Abstract
Chronic myelogenous leukemia (CML) inevitably progresses to a blast phase by mechanisms that are not well understood. The MUC1-C oncoprotein is expressed in CML blasts but not chronic phase cells. The present studies demonstrate that treatment of KU812 and K562 CML cells with a cell-penetrating MUC1-C inhibitor, designated GO-203, is associated with increases in reactive oxygen species (ROS) and depletion of glutathione. GO-203 treatment resulted in the complete downregulation of Bcr-Abl expression and induced cell cycle arrest by a ROS-mediated mechanism that was blocked by the antioxidant N-acetylcysteine. Progression of CML to blast crisis has been linked to dysregulation of Wnt/β-catenin signaling and an arrest of differentiation. The present results show that inhibition of MUC1-C induces ROS-mediated suppression of β-catenin expression and induction of a differentiated myeloid phenotype. Our studies also show that GO-203 treatment is associated with ROS-induced decreases in ATP and loss of survival by late apoptosis/necrosis. These findings demonstrate that inhibition of the MUC1-C oncoprotein in CML cells disrupts redox balance and thereby 1) downregulates expression of both Bcr-Abl and β-catenin and 2) induces terminal myeloid differentiation by ROS-mediated mechanisms.
Keywords: MUC1, CML, Bcr-Abl, ROS, β-catenin
Introduction
Chronic myelogenous leukemia (CML) is a disease of hematopoietic stem cells caused by the t(9:22)(q34:q11) reciprocal translocation and expression of the Bcr-Abl fusion protein.1,2 The constitutively active Bcr-Abl kinase promotes growth and survival in the chronic phase of CML that is characterized by accumulation of cells in the granulocytic lineage.2 Accordingly, inhibitors of the Abl tyrosine kinase function, such as imatinib mesylate, block growth of Bcr-Abl–transformed cells and are highly effective in inducing remissions in patients with CML in chronic phase.3,4 Nonetheless, primitive CML stem cells survive despite treatment with imatinib and acquire drug-resistant mutations within the Bcr-Abl kinase domain.2,5 In this way, the chronic phase of CML inevitably progresses with the accumulation of blasts in the bone marrow and peripheral blood. The blast phase of CML has been attributed to an arrest of differentiation resulting from the acquisition of genetic and epigenetic changes.1,2 Progression to blast crisis has also been attributed to the emergence of progenitor cells with characteristics of stem cells.2 In that line of reasoning, the granulocyte-macrophage progenitor pool has been proposed as the source of stem cells in blast crisis.6,7 Significantly, the self-renewal and leukemic potential of the granulocyte-macrophage pool is decreased by suppression of β-catenin, indicating that dysregulation of β-catenin contributes to CML blast phase.6 In concert with this model, β-catenin deletion markedly reduces the ability of mice to develop Bcr-Abl–induced CML.8 Moreover, imatinib-insensitive CML stem cells in the mouse are dependent on β-catenin for survival.9 Bcr-Abl has been shown to stabilize β-catenin.10 Gene signatures involved in CML progression have also implicated β-catenin as a contributing factor.11 These findings have thus provided support for the importance of the Wnt/β-catenin pathway in the self-renewal of CML stem cells.
MUC1 is expressed in CML blasts, stabilizes the Bcr-Abl protein, and blocks CML cell differentiation.12,13 Following translation as a single polypeptide, MUC1 undergoes autocleavage into 2 subunits that in turn form a heterodimeric complex at the cell membrane.14 The MUC1 N-terminal subunit (MUC1-N) is the mucin component of the heterodimer that contains the characteristic glycosylated tandem repeats. The MUC1 C-terminal subunit (MUC1-C) includes extracellular, transmembrane, and cytoplasmic domains. MUC1-C functions as a cell surface receptor that accumulates in the cytoplasm and is targeted to the nucleus.14,15 The MUC1-C cytoplasmic domain binds directly to the Bcr N-terminal region of Bcr-Abl and thereby blocks Bcr-Abl degradation.12 The MUC1-C cytoplasmic domain also interacts with the Wnt signaling pathway by functioning as a substrate for glycogen synthase kinase 3β (GSK3β).16 and by binding to β-catenin.17 GSK3β phosphorylates β-catenin and thereby targets it for proteosomal degradation.18,19 However, the MUC1-C cytoplasmic domain blocks GSK3β-mediated phosphorylation of β-catenin and in turn stabilizes β-catenin.20 MUC1-C induces anchorage-independent growth and tumorigenicity.20 Significantly, in this regard, disruption of the interaction between the MUC1-C cytoplasmic domain and β-catenin attenuates MUC1-C–induced transformation, indicating that MUC1-C contributes to the malignant phenotype, at least in part, through the stabilization of β-catenin.20 Overexpression of MUC1-C also blocks death in the response of cells to oxidative stress by a mechanism involving regulation of the FOXO3a pathway.21,22 Other studies have demonstrated that blocking MUC1-C with cell-penetrating peptide drugs is associated with disruption of redox balance and an increase in reactive oxygen species (ROS).23 The Wnt/β-catenin pathway is regulated by redox signaling.24 Moreover, low levels of oxidative stress stimulate the interaction between β-catenin and the FOXO transcription factors.25 These findings have supported a model in which MUC1-C regulates β-catenin function through a direct interaction and through redox signaling.
