INTRODUCTION
Neovascular glaucoma (NVG) is an aggressive type of glaucoma, which often results in poor visual outcomes. Most NVG patients have severe underlying systemic and ocular pathology which causes NVG as a late presentation of their primary systemic and/or ocular disease. This makes NVG very difficult to treat. The key to adequate treatment of this devastating ocular disease is an understanding of its pathogenesis, which has recently been better elucidated.
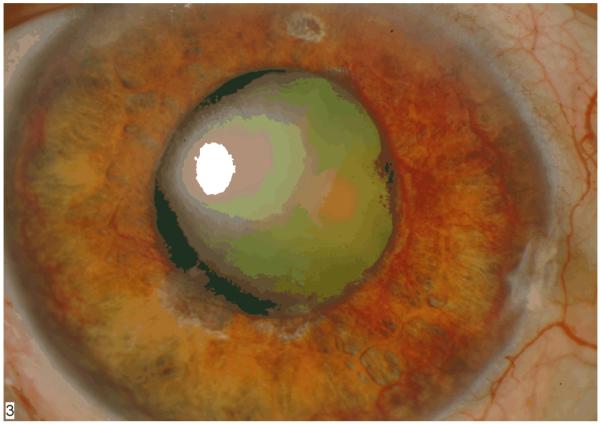
Weiss and colleagues first proposed the term “neovascular glaucoma” in 1963, when they described a severe glaucoma associated with the presence of new iris and angle vessels. (1) These patients present with elevated intraocular pressure (IOP) and neovascularization of the iris and angle, often with hyphema and other ocular findings, such as ectropion uveae (Figure 1). The myriad medical conditions resulting in NVG all lead to a final common pathway of profound retinal ischemia. This ischemia induces retinal production of vasoproliferative factors, including vascular endothelial growth factor (VEGF), which diffuse anteriorly and lead to anterior segment neovascularization of the iris (NVI) and neovascularization of the angle (NVA). Patients with NVI and NVA may have normal IOP in the early stages. If left untreated, the fibrovascular scaffolding of these vessels cross the angle and IOP becomes markedly elevated, though the angle may still be gonioscopically open. Ultimately, in the absence of definitive treatment, these fibrovascular membranes contract and produce synechial angle closure and ectropion uveae with intractable elevation of IOP and damage to the optic nerve with subsequent vision loss.
Figure 1.
NVG with florid NVI and ectropion uveae
The most common conditions resulting in NVG are retinal vein occlusion, diabetic retinopathy, and carotid occlusive disease (including ocular ischemic syndrome) (2, 3). Other conditions resulting in retinal ischemia that can lead to NVG include central retinal artery occlusion, chronic retinal detachment, and intraocular malignancies.
TREATMENT OF NVG
The management of NVG has two main components. The first component is reduction of the IOP by both medical and surgical means. The second component, which is arguably the most critical for effective long-term treatment outcomes, is reduction of the ischemic drive that induces formation of new blood vessels. The mainstay of this treatment component is panretinal photocoagulation (PRP), which reduces the production of vasoproliferative factors from ischemic retina. If performed early during the neovascular process, PRP can induce the regression of both anterior and posterior segment neovascularization. More recently, the use of anti-VEGF agents to induce rapid regression of neovascularization has gained widespread clinical acceptance (3-12).
Prevention of NVG is always preferable to treatment of the consequences of NVG. Patients at high risk for development of NVG, such as those with proliferative diabetic retinopathy or recent CRVO, should be screened carefully at each clinic visit for neovascularization of the iris and angle, even if intraocular pressures are normal. If NVI or NVA is detected, prompt application of PRP and anti-VEGF treatments should be initiated. Treatment of neovascularization, especially if prior to the development of intractable glaucoma, may prevent or delay the development of sight-threatening NVG.
MEDICAL CONTROL OF IOP
Medical control of elevated intraocular pressure includes the topical administration of agents to reduce aqueous production and increase aqueous humor outflow to lower the IOP, such as with beta-blockers, carbonic anhydrase inhibitors, prostaglandin agonists, and alpha-adrenergic agonists. Oral administration of carbonic anhydrase inhibitors is also effective in patients who tolerate this class of medications. Finally, eyes with NVG should also be treated with topical steroids and cycloplegics, particularly if significant intraocular inflammation or hyphema is present.
Medical treatment of the elevated IOP encountered in NVG is temporizing, particularly if the angles are closed by synechiae. Nevertheless, controlling the IOP until more definitive treatment can take effect is important for protecting the optic nerve from damage, decreasing associated pain, and possibly improving vision secondary to IOP-dependent corneal edema.
