Abstract
Radiation exposure to physicians’ hands during interventional procedures with computed tomography (CT) fluoroscopic guidance may be high. A robot was developed that could hold, orient, and advance a needle, with CT fluoroscopic guidance. This robot could be either computer or joystick controlled. Twenty-three robotically guided percutaneous interventions were performed without complication. Physician radiation exposure was negligible during the CT fluoroscopy–guided procedures.
Index terms: Computed tomography (CT), guidance, 70.12119; Computed tomography (CT), radiation exposure, 70.12119; Fluoroscopy, technology; Phantoms; Radiations, exposure to patients and personnel, 70.12119; Radiations, measurement, 70.12119
Computed tomographic (CT) fluoroscopy offers many advantages for performance of interventional procedures. With CT fluoroscopy the trajectory of a needle can be tracked in real time, which allows the physician to make adjustments as necessary. This advantage has made procedures faster, with equivalent or better success rates than those with standard intermittent CT imaging. Gianfelice et al (1) reported faster biopsy procedures, with mean times that declined significantly (P < .0001) from 43 minutes with conventional CT to 28 minutes with CT fluoroscopy, and procedure success rates that increased from 88% to 94%. Likewise, Silverman et al (2) reported mean needle placement times that decreased significantly (P < .005) from 36 minutes with conventional CT to 29 minutes with CT fluoroscopy, with equivalent success rates between the two modalities.
The major limitation of CT fluoroscopy is the relatively high radiation exposure to patient and physician. To make the real-time adjustments in needle trajectory, the physician’s hand is in proximity to the scanning plane. Physician hand exposure has been theoretically and empirically determined to be approximately 2 mGy per procedure (3). Kato et al (4) calculated that, on the basis of an annual dose limit of 500 mSv for the hands, a physician with continuous hand exposure would be limited to performing only four CT fluoroscopic procedures each year. A number of procedural techniques and shields have been suggested to minimize radiation exposure (5). Kato et al (4) and Daly et al (6) used needle holders and Nawfel et al (3) used lead drapes to minimize hand exposure. Others have noted that experience and training may lead to a reduction in exposure (7). Paulson et al (8) recently reported reducing radiation exposure by lowering the milliampere setting and acquiring intermittent spot images during the procedure. Intermittent spot-check imaging has gained greater acceptance, as it generally can allow successful completion of the intervention with a substantial reduction of radiation exposure.
Robots have been introduced into the operating room to hold and move instruments precisely. Robots allow greater precision and accuracy and lack tremor when compared with humans (9,10). Neurosurgeons have used robots to perform stereotactic biopsies on the basis of previously acquired CT images (11-13). Cardiac surgeons use robots to translate gross movements on a magnified image into fine robotic movements in the body (14). One of the advantages of robots that is capitalized in telesurgical applications is the fact that the surgeon does not need to be in the same location as the patient (15). Multiple reports of remote surgery exist (16-18). This advantage of operating remotely may be used to limit radiation exposure in CT fluoroscopy–guided interventional procedures.
The purpose of our study was to evaluate the feasibility of using a robotic system to precisely orient and advance a needle with CT fluoroscopic guidance for various interventional procedures.
Materials and Methods
Robot
The main component of the system used in this study is the PAKY-RCM robot, which was developed at the URobotics Laboratory at our institution (Fig 1). The robot comprises the needle driver (percutaneous access of the kidney, PAKY) and the remote center of motion (RCM) (19).
Figure 1.
