Types of Infections
Hospital-acquired infections are a major challenge to patient safety. It is estimated that in 2002, a total of 1.7 million hospital-acquired infections occurred (4.5 per 100 admissions),1 and almost 99,000 deaths resulted from or were associated with a hospital-acquired infection,1 making hospital-acquired infections the sixth leading cause of death in the United States2; similar data have been reported from Europe.3 The estimated costs to the U.S. health care budget are $5 billion to $10 billion annually.4 Approximately one third or more of hospital-acquired infections are preventable.5
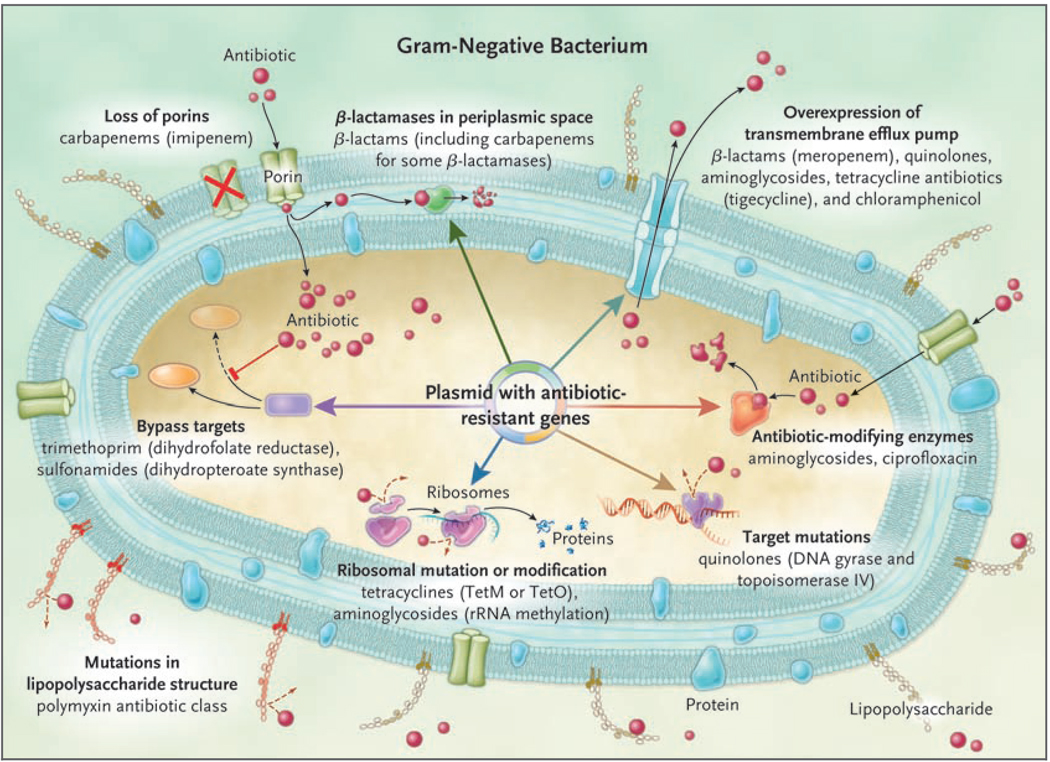
Infections caused by gram-negative bacteria have features that are of particular concern. These organisms are highly efficient at up-regulating or acquiring genes that code for mechanisms of antibiotic drug resistance, especially in the presence of antibiotic selection pressure. Furthermore, they have available to them a plethora of resistance mechanisms, often using multiple mechanisms against the same antibiotic or using a single mechanism to affect multiple antibiotics (Fig. 1). Compounding the problem of antimicrobial-drug resistance is the immediate threat of a reduction in the discovery and development of new antibiotics.6 Several factors have contributed to this decline, including the increasing challenges of screening for new compounds, the high capital costs and long time required for drug development, the growing complexity of designing and performing definitive clinical trials, and the concern about reduced drug longevity due to the emergence of resistance. As a consequence, a perfect storm has been created with regard to these infections: increasing drug resistance in the absence of new drug development.
Figure 1. Mechanisms of Resistance in Gram-Negative Bacteria, and the Antibiotics Affected.
Seven mechanisms of resistance are shown in the gram-negative bacterium, with some being mediated by a mobile plasmid. These mechanisms include the loss of porins, which reduces the movement of drug through the cell membrane; the presence of β-lactamases in the periplasmic space, which degrades the β-lactam; increased expression of the transmembrane efflux pump, which expels the drug from the bacterium before it can have an effect; the presence of antibiotic-modifying enzymes, which make the antibiotic incapable of interacting with its target; target site mutations, which prevent the antibiotic from binding to its site of action; ribosomal mutations or modifications, which prevent the antibiotic from binding and inhibiting protein synthesis; metabolic bypass mechanisms, which use an alternative resistant enzyme to bypass the inhibitory effect of the antibiotic; and a mutation in the lipopolysaccharide, which renders the polymyxin class of antibiotics unable to bind this target. Red spheres indicate antibiotics.
Hospital-acquired infections are most commonly associated with invasive medical devices or surgical procedures. Lower respiratory tract and bloodstream infections are the most lethal; however, urinary tract infections are the most common.