SURGICAL CONTROL OF IOP
Surgical intervention is indicated when medical therapy is inadequate to control IOP, particularly if synechial angle closure from NVA has occurred. Glaucoma surgery is helpful in the early stages to control the IOP until anti-angiogenic panretinal photocoagulation can take effect and for longer term IOP control if the angle is closed secondary to NVA.
Trabeculectomy and other filtering surgeries are moderately successful in the long term in patients with NVG. The success of trabeculectomy success is limited by the severe inflammation encountered with NVG eyes. Trabeculectomy with adjunctive 5-fluorouracil showed high success rates initially, which decreased with longer-term follow up. (13) Trabeculectomy with mitomycin C (MMC) for the IOP lowering in NVG eyes had a success rate of 62.6% at one year, declining to 51.7% by five years of follow-up in a patient cohort where the majority (81.2%) of the NVG cases were secondary to diabetic retinopathy. (14) Risk factors for progressive failure of MMC-treated trabeculectomies included younger age and prior vitrectomy. (14)
In recent years, glaucoma drainage implants (GDI) have gained popularity in the surgical treatment of NVG, as their success is less dependent on control of intraocular inflammation and the failure of a filtering bleb. (15) Figure 2 shows a NVG patient who has undergone recent GDI placement with an associated hyphema from NVI.
Figure 2.
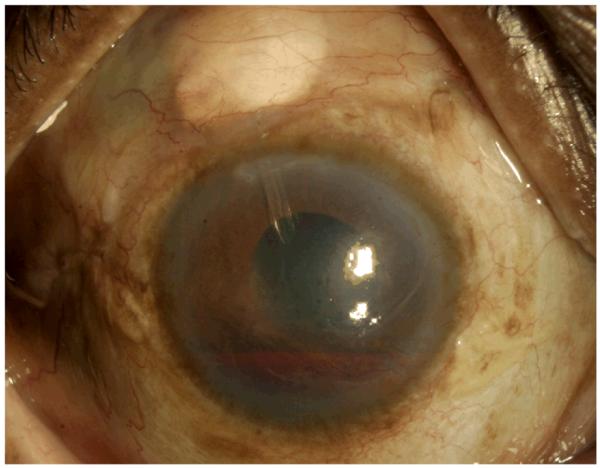
Status post GDI surgery for NVG. Note the presence of hyphema.
Cyclodestructive procedures, such as cyclophotocoagulation (CPC), remain a treatment option, particularly if the eye has little or no useful visual potential. The disadvantage of this surgical option is that it is often difficult to titrate the effect of CPC on the ciliary body and frequently more than one treatment is necessary to attain effective IOP control (16, 17) and excessive laser treatment can lead to hypotony and phthisis. In addition, severe inflammation associated with CPC in these eyes may also lead to hypotony and phthisis.
CONTROL OF THE ISCHEMIC DRIVE FOR NEOVASCULARIZATION
The key to ultimate resolution of the underlying disease process in NVG patients is control of the ischemic drive inducing neovascularization. In many cases, this can be achieved via panretinal photocoagulation (PRP). However, many of these patients have cloudy media, due to a variety of factors such as corneal edema and hyphema, making the fundus view inadequate for placement of PRP. In these cases, medical therapy, as well as anti-angiogenic therapy, as described below, is initiated. A short interval of observation will determine whether or not the view will clear. If it does not, pars plana vitrectomy (PPV) with endolaser (EL) photocoagulation should be strongly considered. Many surgeons will opt for combined GDI placement at the time of PPV and EL, particularly if synechial angle closure is evident or suspected.
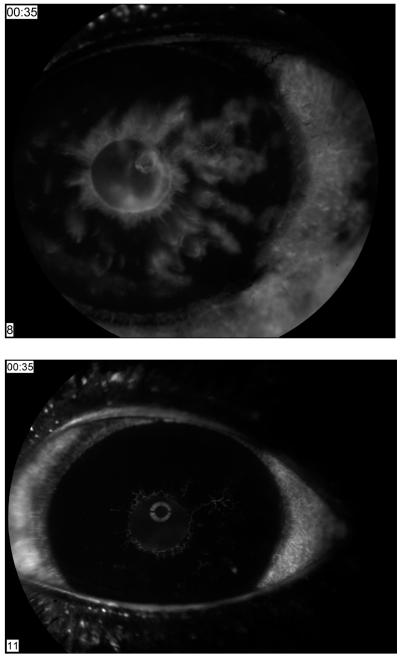
The role of anti-angiogenic agents in the treatment of this disease is currently becoming established. Administration of bevacizumab induces rapid resolution of iris vessels, (5) as demonstrated angiographically. (Figure 3) Published case reports, small case series, and treatment outcome studies of intravitreal bevacizumab for NVG suggested that early diagnosis and treatment of NVG with intravitreal bevacizumab improved NVG outcomes. (6-12) However, bevacizumab-induced regression of neovascularization is often temporary and recurrence is possible, (10) while PRP provides a more permanent reduction of the ischemic angiogenic stimulus.