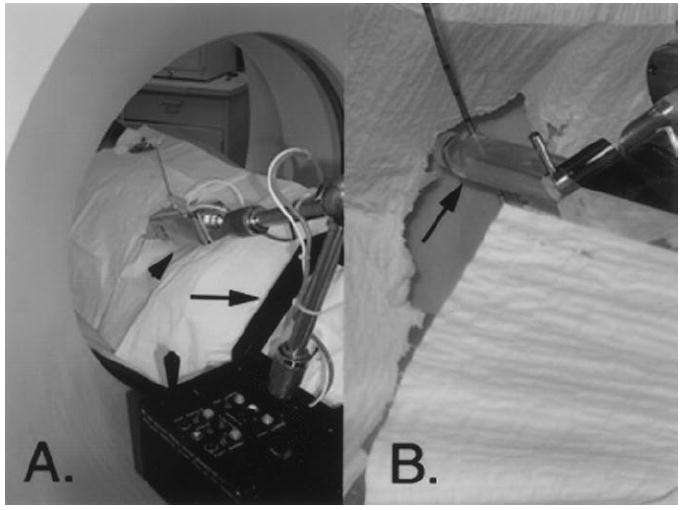
A, The robot. The black frame (arrow) is attached to the bed of the CT scanner. The patient slides his or her legs through the frame. The robotic arm (arrowhead) holds the needle. One joystick controls orientation, and the other controls needle advancement. These commands are separated for safety. The computer workstation is located in the CT control room. B, Close-up view of the sterilizable radiolucent needle holder (arrow) of the robot. The holder creates no scatter artifact on the image. The needle is advanced by a rolling mechanism that propels the needle in forward and reverse directions.
The needle driver, which is sterilizable and radiolucent, is used to guide and actively advance a needle in percutaneous procedures (20). In the needle driver, the needle is tightly held and a rolling dowel mechanism is used to create a friction transmission system that allows needle advancement.
The remote center of motion is a compact robot for clinical applications, in which a fulcrum point is located distal to the mechanism (21,22). Typically, this fulcrum point is at the skin entry site. The robot can precisely orient a surgical instrument or biopsy needle while maintaining the needle tip at the fixed skin entry point. This capability allows the needle to be aimed at any desired trajectory from the skin insertion point. The robot assembly is supported by a passive arm with a frame mounted to the CT table. This arm allows supporting and positioning of the robot in proximity to the target organ. The estimated time for mounting the robot to the CT bed and for subsequent image registration was 15 minutes.
Needle advancement can be computer controlled, in which the robot is registered with the CT image, or manual joystick controlled, in which the physician uses the live CT fluoroscopic images and “drives” the needle to the target with the joystick. There are two joystick controls. One controls the forward-reverse advancement and one controls the trajectory orientation. Separation of these two functions allows increased safety.
The needle holder can accommodate a needle of any size. Eighteen-gauge core biopsy needles and 15-gauge radio-frequency (RF) devices were used in the interventions in this study.
Robot Accuracy Testing
A model was constructed with 22 1-mm-diameter nipple markers dispersed throughout the three-dimensional phantom. The phantom was placed within the CT gantry, and the robotic arm, which was holding an 18-gauge needle, was positioned 5–10 cm from the nipple markers. After robot registration, images were acquired that included the needle tip and each of the target markers. With use of a computer mouse, the needle tip and the target point were defined for the robot computer. Then the robot drove an 18-gauge needle to each marker. Two accuracy measurements were made for each test (Fig 2). First, the targeting accuracy, which was defined as the angle between the ideal trajectory and the actual trajectory of the needle after robotic alignment, was measured from the target to the direction of the needle. Second, the overall error, which was defined as the distance from the target to the tip of the needle after the robot had advanced the needle to the target, was measured.
Figure 2.
