Abstract
West syndrome (WS) is associated with diverse etiological factors. This fact has suggested that there must be a ‘final common pathway’ for these etiologies, which operates on the immature brain to result in WS only at the maturational state present during infancy. Any theory for the pathogenesis of WS has to account for the unique features of this disorder. For example, how can a single entity have so many etiologies? Why does WS arise only in infancy, even when a known insult had occurred prenatally, and why does it disappear? Why is WS associated with lasting cognitive dysfunction? And, importantly, why do these seizures – unlike most others – respond to treatment by a hormone, ACTH? The established hormonal role of ACTH in human physiology is to function in the neuroendocrine cascade of the responses to all stressful stimuli, including insults to the brain. As part of this function, ACTH is known to suppress the production of corticotropin releasing hormone (CRH), a peptide that is produced in response to diverse insults and stressors.
The many etiologies of WS all lead to activation of the stress response, including increased production and secretion of the stress-neurohormone CRH. CRH has been shown, in infant animal models, to cause severe seizures and death of neurons in areas involved with learning and memory. These effects of CRH are restricted to the infancy period because the receptors for CRH, which mediate its action on neurons, are most abundant during this developmental period. ACTH administration is known to inhibit production and release of CRH via a negative feedback mechanism. Therefore, the efficacy of ACTH for WS may depend on its ability to decrease the levels of the seizure-promoting stress-neurohormone CRH.
This CRH-excess theory for the pathophysiology of WS is consistent not only with the profile of ACTH effects, but also with the many different ‘causes’ of WS, with the abnormal ACTH levels in the cerebrospinal fluid of affected infants and with the spontaneous disappearance of the seizures. Furthermore, if CRH is responsible for the seizures, and CRH-mediated neuronal injury contributes to the worsened cognitive outcome of individuals with WS, then drugs which block the actions of CRH on its receptors may provide a better therapy for this disorder.
Keywords: (ACTH), Corticotropin, (CRF), Corticotropin releasing hormone, Seizures, Melanocortin receptors, Glucocorticoid receptors, Amygdala, Hippocampus, Stress, Development
1. Introduction
West syndrome (WS) is an age-specific disorder of brain excitability, with diverse genetic, teratogenic, perinatal and postnatally acquired etiological factors. Indeed, the characteristic aspects of this disorder, which help to distinguish it from most other epilepsies are: (1) the large number and variability of the predisposing factors, and (2) the fact that regardless of the time of onset of the provoking etiology (i.e. conception, intrauterine, prenatal, perinatal or postnatal), WS commence at a distinct developmental age (usually during the third to seventh postnatal month). These observations have led to the notion that there must be a ‘final common pathway’ for the etiologies, leading to WS; in addition, this final common pathway must be operative only during the state of brain maturation that occurs during infancy.
Theories for the mechanisms of the development of WS have included those invoking abnormal immune function, brainstem dysfunction [1], developmental arrest [2] and cortical microdysplasia [3,4]. Availability of new imaging modalities has revealed many more WS infants with brain malformations, but dysgenesis has also been described in autopsied brains of normal individuals [5]. Indeed, the majority of infants with symptomatic WS have etiologies that do not involve cortical dysplasias or other structural anomalies, such as infections, asphyxia or metabolic and chromosomal/genetic abnormalities.
Pathogenetic theories for WS must account for most, if not all, of the unique features of this disorder. For example, how can a single entity have so many etiologies? Why does WS arise only in infancy, even when the known provoking insult had occurred prenatally? Why does WS disappear upon further brain maturation? Why is WS associated with lasting cognitive dysfunction, and why do these seizures – unlike most others – respond to high doses of ACTH (in 86–88% of cases [6]).
