Equine infectious anemia virus (EIAV) infection and disease
Equine infectious anemia (EIA) is a worldwide disease of Equids that was first identified in 1843. In 1904, the infectious organism that caused EIA was identified as a “filterable agent,” making EIA the first animal disease to be assigned a viral etiology. In the 1970s EIAV was characterized as a retrovirus and assigned to the lentivirus subfamily along with visna virus of sheep and caprine arthritis encephalitis virus of goats [1]. With the discovery of HIV-1 in 1983 and its classification as a lentivirus (based largely on similar morphology and serological cross reactivity with EIAV), there was a rapid serial discovery of related animal lentiviruses, including simian, bovine, and feline immunodeficiency viruses [2]. These animal lentivirus systems have played an important role in elucidating the mechanisms of persistence and pathogenesis, and in evaluating strategies for vaccine control of infection and disease.
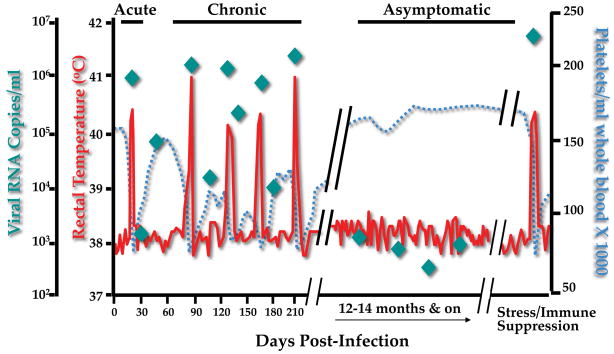
In general, lentiviral disease is characterized by persistent infection that produces progressively degenerative pathologies that lead to death of the host in the absence of therapeutic intervention. As implied by the name “lenti” virus, these diseases are typically slow in their manifestation and can be inapparent for years. In distinct contrast, EIAV infection of horses is characterized by a remarkably dynamic profile distinguished during the first year by a rapid onset of consecutive disease cycles and associated waves of viremia at irregular intervals separated by weeks or months [1–3]. (Figure 1) Disease episodes include clinical manifestations of fever, diarrhea, edema, thrombocytopenia, and anemia, but no evident immune suppression, perhaps due to the exclusive macrophage tropism of EIAV. Following this initial chronic stage of disease, however, most infected horses progress to a long-term asymptomatic state that can last for decades, despite the fact that the horses remain inapparent carriers of highly virulent EIAV. Importantly, natural inapparent carriers are remarkably resistant to frequent exposure of variant EIAV strains that are transmitted by horsefly bites in the field. Thus, EIAV uniquely offers a natural model for the immunologic control of a lentivirus infection and disease, and the virus-specific immune responses present in inapparent carriers appears to offer the enduring broadly protective immunity against virus exposure that has been the elusive goal of AIDS vaccine research for the past 30 years.
Figure 1. Diagram of EIAV clinical disease.
Clinical profile associated with EIAV infection of horses indicating the characteristic stages of EIA. Febrile episodes are defined as rectal temperatures above 39°C (103° F), and thrombocytopenia is defined as platelet levels below 105,000/μl of blood.
Lessons from experimental EIAV infections
The defined nature of the sequential disease episodes and associated waves of viremia during the chronic stage of disease have been shown to result from the progressive evolution of viral envelope (Env) proteins and the chronological selection of antigenic variants that allow temporary escape from host immunity. Detailed analyses of the evolution of EIAV Env quasispecies during serial disease cycles reveal a unique Env population associated with each disease episode [3]. Longitudinal analyses of Env populations reveal an ongoing evolution in inapparent carriers, despite the very low levels of detectable virus replication. These observations of infection and disease reflect a dynamic battle between a constantly evolving virus population and host immune response, where alternate temporary successes by either component during the first year post infection (chronic disease) progresses to a state where the host immune system achieves an effective long-term control of virus replication, even with ongoing variation in the viral reservoir [3].
In the search for a natural model for protective immunity to lentiviral infections to serve as a guide for AIDS vaccine development, the EIAV system offers several important lessons. First, the persistence of EIAV infection is due to random mutations during viral genomic replication that continually present new genomic and protein sequences that can potentially provide a selective advantage for the virus. In this regard, lentiviruses are perpetual genetic engineers with the ability to rapidly overcome factors (antibodies, CTL, etc) that can suppress virus replication [1–3]. This is the bad news. The good news, and the second important lesson, is that the host immune system is evidently able to rapidly recognize new viral variants and mount new immune responses that eliminate the targeted viral variants and eventually establish long-term control. The third lesson is that the establishment of the enduring broadly protective immunity observed in inapparent carriers requires a complex and lengthy maturation of virus-specific immune responses [1, 4]. In this regard, characterization of the progression of Env-specific antibody responses in experimentally infected horses reveals a characteristic evolution of the qualitative properties (neutralization, conformational dependence, and avidity) of Env-specific serum antibodies during the first 6–8 months post infection, despite the fact that the quantitative level (binding titer) of Env-specific serum antibodies is observed at about 2 months post infection. This immune control of EIAV infection and disease requires a necessary maturation of immune responses (humoral and cellular) that is then maintained indefinitely in the inapparent carrier. A similar characteristic maturation of Env-specific serum antibody responses to SIV, SHIV, FIV, and HIV-1 infections has been observed and temporally correlated with the establishment of steady state levels of virus replication in the infected animal or person [5].