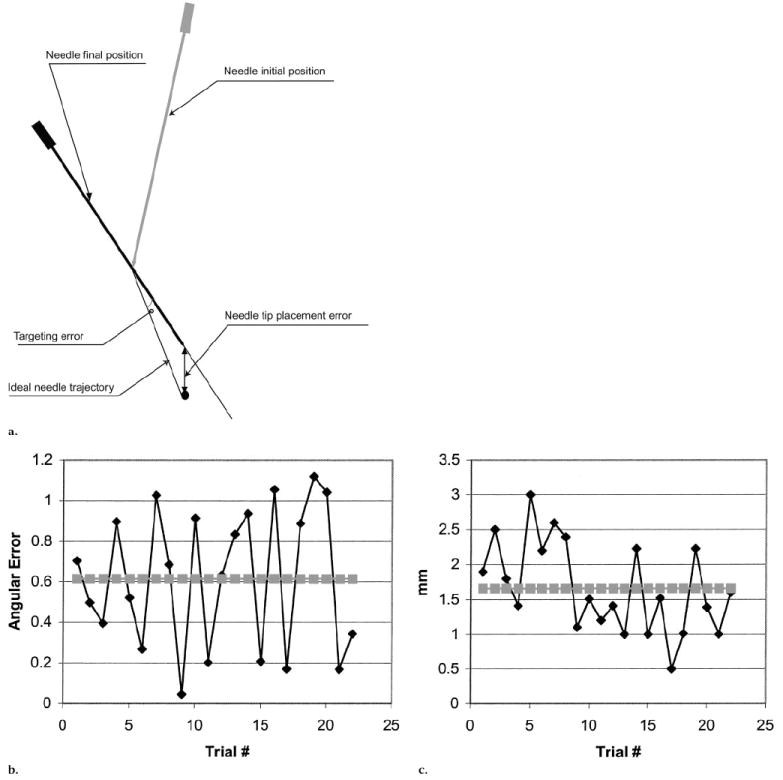
(a)Diagram shows the needle at its initial position and at its final position. The error in trajectory is given by the angle, in degrees, from the ideal trajectory to the final trajectory. The overall error is the distance from the needle tip at the final position to the target. (b) The angular error (◆), which indicates the difference between the ideal trajectory and the robot-chosen trajectory, is given for each of the 22 individual attempts. An average error (■) is then plotted. (c) The distance between the final needle tip position and the target (tip-target error [◆]) is plotted for each of the 22 individual attempts. The tip-target average error (■) is also graphed.
Patients
Sixteen patients (11 men and five women; age range, 49–90 years; mean age, 69 years) were scheduled for 23 CT-guided procedures. These consecutive procedures were performed by one of the authors (S.B.S.). The procedures were RF ablation (n = 11), core needle biopsy (n = 10), nephrostomy tube placement (n = 1), and neobladder access (n = 1). All patients provided informed consent as part of the protocol, which was approved by the institutional review board and was aimed at testing the feasibility and accuracy of targeting with the robot. All procedures were performed by at least one of the physician authors with technical assistance from at least one of the engineer authors. As part of the patient evaluation, the size of the target and appropriate needle tip location were assessed by the one or two radiologists present to perform the procedure.
Entry Site Selection
The patients were placed on the CT fluoroscopy table (Somatom Plus 4 with CARE Vision; Siemens Medical Systems, Iselin, NJ). Patients were placed supine or prone, depending on the expected skin entry site. The patient’s legs were slid into a frame that was attached to the table and from which the robot arm extended. A nonenhanced breath-hold spiral CT scan was obtained to help localize the lesion and plan the procedure. The appropriate section for needle entry was selected, and the table was moved to that position. Metallic nipple markers were placed on the patient’s skin at the selected section, with use of the CT scanner laser light for guidance. Another single-breath-hold image was acquired at the selected section. The nipple markers that were visible on the CT image allowed selection of the appropriate skin entry site. Each patient’s skin was cleaned with povidone iodine, and lidocaine was administered locally over the planned entry site. A small dermotomy was made in the skin at the appropriate entry site.
Registration
Use of a number of image registration methods is possible (23). With the registration method used in this study, we took advantage of the laser light that is incorporated in the section-selection component of all CT scanners.
The mechanical arm with the sterilized needle holder, which contained the appropriate needle for the given procedure, was manually placed so that the tip of the needle was in the skin dermotomy site (Fig 1). The needle shaft was moved to reflect the CT laser light (Fig 3, A). This point was noted to the computer. Then a second position, with the needle tip still in the entry site, was selected. This second position was selected to also reflect the laser light along the needle shaft. The second position was noted to the computer (Fig 3, B). Thus, the two needle positions that reflected the laser light were used to identify a plane for image registration. The third coordinate came from the position of the CT scanner table.