2. Response to hormones as a clue to the pathophysiology of WS
The distinctive efficacy of ACTH for this seizure disorder has been a focus of intense speculation vis a vis the potential mechanisms of action of this hormone. It has commonly been considered that understanding the mechanisms of ACTH efficacy for WS may provide critical clues to the understanding of WS itself. For example, ACTH may accelerate central nervous system (CNS) myelination and dendritic formation, and thus may shorten a hypothetical period of vulnerability to WS [2]. ACTH may also act as a direct anticonvulsant, via GABAergic or other mechanisms [7]. However, it is the hormonal actions of ACTH (as opposed to other potential effects) that have been shown to be necessary for efficacy, since analogs of ACTH without hormonal effects were not helpful for WS [8,9]. In addition, the profile of pharmacokinetic actions of ACTH has been quite atypical for a straightforward anticonvulsant: response has typically been rapid (median response time was 2 days in a controlled study [6]); the response has been ‘all-or-none’, with complete suppression of all spasms, and often the response to ACTH has been permanent, even upon withdrawal of the hormone. All these are not consistent with conventional anticonvulsant properties [1,6,10,11].
3. The many etiologies of WS do involve final common pathways which include ACTH and steroid hormones
WS, at least the symptomatic cases, occur in the context of an insulted and stressed developing brain [10]. The many etiologies of WS all lead to activation of the stress response, including the stress-neurohormone corticotropin releasing hormone (CRH). CRH has been shown, in infant animal models, to cause severe seizures and death of neurons in areas involved with learning and memory [12,13]. These effects of CRH are largely restricted to the infancy period because the receptors for CRH, which mediate its actions on neurons, are most abundant during this developmental period [14,15]. The mechanisms by which CRH increases excitability of limbic neurons have been examined in vitro e.g. [16,17], and involve a suppression of after-hyperpolarization and a potentiation of glutamatergic neurotransmission. Thus, the possibility that WS results from ‘excessive’ levels of CRH in limbic synapses, which are a result of activation of the production and secretion of CRH as part of the CNS stress-response to the etiologies of WS has been suggested [10,11,15].
4. Infants with WS have abnormal ACTH and steroid hormone cerebrospinal fluid (CSF) levels
Data from human infants with WS support a disruption of the CRH-ACTH stress cascade in the brains of these infants. High brain CRH levels are expected to reduce CSF levels of ACTH and of steroids. This is due to the fact that chronic activation of CRH receptors by CRH leads to their desensitization [18], which decreases ACTH release. Indeed, several groups have independently reported reduced ACTH levels in CSF of WS patients, compared with age matched controls [19–22]. These data are consistent with enhanced levels of endogenous CRH in brains of infants with WS. As will be discussed below, increased CRH levels at brain synapses, particularly in hippocampus and amygdala, should promote increased excitability, potentially leading to the chronically abnormal neuronal activity (hypsarrhythmia) and to the clinical spasms characteristic of WS.
5. Excess CRH provokes limbic seizures involving amygdala and hippocampus
Studies of mechanisms of WS in human infants are confounded by the inability to directly study brains of infants who are experiencing WS. Autopsied brains may reflect also the pathology of the underlying provoking etiology. In addition, tools for functional evaluation of the processes causing WS or those involved in the abnormal electroencephalogram (EEG) or the spasms, are limited. Therefore, attempts have been made to use immature animals to model this disorder. To test the possibility that excess CRH (endogenously produced by stressful insults to the brain) increases excitability and may lead to seizures, the immature rat was used [12,15]. CRH is chemically identical in rat and human, and the regulation of and receptors for the peptide are exceptionally similar. In addition, much is known about the distribution and regulation of CRH in the infant rat [23–27] particularly in response to stressful insults. It should be noted that correlation of the state of brain maturation of human infants and neonatal rats can only be estimated [28], but in general, the first 2 postnatal weeks in the rat approximate the neonatal and infancy period in the immature human.