The present studies demonstrate that inhibition of MUC1-C in CML cells with the cell-penetrating peptide, GO-203, is associated with increases in ROS. GO-203 contains a poly Arg cell transduction domain linked to the CQCRRKN sequence ([R]9-CQCRRKN; all D-amino acids) that binds directly to the MUC1-C cytoplasmic domain.26 GO-203 treatment was also associated with pronounced suppression of Bcr-Abl expression and cell cycle arrest by a ROS-dependent mechanism. The results further demonstrate that inhibition of MUC1-C with GO-203 results in ROS-mediated downregulation of β-catenin and the induction of terminal CML differentiation.
Results
Inhibition of MUC1-C disrupts redox balance in CML cells
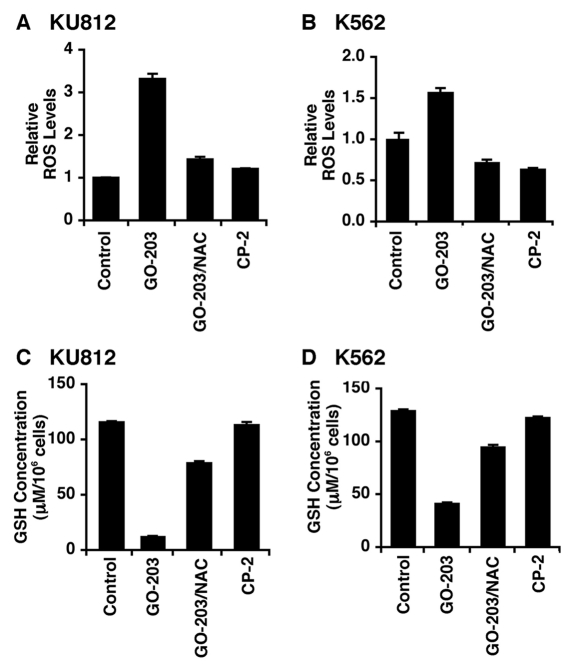
The MUC1-C oncoprotein protects against increases in intracellular ROS.21,22,27,28 In this capacity, treatment of KU812 CML cells for 48 hours with the MUC1-C inhibitor, GO-203, was associated with a marked increase in ROS, and this response was attenuated by the antioxidant NAC (Fig. 1A). By contrast, CP-2, a GO-203 analog that is ineffective in inhibiting MUC1-C, had little effect on ROS levels (Fig. 1A). Similarly, K562 CML cells responded to GO-203 with increases in ROS that were blocked by NAC treatment (Fig. 1B). Oxidative stress results in depletion of intracellular GSH levels.29 Accordingly, the GO-203–induced ROS increase in KU812 cells was associated with decreases in GSH that were in part reversed by NAC, a precursor for GSH synthesis (Fig. 1C). Treatment of K562 cells with GO-203 was also associated with depletion of GSH and a NAC-mediated reversal of this response (Fig. 1D). These findings indicated that GO-203 disrupts redox balance in CML cells and that NAC can be used to discriminate GO-203–conferred responses mediated by increases in ROS.
Figure 1.
GO-203 disrupts redox balance in CML cells. (A-D) KU812 (A, C) and K562 (B, D) cells were treated with 5 µM GO-203 or 5 µM CP-2 each day for 2 days. The GO-203–treated cells were also incubated concurrently with 5 mM NAC. (A and B) Oxidation of c-H2DCFDA to DCF was determined by flow cytometry. The results are expressed as the relative ROS level (mean ± SD for 3 determinations) as compared to that of control cells (assigned a value of 1). (C and D) Cells were analyzed for GSH levels. The results (mean ± SD of 3 determinations) are expressed as GSH levels/106 cells (µM).