Figure 3.
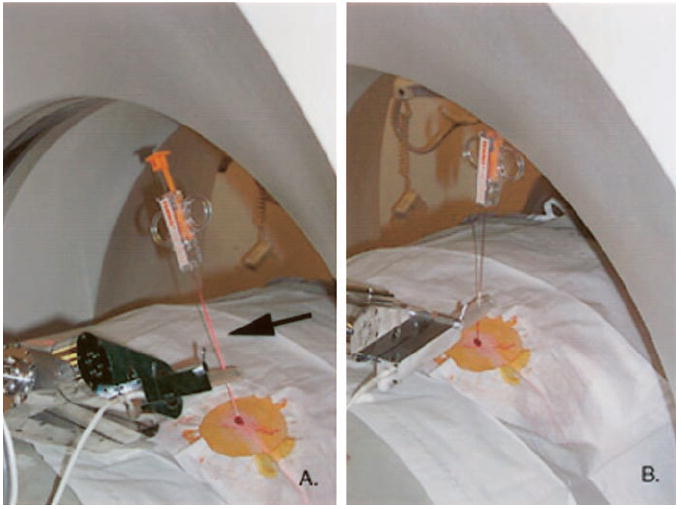
Registration method for aligning the robot’s coordinate system with that of the image involves use of the plane of the laser light in the CT scanner. A, Needle in the plane of the laser, with reflection on the shaft (arrow). B, Laser beam reflects on the shaft but in a different position. These two positions define the section plane. The third coordinate comes from the CT table position, which is known by the CT scanner.
Target Selection
Next, a single-breath-hold image was obtained that showed the tip of the needle. The image was transferred to the computer workstation. By using a computer mouse, the physician indicated the tip of the needle and the shaft to the computer (Fig 4, A). Then, a breath-hold image was obtained that showed the target, which was not always in the same image as the needle tip. The image was transferred to the computer workstation, and the target position was again indicated with the computer mouse (Fig 4, B).
Figure 4.
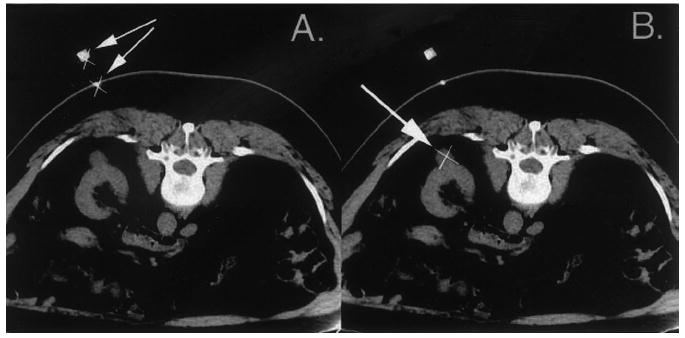
Two images sent by the physician to the computer workstation. A, Image used to indicate the tip (×) and shaft (arrows) of the needle at the skin. B, Image used to indicate the target (×, arrow), an exophytic renal mass. The target is in the same image as is the needle tip, but it does not need to be.
Robot Needle Insertion
The robot first moved the needle to the correct trajectory and then, with CT fluoroscopic guidance (50 mA, 120 kVp), advanced the needle to its target location, without physician radiation exposure. Movement and advancement were accomplished during one breath hold. The target was the mass for the core biopsies, the collecting system for the nephrostomy tube placement, and the neobladder for the access procedure. In the case of RF ablations, the robot moved to the correct angle for placement but did not advance the probe. The ablation probe was pushed manually to the correct depth because the advancement mechanism of the robot was found to strip the insulation off the RF probe. Manual advancement was performed by using the spot-check method of CT fluoroscopy. Joystick control was used if fine-tuning of position was necessary, as occurred in cases of respiratory motion where the lesion moved in and out of the target coordinates. The combination of joystick control with CT fluoroscopy allowed advancement of the needle to be timed with the respiratory motion.