In an immature rat, particularly during the stage of brain development that is comparable to that of human infants, administration of minute amounts of CRH directly into the CSF causes prolonged and severe seizures [12,15,29]. These seizures involve activation of CRH receptors in amygdala and hippocampus. Therefore, it is reasonable to assume that increased levels of endogenous CRH in amygdala and hippocampus would also increase excitability in the amygdala–hippocampal limbic circuit. Interestingly, CRH is highly expressed in both immature and adult central nucleus of the amygdala [30,25]. Recently, robust expression of CRH in hippocampal neurons of the immature rat has been demonstrated as well [23,24], although levels of CRH in mature hippocampus are rather low [31]. In addition, recurrent stress has been shown to increase CRH levels in the amygdala of the infant rat [25], and some stresses also increase CRH expression in immature hippocampus [27]. Therefore, the following scenario is proposed: The many etiologies of WS share the common characteristic of being ‘stressful’ to the immature brain, in the sense of activating the intrinsic stress-response. While stress does not increase CRH synthesis in the neonate [26], CRH levels in amygdala, and potentially in hippocampus, would be enhanced in the infant. This would lead to excess activation of CRH receptors, which are particularly abundant during infancy in amygdala and hippocampus (see above), and to hyperexcitability and seizures. Drastic reduction in the abundance of CRH receptors, occurring with maturation, would be responsible for the reduction in this peptide-mediated hyperexcitability later in life [15].
6. The hormonal effects of ACTH – acting via adrenal steroids – modulate excitability via altering CRH expression
Earlier studies have focused on the hormonal action of ACTH, specifically its induction of steroid release, as the key mechanism of its efficacy for WS. This view was supported by several facts. First, steroids by themselves are effective in a significant portion of infants with WS. In addition, ACTH fragments that did not release steroids were not effective for WS. (It is now known, however, that these fragments also do not activate the ACTH/melanocortin receptors in the brain, see below.) While the full spectrum of the mechanisms of action of steroids in ameliorating WS is unknown (see for example, Refs. [32,33]), these hormones reduce CRH expression in some brain regions, notably in brainstem [34], a region suspected to be involved in WS [1]. However, steroids do not reduce CRH levels in limbic regions such as amygdala and hippocampus.
7. ACTH may also act directly, independent of steroids, to modulate excitability by suppressing the production of CRH in limbic regions
The possibility that ACTH would act directly on CNS neurons is supported by the fact that ACTH is found in the brain (in addition to its well-known presence, in large quantities, in the anterior pituitary). For example, neurons containing ACTH have been localized to a number of CNS regions, particularly the hypothalamus, and ACTH-immunoreactive cell bodies or fibers have also been described in amygdala, cerebral cortex, brainstem and cerebellum [35]. In contrast to pituitary ACTH, the functions of CNS–ACTH have not been well defined. Evidence from both human and animal studies has suggested that CNS–ACTH may function as a neurotransmitter or neuromodulator [36,37]. Indeed, central physiological roles for ACTH, including modulation of learning and memory processes and facilitation of arousal states have been suggested, but the mechanisms for these actions of ACTH have remained unclear [36,37]. Recently, the ‘cellular machinery’ through which ACTH may directly influence neurons has been uncovered: specific and novel receptors, the melanocortin receptor family consisting of several members have been shown to bind ACTH [38,39].
In specific brain regions, such as the excitable, seizure-prone amygdala, ACTH and CRH are found in close proximity. For example, ACTH-immunoreactive fibers have been demonstrated in the central nucleus of the amygdala, a major location of CRH-expressing neurons. This is consistent with interactions between the ACTH- and CRH-expressing neuronal systems [40,18,41]. As mentioned above, CRH production in the central nucleus is activated when diverse stressors or brain insults induce the neuroendocrine stress cascade. In view of these facts, the possibility that ACTH may act to suppress stress-induced CRH expression in the central nucleus of the amygdala, and the resulting reduced excitability [12,6] has been suggested. This process may apply not only to the rat model, but may provide a potential mechanism for the efficacy of ACTH in WS.
