existence of insulin resistance in the ZF hepatocytes plays a role here or not deserves further study.
Our results demonstrated that berberine hydrochloride dose-dependently induced phosphorylation of AMPKα in primary hepatocytes without or with insulin. The phosphorylation of AMPKα in primary hepatocytes correlated with an increase of the phosphorylation of its substrate ACC, demonstrating the elevation of AMPK activity after berberine hydrochloride treatment in primary hepatocytes (Fig. 6). This activation of AMPK was not affected by the presence of insulin, which induces phosphorylation of Akt, a downstream kinase mediating insulin signaling pathway. It has been shown that berberine activated AMPK in 3T3-L1 adipocytes [13] and in L6 myotubes [37]. Constitute activation of AMPK in mouse liver reduced Srebp-1c expression [38]. This AMPK activation mediated by berberine hydrochloride treatment might have contributed to the attenuation of insulin-induced Srebp-1c expression in the presence of berberine hydrochloride (Fig. 2, 4 and 5). Reduction of plasma triglyceride and cholesterol levels were observed in hypercholesterolemic patients treated with berberine [21]. Given the critical role of SREBP-1c in hepatic lipogenesis [11], this antagonization of berberine hydrochloride to insulin-induced Srebp-1c expression might have contributed to the hypolipidemic effects of berberine. However, it is worth to note that in ZL hepatocytes, berberine hydrochloride at 10 and 25 µM potentiated and attenuated insulin-suppressed G6pc expression, respectively (Fig. 4). One explanation is that the degree of AMPKα phosphorylation might have played a role here. As shown in Fig. 6, phosphorylation of AMPKα was further enhanced with the rise of berberine hydrochloride concentrations from 10 µM to 30 µM. The additional phosphorylation of AMPKα might have changed the dynamic of AMPK activation, which might have contributed negatively to insulin's effects on G6pc expression. It has been reported that AMPK phosphorylated raptor, a component of mTORC1 complex. This phosphorylation resulted in suppression of its activity, which is responsible for the inhibition of mTORC1 and cell-cycle arrest induced by energy stress [39]. Alternatively, another signaling pathway might have been activated by 25 µM berberine hydrochloride, which might have contributed to the phenomenon. The activation of AMPK alone may not be enough to explain all the berberine hydrochloride effects. It has been shown that metformin, another antidiabetic drug that can activate AMPK [20], still suppressed hepatic gluconeogenesis in mice without AMPK catalytic subunits (AMPKα knockout) [40]. Given the different functions of these four genes in glucose and lipid metabolism, it is possible that AMPK activation may not be the only reason for the observed effects of berberine on these four genes' expression in primary hepatocytes. The mechanisms by which berberine hydrochloride affect hepatic genes involved in glucose metabolism remain to be investigated.
In summary, we demonstrated that berberine hydrochloride regulated the transcription of representative genes involved in glucose and fatty acid synthesis in hepatocytes from either insulin sensitive rats (SD and FL) or resistant (ZF) rats. It suggests that the beneficial effects of berberine on diabetes and hyperlipidemia [13-16] may have something to do, at least in part, with the berberine-regulated transcription of hepatic genes involved in glucose and lipid metabolism. The berberine-mediated AMPK activation may contribute in part to its effects on insulin-regulated gene expression. Further understanding of the activation mechanism of berberine in hepatocytes may provide us with new tools to combat metabolic diseases.
Acknowledgments
This work was partially supported by start-up fund from the University of Tennessee at Knoxville (to G.C.), Scientist Development Grant from American Heart Association (09SDG2140003, to G.C.), research grant from Allen Foundation Inc (to G.C.), and Chinese Scholarship Counsel funds (to Y.L., W.C. and Y.L.).
Abbreviations
- Gck
glucokinase gene
- G6pc
glucose 6-phosphotase catalytic subunits gene
- Pck1
cytosolic form of phosphoenolpyruvate carboxyl kinase gene
- Srebp-1c
sterol regulatory element-binding protein 1c gene
- SD
Sprague-Dawley
- ZL
Zucker lean
- ZF
Zucker fatty.