Procedure Assessment
Procedures were assessed with CT fluoroscopy of the needle tip in the target tissue. In addition, for the cases of neobladder access and nephrostomy tube placement, urine return was evidence of successful targeting. Pathologic findings were correlated with imaging findings for the 10 biopsies performed with the robot.
Results
In Vitro Results
Findings in the phantom studies showed a mean angular error of 0.61° (Fig 2b). This was the error angle between the ideal trajectory to the target and the actual trajectory selected for the robot. Findings also showed a mean distance error between the target and the ultimate needle tip position of 1.66 mm (Fig 2c).
Patient Results
All interventional procedures were performed successfully without complication. These procedures included 10 percutaneous core biopsies (kidney, n = 7; lung, n = 2; liver, n = 1), 11 RF ablations (kidney, n = 9; spine, n = 2), nephrostomy tube placement (n = 1), and neobladder access (n = 1) (Fig 5, Table).
Figure 5.
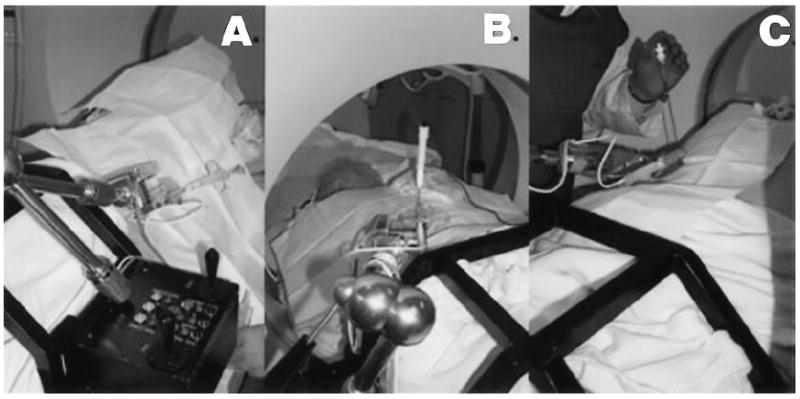
Examples of the robot position for three CT interventional procedures: A, percutaneous biopsy; B, RF ablation; and C, nephrostomy tube placement. The physician in the room is finishing the procedure, with the working site outside of the gantry.
Table.
Summary of Interventional Procedures and Findings with the Robot
| Patient No. | Target | Findings* | Largest Diameter of Target (cm) |
|---|---|---|---|
| 1 | Kidney biopsy | Atypia | 2.5 |
| 2 | Nephrostomy | Urine | 5.0 |
| 3 | Kidney biopsy and RF ablation | Atypia | 1.0 |
| 4 | Two spinal RF ablations | NA | 3.0, 2.5 |
| 5 | Kidney RF ablation | NA | 2.5 |
| 6 | Kidney biopsy and RF ablation | Focal scarring | 2.5 |
| 7 | Neobladder access | Urine | 8.0 |
| 8 | Kidney biopsy and RF ablation | Inflammation | 2.0 |
| 9 | Kidney biopsy and RF ablation | Inflammation | 2.0 |
| 10 | Kidney biopsy and RF ablation | Atypia | 2.5 |
| 11 | Liver biopsy | Neoplasm | 3.0 |
| 12 | Kidney RF ablation | NA | 2.5 |
| 13 | Lung biopsy | Non–small cell lung cancer | 3.0 |
| 14 | Lung biopsy | Plasma cells, fibroblast proliferation, and granulation tissue | 2.0 |
| 15 | Kidney RF ablation | NA | 2.0 |
| 16 | Muscle biopsy and RF ablation | Leiomyosarcoma | 2.0 |
Note.—NA = not applicable.
In each case, success was defined as hitting the target correctly with the needle tip. Target size ranged from 1.0 cm for a lesion at biopsy to 8.0 cm for the neobladder.