8. Evidence for direct effects of ACTH to suppress CRH production in the amygdala
Using infant rats, ACTH, in the form and doses (80 IU/kg) typically used for therapy of WS in the United States (ACTHARGEL, Rhone-Poulenc Rorer, Collegeville, PA), was administered into the peritoneal cavity. These high doses were used not only because of results of clinical trials [6,42], but also because of the known limited blood brain barrier penetration of this hormone [43,44]. The effects of ACTH in the presence and in the absence of endogenous steroids, and with blockade of steroid or ACTH/melanocortin receptors on the levels of CRH expression in the amygdala were examined in infant rats. In addition, ACTH4–10, an analog that binds melanocortin receptors but does not induce steroid secretion from the adrenal, was also tested, and infused directly into the cerebral ventricles [45]. Additional experiments blocked steroid receptors using RU 38486 to distinguish direct action of ACTH from those related to ACTH-induced steroid secretion. Finally, melanocortin receptors were blocked using a specific antagonist (SHU9119, courtesy Dr K. Yagaloff, Roche).
In essence, administration of ACTH down-regulated CRH expression in the central nucleus significantly [45]. Steroids were not required for the ACTH-induced down-regulation of CRH expression because it occurred also in the absence of endogenous steroids: in adrenalectomized rats –as in intact littermates – ACTH resulted in a significant suppression of CRH expression. It should be noted that adrenalectomy – eliminating steroids and the consequent negative feedback on ACTH secretion – resulted in high levels of intrinsic, endogenous ACTH (Fig. 1). These ACTH levels, by themselves, were sufficient to significantly suppress CRH expression. The effect of ACTH on CRH expression in amygdala was central: similar to systemically administered ACTH, the analog ACTH4–10 infused into the CSF resulted in a significant down-regulation of CRH expression, despite the fact that doses of the ACTH4–10 was relatively small and was therefore unlikely to reach the adrenal when given centrally. In addition, this ACTH analog does not stimulate corticosterone secretion from the adrenal and did not raise plasma steroid levels in the experiments described here. Therefore, it is most likely that ACTH4–10 and ACTH itself reduce CRH expression in amygdala via a central, rather than a steroid-mediated peripheral mechanism.
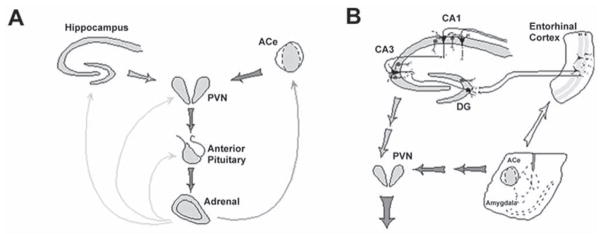
Fig. 1.
The neuroendocrine (A) and limbic (B), stress-activated CRH–ACTH-steroid loops. (A) Stress-conveying signals rapidly activate CRH-expressing neurons of the central nucleus of the amygdala (ACe). Rapid CRH release in the ACe activates CRH-expression in the hypothalamic paraventricular nucleus (PVN) to secrete CRH into the hypothalamo-pituitary portal system, inducing ACTH and glucocorticoid (steroid) secretion from the pituitary and adrenal, respectively. Steroids exert a negative feedback on the production of CRH in the hypothalamus (directly and via the hippocampus), yet activate CRH gene expression in the amygdala, potentially promoting further CRH release and seizure-promoting actions in this region. (B) CRH-expressing GABAergic interneurons (dark cells) in the principal cell layers of the hippocampal CA1, CA3 and the dentate gyrus (DG) are positioned to control excitability of the pyramidal and granule cells, respectively. These neurons may be influenced by stress-evoked release of CRH from the ACe, via connections in the entorhinal cortex. For both panels, thick and thin arrows denote established or putative potentiating and inhibitory actions, respectively. Arrows do not imply mono-synaptic connections. (Modified and published with permission, from Baram and Hatalski [15]).
Indeed, blocking melanocortin receptors demonstrated that their occupancy and activation was required for the action of ACTH on amygdala. In contrast, when RU 38486, a glucocorticoid receptor blocker was given to intact animals together with ACTH, it failed to block the effects of ACTH administration on CRH expression in amygdala. These data indicate that activation of melanocortin receptors, but not of glucocorticoid receptors, is required for the actions of ACTH on CRH expression in the central nucleus of the amygdala.