GO-203 downregulates Bcr-Abl and induces cell cycle arrest
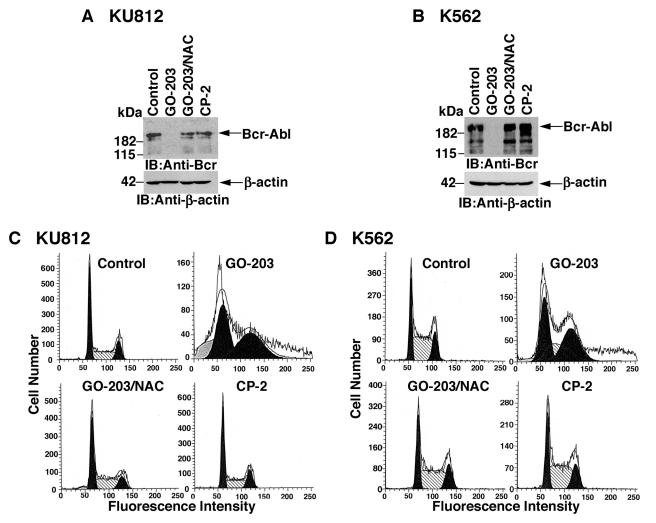
The stability of Bcr-Abl is decreased by oxidative stress.30 In this context, treatment of KU812 cells with GO-203 was associated with marked downregulation of Bcr-Abl levels (Fig. 2A). In addition, this response was attenuated by NAC (Fig. 2A). K562 cells also responded to GO-203 with ROS-induced decreases in Bcr-Abl expression (Fig. 2B). By contrast, CP-2 treatment had no apparent effect on Bcr-Abl levels (Fig. 2A and 2B). Bcr-Abl promotes the growth of CML cells, and by extension, GO-203 treatment was associated with accumulation of KU812 cells in G1 and G2/M phases (Fig. 2C). The arrest of KU812 cell cycle progression was reversed by NAC, consistent with the effects of this antioxidant on Bcr-Abl expression (Fig. 2C). GO-203–induced arrest of K562 cell cycle progression in G1 and G2/M phases was also attenuated by NAC (Fig. 2D). These findings indicate that CML cells respond to GO-203 with ROS-mediated downregulation of Bcr-Abl expression and cell cycle arrest.
Figure 2.
GO-203 treatment decreases Bcr-Abl levels and induces cell cycle arrest. (A-D) KU812 (A, C) and K562 (B, D) cells were treated with 5 µM GO-203 or 5 µM CP-2 each day for 2 days. The GO-203–treated cells were also incubated in the presence of 5 mM NAC. (A and B) Lysates were immunoblotted with the indicated antibodies. (C and D) Cells were fixed and analyzed for cell cycle distribution by flow cytometry.
Inhibition of MUC1-C results in ROS-mediated downregulation of β-catenin and induction of differentiation
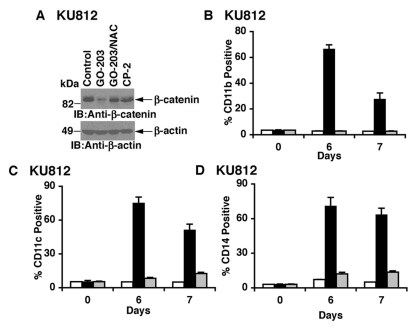
Dysregulation of β-catenin contributes to the development of CML.6 The MUC1-C cytoplasmic domain stabilizes β-catenin and has thereby been shown to contribute to transformation.20 Based on these premises, we asked if inhibition of MUC1-C with GO-203 affects the Wnt/β-catenin pathway. Significantly, treatment of KU812 cells with GO-203 was associated with downregulation of β-catenin expression (Fig. 3A). Moreover, NAC attenuated this response, consistent with suppression of β-catenin by a ROS-dependent mechanism (Fig. 3A). Dysregulation of β-catenin contributes to the arrest of differentiation associated with CML blast crisis.6 In that line of reasoning, treatment of KU812 cells with GO-203, but not CP-2, resulted in induction of the myeloid/monocytic marker CD11b (Fig. 3B). Consistent with the induction of myeloid differentiation, GO-203 treatment was also associated with increased expression of CD11c (Fig. 3C) and CD14 (Fig. 3D).
Figure 3.
Downregulation of β-catenin and induction of myeloid differentiation in the response of KU812 cells to GO-203 treatment. (A) KU812 cells were treated with 5 µM GO-203 or 5 µM CP-2 each day for 2 days. The GO-203–treated cells were also incubated in the presence of 5 mM NAC. Lysates were immunoblotted with the indicated antibodies. (B-D) KU812 cells were treated with 5 µM GO-203 or 5 µM CP-2 each day. Cells harvested at the indicated times were analyzed for expression of CD11b (B), CD11c (C), and CD14 (D). The results (mean ± SD of 3 determinations) are presented as the percentage of control (open bars), GO-203–treated (solid bars), and CP-2–treated (shaded bars) cells expressing the indicated markers.