Recent data from the U.S. National Healthcare Safety Network indicate that gram-negative bacteria are responsible for more than 30% of hospital-acquired infections, and these bacteria predominate in cases of ventilator-associated pneumonia (47%) and urinary tract infections (45%).7 In intensive care units (ICUs) in the United States, gram-negative bacteria account for about 70% of these types of infections, and similar data are reported from other parts of the world.8 A range of gram-negative organisms are responsible for hospital-acquired infections, the Enterobacteriaceae family being the most commonly identified group overall (see the table in the Supplementary Appendix, available with the full text of this article at NEJM.org). Unfortunately, multidrug-resistant organisms, including Pseudomonas aeruginosa, Acinetobacter baumannii, and extended-spectrum β-lactamase (ESBL)–producing or carbapenemase-producing Enterobacteriaceae, are increasingly being reported worldwide.
Pneumonia
Hospital-acquired pneumonia is the most common life-threatening hospital-acquired infection, and the majority of cases are associated with mechanical ventilation. Ventilator-associated pneumonia occurs in approximately 10 to 20% of patients who are on ventilators for longer than 48 hours and is associated with significant increases in length of hospital stay, mortality, and costs.9 Gram-negative organisms predominate in hospital-acquired pneumonia, particularly P. aeruginosa, A. baumannii, and the Enterobacteriaceae.8 Between 1986 and 2003, acinetobacter species were the only gram-negative organisms that increased significantly as a cause of pneumonia in ICUs in the United States.8 Unfortunately, the resistance of these organisms to antibiotics, particularly to carbapenems, has posed important therapeutic challenges. In a recent survey, 26.4% of 679 P. aeruginosa isolates and 36.8% of 427 A. baumannii isolates that caused ventilator-associated pneumonia were resistant to carbapenems (imipenem or meropenem).7 Similar data have been reported from other parts of the world, with countries such as Greece reporting rates of carbapenem resistance of up to 85% among ICU isolates.10 Of greatest concern are reports of infections caused by organisms that are resistant to all currently available antibiotics, including the polymyxins.11,12
A more recent clinical entity that physicians need to be aware of is health care–associated pneumonia — that is, cases of pneumonia acquired in the community by patients who have had direct or indirect contact with a health care or long-term care facility and are subsequently hospitalized. Such patients are more likely to have a coexisting illness and to receive inactive empirical antibiotic therapy and are at greater risk for death than patients who have true community-acquired pneumonia.13,14 As a consequence, antibiotics with a broader spectrum of coverage — particularly those with activity against P. aeruginosa, other multidrug-resistant gram-negative bacilli, and drug-resistant Staphylococcus aureus14 — should be considered for patients who have defined risk factors and who present to the emergency room with pneumonia (Table 1).15,16 In order to minimize the overuse of broad-spectrum antibiotics, further research is required to determine the true predictive value of each of these risk factors for resistant bacteria.17 Recent hospitalization or antibiotic exposure and residence in a long-term care facility should be considered the most important risk factors.
Table 1.
Risk Factors for Health Care–Associated Infections and Infection with Drug-Resistant Bacteria.*
| Risk factors for health care–associated infections |
| Hospitalization for ≥2 days in preceding 90 days |
| Residence in a nursing home or long-term care facility |
| Home infusion therapy, including antimicrobial agents |
| Long-term dialysis within 30 days |
| Home wound care |
| Family member with multidrug-resistant pathogen |
| Risk factors for infection with drug-resistant bacteria |
| Antimicrobial therapy in preceding 90 days |
| Current hospitalization for ≥5 days |
| High frequency of antibiotic resistance in the community or in the specific hospital unit |
| Immunosuppression |
Risk factors are from the Infectious Diseases Society of America and the American Thoracic Society guidelines.15
Apart from being associated with increased morbidity and mortality, suspected hospital-acquired pneumonia in the ICU can lead to the inappropriate use of antibiotic drugs, contributing to bacterial drug resistance and increases in toxic effects and health care costs. To optimize the appropriateness of antibiotic use, physicians must be aware of the management paradigms for hospital-acquired pneumonia (Table 2). The diagnosis of ventilator-associated pneumonia remains challenging, with no easily obtained reference standard. Apart from clinical criteria, microbiologic assessment is important to help guide therapy. For patients in whom ventilator-associated pneumonia is suspected, a sample from the lower respiratory tract should be obtained by means of endotracheal aspiration, bronchoalveolar lavage, or a protected specimen brush (depending on the resources available)18,19 for microscopy and culture before antibiotics are administered. Although each sampling method has its limitations, the most important point is to obtain the sample in a timely manner. The alternative techniques appear to be associated with similar outcomes, on the basis of recent systematic reviews.20,21 When the patient is severely ill, the administration of empirical antibiotic therapy should not be delayed on account of the diagnostic procedure.15
Table 2.