9. What is the relevance of experimental approaches to the treatment of WS?
ACTH, particularly in high doses, has been shown to provide efficacy for WS (86–88%), that is greater than that of steroids. Indeed, controlled prospective and blinded studies demonstrated a clear superiority of high doses of ACTH over steroids [6,46]. These clinical data raise several issues: first, if ACTH acts via steroids, then maximal doses of both agents should have similar effects; second, once doses of ACTH that suffice to maximally release endogenous steroids are used, then higher doses should not have additional benefits. The enhanced potency of ACTH compared with steroids, and the superiority of extremely high doses of ACTH compared with lower ones, are consistent with direct, steroid-independent actions of ACTH within the CNS. The studies described here demonstrate direct actions of systemically or centrally administered ACTH on the brain that are independent of adrenal steroids. They also suggest that ACTH-induced reduction of CRH expression in amygdala may provide a mechanism for the efficacy of ACTH in WS.
These studies also help explain the clinical finding by many authors that very high doses of the gel-form of ACTH are superior to lower doses. In contrast, the Japanese and European formulations may act at much lower doses. The native ACTH penetrates the blood–brain barrier poorly, and a depot form may do so even worse. If the action of ACTH require that the hormone reach and interact the brain cells, then very high doses may be required, so that the 0.1–1% penetration rate will still lead to clinical effects [43,44].
10. The direct actions of ACTH on neurons suggest new medications for treating WS
The effects of ACTH on amygdala neurons involved activation of specific melanocortin receptors. Melanocortin receptors, a family of transmembrane G-protein coupled receptors, have been found in diverse brain regions, particularly those associated with effects of ACTH. For example, in substantia nigra they may mediate ACTH-induced grooming, whereas in amygdala they may be involved in the effects of ACTH on learning and memory. The data reviewed in this chapter demonstrate that ACTH may activate melanocortin receptors to reduce the production of a pro-convulsant compound (CRH). This may suggest that other molecules that activate these receptors may be useful as anticonvulsants for WS and other ACTH-responsive epilepsies.
Interestingly, the (4–10) fragment of ACTH that does not release adrenal steroids also led to the depression of CRH expression. Earlier clinical studies have shown that shorter fragments (4–9) ACTH that failed to release adrenal steroids were not helpful for WS [8,9]. Currently available information explains this apparent discrepancy: new information about the binding of ACTH fragments to melanocortin receptors shows that while the 4–10 fragment used here activates these receptors, the 4–9 fragment does not [39,47]. Again, these data are consistent with activation of melanocortin receptors as the mechanism for the observed effects of ACTH on amygdala neurons.
11. Conclusions
WS is a disorder with innumerable etiologies, all of which seem to act as insults activating the intrinsic responses of the developing brain to stress. These responses include increased production and secretion of a highly excitatory peptide, CRH. ACTH acts to reduce CRH expression in different brain regions via two mechanisms: the first is the ‘conventional’ activation of steroid release, and the second involves direct actions on neurons, by activation of melanocortin receptors, leading to decreased production of the pro-convulsant peptide CRH.
Importantly, the data reviewed here point out potential new therapeutic agents for WS which either activate melanocortin receptors or block the seizure-promoting action of CRH.
Acknowledgments
This work is supported by NIH RO1 NS28912 and R41 HD34975 (T.Z.B.) and a System-wide University of California Biotechnology oriented predoctoral award (K.L.B.). The authors thank M. Hinojosa for excellent editorial assistance.