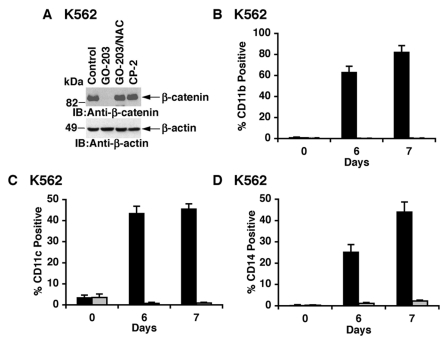
As confirmation of the effects of inhibiting MUC1-C in KU812 cells, treatment of K562 cells with GO-203 was similarly associated with ROS-mediated downregulation of β-catenin levels (Fig. 4A). Moreover, GO-203 induced the expression of CD11b (Fig. 4B), CD11c (Fig. 4C), and CD14 (Fig. 4D). Importantly, the induction of these markers of myeloid differentiation was blocked by NAC, consistent with dependence on ROS signaling. These findings thus supported a model in which inhibition of MUC1-C with GO-203 results in downregulation of β-catenin signaling and the induction of a differentiated CML phenotype.
Figure 4.
GO-203 downregulates β-catenin and induces myeloid differentiation of K562 cells. (A) K562 cells were treated with 5 µM GO-203 or 5 µM CP-2 each day for 2 days. The GO-203–treated cells were also incubated in the presence of 5 mM NAC. Lysates were immunoblotted with the indicated antibodies. (B-D) K562 cells were treated with 5 µM GO-203 in the absence and presence of NAC. Cells harvested at the indicated times were analyzed for expression of CD11b (B), CD11c (C), and CD14 (D). The results (mean ± SD of 3 determinations) are presented as the percentage of GO-203–treated cells in the absence (solid bars) and presence of NAC (shaded bars) expressing the indicated markers.
Inhibition of MUC1-C induces ROS-mediated depletion of ATP and late apoptosis/necrosis
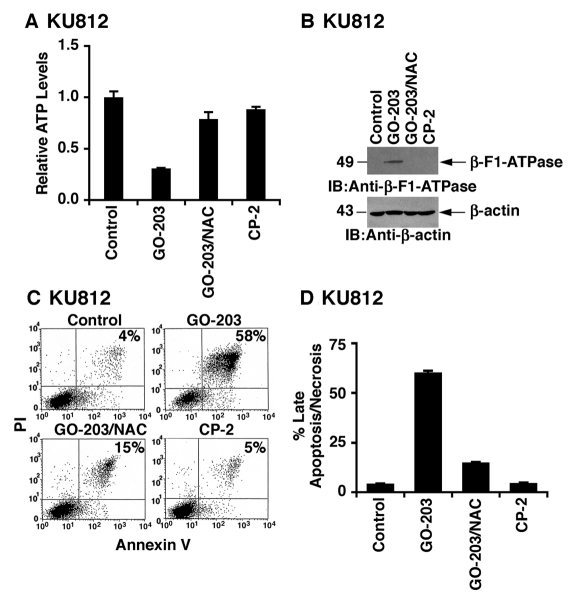
Mitochondria are a major source of ROS that is dependent on the extent of oxidative phosphorylation.31 In turn, ROS can affect mitochondrial ATP production.31 In this way, the GO-203–induced redox imbalance in KU812 cells was associated with depletion of ATP (Fig. 5A). This response to GO-203 was reversed by NAC, confirming the involvement of oxidative stress (Fig. 5A). As a control, treatment with CP-2 had little if any effect on ATP levels (Fig. 5A). ATP production requires the molecular subunits of the H+-ATP synthase. However, downregulation of the β-catalytic subunit of the H+-ATP synthase (β-F1-ATPase) in malignant cells can limit the flux of electrons along the respiratory chain and therefore the generation of ROS and ATP.32,33 Of potential relevance to the relationship between inhibition of MUC1-C and oxidative stress, GO-203 treatment was associated with upregulation of the β-F1-ATPase, and this induction was reversed by NAC (Fig. 5B). In concert with GO-203–induced increases in ROS and decreases in ATP, treatment of KU812 cells with the MUC1-C inhibitor resulted in the induction of annexin V and PI staining, consistent with the induction of late apoptosis/necrosis (Fig. 5C). Moreover, GO-203–induced death was attenuated by NAC (Fig. 5C). These results were confirmed in repetitive experiments and further demonstrated that CP-2 has no effect on viability (Fig. 5D). Treatment of KU812 cells with GO-203 and the caspase inhibitor zVAD-fmk was associated with a significant decrease in the induction of late apoptosis/necrosis (Suppl. Fig. S1A). By contrast, zVAD-fmk had little effect on GO-203–induced downregulation of Bcr-Abl and β-catenin (Suppl. Fig. S1B), indicating that these responses are not a secondary effect of cell death.
