Abstract
Adults can improve their performance on many perceptual tasks with training, but when does the response to training become mature? To investigate this question, we trained 11-year-olds, 14-year-olds and adults on a basic auditory task (temporal-interval discrimination) using a multiple-session training regimen known to be effective for adults. The adolescents all began with performance in the adult range. However, while all of the adults improved across sessions, none of the 11-year-olds and only half of the 14-year-olds did. The adolescents who failed to learn did so even though the 10-session training regimen provided twice the number of sessions required by adults to reach asymptotic performance. Further, over the course of each session, the performance of the adults was stable but that of the adolescents, including those who learned, deteriorated. These results demonstrate that the processes that underlie perceptual learning can continue to develop well into adolescence.
Introduction
One of the most remarkable aspects of human sensory perception is that it can be improved with practice even in adulthood, resulting in more skilled perceptual performance than is typically reached through development alone. In other words, adults are fully developed in terms of their initial performance on a given perceptual skill yet they are able to refine that performance if provided with the right experience. Of interest here is when during development mature perceptual learning emerges. While there has been considerable interest in the honing of perceptual skills in adults (for reviews see Fahle, 2009; Ahissar, Nahum, Nelken & Hochstein, 2009; Wright & Zhang, 2009), virtually nothing is known about the time course over which adult-like learning appears. Any quantifiable changes in perceptual learning during development would need to be incorporated into attempts to identify the neural correlates of these improvements. Here we report that a multiple-session training regimen that yielded perceptual learning in adults did not do so in adolescents, indicating that perceptual learning processes can have a prolonged maturational course.
Mature perceptual learning is, by definition, the improvement pattern that results from training in adults. Thus, immature learning would be indicated by any difference in the response to the same training regimen between adults and younger populations. However, this direct comparison has been made only rarely and never for more than one session of training (Fahle & Daum, 1997; Halliday, Taylor, Edmondson-Jones & Moore, 2008). Most developmental studies of perceptual learning have included only children and therefore do not provide a within-experiment comparison to adults (Edwards, Giaschi, Low & Edgell, 2005; Merzenich, Jenkins, Johnston, Schreiner, Miller & Tallal, 1996; Soderquist & Moore, 1970). Across-experiment comparisons between child and adult learning also are impeded because the child studies typically employed training regimens that differed from those used for adults.
In the present investigation we trained adolescents and adults with the same multiple-session training regimen and compared their performance within as well as across these sessions. Within-session behavior demonstrates the immediate response to training. It therefore reflects elements of the initial stage of learning, acquisition, during which the specific experiences that can lead to permanent improvement are provided (e.g. Banai, Ortiz, Oppenheimer & Wright, 2010; Censor, Karni & Sagi, 2006; Mednick, Nakayama & Stickgold, 2003). Across-session behavior provides an assessment of the delayed response to training. Improvements on many perceptual tasks continue over multiple training sessions and occur only between and not within sessions. Thus, the experiences gained during acquisition are maintained and can even be augmented between sessions. These across-session (delayed) improvements are attributed to a later stage of learning, consolidation, which occurs after each training session and is characterized by the transfer of the acquired skill from labile, short-term memory to more stable long-term memory (e.g. Banai et al., 2010; Censor, Karni & Sagi, 2006; Mednick et al., 2003).
More specifically, we compared the immediate and delayed responses to 10 sessions of training on auditory temporal-interval discrimination between adolescents and adults. To reduce the potential contribution of differences in starting performance to learning outcomes, we included only those adolescents who had adult-like naïve performance. We chose to train auditory temporal-interval discrimination because improvements on this task accumulate across sessions in adults (Banai et al., 2010; Karmarkar & Buonomano, 2003; Wright, Buonomano, Mahncke & Merzenich, 1997; Wright & Sabin, 2007) and therefore occur only if acquisition and consolidation are both successful. This task also provides a clear standard of mature performance, as both naïve performance and learning on it are relatively uniform across adults. We used a 10-day training regimen because it provided additional iterations of the acquisition and consolidation cycle beyond the first five over which most adult learning on temporal-interval discrimination occurs. It therefore enhanced the opportunity to document potential differences in the initial onset or time course of learning between adults and adolescents. Finally, we chose to examine adolescents because differences in learning between this population and adults would be a powerful demonstration that perceptual learning can mature quite late in development.