References
- 1.Hrachovy RA, Frost JD. Infantile spasms. Pediatr Clin North Am. 1989;36:311–329. doi: 10.1016/s0031-3955(16)36651-2. [DOI] [PubMed] [Google Scholar]
- 2.Riikonen R. Infantile spasms: some new theoretical aspects. Epilepsia. 1983;24:159–168. doi: 10.1111/j.1528-1157.1983.tb04875.x. [DOI] [PubMed] [Google Scholar]
- 3.Vinters HV, Fisher RS, Cornford ME. Morphological substrates of infantile spasms: studies based on surgically resected cerebral tissue. Childs Nerv Syst. 1992;8:8–17. doi: 10.1007/BF00316556. [DOI] [PubMed] [Google Scholar]
- 4.Dalla Bernadina B, Dulac O. Introduction to etiology. In: Dulac O, Chugani H, Dalla Bernadina B, editors. Infantile spasms and West syndrome. London: W.B. Saunders; 1994. pp. 166–171. [Google Scholar]
- 5.Lyon G, Gastaut H. Considerations on the significance attributed to unusual cerebral histological findings recently described in eight patients with primary generalized epilepsy. Epilepsia. 1985;26:365–367. doi: 10.1111/j.1528-1157.1985.tb05664.x. [DOI] [PubMed] [Google Scholar]
- 6.Baram TZ, Mitchell WG, Tournay A, Snead OC, III, Hanson RA, Horton EG. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97:375–379. [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes GL, Weber DA. Effects of ACTH on seizure susceptibility in the developing brain. Ann Neurol. 1986;1:82–88. doi: 10.1002/ana.410200114. [DOI] [PubMed] [Google Scholar]
- 8.Pentella K, Bachman DS, Sandman CA. Trial of an ACTH 4–9 analogue in children with intractable seizures. Neuropediatrics. 1982;13:59–62. doi: 10.1055/s-2008-1059598. [DOI] [PubMed] [Google Scholar]
- 9.Willig RP, Lagenstein I. Use of ACTH fragments in children with infantile spasms. Neuropediatrics. 1982;13:55–58. doi: 10.1055/s-2008-1059597. [DOI] [PubMed] [Google Scholar]
- 10.Baram TZ. Pathophysiology of massive infantile spasms: perspective on the putative role of the brain adrenal axis. Ann Neurol. 1993;33:231–236. doi: 10.1002/ana.410330302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baram TZ, Mitchell WG, Brunson K, Haden E. Infantile spasms: hypothesis-driven therapy and pilot human infant experiments using corticotropin-releasing hormone receptor antagonists. Dev Neurosci. 1999;21:281–289. doi: 10.1159/000017407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baram TZ, Hirsch E, Snead OC, III, Schultz L. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. NeuroReport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Dev Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- 17.Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:78–84. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauger RL, Irwin MR, Lorang M, Aguilera G, Brown MR. High intracerebral levels of CRH result in CRH receptor downregulation in the amygdala and neuroimmune desensitization. Brain Res. 1993;616:283–292. doi: 10.1016/0006-8993(93)90219-d. [DOI] [PubMed] [Google Scholar]
- 19.Nalin A, Facchinetti F, Galli V, Petraglia F, Storchi R, Genazzani AR. Reduced ACTH content in cerebrospinal fluid of children affected by cryptogenic infantile spasms with hypsarrhythmia. Epilepsia. 1985;26:446–449. doi: 10.1111/j.1528-1157.1985.tb05678.x. [DOI] [PubMed] [Google Scholar]
- 20.Baram TZ, Mitchell WG, Snead OC, III, Horton EJ, Saito M. Brain adrenal axis hormones are altered in the CSF of infants with massive infantile spasms. Neurology. 1992;42:1171–1175. doi: 10.1212/wnl.42.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baram TZ, Mitchell WG, Snead OC, III, Horton EJ. Corticotropin and cortisol are increased in the cerebrospinal fluid of infants with massive infantile spasms. Pediatr Neurol. 1995;13:108–110. doi: 10.1016/0887-8994(95)00121-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heiskala H. CSF ACTH and beta-endorphin in infants with West syndrome and ACTH therapy. Brain Dev. 1997;5:339–342. doi: 10.1016/s0387-7604(97)00026-0. [DOI] [PubMed] [Google Scholar]