Diagnostic Criteria and Management Guidelines for Ventilator-Associated Pneumonia.*
| Diagnostic criteria |
| Presence of a new or progressive infiltrate on chest radiography and two of the following three clinical features: |
| Temperature >38°C (100.4°F) |
| Leukocytosis or leucopenia |
| Purulent respiratory secretions |
| Positive respiratory culture |
| For quantitative cultures, a bacterial density of at least |
| 106 CFU/ml for an endotracheal aspirate |
| 104 CFU/ml for a bronchoalveolar-lavage specimen |
| 103 CFU/ml for a protected-specimen brush |
| For semiquantitative cultures, at least moderate growth of bacteria |
| Key management steps |
| Make the appropriate diagnosis |
| Use local antimicrobial-susceptibility data and the length of the hospital stay before pneumonia developed to determine the most effective empirical antibiotic coverage |
| Reassess the patient and recheck culture results at 48 to 72 hours, with the goal of tailoring antibiotic therapy to the susceptibilities of the cultured bacteria |
| Initiate a short course of therapy (8 days) for most organisms with the exception of nonfermenting gram-negative organisms (e.g., Pseudomonas aeruginosa), for which a course of 15 days is recommended |
| Implement a bundled prevention program for ventilator-associated pneumonia |
CFU denotes colony-forming units.
To assist the treating physician in determining whether a cultured organism signifies colonization or infection, it has been recommended that quantitative cultures be obtained, either by measuring the colony-forming units (CFU) per milliliter or by grading the bacterial growth as light, moderate, or heavy (semiquantified approach). In bronchoalveolar-lavage fluid, a cutoff value of less than 104 CFU per milliliter is more likely to indicate colonization; however, this information needs to be interpreted on the basis of the patient’s clinical state. Quantitative culture results are subject to possible sampling variability, and there is no evidence that quantitative cultures, as compared with qualitative cultures, are associated with reductions in mortality, the length of the ICU stay, the duration of mechanical ventilation, or the need to adjust antibiotic therapy.20 Nevertheless, quantitative cultures are more helpful in differentiating between colonization and true infection and thus are less likely to lead to unnecessary antibiotic therapy. To further improve such differentiation in patients with ventilator-associated pneumonia, promising biomarkers are being studied in combination with clinical and microbiologic factors. These biomarkers include procalcitonin, C-reactive protein, and soluble triggering receptor expressed on myeloid cells (sTREM-1).22,23
Once a diagnosis of pneumonia has been made, empirical antibiotic therapy needs to be tailored to the institution’s microbial ecology and the length of time the patient was in the hospital before pneumonia developed. With a hospital stay of 5 days or longer, as compared with a shorter stay, the patient is at greater risk for infection with more resistant pathogens, and empirical treatment with broad-spectrum antimicrobial agents should be prescribed (see discussion of treatment below). Growing evidence suggests that early, appropriate antibiotic therapy improves outcomes,24,25 and such therapy should therefore be a goal; however, this strategy needs to be coupled with an early reassessment of both diagnosis and therapy, usually within 48 to 72 hours. In the majority of cases, the antibiotic coverage can then be reduced to a more targeted regimen based on the results of respiratory cultures or even discontinued, if an alternative diagnosis is identified.26 When respiratory cultures are not available, therapy needs to be tailored to the most likely causative organisms for the given institution, with close monitoring for clinical failure, recently defined as lack of improvement in the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen and persistence of fever after 3 days of treatment.27
When definitive antibiotic therapy is warranted, a relatively short course (8 days) should be prescribed for patients with uncomplicated ventilator-associated pneumonia who receive appropriate antibiotic therapy initially.28 For patients infected with nonfermenting gram-negative organisms such as P. aeruginosa, however, the rate of relapse is higher with short-course therapy, and thus the longer course of therapy (15 days) should be prescribed. Finally, the importance of preventive measures for ventilator-associated pneumonia deserves specific mention, particularly a bundled approach (Table 3).5 Institutions that adhere to such measures report a significant reduction in the rates of ventilator-associated pneumonia.9
Table 3.