In four cases, the target was not met adequately, and fine-tuning adjustment with joystick control was required to ultimately reach the target. The four cases included patients 1, 3, 8, and 14, with target diameters of 2.5, 1.0, 2.0, and 2.0 cm, respectively. The remaining cases were targeted satisfactorily, on the basis of image interpretation.
Discussion
The use of CT fluoroscopy has made interventional procedures faster with equivalent or better success rates than those with standard intermittent CT imaging (1,2). However, the major limitation of the modality is the relatively high radiation exposure to patient and physician (3,4). Robots have been used in surgery to help solve problems of holding and controlling instruments (8-11). In this article, we describe the use of a robot that can hold, orient, and advance a needle, with CT fluoroscopic guidance, to a target lesion.
In this study, the robotic arm held and advanced the needle. Since the physician’s hands did not need to be in the scanning plane at all, physician radiation exposure was dramatically reduced. In fact, radiation exposure to the physician can be completely eliminated if the joystick and computer are placed in the control room, where the physician can view the technologist’s monitor to perform the procedure.
Radiation exposure to the patient can also be reduced with use of the robot. Since the computer can reliably advance the robot’s needle to the target, continuous imaging may not be necessary as long as respiration is relatively controlled. This will significantly reduce patient radiation exposure. This method of allowing the computer to advance the needle without continuous CT fluoroscopy may also be useful in cases where CT fluoroscopy is not available. Once the needle is placed by the robot, a subsequent image can be acquired to assess position. Any inaccuracies in registration or movement due to respiration can be updated with the joystick manual control.
One of the challenges in standard CT fluoroscopy–guided procedures is that the entry site may not be in the same plane as that of the lesion. Multiple manual manipulations are often necessary to determine the correct needle trajectory. In the system used for this study, this challenge can be avoided. The needle tip or entry site for registration and the lesion can be in different scanning planes. The ability to have the computer advance the needle tip to the plane of the lesion can avoid having to “hunt” for the needle tip.
The limitation to use of the robot is the extra preparation time. The robot needs to be attached to the CT table, and registration must be performed. In the future, if the robot can be mounted on the CT table, registration could be performed during installation and not have to be repeated with each additional patient. In the current situation, however, approximately 15 minutes of extra preparation time are needed for frame mounting and robot registration.
Another limitation relates to the mechanism of needle advancement by the robot. Since the robot has a friction rolling mechanism to advance the needle, it will strip off the insulation of RF ablation probes. In these cases, we advanced the needle manually for the known distance when the angle of trajectory was selected by the robot. We are developing a new mechanism of advancement to avoid this issue.
Use of the robot will limit the tactile feel that an interventionalist often relies on when performing procedures. Many researchers have applied haptic interfaces to simulate the force feedback that a physician may sense when performing a procedure such as angioplasty or laparoscopy (24,25). These interfaces have been focused on virtual reality educational simulations. Some of these techniques may be incorporated into the joystick control of the robot, which remains an area for further investigation.
The robot may also be useful with standard CT scanners without CT fluoroscopy. In these cases, the robot can advance the needle to the target, which would obviate the repeated scanning and hunting for the needle tip that occur in conventional procedures. Use of the robot can potentially save procedure time and hopefully improve accuracy.
The robot itself is relatively easy to use without special training. It is driven by a personal computer, and touch screen functions are included. While we used a research prototype system, a commercial unit might cost in the range of $20,000. The steps simply include the technologist transferring the registration images to the computer and the radiologist using the mouse to select the needle tip and target.
Last, several interventional tools, such as the RF devices, do not fit well into the CT gantry. It is conceivable that a shorter needle might be used to “register” the robot for the correct trajectory; then, out of the CT gantry, the appropriate device could be placed into the robot and advanced to the target.
In summary, CT fluoroscopy is a helpful tool for performing interventional procedures with image guidance. The limitation of added radiation exposure to the physician can be dramatically reduced by using a robotically controlled needle driver, as described in this study.
Acknowledgments
We appreciate the contributions of Russ Taylor, PhD, and Louis Whitcomb, PhD, who provided the initial development of the robot.