Figure 5.
Depletion of ATP and induction of late apoptosis/necrosis in GO-203–treated KU812 cells. (A-D) KU812 cells were treated with 5 µM GO-203 or 5 µM CP-2 each day for 2 days. The GO-203–treated cells were also incubated in the presence of 5 mM NAC. Intracellular ATP levels (mean ± SD of 3 determinations) are expressed relative to that obtained for untreated cells (A). Lysates were immunoblotted with the indicated antibodies (B). Cells were stained with PI/annexin V and analyzed by flow cytometry (C). The results (mean ± SD of 3 determinations) are expressed as the percentage of cells with late apoptosis/necrosis (D).
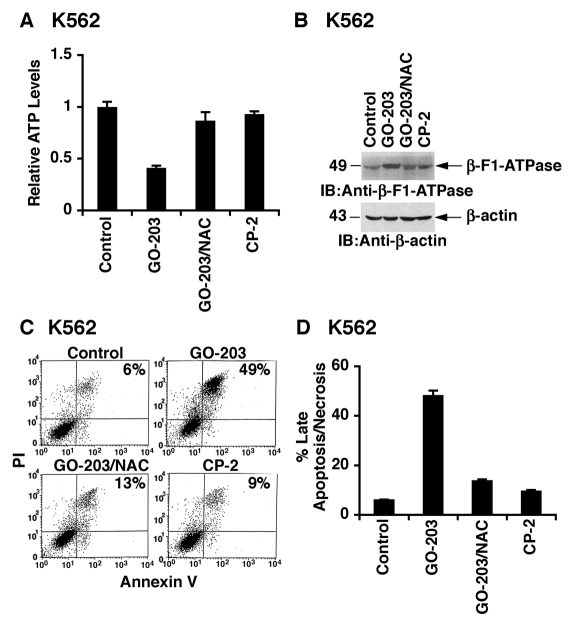
By extension to K562 cells, GO-203 treatment was associated with decreases in ATP levels (Fig. 6A) and upregulation of the β-F1-ATPase (Fig. 6B). As found in KU812 cells, GO-203–induced depletion of ATP (Fig. 6A) and expression of the β-F1-ATPase (Fig. 6B) were reversed by NAC. GO-203 treatment also resulted in the induction of annexin V and PI staining that was attenuated by NAC (Fig. 6C). Repetitive experiments confirmed that GO-203 confers a ROS-mediated induction of late apoptosis/necrosis (Fig. 6D).
Figure 6.
GO-203 treatment depletes ATP levels and induces late apoptosis/necrosis in K562 cells. (A and B) K562 cells were treated with 5 µM GO-203 or 5 µM CP-2 each day for 2 days. The GO-203–treated cells were also incubated in the presence of 5 mM NAC. Intracellular ATP levels (mean ± SD of 3 determinations) are expressed relative to that obtained for untreated cells (A). Lysates were immunoblotted with the indicated antibodies (B). Cells were stained with PI/annexin V and analyzed by flow cytometry (C). The results (mean ± SD of 3 determinations) are expressed as the percentage of cells with late apoptosis/necrosis (D).
Discussion
Inhibition of MUC1-C in CML cells disrupts redox balance
Previous work had shown that Bcr-Abl promotes redox imbalance in CML cells by increasing ROS production.34-36 These observations supported the concept that CML cells become particularly sensitive to agents that further induce oxidative stress.30,37 The present results demonstrate that treatment of CML cells with an inhibitor of the MUC1-C oncoprotein is associated with increases in ROS and depletion of GSH, consistent with disruption of redox balance. MUC1 had been shown to protect cells from oxidative stress associated with exposure to hydrogen peroxide,21,22 hypoxia,27 and glucose deprivation,28 suggesting that MUC1-C inhibitors could potentially block this protective effect with a resulting increase in ROS levels. CML cells were thus treated with the cell-penetrating GO-203 peptide that binds directly to the MUC1-C cytoplasmic domain and blocks its function.26 The GO-203–induced disruption of redox balance was reversed by NAC, a precursor of GSH synthesis, providing a model to assess the cellular effects of GO-203 attributable to increases in ROS. Indeed, GO-203 treatment was associated with complete downregulation of Bcr-Abl expression that was abrogated by NAC, supporting a ROS-mediated mechanism. Studies with the natural compound PEITC similarly showed that disruption of CML cell redox balance promotes Bcr-Abl degradation and that this response is reversed by NAC.30 How increases in ROS induce Bcr-Abl degradation is not known; nonetheless, dependence of CML cell growth on Bcr-Abl signaling invoked the possibility that GO-203–induced disruption of redox balance would affect cell cycle progression. Based on these premises, we found that GO-203 treatment blocks CML cells in G1 and G2/M phases and that this response is attenuated by NAC. These findings thus provided support for inhibition of MUC1-C as a strategy to downregulate Bcr-Abl and block proliferation of CML cells.