References
- 1.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The Disease Burden Associated With Overweight and Obesity. JAMA. 1999 Oct 27;282:1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 2.McGarry JD. Banting Lecture 2001: Dysregulation of Fatty Acid Metabolism in the Etiology of Type 2 Diabetes. Diabetes. 2002 Jan 1;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Friedrich MJ. Tight Blood Glucose Control Pays Off in Reduced Cardiovascular Risk in Diabetes. JAMA. 2006 Sep 27;296:1455–6. doi: 10.1001/jama.296.12.1455. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien RM GD. Regulation of gene expression by insulin. Physiol Rev. 1996 Oct 1;76:1109–61. doi: 10.1152/physrev.1996.76.4.1109. [DOI] [PubMed] [Google Scholar]
- 5.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and Increased SREBP-1c Lead to Mixed Insulin Resistance and Sensitivity in Livers of Lipodystrophic and ob/ob Mice. Molecular Cell. 2000 Jul;6:77–86. [PubMed] [Google Scholar]
- 6.Spiegelman BM, Flier JS. Obesity and the Regulation of Energy Balance. Cell. 2001 Feb 23;104:531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 7.Iynedjian PB. Mammalian glucokinase and its gene. Biochem J. 1993 Jul 1;293:1–16. doi: 10.1042/bj2930001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnuson MA, Andreone TL, Printz RL, Koch S, Granner DK. Rat Glucokinase Gene: Structure and Regulation by Insulin. PNAS. 1989 Jul 1;86:4838–42. doi: 10.1073/pnas.86.13.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson RW, Patel YM. Regulation of Phosphoenolpyruvate Carboxykinase (GTP) Gene expression. Annual Review of Biochemistry. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- 10.Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. PNAS. 1999 Nov 23;96:13656–61. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002 May;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs PJ, Seddon K.R. Berberine. Alternative Medicine Review. 2000 Apr;5:175–7. [PubMed] [Google Scholar]
- 13.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK. et al. Berberine, a Natural Plant Product, Activates AMP-Activated Protein Kinase With Beneficial Metabolic Effects in Diabetic and Insulin-Resistant States. Diabetes. 2006 Aug;55:2256–64. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 14.Leng SH, Lu FE, Xu LJ. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol Sin. 2004 Apr;25:496–502. [PubMed] [Google Scholar]
- 15.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008 May;57:712–7. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J. et al. Treatment of Type 2 Diabetes and Dyslipidemia with the Natural Plant Alkaloid Berberine. J Clin Endocrinol Metab. 2008 Jul 1;93:2559–65. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Wei J, Xue R, Wu JD, Zhao W, Wang ZZ, Wang SK, Zhou ZX, Song DQ. et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010 Feb;59:285–92. doi: 10.1016/j.metabol.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008 Jan 1;294:E148–E156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, Issandou M. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006 Jun 1;47:1281–8. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001 Oct 15;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S. et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004 Dec;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 22.Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, Issandou M. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006 Jun 1;47:1281–8. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Xia X, Yan J, Shen Y, Tang K, Yin J, Zhang Y, Yang D, Liang H, Ye J, Weng J. Berberine Improves Glucose Metabolism in Diabetic Rats by Inhibition of Hepatic Gluconeogenesis. PLoS ONE. 2011 Feb 3;6:e16556. doi: 10.1371/journal.pone.0016556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aleixandre de Arti±ano A, Miguel Castro M. Experimental rat models to study the metabolic syndrome. British Journal of Nutrition. 2009;102:1246–53. doi: 10.1017/S0007114509990729. [DOI] [PubMed] [Google Scholar]