Lessons from experimental EIAV vaccines
Paralleling studies in SIV and other animal lentivirus models, a diverse array of EIAV vaccine strategies have been evaluated for their ability to elicit enduring broadly protective immunity against natural routes of viral exposure. These vaccines have included immunizations based on various attenuated virus constructs, inactivated virus particles, protein subunits, DNA vaccines, and live vectors. The results of these various vaccine trials have indicated a remarkable breadth of efficacy, ranging from apparently sterilizing immunity to severe immune enhancement of virus replication and disease in experimentally immunized horses. Thus, the first lesson from EIAV vaccine trials is that virus-specific immune responses are a double edged sword that can contribute to either control or enhancement of virus replication or disease, apparently depending largely on the nature of the Env immunogen used in the vaccine. The second important lesson from EIAV vaccine trails is the general association of the nature of Env-specific immune responses with the protective efficacy of the particular experimental vaccine. In general, immature immune responses were associated with lack of protection or enhancement by the experimental vaccine, while mature immune responses were necessary for protective vaccine immunity. While a reliable immune correlate of vaccine protection remains to be defined, Env-specific antibody avidity has emerged as the most statistically significant indicator of vaccine efficacy [3]. Importantly, similar patterns of vaccine efficacy and immune correlates have been reported for FIV and SIV models, indicating general principles from these animal lentivirus models that may be important considerations in AIDS vaccine development [4].
Among the various immunization strategies, attenuated virus vaccines provide the highest level of vaccine protection, presumably due to the continuous antigen presentation and optimized maturation of host immune responses. Attenuated EIAV vaccines have been the most closely examined to elucidate the requirements for protective vaccine development. The first lesson from these studies is that there is an inverse relationship between the level of virus attenuation and the level of protection, indicating that the attenuated virus must achieve a critical level of virus replication to sufficiently drive the maturation of host immune responses to achieve protection. The second lesson from attenuated viruses is that the best vaccine may achieve an apparent 100% protection from homologous virus challenge, but thorough analyses reveal only 50% protection from challenge virus infection. The third lesson is that the viral Env protein is a predominant determinant of the efficacy of an attenuated EIAV vaccine. There is a direct inverse relationship between degree of variation between vaccine and challenge virus Env proteins, such that all protection is lost when the two Env species differ by about 20%, the average variation observed among clade-specific HIV-1 Env proteins. Finally, the level of protection observed with the vaccine correlates with the level of divergence (not diversity) of the viral Env, indicating the importance of exposure to variant Env species to achieve the most effective vaccine immunity [6].
Translation to HIV/AIDS vaccines
While attenuated HIV-1 vaccines are not a practical option for human AIDS vaccines due to obvious safety concerns, the lesson learned from trials of attenuated animal lentivirus vaccines provide important guidelines for AIDS vaccine development. In this regard, HIV-vaccine strategies should be designed to expose the immune system to diverse Env species over prolonged periods of time to sufficiently drive the maturation of vaccine immune responses to achieve enduring broadly protective immunity. Single shot protein vaccines do not appear to be a viable option. Thus the emphasis should be shifted from protein subunit vaccines, to immunizations using vectors that can provide an extended exposure to critical viral immunogens, including the appropriately engineered Env immunogens that can elicit broadly reactive immunity (humoral and cellular) to diverse strains of HIV-1. In light of the ongoing evolution of HIV-1 populations, it is likely that control of HIV-1 infection and disease will require periodic updates of vaccine Env immunogens and re-immunizations, as currently performed for influenza virus vaccines. While immune control of evolving HIV populations may be a biological horse race, the EIAV system suggests that the immune system can indeed be prompted to win this critical contest.
Bibliography
- 1.Craigo JK, Montelaro RC. Encyclopedia of Virology. 3. Vol. 2. Academic Press; 2008. Equine Infectious Anemia Virus (Retroviridae) pp. 167–174. [Google Scholar]
- 2.Craigo JK, Leroux C, Montelaro RC. Latent Infection by Ungulate Lentiviruses. In: Brown A, editor. Latent Infection by HIV and Other Lentiviruses: New Approaches and Treatment Challenges. Transworld Research Network; 2008. pp. 1–20. [Google Scholar]
- 3.Craigo JK, Montelaro RC. EIAV envelope diversity: shaping viral persistence and encumbering vaccine efficacy. Curr HIV Res. 2010;8:81–86. doi: 10.2174/157016210790416398. [DOI] [PubMed] [Google Scholar]
- 4.Craigo JK, Montelaro RC. Lentivirus Tropism and Disease. In: Desport M, editor. Lentiviruses and Macrophages: Molecular and Cellular Interactions. Horizon Academic Press; 2010. pp. 1–23. [Google Scholar]
- 5.Montelaro RC, Cole KS, Hammond SA. Maturation of immune responses to lentivirus infection: implications for AIDS vaccine development. AIDS Res Hum Retroviruses. 1998;14 (Suppl 3):S255–S259. [PubMed] [Google Scholar]
- 6.Craigo JK, Barnes S, Cook SJ, Issel CJ, Montelaro RC. Divergence, not diversity of an attenuated equine lentivirus vaccine strain correlates with protection from disease. Vaccine. 2010;28:8095–8104. doi: 10.1016/j.vaccine.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]