Materials and methods
Listeners
Eleven-year-olds (n = 11, five females, mean age = 11.4 years, range = 11 years 0 months to 12 years 0 months), 14-year-olds (n = 12, five females, mean age = 14.4 years, range = 13 years 11 months to 14 years 11 months) and adults (n = 15, nine females, mean age = 21.2 years, range = 18–26 years) served as listeners in this cross-sectional study. These listeners had normal hearing, reported no past or present language or learning problems, and had no prior experience with psychoacoustic tasks. All listeners were paid for their participation. All data were collected in accordance with Northwestern University policies on the conduct of research with human subjects and with approval of the Institutional Review Board. Subjects were recruited through fliers posted on the Northwestern University campus and through letters to parents or guardians sent home from a local school.
Organization of experiment
The experiment consisted of three parts: pre-training test, training phase, and post-training test. During the pre- and post-training tests, all listeners (n = 38) were tested on the condition used in training and five other conditions not reported here (300 trials per condition; five threshold estimates). Some listeners (n = 19), referred to as controls, completed both the pre- and post-training tests but did not undergo training. Others (n = 19), referred to as the trained listeners, participated in the pre-training test, the training phase, and the post-training test. During the training phase, these listeners practiced a single temporal-interval-discrimination condition for 900-trials (15 threshold estimates; 1 to 1.5 hours) per day for 10 days. The pre- and post-training tests were separated by an average of 14.6 days (SD = 4.9) for the controls and 17.1 days (SD = 2.2) for the trained listeners. To reduce the potential contribution of differences in starting (naïve) performance to learning outcomes, we selected only those 11- and 14-year-olds whose performance on the target condition at the pre-training test was within 2 standard deviations of the adult average. The trained groups consisted of six adults, eight 14-year-olds and five 11-year-olds. The control groups consisted of nine adults, four 14-year-olds and six 11-year-olds.
Trained condition
The trained condition was a temporal-interval discrimination task with a 100-ms, 1-kHz standard. In each presentation of a two-presentation forced-choice trial, two brief 1-kHz tones were presented. In one randomly chosen presentation, the tones were separated by a standard interval (t = 100 ms), while in the other presentation the tones were separated by a longer comparison interval (t + Δt). The listener was asked to select the comparison (longer) interval. The interval was measured from the onset of the first tone to the onset of the second tone. The onsets of the initial tones in the first and second presentations were separated by 900 ms. Stimulus generation was as in Wright and Sabin (2007).
Procedure
We estimated discrimination thresholds using an adaptive, two-alternative, forced-choice procedure with feedback. Listeners pressed a key on a computer keyboard or used a mouse to click a button on a computer display to indicate which of the two randomly selected presentations contained the longer temporal interval. Throughout the experiment, each condition was described verbally and practice trials were provided immediately before testing began on that condition. All listeners indicated that they understood the task and could discriminate between practice trials. During testing, written instructions were provided on the computer display. Visual feedback (correct/incorrect) appeared on the computer screen after each trial during all phases of the experiment. Threshold estimates were calculated over blocks of 60 trials using a 3-down/1-up adaptive rule. The Δt decreased after three consecutive correct responses and increased after one incorrect response. When the change in Δt switched from decreasing to increasing, or vice versa, the value at which that change occurred was labeled a reversal. The first three reversals were discarded, and the mean of the largest remaining even number of reversals was calculated. This procedure yielded an estimate of the value of Δt that the listener could successfully discriminate on 79.4% of trials, defined here as threshold (Levitt, 1971). Blocks that contained fewer than seven total reversals were excluded from the analyses. On the first trial of every block, the listener was forced to guess because the comparison temporal interval was equal to that of the standard. The step size was 10 ms until the third reversal, and 1 ms thereafter.