Evidence-Based Guidelines for the Prevention of Hospital-Acquired Infections.*
| Ventilator-Associated Pneumonia | Central Venous Catheter–Associated Bloodstream Infection | Catheter-Associated Urinary Tract Infection |
|---|---|---|
| Follow effective hand-hygiene procedures Educate health care personnel who care for patients on ventilators about the local epidemiology of VAP, risk factors, and outcomes Implement policies and practices for disinfection, sterilization, and maintenance of respiratory equipment according to evidence-based standards29 Provide regular antiseptic oral care according to product guidelines Ensure that patient is in a semirecumbent position, unless contraindicated Use noninvasive ventilation in appropriately selected patients with respiratory failure† Conduct active surveillance for VAP and institute preventive measures In hospitals with suboptimal infection control using basic practices, use an endotracheal tube with in-line and subglottic suctioning for all eligible patients |
Before insertion, educate health care personnel involved with central venous catheterization about prevention of infection Use a catheter-insertion checklist to ensure adherence to infection-prevention practices Follow hand-hygiene procedures before catheter insertion or manipulation Avoid use of the femoral vein in adults Use an all-inclusive catheter kit and maximal sterile-barrier precautions during catheter insertion Use a chlorhexidine-based antiseptic for skin preparation in patients >2 mo of age After insertion, disinfect catheter hubs, needleless connectors, and injection ports before accessing the catheter; remove nonessential catheters For nontunneled catheters in adults, change transparent dressings and perform site care with a chlorhexidine antiseptic every 5 to 7 days (every 2 days for gauze dressings), or more frequently if dressing is soiled, loose, or damp‡ Replace administration sets not used for blood products or lipids at intervals not longer than 96 hr Use antimicrobial ointments for hemodialysis-catheter insertion sites Perform surveillance for bloodstream infection In hospitals with suboptimal infection control using basic practices, bathe ICU patients >2 mo of age with a chlorhexidine preparation daily, use antiseptic-impregnated or antimicrobial-impregnated catheters for adult patients, use chlorhexidine-containing sponge dressings in patients >2 mo of age, and use antimicrobial locks for central venous catheters |
Implement written catheter-care protocols, including guidelines on catheter insertion Insert urinary catheter only when necessary and leave in only as long as indicated Consider other methods for management, including condom catheters or in-and-out catheterization, as appropriate Maintain a sterile, continuously closed drainage system Do not disconnect the catheter and drainage tube unless the catheter must be irrigated Maintain unobstructed urine flow Empty the collecting bag regularly, using a separate collecting container for each patient, and take care not to let the drainage spigot touch the collecting container Cleaning the meatal area with antiseptic solutions is unnecessary; routine hygiene is appropriate Do not routinely use silver-coated or other antibacterial Catheters Do not screen for asymptomatic bacteriuria in catheterized patients Avoid catheter irrigation if possible Do not use systemic antibacterial agents routinely as Prophylaxis |
These guidelines have been adapted from recent guidelines published by professional societies and organizations committed to improving patient safety and quality of care.5 We report only those strategies with grade I quality of evidence (i.e., from one or more properly randomized, controlled trials) or grade II quality of evidence (i.e., from one or more well-designed, nonrandomized clinical trials). ICU denotes intensive care unit, and VAP ventilator-associated pneumonia.
This strategy is considered grade I in the American Thoracic Society guidelines15 but grade III in the guidelines published more recently by the collaborative group of societies and organizations.5
Central venous or arterial catheters should not be routinely replaced.
Bloodstream Infection
Infection of the bloodstream remains a life-threatening occurrence and is most commonly associated with the presence of a central vascular catheter but may also be associated with a gram-negative infection in other areas of the body, such as the lung, genitourinary tract, or abdomen. Approximately 30% of hospital-acquired bloodstream infections in ICUs in the United States are due to gram-negative organisms,8 although this proportion is lower when hospital-wide data are examined.7
Given an adequate portal of entry, almost any gram-negative organism can cause bloodstream infection; however, the most common organisms include klebsiella species, Escherichia coli, enter-obacter species, and P. aeruginosa. As described above for organisms that cause hospital-acquired pneumonia, resistance is an emerging problem, particularly resistance against extended-spectrum cephalosporins and carbapenems. For example, of bloodstream isolates of Klebsiella pneumoniae from hospitals throughout the United States, 27.1% (from 483 isolates tested) were resistant to third-generation cephalosporins and 10.8% (from 452 isolates tested) were resistant to carbapenems.7 Higher rates of resistance are reported from parts of Europe.10
The most recent challenge has been the spread of carbapenemase-producing Enterobacteriaceae.30 The β-lactamase responsible for this phenotype, also known as K. pneumoniae carbapenemase, or KPC, confers reduced susceptibility to all cephalosporins (including cefepime), monobactams (aztreonam), and the carbapenems.30 Carbapenemase-producing Enterobacteriaceae have now been identified in hospitals in at least 20 states in the United States, as well as in other parts of the world, including South America, Israel, China, and, less commonly, Europe.30 The genetic relatedness of the strains responsible for outbreaks within and between countries highlights the importance of strict infection control to prevent ongoing dissemination.31 These β-lactamases are encoded on mobile genetic elements, mostly plasmids and transposons, which probably explains their spread among gram-negative genera. Furthermore, they often coexist with other resistance genes, including the most widespread of the ESBLs (the blaCTX-M-15 gene), aminoglycoside plasmid-mediated quinolone-resistance genes (qnrA and qnrB),30 thus leaving the physician with few therapeutic options. As has been described for the nonfermenting gram-negative organisms, K. pneumoniae strains that are resistant to all currently available antibiotics, including the polymyxins, have been reported.10
As with hospital-acquired pneumonia, delays in the administration of appropriate antibiotic therapy are associated with excess mortality among patients with hospital-acquired bloodstream infection,32 although the data reflect predominantly gram-positive infections. Data on the clinical effect of initial therapy for gram-negative bloodstream infection are more heterogeneous. Empirical antibiotic coverage for gram-negative bacteria should be considered for patients who are immunosuppressed, those in the ICU, those with a femoral catheter, those with gram-negative bacterial infection at another anatomical site (particularly the lung, genitourinary tract, or abdomen), and those with other risk factors for resistant organisms (Table 1). Moreover, patients who present at the hospital with suspected bloodstream infection who have health care–associated risk factors should be treated initially with broad-spectrum empirical antibiotics, pending the results of blood cultures.33 Detailed guidelines for the management of central vascular catheter–related bloodstream infections have recently been published.34
Prevention of bloodstream infections associated with central catheters is of paramount importance. Adherence to evidence-based interventions has proved highly successful (Table 3),35 and hospitals worldwide should be adopting such cost-effective, preventive measures. Evidence is also emerging in support of other interventions, such as the use of catheters impregnated with an antiseptic, an antibiotic, or both36 or the use of chlorhexidine-impregnated dressings37; however, when the described interventions for best practice are adhered to, the cost-effectiveness of these interventions is less clear.