D.S. and L.R.K. have a financial arrangement with ImageGuide, a company organized for possible sale of the robot described in this article.
Abbreviation
- RF
radio frequency
Footnotes
Author contributions: Guarantor of integrity of entire study, S.B.S.; study concepts and design, S.B.S., M.E.B., L.R.K., D.S.; literature research, S.B.S., A.P.; clinical studies, S.B.S., M.E.B., L.R.K.; experimental studies, S.B.S., A.P., D.S.; data acquisition, S.B.S., A.P., D.S.; data analysis/interpretation, S.B.S., M.E.B., A.P., D.S.; manuscript preparation, S.B.S., A.P., D.S.; manuscript definition of intellectual content, S.B.S.; manuscript editing, revision/review, and final version approval, all authors.
Supported in part by National Science Foundation Cooperative Agreement EEC 9731478, Engineering Research Center for Computer-Integrated Surgical Systems and Technology; and National Cancer Institute grant 1R21CA088232-01A1.
References
- 1.Gianfelice D, Lepanto L, Perreault P, Chartrand-Lefebvre C, Milette PC. Value of CT fluoroscopy for percutaneous biopsy procedures. J Vasc Interv Radiol. 2000;11:879–884. doi: 10.1016/s1051-0443(07)61805-3. [DOI] [PubMed] [Google Scholar]
- 2.Silverman SG, Tuncali K, Adams DF, Nawfel RD, Zou KH, Judy PF. CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology. 1999;212:673–681. doi: 10.1148/radiology.212.3.r99se36673. [DOI] [PubMed] [Google Scholar]
- 3.Nawfel RD, Judy PF, Silverman SG, Hooton S, Tuncali K, Adams DF. Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology. 2000;216:180–184. doi: 10.1148/radiology.216.1.r00jl39180. [DOI] [PubMed] [Google Scholar]
- 4.Kato R, Katada K, Anno H, Suzuki S, Ida Y, Koga S. Radiation dosimetry at CT fluoroscopy: physician’s hand dose and development of needle holders. Radiology. 1996;201:576–578. doi: 10.1148/radiology.201.2.8888264. [DOI] [PubMed] [Google Scholar]
- 5.Nickoloff EL, Khandji A, Dutta A. Radiation doses during CT fluoroscopy. Health Phys. 2000;79:675–681. doi: 10.1097/00004032-200012000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Daly B, Krebs TL, Wong-You-Cheong JJ, Wang SS. Percutaneous abdominal and pelvic interventional procedures using CT fluoroscopy guidance. AJR Am J Roentgenol. 1999;173:637–644. doi: 10.2214/ajr.173.3.10470894. [DOI] [PubMed] [Google Scholar]
- 7.Gianfelice D, Lepanto L, Perreault P, Chartrand-Lefebvre C, Milette PC. Effect of the learning process on procedure times and radiation exposure for CT fluoroscopy-guided percutaneous biopsy procedures. J Vasc Interv Radiol. 2000;11:1217–1221. doi: 10.1016/s1051-0443(07)61367-0. [DOI] [PubMed] [Google Scholar]
- 8.Paulson EK, Sheafor DH, Enterline DS, Mc-Adams HP, Yoshizumi TT. CT fluoroscopy-guided interventional procedures: techniques and radiation dose to radiologists. Radiology. 2001;220:161–167. doi: 10.1148/radiology.220.1.r01jl29161. [DOI] [PubMed] [Google Scholar]
- 9.Kavoussi LR, Moore RG, Adams JB, Partin AW. Comparison of robotic versus human laparoscopic camera control. J Urol. 1995;154:2134–2136. [PubMed] [Google Scholar]
- 10.Fadda M, Marcacci M, Toksvig-Larsen S, Wang T, Meneghello R. Improving accuracy of bone resections using robotics tool holder and a high speed milling cutting tool. J Med Eng Technol. 1998;22:280–284. doi: 10.3109/03091909809010012. [DOI] [PubMed] [Google Scholar]