GO-203 treatment downregulates β-catenin expression by a ROS-dependent mechanism
The available evidence from mouse models and human CML cells indicates that dysregulation of β-catenin contributes to CML blast phase.6,8,9,11 The MUC1-C cytoplasmic domain binds directly to β-catenin and thereby attenuates β-catenin degradation.20 The MUC1-C cytoplasmic domain also functions as a substrate for GSK3β phosphorylation and thereby blocks GSK3β-mediated phosphorylation of β-catenin.16,20 Consistent with the decrease in β-catenin levels, GO-203 disrupts the interaction between MUC1-C and β-catenin, thus releasing β-catenin for phosphorylation by GSK3β and proteosomal destruction (unpublished data). In concert with the importance of GSK3β→β-catenin signaling, GSK3β suppresses the generation of malignant CML stem cells by a β-catenin–dependent mechanism.38 Oxidative stress has been linked to activation and inhibition of the Wnt/β-catenin pathway, depending on the timing of ROS signaling.24,39 Moreover, oxidative stress regulates β-catenin function by stimulating the interaction between β-catenin and FOXO transcription factors.25 In this capacity, β-catenin enhances FOXO activity and promotes a protective response to oxidative stress.25,40 Notably, the MUC1-C cytoplasmic domain interacts with β-catenin17,20 and also regulates the FOXO3a signaling pathway in a survival response to oxidative stress.22 The present results indicate that inhibition of MUC1-C with GO-203 subverts this survival response with increases in ROS and downregulation of β-catenin. Of potential importance to CML, suppression of β-catenin depletes the granulocyte-macrophage progenitor pool that contributes to CML blast phase.6 Other studies have shown that β-catenin is necessary for CML stem cell self-renewal and survival.8,9 Based on these observations and the present results, inhibition of MUC1-C and the resultant ROS-mediated downregulation of β-catenin thus represent a potential approach for treating CML in blast phase by decreasing self-renewal and reversing the arrest in differentiation.
Inhibition of MUC1-C induces ROS-mediated terminal differentiation
The finding that MUC1 is expressed in CML blasts and not chronic phase cells lends support to the premise that upregulation of MUC1-C contributes to the genetic or epigenetic changes that block differentiation and increase self-renewal capacity.12 Indeed, silencing MUC1 in KU812 and K562 cells is associated with decreases in self-renewal and a more differentiated erythroid phenotype.12 Moreover, inhibition of MUC1-C results in arrest of KU812 and K562 cell growth, induction of myeloid differentiation, and loss of survival,13 indicating that blocking MUC1-C function has additional effects not found with MUC1-C silencing. The present results demonstrate that inhibition of MUC1-C with GO-203 in CML cells induces ROS-mediated myeloid differentiation, ATP depletion, and late apoptotic/necrotic death, consistent with the induction of terminal differentiation. Other studies have shown that AML cells are also sensitive to the effects of GO-203, indicating that the findings are not restricted to CML cells.41 ROS have been shown to play a role in inducing myeloid differentiation of normal hematopoietic cells42 and leukemic blasts43-45 by mechanisms that have largely remained unclear. The finding that ataxia telangiectasia mutated (ATM)–deficient mice develop bone marrow failure that is reversed by NAC provided one link between redox imbalance and loss of hematopoietic cell self-renewal.46 Other studies have shown that FOXO family members protect against oxidative stress and that FOXO-deficient mice exhibit decreases in the hematopoietic cell compartment, which are also restored by NAC.42 MUC1-C interacts with ATM47 and FOXO22 signaling, and thus, inhibition of MUC1-C with GO-203 could induce oxidative stress by affecting these pathways. MUC1-C also localizes to the mitochondrial outer membrane.48,49 ROS are in large part generated by mitochondria; therefore, further studies will be needed to more precisely define how treatment with GO-203 induces oxidative stress. The observation that GO-203 treatment induces β-F1-ATPase expression could be a contributing factor in increasing ROS and depleting ATP and also warrants further investigation. The present findings thus demonstrate that inhibition of MUC1-C represents a novel strategy to disrupt redox balance in CML cells and thereby 1) decrease Bcr-Abl levels, 2) downregulate β-catenin expression, and 3) induce terminal myeloid cell differentiation.