Urinary Tract Infection
Gram-negative organisms predominate in hospital-acquired urinary tract infections, almost all of which are associated with urethral catheterization. After the second day of catheterization, it is estimated that the risk of bacteriuria increases by 5 to 10% per day. The majority of cases of bacteriuria are asymptomatic, and the most effective management is removal of the catheter rather than antibiotic treatment. In rare cases, local and systemic complications ensue, and antibiotic treatment should be initiated for asymptomatic bacteriuria in patients who are about to undergo urologic surgery or implantation of a prosthesis.38 Such therapy should also be considered in immunocompromised patients. Bloodstream infection appears to be a well-defined but rare complication of catheter-associated urinary tract infection.39
Recent U. S. data indicate that E. coli is the most common etiologic gram-negative organism, followed in descending order of frequency by P. aeruginosa, klebsiella species, enterobacter species, and A. baumannii.7 Uropathogenic E. coli strains infect the urinary tract through a range of mechanisms, including specialized adhesins, fimbriae, biofilm, and aversion of host responses.40 The emergence of resistance to quinolones and extended-spectrum cephalosporins remains a considerable challenge, since these agents are often used as first-line therapy. Traditionally, the SHV-type and TEM-type ESBLs have predominated among hospital-acquired organisms, and this is still the case in the United States. The epidemiology of ESBLs is changing, however, and CTX-M–type ESBLs are becoming more common worldwide. In particular, CTX-M-15 is the most widespread, and this β-lactamase has frequently been associated with a uropathogenic E. coli clone known as sequence type 131.41 Unfortunately, the plasmids carrying these ESBL genes often carry resistance determinants targeting fluoroquinolones as well. To reduce the morbidity associated with hospital-acquired urinary tract infections and prevent the dissemination of drug-resistant gram-negative organisms, adherence to evidence-based prevention guidelines is strongly recommended (Table 3). Until further data are available, we do not recommend the use of antibiotic-impregnated or silver-coated urinary catheters.
Treatment Options
The importance of knowing local antimicrobial susceptibility to direct empirical antibiotic therapy cannot be overemphasized. Recommendations regarding empirical therapy for the hospital-acquired infections discussed here and definitive therapy for infections caused by drug-resistant gram-negative organisms are presented in Tables 4 and 5, respectively.
Table 4.
Recommended Empirical Therapy to Cover Gram-Negative Organisms That Cause Hospital-Acquired Infections.*
| Hospital-Acquired Infection | Recommended Therapy and Dosage |
|---|---|
| Hospital-acquired pneumonia (includes VAP and HCAP) | |
| Length of hospital stay <5 days before pneumonia developed† | One of the following regimens: ceftriaxone, 1 g given intravenously every 24 hr; ampicillin–sulbactam, 3 g given intravenously every 6 hr; levofloxacin, 750 mg given orally or intravenously every 24 hr; moxifloxacin, 400 mg given orally or intravenously every 24 hr; or ertapenem, 1 g given intravenously every 24 hr |
| Length of hospital stay ≥5 days before pneumonia developed or diagnosis of HCAP | One of the following antipseudomonal β-lactam regimens: cefepime, 2 g given intravenously every 12 hr; ceftazidime, 2 g given intravenously every 8 hr; piperacillin–tazobactam, 4.5 g given intravenously every 6–8 hr; ticarcillin–clavulanate, 3.1 g given intravenously every 6 hr; meropenem, 1–2 g given intravenously every 8 hr; imipenem, 500 mg given intravenously every 6 hr; doripenem, 500 mg given intravenously every 8 hr or as a 1-hr or 4-hr infusion; or aztreonam, 1 g given intravenously every 8 hr‡ |
| Plus one of the following regimens: ciprofloxacin, 400 mg given intravenously every 8–12 hr; levofloxacin, 750 mg given intravenously every 24 hr; gentamicin or tobramycin, 5–7 mg/kg of body weight given intravenously every 24 hr; or amikacin, 15–20 mg/kg given intravenously every 24 hr | |
| Bloodstream infections (including health care–associated infections) | Same as for hospital-acquired pneumonia |
| Catheter-associated urinary tract infection | One of the following regimens: cefepime, 1 g given intravenously every 12 hr; ceftazidime, 1 g given intravenously every 8 hr; piperacillin–tazobactam, 3.75 g given intravenously every 8 hr; meropenem, 500 mg given intravenously every 8 hr; imipenem, 500 mg given intravenously every 8 hr; aztreonam, 500 mg given intravenously every 8 hr‡; ciprofloxacin, 400 mg given orally or intravenously every 12 hr; or gentamicin, 5 to 7 mg/kg given intravenously every 24 hr |
Therapy for staphylococcus or legionella species, which may also require initial empirical coverage, is not described in this article. These recommendations are based on the treatment of adults with normal renal function.15 HCAP denotes health care–associated pneumonia, and VAP ventilator-associated pneumonia.