Figure 3.
Iris fluorscein angiography showing rapid of NVI after administration of Bevacizumab
If bevacizumab is administered while the anterior chamber angle is open at the time of NVG diagnosis and prior to the formation of PAS and angle closure, some have postulated that IOP may be controlled without the need for surgical procedures. (8) Iliev et al reported the use of intravitreal bevacizumab for NVG in six eyes. Three eyes with PAS at the time of NVG diagnosis required treatment with CPC laser to control IOP, while three eyes without PAS did not require CPC. (6) Similarly, Gheith et al reported the use of intravitreal bevacizumab for NVG in six eyes; those eyes with PAS at the time of presentation subsequently required glaucoma surgery for adequate IOP control, while those eyes without PAS required only topical medication to control IOP. (10) Eyes with NVG and open angles have a delayed requirement for GDI by at least six months, when compared with NVG eyes with closed angles. (3) Eyes with NVG and closed angles have minimal IOP lowering response compared with eyes with open angles when NVI is treated with intravitreal bevacizumab, although eyes with closed angles have higher initial IOPs prior to anti-VEGF treatment. (4) In addition, the majority (93%) of these closed angle eyes, even with regression of NVI induced by intravitreal bevacizumab, required glaucoma surgery, compared with seventeen percent of open angle NVG eyes treated similarly. (4)
Several authors have advocated the use of bevacizumab as an adjunct to glaucoma surgery in order to facilitate surgery and improve long-term outcomes. (18-20) Our group undertook a large study of NVG patients, comparing those eyes treated with and without intravitreal bevacizumab. In our study, the cumulative proportion of eyes receiving a GDI was initially less in eyes receiving intravitreal bevacizumab compared with those eyes not treated with bevacizumab; however, the cumulative proportions were similar with longer follow-up (Olmos et al, submitted). This illustrates the importance of prompt, definitive, treatment and careful long-term follow-up in those eyes injected with bevacizumab, regardless of initial angle status and initial IOP lowering. The majority of patients, if followed long enough, require surgical intervention for the control of IOP. Eighty percent of patients in our study ultimately received a glaucoma drainage implant.
The ideal treatment outcome in NVG patients is prevention of synechial angle closure with a combination of angi-angiogenic treatment and PRP. (21, 22) However, this is rarely occurs, as NVG patients often present late in the course of their disease, missing the narrow therapeutic window for prevention of angle closure and the need for glaucoma surgery. In patients with regressed NVA observed gonioscopically, angles may appear open, but transparent residual ghost vessel bodies may be present and form synechial membranes with closed angles that are recalcitrant to IOP treatment and require glaucoma surgery. Thus, close observation of regressed NVA is important for treating incipient glaucoma prior to significant optic nerve damage.
COMPLICATIONS OF NVG TREATMENT
The treatment of neovascular glaucoma can be fraught with complications. Eyes with this condition often present with pain, significantly decreased vision, and marked intraocular inflammation. In addition, neovascular vessels are prone to bleeding with even minor manipulation due to their friable nature.
Intravitreal injections of anti-VEGF agents often result in marked acute IOP elevation, necessitating either pre- or post-injection anterior chamber (AC) paracentesis. Paracentesis can be complicated by hyphema, given the presence of the friable iris vessels. Hyphemas resulting from AC paracentesis are typically managed medically with topical steroids and cycloplegics unless sustained significantly elevated IOP in association with hyphema may cause corneal blood staining.
Surgical intervention, such as the placement of a glaucoma drainage implant, can also lead to hyphema as a result of iris and globe manipulation during surgery and intraocular trauma to neovascular vessels. (Figure 2) Hyphema can impair visualization during surgery and lead to longer operative times, as well as cause blood clots which can occlude the tube tip of a GDI. Our preference is to place superior GDIs in NVG patients to decrease the risk of GDI tube tip occlusion by hyphema. In addition, hyphema can lead to clot formation at the valve junction in valved GDIs that can compromise early IOP control. A recent study found that intravitreal bevacizumab was a useful preparatory step to safely implant an aqueous shunting tube in NVG. (19) Our clinical impression is that administration of anti-VEGF agents pre-operatively (within one week of surgery) reduces such bleeding complications by causing rapid regression of the neovascular vessels, which tend to be more fragile and leaky.