Materials and Methods
Cell culture
Human KU812 and K562 CML cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Cellgro, Manassas, VA), 100 units/mL penicillin, 100 µg/mL streptomycin, and 2 mM L-glutamine. Cells were treated with the cell-penetrating GO-203 ([R]9-CQCRRKN;D-amino acids) and CP-2 ([R]9-AQARRKN; D-amino acids) peptides26 (AnaSpec, Fremont, CA), N-acetylcysteine (NAC) (Calbiochem, La Jolla, CA), and the caspase inhibitor zVAD-fmk (BD Pharmingen, Franklin Lakes, NJ).
Measurement of ROS levels
Cells were incubated with 5 µM carboxy-H2DCFDA (Molecular Probes, Eugene, OR) for 20 minutes at 37°C.Fluorescence of oxidized DCF was measured at an excitation wavelength of 480 nm and an emission wavelength of 525 nm using a flow cytometer (BD Biosciences, Franklin Lakes, NJ).
Determination of GSH concentrations
Cell lysates were prepared as described22 and analyzed for GSH levels using the Bioxytech GSH-400 kit (OXIS International, Beverly Hills, CA).
Immunoblot analysis
Cell lysates were prepared as described.27 Soluble proteins were analyzed by immunoblotting with anti-Bcr (Santa Cruz Biotechnology, Santa Cruz, CA), anti-β-catenin (BD Biosciences), anti-β-F1-ATPase (Molecular Probes), and anti-β-actin (Sigma-Aldrich, St. Louis, MO).
Analysis of cell cycle distribution and death
Cells were harvested, washed with PBS, fixed with 80% ethanol, and incubated in PBS containing 30 µg/mL RNase and 5 µg/mL propidium iodide (PI) as described.13 Cell cycle distribution was determined by flow cytometry for relative DNA content. For analysis of apoptosis and necrosis, cells were incubated with PI/Annexin V-FITC (BD Biosciences) and analyzed by flow cytometry.
FACS analysis
Cells were incubated with FITC-conjugated antibodies against human CD11b, CD11c, or CD14 (Novus Biologicals, Littleton, CO). Reactivity was analyzed by FACScan (Becton, Dickinson and Company, Franklin Lakes, NJ).
Measurement of ATP levels
Cell lysates were analyzed for ATP levels using an ATP Determination kit (Molecular Probes).
Supplementary Material
Footnotes
Supplementary material for this article is available on the Genes & Cancer website at http://ganc.sagepub.com/supplemental.
D. Kufe is an equity holder of and consultant to Genus Oncology.
This work was supported by the National Cancer Institute [grant #CA42802] and the Leukemia Lymphoma Society [grant #6074-09]
References
- 1. Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7:441-53 [DOI] [PubMed] [Google Scholar]
- 2. Savona M, Talpaz M. Getting to the stem of chronic myeloid leukaemia. Nat Rev Cancer. 2008;8:341-50 [DOI] [PubMed] [Google Scholar]
- 3. Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561-6 [DOI] [PubMed] [Google Scholar]
- 4. Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808-17 [DOI] [PubMed] [Google Scholar]
- 5. Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876-80 [DOI] [PubMed] [Google Scholar]
- 6. Jamieson CH, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657-67 [DOI] [PubMed] [Google Scholar]
- 7. Minami Y, Stuart SA, Ikawa T, et al. BCR-ABL-transformed GMP as myeloid leukemic stem cells. Proc Natl Acad Sci U S A. 2008;105:17967-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao C, Blum J, Chen A, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu Y, Chen Y, Douglas L, Li S. Beta-catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia. 2009;23:109-16 [DOI] [PubMed] [Google Scholar]
- 10. Coluccia AM, Vacca A, Dunach M, et al. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26:1456-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McWeeney SK, Pemberton LC, Loriaux MM, et al. A gene expression signature of CD34+ cells to predict major cytogenetic response in chronic-phase chronic myeloid leukemia patients treated with imatinib. Blood. 2010;115:315-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawano T, Ito M, Raina D, et al. MUC1 oncoprotein regulates Bcr-Abl stability and pathogenesis in chronic myelogenous leukemia cells. Cancer Res. 2007;67:11576-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yin L, Ahmad R, Kosugi M, et al. Terminal differentiation of chronic myelogenous leukemia cells is induced by targeting of the MUC1-C oncoprotein. Cancer Biol Ther. 2010;10:483-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kufe D. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell. 2007;27:992-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3β with the DF3/MUC1 carcinoma-associated antigen and β-catenin. Mol Cell Biol. 1998;18:7216-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and β-catenin in cell adhesion.J Biol Chem. 1997;272:12492-4 [DOI] [PubMed] [Google Scholar]
- 18. Orford K, Crockett C, Jensen J, Weissman A, Byers S. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735-8 [DOI] [PubMed] [Google Scholar]