These recommendations apply when there are no risk factors for a multidrug-resistant pathogen.
Aztreonam is an alternative for patients who are allergic to β-lactams except when allergy is to ceftazidime, in which case a cross-reaction with aztreonam can occur.
Table 5.
Recommended Definitive Therapy for Serious Infections, Including VAP and Bloodstream Infections, Caused by Drug-Resistant Gram-Negative Bacteria.*
| Drug-Resistant Pathogen | Recommended Therapy |
|---|---|
| Extended-spectrum β-lactamase-producing Enterobacteriaceae | Meropenem, 1–2 g given intravenously every 8 hr; or imipenem, 500 mg given intravenously every 6 hr; doripenem, 500 mg given intravenously every 8 hr or as a 1-hr or 4-hr infusion |
| Carbapenemase-producing Enterobacteriaceae | Colistin, 2.5–5.0 mg of colistin base/kg of body weight/day given in 2 to 4 divided doses† (equivalent to 6.67–13.3 mg of colistimethate sodium/kg /day); or tigecycline, 100 mg given intravenously as a loading dose, then 50 mg given intravenously every 12 hr |
| Carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii | For P. aeruginosa: Colistin as for carbapenemase-producing Enterobacteriaceae For A. baumannii: Colistin as for carbapenemase-producing Enterobacteriaceae; or ampicillin–sulbactam, up to 6 g of sulbactam given intravenously per day; or tigecycline, 100-mg intravenous loading dose, then 50 mg given intravenously every 12 hr‡ Possible alternatives: Extended infusion of meropenem, 1–2 g given as an intravenous infusion over a 3-hr period every 8 hr; of doripenem, 500 mg–1 g given as an intravenous infusion over a 4-hr period every 8 hr; or of imipenem, 1 g given as an intravenous infusion over a 3-hr period every 8 hr; combination therapy with a nontraditional antibiotic, including rifampin, minocycline or doxycycline, or azithromycin§ For pneumonia: Nebulized colistimethate sodium, 1 million to 3 million IU/day in divided doses (diluted in sterile normal saline), administered with a conventional nebulizer; or nebulized aminoglycosides |
The bacteria listed here are often resistant to fluoroquinolones and aminoglycosides; however, these agents can be used if the bacteria are susceptible to them.
This regimen is based on the current parenteral formulation in the United States (Coly-Mycin M Parenteral [Parkdale Pharmaceuticals]).
This regimen would not be recommended for bloodstream infection, owing to low serum drug levels.
Use of these antibiotics is based on in vitro data and animal models and on clinical case reports and studies of small series of patients.
The polymyxins (colistin and polymyxin B) are an older antibiotic class that has seen a resurgence of use in recent years and deserves mention. Discovered in the late 1940s, polymyxins have specificity for lipopolysaccharides on the outer cell membrane of gram-negative bacteria. Organisms inherently resistant to polymyxins include serratia, proteus, Stenotrophomonas maltophilia, Burkholderia cepacia, and flavobacterium. Their use was initially hampered by nephrotoxicity and then rapidly declined with the advent of newer antibiotics. However, this class of antibiotic has been reinstated as a key therapeutic option for carbapenem-resistant organisms, particularly P. aeruginosa, A. baumannii, and carbapenemase-producing Enterobacteriaceae (Table 4). It is still a challenge to determine the appropriate dosage, since the polymyxins were never subjected to the rigorous drug-development process we now expect for new antimicrobial agents.42 Despite in vitro data suggesting that colistin’s antimicrobial activity is dependent on the peak blood concentration and that its effectiveness could be enhanced by once-daily administration, selection for colistin-resistant mutants, regrowth, and increased toxicity in an animal model have been reported with this dosing frequency. 43,44 Therefore, two to four divided doses per day are currently recommended.
Recently licensed agents with activity against gram-negative bacteria include tigecycline, which is a parenteral glycylcycline antibiotic, and doripenem, which is a parenteral carbapenem that appears to have activity similar to that of meropenem. Tigecycline, a minocycline derivative with a broader spectrum of activity, is approved for the treatment of complicated skin, soft-tissue, and intraabdominal infections. In vitro activity of tigecycline against a range of troublesome gram-negative organisms, including ESBL-producing and carbapenemase-producing Enterobacteriaceae, acinetobacter species, and Stenotrophomonas maltophilia, has been reported (P. aeruginosa and proteus species are intrinsically resistant to the drug). Clinical experience with treating these multidrug-resistant bacteria remains limited, however. The urine concentrations of tigecycline are low, so it is not suitable for the treatment of urinary tract infections. Furthermore, it was shown to be inferior to imipenem–cilastatin for the treatment of ventilator-associated pneumonia in a randomized, double-blind trial.45 Given its rapid movement from the bloodstream into tissues after administration, peak tigecycline serum levels are low (0.63 µg per milliliter) with standard dosing (a 100-mg loading dose followed by 50 mg every 12 hours). Thus, its use for bloodstream infection due to organisms with a minimum inhibitory concentration of 1 µg per milliliter or more also remains limited and requires caution.46
There is still much debate about the role of combination therapy versus monotherapy for gram-negative infections. The results of earlier studies and meta-analyses are difficult to interpret, but more recent evidence is starting to clarify this issue. For empirical treatment, combination therapy improves the likelihood that a drug with in vitro activity against the suspected organism is being administered (often defined as appropriate therapy).47 This effect is more pronounced in institutions with a greater prevalence of multidrug-resistant organisms. The antibiotics selected for the combination, however, need to be tailored to local susceptibility data, because the benefits can be lost in the presence of high cross-resistance, such as to fluoroquinolones and third-generation cephalosporins. When the antibiotic susceptibilities of the infecting organism are known, monotherapy and combination therapy have similar outcomes, including rates of emergence of resistance and recurrence of infection.48 Exceptions include monotherapy with aminoglycosides for P. aeruginosa, which is inferior to any other monotherapy regimen, and possibly monotherapy for patients who have cystic fibrosis. Therefore, we recommend institution-tailored combination therapy for the empirical treatment of serious hospital-acquired gram-negative infections, followed by de-escalation to monotherapy once susceptibilities have been determined. Although clinicians have historically preferred dual therapy for serious pseudomonal infections, the data support single-agent therapy as long as an active β-lactam can be chosen.