- 23.Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin-releasing hormone (CRH)-containing neurons in the immature rat hippocampal formation: light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Bender RA, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatio-temporal analysis. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatalski CG, Brunson KL, Tantayanubutr B, Chen Y, Baram TZ. Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin-releasing hormone expression in the immature rat. Neuroscience. 2000;101:571–580. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlieb A, Keydar Y, Epstein HT. Rodent brain growth stages: an analytical review. Biol Neonate. 1977;32:166–176. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- 29.Baram TZ, Schultz L. Corticotropin-releasing hormone is a rapid and potent convulsant in the infant rat. Dev Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 31.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 32.Karst H, Wadman WJ, Joels M. Corticosteroid receptor-dependent modulation of calcium currents in rat hippocampal CA1 neurons. Brain Res. 1994;649:234–242. doi: 10.1016/0006-8993(94)91069-3. [DOI] [PubMed] [Google Scholar]
- 33.Pavlides C, Kimura A, Magarinos AM, McEwen BS. Hippocampal homosynaptic long-term depression/depotentiation induced by adrenal steroids. Neuroscience. 1995;68:379–385. doi: 10.1016/0306-4522(95)94332-s. [DOI] [PubMed] [Google Scholar]
- 34.Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilcher WH, Joseph SA. Co-localization of CRF-ir perikarya and ACTH-ir fibers in rat brain. Brain Res. 1984;299:91–102. doi: 10.1016/0006-8993(84)90791-1. [DOI] [PubMed] [Google Scholar]
- 36.Pranzatelli MR. On the molecular mechanism of adrenocorticotrophic hormone in the CNS: neurotransmitters and receptors. Exp Neurol. 1994;125:142–161. doi: 10.1006/exnr.1994.1018. [DOI] [PubMed] [Google Scholar]
- 37.de Wied D. Behavioral effects of neuropeptides related to ACTH, MSH, and βLPH. Ann NY Acad Sci. 1977;297:263–275. doi: 10.1111/j.1749-6632.1977.tb41859.x. [DOI] [PubMed] [Google Scholar]
- 38.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:543–546. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 39.Adan RAH, Gispen WH. Brain melanocortin receptors: from cloning to function. Peptides. 1997;8:1279–1287. doi: 10.1016/s0196-9781(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 40.Wu HC, Chen KY, Lee WY, Lee EHY. Antisense oligonucleotides to corticotropin-releasing factor impair memory retention and increase exploration in rats. Neuroscience. 1997;78:147–153. doi: 10.1016/s0306-4522(96)00533-7. [DOI] [PubMed] [Google Scholar]
- 41.Joseph SA, Pilcher WH, Knigge KM. Anatomy of the corticotropin-releasing factor and opiomelanocortin systems of the brain. Fed Proc. 1985;44:100–107. [PubMed] [Google Scholar]
- 42.Snead OC, III, Benton JW, Hosey LC, Swann JW, Spink D, Martin D, et al. Treatment of infantile spasms with high-dose ACTH: efficacy and plasma levels of ACTH and prednisone. Neurology. 1989;39:1027–1031. doi: 10.1212/wnl.39.8.1027. [DOI] [PubMed] [Google Scholar]
- 43.Nicholson WE, Liddle RA, Puett D, Liddle GW. Adrenocorticotropic hormone biotransformation, clearance, and catabolism. Endocrinology. 1978;103:1344–1351. doi: 10.1210/endo-103-4-1344. [DOI] [PubMed] [Google Scholar]
- 44.Mezey E, Palkovitz M, de Kloet ER, Verhoef J, de Wied D. Evidence for pituitary-brain transport of a behaviorally potent ACTH analog. Life Sci. 1978;22:831–838. doi: 10.1016/0024-3205(78)90606-9. [DOI] [PubMed] [Google Scholar]
- 45.Brunson KL, Khan N, Eghbal-Ahmadi M, Baram TZ. ACTH acts directly on amygdala neurons to down-regulate corticotropin releasing hormone gene expression. Ann Neurol. 2001;29:304–312. [PMC free article] [PubMed] [Google Scholar]
- 46.Hrachovy RA, Frost JD, Kellaway P, Zion TE. Double-blind study of ACTH vs. prednisone therapy in infantile spasms. J Pediatr. 1983;103:641–645. doi: 10.1016/s0022-3476(83)80606-4. [DOI] [PubMed] [Google Scholar]
- 47.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]