- 19. Liu C, Li Y, Semenov M, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837-47 [DOI] [PubMed] [Google Scholar]
- 20. Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks GSK3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005;65:10413-22 [DOI] [PubMed] [Google Scholar]
- 21. Yin L, Kufe D. Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress.J Biol Chem. 2003;278:35458-64 [DOI] [PubMed] [Google Scholar]
- 22. Yin L, Huang L, Kufe D. MUC1 oncoprotein activates the FOXO3a transcription factor in a survival response to oxidative stress. J Biol Chem. 2004;279:45721-7 [DOI] [PubMed] [Google Scholar]
- 23. Raina D, Ahmad R, Joshi M, et al. Direct targeting of the MUC1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Funato Y, Michiue T, Asashima M, Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501-8 [DOI] [PubMed] [Google Scholar]
- 25. Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181-4 [DOI] [PubMed] [Google Scholar]
- 26. Yin L, Ahmad R, Kosugi M, et al. Survival of human multiple myeloma cells is dependent on MUC1 C-terminal transmembrane subunit oncoprotein function. Mol Pharm. 2010;78:166-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yin L, Kharbanda S, Kufe D. Mucin 1 oncoprotein blocks hypoxia-inducible factor 1 alpha activation in a survival response to hypoxia.J Biol Chem. 2007;282:257-66 [DOI] [PubMed] [Google Scholar]
- 28. Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein promotes autophagy in a survival response to glucose deprivation. Int J Oncol. 2009;34:1691-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303-14 [DOI] [PubMed] [Google Scholar]
- 30. Zhang H, Trachootham D, Lu W, et al. Effective killing of Gleevec-resistant CML cells with T315I mutation by a natural compound PEITC through redox-mediated mechanism. Leukemia. 2008;22:1191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koopman WJ, Nijtmans LG, Dieteren CE, et al. Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation. Antioxid Redox Signal. 2010;12:1431-70 [DOI] [PubMed] [Google Scholar]
- 32. Cuezva JM, Krajewska M, de Heredia ML, et al. The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 2002;62:6674-81 [PubMed] [Google Scholar]
- 33. Shin YK, Yoo BC, Chang HJ, et al. Down-regulation of mitochondrial F1F0-ATP synthase in human colon cancer cells with induced 5-fluorouracil resistance. Cancer Res. 2005;65:3162-70 [DOI] [PubMed] [Google Scholar]
- 34. Sattler M, Verma S, Shrikhande G, et al. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275:24273-8 [DOI] [PubMed] [Google Scholar]
- 35. Kim JH, Chu SC, Gramlich JL, et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717-23 [DOI] [PubMed] [Google Scholar]
- 36. Koptyra M, Falinski R, Nowicki MO, et al. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood. 2006;108:319-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trachootham D, Zhou Y, Zhang H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241-52 [DOI] [PubMed] [Google Scholar]
- 38. Abrahamsson AE, Geron I, Gotlib J, et al. Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci U S A. 2009;106:3925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shin SY, Kim CG, Jho EH, et al. Hydrogen peroxide negatively modulates Wnt signaling through downregulation of beta-catenin. Cancer Lett. 2004;212:225-31 [DOI] [PubMed] [Google Scholar]
- 40. Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem. 2008;283:9224-30 [DOI] [PubMed] [Google Scholar]
- 41. Yin L, Wu Z, Avigan D, et al. MUC1-C oncoprotein suppresses reactive oxygen species-induced terminal differentiation of acute myelogenous leukemia cells. Blood. 2011;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325-39 [DOI] [PubMed] [Google Scholar]
- 43. Ikeda T, Sporn M, Honda T, Gribble G, Kufe D. The novel triterpenoid CDDO induces apoptosis by disruption of intracellular redox balance. Cancer Res. 2003;63:5551-8 [PubMed] [Google Scholar]
- 44. Konopleva M, Tsao T, Ruvolo P, et al. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99:326-35 [DOI] [PubMed] [Google Scholar]
- 45. Callens C, Coulon S, Naudin J, et al. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J Exp Med. 2010;207:731-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997-1002 [DOI] [PubMed] [Google Scholar]
- 47. Huang L, Liao X, Beckett M, et al. MUC1-C oncoprotein interacts directly with ATM and promotes the DNA damage response to ionizing radiation. Genes Cancer. 2010;1:239-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ren J, Agata N, Chen D, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anti-cancer agents. Cancer Cell. 2004;5:163-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ren J, Raina D, Chen W, Li G, Huang L, Kufe D. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res. 2006;4:873-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.