Other strategies currently used to treat multidrug-resistant gram-negative infections include prolonged infusion (3 to 4 hours) or continuous infusion of β-lactams and aerosolized antibiotics for the treatment of ventilator-associated pneumonia. These strategies are particularly useful for infections caused by multidrug-resistant organisms (Table 5). For example, according to pharmacokinetic and pharmacodynamic data in hospitalized patients, prolonging the infusion of β-lactams such as cefepime, piperacillin–tazobactam, and the carbapenems significantly improves bactericidal target attainment (i.e., time above the minimum inhibitory concentration for at least 50% for cefepime and piperacillin–tazobactam and 40% for the carbapenems), especially for organisms with an elevated minimum inhibitory concentration (8 to 16 µg per milliliter).49 Furthermore, the emergence of resistance has been prevented in in vitro models.50 Clinical data for extended-infusion β-lactams remain sparse, with some retrospective studies showing improved outcomes, but results of prospective trials are less consistent. Nebulized antibiotics such as tobramycin, amikacin, and colistimethate sodium attempt to minimize systemic toxicity and improve drug delivery at the site of infection. For severe or refractory cases of pneumonia or those caused by highly drug-resistant organisms, nebulized antibiotics given as an adjunct to systemic antibiotics should be thought of as a therapeutic option (Table 5). Respiratory toxicity such as bronchospasm has been reported and may be diminished or prevented by the administration of bronchodilators before dosing. Moreover, a recent Food and Drug Administration alert informed physicians about the importance of using aerosolized colistimethate sodium soon after preparation to prevent lung toxicity from the active colistin form. Prospective studies should be focused on determining the clinical benefits and safety of nebulized antibiotics and extended-infusion β-lactams, especially for infections caused by nonfermenting gram-negative bacteria.
Acknowledgments
Dr. Peleg reports receiving consulting fees from Abbott Molecular and Ortho–McNeil–Janssen; and Dr. Hooper, serving on scientific advisory boards for Pfizer and Novexel, receiving consulting fees from Cubist, Johnson & Johnson, and Mutabilis, and receiving lecture fees from Daiichi–Sankyo.
We thank Howard Gold and David Paterson for their critical review of an earlier version of the manuscript.
Footnotes
No other conflict of interest relevant to this article was reported. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 3.Chopra I, Schofield C, Everett M, et al. Treatment of health-care-associated infections caused by Gram-negative bacteria: a consensus statement. Lancet Infect Dis. 2008;8:133–139. doi: 10.1016/S1473-3099(08)70018-5. [DOI] [PubMed] [Google Scholar]
- 4.Stone PW, Hedblom EC, Murphy DM, Miller SB. The economic impact of infection control: making the business case for increased infection control resources. Am J Infect Control. 2005;33:542–547. doi: 10.1016/j.ajic.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29 Suppl 1:S12–S21. doi: 10.1086/591060. [DOI] [PubMed] [Google Scholar]
- 6.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 7.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [Erratum, Infect Control Hosp Epidemiol 2009; 30:107.] [DOI] [PubMed] [Google Scholar]
- 8.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis WR. The Lowbury Lecture: the United States approach to strategies in the battle against healthcare-associated infections, 2006: transitioning from benchmarking to zero tolerance and clinician accountability. J Hosp Infect. 2007;65 Suppl 2:3–9. doi: 10.1016/S0195-6701(07)60005-X. [DOI] [PubMed] [Google Scholar]
- 10.Souli M, Galani I, Giamarellou H. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveill. 2008;13(47):19045. pii. [PubMed] [Google Scholar]
- 11.Paterson DL, Lipman J. Returning to the pre-antibiotic era in the critically ill: the XDR problem. Crit Care Med. 2007;35:1789–1791. doi: 10.1097/01.CCM.0000269352.39174.A4. [DOI] [PubMed] [Google Scholar]
- 12.Valencia R, Arroyo LA, Conde M, et al. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol. 2009;30:257–263. doi: 10.1086/595977. [DOI] [PubMed] [Google Scholar]
- 13.Carratalà J, Mykietiuk A, Fernández-Sabé N, et al. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med. 2007;167:1393–1399. doi: 10.1001/archinte.167.13.1393. [DOI] [PubMed] [Google Scholar]
- 14.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large U.S. database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [Erratum, Chest 2006;129: 831.] [DOI] [PubMed] [Google Scholar]
- 15.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 16.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care-associated pneumonia. Arch Intern Med. 2008;168:2205–2210. doi: 10.1001/archinte.168.20.2205. [DOI] [PubMed] [Google Scholar]
- 18.The Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355:2619–2630. doi: 10.1056/NEJMoa052904. [DOI] [PubMed] [Google Scholar]