- 11.Koyama H, Uchida T, Funakubo H, Takakura K, Fankhauser H. Development of a new microsurgical robot for stereotactic neurosurgery. Stereotact Funct Neurosurg. 1990;54:462–467. doi: 10.1159/000100255. [DOI] [PubMed] [Google Scholar]
- 12.Kwoh YS, Hou J, Jonckheere EA, Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng. 1988;35:153–160. doi: 10.1109/10.1354. [DOI] [PubMed] [Google Scholar]
- 13.Fankhauser H, Glauser D, Flury P, et al. Robot for CT-guided stereotactic neurosurgery. Stereotact Funct Neurosurg. 1994;63:93–98. doi: 10.1159/000100300. [DOI] [PubMed] [Google Scholar]
- 14.Autschbach R, Onnasch JF, Falk V, et al. The Leipzig experience with robotic valve surgery. J Card Surg. 2000;15:82–87. doi: 10.1111/j.1540-8191.2000.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee BR, Png DJ, Liew L, et al. Laparoscopic telesurgery between the United States and Singapore. Ann Acad Med Singapore. 2000;29:665–668. [PubMed] [Google Scholar]
- 16.Link RE, Schulam PG, Kavoussi LR. Telesurgery: remote monitoring and assistance during laparoscopy. Urol Clin North Am. 2001;28:177–188. doi: 10.1016/s0094-0143(01)80020-3. [DOI] [PubMed] [Google Scholar]
- 17.Cheah WK, Lee B, Lenzi JE, Goh PM. Telesurgical laparoscopic cholecystectomy between two countries (abstr) Surg Endosc. 2000;14:1085. doi: 10.1007/s004640000099. [DOI] [PubMed] [Google Scholar]
- 18.Marescaux J, Smith MK, Folscher D, Jamali F, Malassagne B, Leroy J. Telerobotic laparoscopic cholecystectomy: initial clinical experience with 25 patients. Ann Surg. 2001;234:1–7. doi: 10.1097/00000658-200107000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoianovici D, Witcomb LL, Anderson JH, Taylor RH, Kavoussi LR. 1998 MICCAI, Lecture Notes in Computer Science. Vol. 1496. Heidelberg, Germany: Springer-Verlag; 1998. A modular surgical robotic system for image guided percutaneous procedures; pp. 404–410. [Google Scholar]
- 20.Stoianovici D, Cadedu JA, Demaree RD, et al. 1997 CVRMed-MrCas, Lecture Notes in Computer Science. Vol. 1205. Berlin, Germany: Springer-Verlag; 1997. An efficient needle injection technique and radiological guidance method for percutaneous procedures; pp. 295–298. [Google Scholar]
- 21.Stoianovici D, Lee BR, Bishoff JT, et al. Robotic telemanipulation for percutaneous renal access (abstr) J Endourol. 1998;12:S201. [Google Scholar]
- 22.Stoianovici D, Witcomb LL, Anderson JH, Taylor RH, Kavoussi LR. 1998 MICCAI, Lecture Notes in Computer Science. Vol. 1496. Berlin, Germany: Springer-Verlag; 1998. A modular surgical robotic system for image guided percutaneous procedures; pp. 404–410. [Google Scholar]
- 23.Susil RC, Anderson J, Taylor RH. 1999 MICCAI, Lecture Notes in Computer Science. Vol. 1679. Berlin, Germany: Springer-Verlag; 1999. A single image registration method for CT guided interventions; pp. 798–808. [Google Scholar]
- 24.Barnes SZ, Morr DR, Oggero E, Pagnacco G, Berme N. The realization of a haptic (force feedback) interface device for the purpose of angioplasty surgery simulation. Biomed Sci Instrum. 1997;33:19–24. [PubMed] [Google Scholar]
- 25.Baur C, Guzzoni D, Georg O. VIRGY: a virtual reality and force feedback based endoscopic surgery simulator. Stud Health Technol Inform. 1998;50:110–116. [PubMed] [Google Scholar]