Sustained elevated intraocular pressure during or after surgical intervention such as GDI placement or vitrectomy can “snuff” the already damaged optic nerves of these patients. This is particularly notable in patients who have suffered from years of optic nerve damage due to other types of glaucoma prior to developing NVG. This risk should be discussed with the patient pre-operatively. Aggressive medical management of IOP should also be instituted pre-operatively in order to minimize this potential complication.
CONCLUSIONS
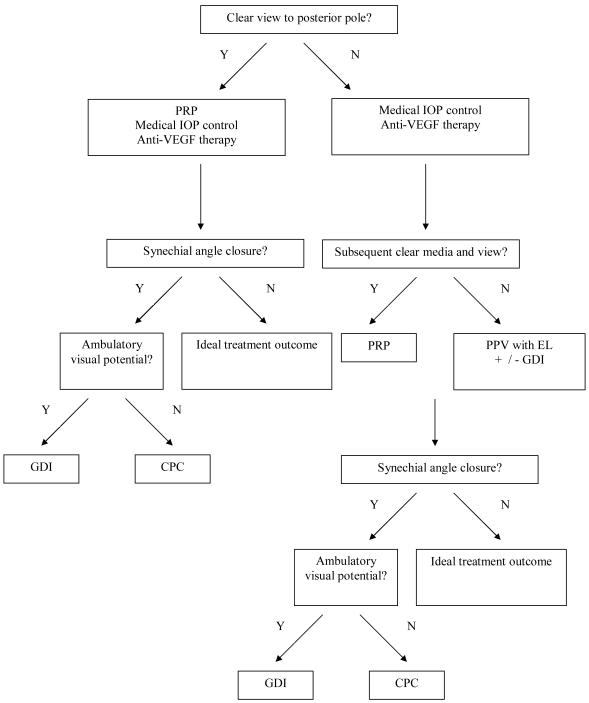
NVG represents a therapeutic challenge. However, by understanding its pathogenesis and applying those principles towards its treatment, NVG can be successfully treated when diligent clinical attention is applied. Figure 4 presents a proposed treatment algorithm for NVG. As guided by evidence-based studies reviewed herein, the current standard of care for NVG at our institution typically includes administration of intravitreal bevacizumab at the time of diagnosis of NVG or prior to glaucoma surgery for NVG, prompt application of PRP if an adequate view of the posterior pole exists, or application of endolaser during PPV (if planned in association with retinal surgery with or without the need for glaucoma surgery), and lowering of IOP medically and via placement of a GDI as necessary.
Figure 4.
Proposed Treatment Algorithm for Neovascular Glaucoma
Acknowledgments
FUNDING RKL is supported by NIH NEI grant EY016775. The Bascom Palmer Eye Institute is supported by an unrestricted research grant from Research to Prevent Blindness and NIH Center Grant EY014801.
Footnotes
The authors have no relevant financial interests in any of the products discussed in this article.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Weiss DI, Shaffer RN, Nehrenberg TR. Neovascular glaucoma complicating carotid-cavernous fistula. Arch Ophthalmol. 1963;69:304. doi: 10.1001/archopht.1963.00960040310007. [DOI] [PubMed] [Google Scholar]
- 2.Allingham RR, Damji K, Freedman S, et al., editors. Shields’ Textbook of Glaucoma. ed 5 Lippincott Williams & Wilkins; Philadelphia: 2005. Glaucomas Associated with Disorders of the Retina, Vitreous, and Choroid; pp. 328–341. [Google Scholar]
- 3.Moraczewski AL, Lee RK, Palmberg PF, et al. Outcomes of treatment of neovascular glaucoma with intravitreal bevacizumab. Br J Ophthalmol. 2009;93:589–593. doi: 10.1136/bjo.2008.151472. [DOI] [PubMed] [Google Scholar]
- 4.Wakabayashi T, Oshima Y, Sakaguchi H, et al. Intravitreal bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to ischemic retinal disease in 41 consecutive cases. Ophthalmology. 2008;115(9):1571–80. doi: 10.1016/j.ophtha.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single bevacizumab (Avastin) injection. Retina. 2006;26:354–6. doi: 10.1097/00006982-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Iliev ME, Domig D, Wolf-Schnurrbursch U, et al. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006;142:1054–6. doi: 10.1016/j.ajo.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 7.Kahook MY, Schuman JS, Noecker RJ. Intravitreal bevacizumab in a patient with neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2006;37:144–6. [PubMed] [Google Scholar]
- 8.Mason JO, III, Albert MA, Jr, Mays A, et al. Regression of neovascular iris vessels by intravitreal injection of bevacizumab. Retina. 2006;26:839–41. doi: 10.1097/01.iae.0000230425.31296.3b. [DOI] [PubMed] [Google Scholar]