- 19.Fagon JY, Chastre J, Wolff M, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia: a randomized trial. Ann Intern Med. 2000;132:621–630. doi: 10.7326/0003-4819-132-8-200004180-00004. [DOI] [PubMed] [Google Scholar]
- 20.Berton DC, Kalil AC, Cavalcanti M, Teixeira PJ. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst Rev. 2008;4:CD006482. doi: 10.1002/14651858.CD006482.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Rea-Neto A, Youssef NC, Tuche F, et al. Diagnosis of ventilator-associated pneumonia: a systematic review of the literature. Crit Care. 2008;12(2):R56. doi: 10.1186/cc6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duflo F, Debon R, Monneret G, Bienvenu J, Chassard D, Allaouchiche B. Alveolar and serum procalcitonin: diagnostic and prognostic value in ventilator-associated pneumonia. Anesthesiology. 2002;96:74–79. doi: 10.1097/00000542-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 24.Kollef KE, Schramm GE, Wills AR, Reichley RM, Micek ST, Kollef MH. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest. 2008;134:281–287. doi: 10.1378/chest.08-1116. [DOI] [PubMed] [Google Scholar]
- 25.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 26.Joffe AR, Muscedere J, Marshall JC, Su Y, Heyland DK. The safety of targeted antibiotic therapy for ventilator-associated pneumonia: a multicenter observational study. J Crit Care. 2008;23:82–90. doi: 10.1016/j.jcrc.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Shorr AF, Cook D, Jiang X, Muscedere J, Heyland D. Correlates of clinical failure in ventilator-associated pneumonia: insights from a large, randomized trial. J Crit Care. 2008;23:64–73. doi: 10.1016/j.jcrc.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 versus 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 29.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 30.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 31.Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother. 2009;53:818–820. doi: 10.1128/AAC.00987-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 33.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 34.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 36.Casey AL, Mermel LA, Nightingale P, Elliott TS. Antimicrobial central venous catheters in adults: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:763–776. doi: 10.1016/S1473-3099(08)70280-9. [DOI] [PubMed] [Google Scholar]
- 37.Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301:1231–1241. doi: 10.1001/jama.2009.376. [DOI] [PubMed] [Google Scholar]
- 38.Tenke P, Kovacs B, Bjerklund Johansen TE, Matsumoto T, Tambyah PA, Naber KG. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents. 2008;31 Suppl 1:S68–S78. doi: 10.1016/j.ijantimicag.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 39.Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch Intern Med. 2000;160:678–682. doi: 10.1001/archinte.160.5.678. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev. 2008;21:26–59. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, et al. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother. 2008;61:273–281. doi: 10.1093/jac/dkm464. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 43.Wallace SJ, Li J, Nation RL, et al. Subacute toxicity of colistin methanesulfonate in rats: comparison of various intravenous dosage regimens. Antimicrob Agents Chemother. 2008;52:1159–1161. doi: 10.1128/AAC.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother. 2008;61:636–642. doi: 10.1093/jac/dkm511. [DOI] [PubMed] [Google Scholar]
- 45.Press release of Wyeth Pharmaceuticals; Collegeville, PA: 2007. Jul 6, [Accessed April 16, 2010]. Wyeth to file for FDA approval of Tygacil for the treatment of patients with community-acquired pneumonia. at http://www.wyeth.com/irj/portal/news/archive?nav=display&navTo=/wyeth_ html/home/news/pressreleases/2007/1184074360162.html.) [Google Scholar]
- 46.Peleg AY, Potoski BA, Rea R, et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother. 2007;59:128–131. doi: 10.1093/jac/dkl441. [DOI] [PubMed] [Google Scholar]
- 47.Garnacho-Montero J, Sa-Borges M, Sole-Violan J, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med. 2007;35:1888–1895. doi: 10.1097/01.CCM.0000275389.31974.22. [DOI] [PubMed] [Google Scholar]
- 48.Heyland DK, Dodek P, Muscedere J, Day A, Cook D. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med. 2008;36:737–744. doi: 10.1097/01.CCM.0B013E31816203D6. [DOI] [PubMed] [Google Scholar]
- 49.Jaruratanasirikul S, Sriwiriyajan S, Punyo J. Comparison of the pharmacodynamics of meropenem in patients with ventilator-associated pneumonia following administration by 3-hour infusion or bolus injection. Antimicrob Agents Chemother. 2005;49:1337–1339. doi: 10.1128/AAC.49.4.1337-1339.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tam VH, Schilling AN, Neshat S, Poole K, Melnick DA, Coyle EA. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:4920–4927. doi: 10.1128/AAC.49.12.4920-4927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]