- 25.Unger RH. How obesity causes diabetes in Zucker diabetic fatty rats. Trends in Endocrinology and Metabolism. 1997 Sep;8:276–82. doi: 10.1016/s1043-2760(97)00094-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen G. Liver lipid molecules induce PEPCK-C gene transcription and attenuate insulin action. Biochemical and Biophysical Research Communications. 2007 Sep 28;361:805–10. doi: 10.1016/j.bbrc.2007.07.108. [DOI] [PubMed] [Google Scholar]
- 27.Bushnell DA, Cramer P, Kornberg RD. Structural basis of transcription: alpha -Amanitin-RNA polymerase II cocrystal at 2.8 A resolution. PNAS. 2002 Feb 5;99:1218–22. doi: 10.1073/pnas.251664698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner N, Li JY, Gosby A, To SWC, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW. et al. Berberine and Its More Biologically Available Derivative, Dihydroberberine, Inhibit Mitochondrial Respiratory Complex I. Diabetes. 2008 May;57:1414–8. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- 29.Pan Gy, Wang GJ, Liu XD, Fawcett JP, Xie YY. The Involvement of P-Glycoprotein in Berberine Absorption. Pharmacology & Toxicology. 2002;91:193–7. doi: 10.1034/j.1600-0773.2002.t01-1-910403.x. [DOI] [PubMed] [Google Scholar]
- 30.Cheng Z, Chen AF, Wu F, Sheng L, Zhang HK, Gu M, Li YY, Zhang LN, Hu LH. et al. 8,8-Dimethyldihydroberberine with improved bioavailability and oral efficacy on obese and diabetic mouse models. Bioorganic & Medicinal Chemistry. 2010 Aug 15;18:5915–24. doi: 10.1016/j.bmc.2010.06.085. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y, Ou TM, Hou JQ, Lu YJ, Tan JH, Gu LQ, Huang ZS. 9-N-Substituted berberine derivatives: Stabilization of G-quadruplex DNA and down-regulation of oncogene c-myc. Bioorganic & Medicinal Chemistry. 2008 Aug 15;16:7582–91. doi: 10.1016/j.bmc.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Wang YX, Wang YP, Zhang H, Kong WJ, Li YH, Liu F, Gao RM, Liu T, Jiang JD, Song DQ. Synthesis and biological evaluation of berberine analogues as novel up-regulators for both low-density-lipoprotein receptor and insulin receptor. Bioorganic & Medicinal Chemistry Letters. 2009 Nov 1;19:6004–8. doi: 10.1016/j.bmcl.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 33.Yang P, Song DQ, Li YH, Kong WJ, Wang YX, Gao LM, Liu SY, Cao RQ, Jiang JD. Synthesis and structure-activity relationships of berberine analogues as a novel class of low-density-lipoprotein receptor up-regulators. Bioorganic & Medicinal Chemistry Letters. 2008 Aug 15;18:4675–7. doi: 10.1016/j.bmcl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 34.He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and Insulin Suppress Hepatic Gluconeogenesis through Phosphorylation of CREB Binding Protein. Cell. 2009 May 15;137:635–46. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. PNAS. 2010 Feb 23;107:3441–6. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakuma T, Lee Y, Higa M, Wang Zw, Pan W, Shimomura I, Unger RH. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proceedings of the National Academy of Sciences of the United States of America. 2000 Jul 18;97:8536–41. doi: 10.1073/pnas.97.15.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Z, Pang T, Gu M, Gao AH, Xie CM, Li JY, Nan FJ, Li J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochimica et Biophysica Acta (BBA) - General Subjects. 2006 Nov;1760:1682–9. doi: 10.1016/j.bbagen.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Maika S, Craddock L, King JA, Liu ZM. Chronic activation of AMP-activated protein kinase-alpha1 in liver leads to decreased adiposity in mice. Biochemical and Biophysical Research Communications. 2008 May 30;370:248–53. doi: 10.1016/j.bbrc.2008.03.094. [DOI] [PubMed] [Google Scholar]
- 39.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Molecular Cell. 2008 Apr 25;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010 Jul 1;120:2355–69. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]