- 9.Paula JS, Jorge R, Costa RA, et al. Short-term results of intravitreal bevacizumab (Avastin) on anterior segment neovascularization in neovascular glaucoma. Acta Ophthalmol Scand. 2006;84:556–7. doi: 10.1111/j.1600-0420.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 10.Gheith ME, Siam GA, de Barros DS, et al. Role of intravitreal bevacizumab in neovascular glaucoma. J Ocul Pharmacol Ther. 2007;23:487–91. doi: 10.1089/jop.2007.0036. [DOI] [PubMed] [Google Scholar]
- 11.Chilov MN, Grigg JR, Playfair TJ. Bevacizumab (Avastin) for the treatment of neovascular glaucoma. Clin Exp Ophthalmol. 2007;35:494–6. doi: 10.1111/j.1442-9071.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 12.Yazdani S, Hendi K, Pakravan M, et al. Intravitreal bevacizumab for neovascular glaucoma: a randomized controlled trial. J Glaucoma. 2009;18(8):632–7. doi: 10.1097/IJG.0b013e3181997211. [DOI] [PubMed] [Google Scholar]
- 13.Tsai JC, Feuer WJ, Parrish RK, 2nd, et al. 5-Fluorouracil filtering surgery and neovascular glaucoma. Long-term follow-up of the original pilot study. Ophthalmology. 1995;102(6):887–92. doi: 10.1016/s0161-6420(95)30938-4. [DOI] [PubMed] [Google Scholar]
- 14.Takihara Y, Inatani M, Fukushima M, et al. Trabeculectomy with mitomycin C for neovascular glaucoma: prognostic factors for surgical failure. Am J Ophthalmol. 2009;147(5):912–8. doi: 10.1016/j.ajo.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Park UC, Park KH, Kim DM, et al. Ahmed Glaucoma Valve Implantation for Neovascular Glaucoma after Vitrectomy for Proliferative Diabetic Retinopathy. J Glaucoma. 2010 doi: 10.1097/IJG.0b013e3181f3eb06. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 16.Leszczynski R, Domanksi R, Forminksa-Kapuscik M, et al. Contact transscleral cyclophotocoagulation in the treatment of neovascular glaucoma: a five-year follow-up. Med Sci Monit. 2009;15(3):BR84–7. [PubMed] [Google Scholar]
- 17.Pokroy R, Greenwald Y, Pollack A, et al. Visual loss after transscleral diode laser cyclophotocoagulation for primary open-angle and neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2008;39(1):22–9. doi: 10.3928/15428877-20080101-09. [DOI] [PubMed] [Google Scholar]
- 18.Saito Y, Higashide T, Takeda H, et al. Beneficial effects of preoperative intravitreal bevacizumab on trabeculectomy outcomes in neovascular glaucoma. Acta Ophthalmologica. 2010;88(1):96–102. doi: 10.1111/j.1755-3768.2009.01648.x. [DOI] [PubMed] [Google Scholar]
- 19.Takihara Y, Inatani M, Kawaji T, et al. Combined Intravitreal Bevacizumab and Trabeculectomy with Mitomycin C Versus Trabeculectomy With Mitomycin C Alone for Neovascular Glaucoma. J Glaucoma. 2010 doi: 10.1097/IJG.0b013e3181d9ce12. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 20.Eid TM, Radwan A, El-Manawy W, et al. Intravtireal bevacizumab and aqueous shunting surgery for neovascular glaucoma: safety and efficacy. Can J Ophthal. 2009;44(4):451–6. doi: 10.3129/i09-108. [DOI] [PubMed] [Google Scholar]
- 21.Ciftci S, Sakalar YB, Unlu K, et al. Intravitreal bevacizumab combined with panretinal photocoagulation in the treatment of open angle neovascular glaucoma. Eur J Ophthal. 2009;19(6):1028–33. doi: 10.1177/112067210901900620. [DOI] [PubMed] [Google Scholar]
- 22.Ehlers JP, Spirn MJ, Lam A, et al. Combination intravitreal bevacizumab/panretinal photocoagulation versus panretinal photocoagulation alone in the treatment of neovascular glaucoma. Retina. 2008;28(5):696–702. doi: 10.1097/IAE.0b013e3181679c0b. [DOI] [PubMed] [Google Scholar]