Results
Across-session performance
Although the listeners in all three age groups began with adult-like performance on the trained temporal-interval discrimination condition, the effectiveness of the training increased between 11 years of age and adulthood. None of the three control groups showed significant improvement between the pre- and post-training tests (Figure 1a–c, open squares, paired t-tests: |t(3, 5 or 8)| ≤ 1.30 p ≥.29). Of the trained groups (filled triangles), only the adults improved across the 10 training sessions. The daily mean thresholds of the adult group decreased during the training phase (linear regression of threshold on log of training session: slope = −4.22, t(58) = −4.96, p < .01) and improved more between the pre- and post-training tests than those of the adult controls (ANOVA, repeated on test, 2 group × 2 test interaction: F(1, 13) = 7.23, p = .02). In contrast, the 14-year-old group did not improve across training sessions (regression: t(78) = 0.94, p = .35) or differ from same-age controls in the change between the pre- and post-training tests (2 × 2 interaction: F(1, 10) < 0.01, p = .96). Finally, the 11-year-old group actually got worse across the 10 training sessions (regression: slope = 23.36, t(48) = 2.64, p = .03), though not enough as to differentiate their performance between the pre- and post-training tests from that of same-age controls (2 × 2 interaction: F(1, 9) = 0.27, p = .62). In addition, the three trained groups differed in their patterns of performance during the training phase (ANOVA, repeated on session, 3 group × 10 session interaction: F(18, 144) = 2.08, p < .01), but while the 11-year-olds differed from the adults (2 × 10 interaction: F(9, 81) = 4.51, p < .01), the 14-year-olds differed from neither the 11-year-olds nor the adults (both F(9, 99) and F(9, 108) ≤ 1.19, p ≥.15).
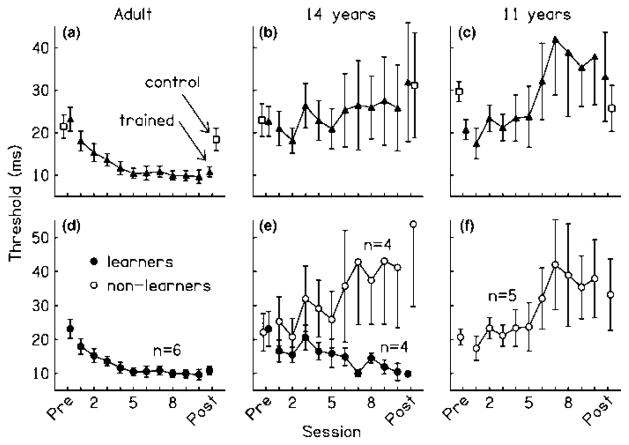
Figure 1.
Across-session performance of adults, 14-year-olds, and 11-year-olds. (a–c) Average group results: Mean temporal-interval discrimination thresholds (Δt for 79.4% correct) for the trained (filled triangles) and control groups (open squares) at the pre- and post-training tests, and the trained groups during the training phase. Results are shown separately for the adults (a), 14-year-olds (b), and 11-year-olds (c). Only the trained adults learned gradually across training sessions and improved more than same-age controls between the pre- and post-training tests. (d–f) Learners vs. non-learners: Mean temporal-interval discrimination thresholds for each trained group, divided into learners (open circles) and non-learners (filled circles) based on each listener’s training phase performance. The proportion of learners in each group became greater with increasing age. Error bars indicate ± one standard error. Trained groups: adult, n = 6, all learners; 14-year-olds, n = 8, 4 learners; 11-year-olds, n = 5, all non-learners. Control groups: adult, n = 9; 14-year-olds, n = 4; 11-year-olds, n = 6.
The improved effectiveness of training with increasing age was also apparent at the individual level. Learning in a given listener was indicated by a significant and negative slope resulting from the linear regression of individual threshold estimates on the log of the session number. By this definition, all of the adults, but none of the 11-year-olds, improved across the 10 training sessions (Figure 1d–f; adults: slope ≤ −2.55, t(148) ≤ −2.28, p ≤.02; 11-year-olds: either slope ≥ 0 or p ≥.05). Of the 14-year-olds, half learned (learners: filled circles, slope ≤ −3.60, t(148) ≤ −2.35, p ≤ .02), while the other half did not (non-learners: open circles, either slope ≥ 0 or p ≥ .05). These differences in the proportion of learners in each group are unlikely to have occurred by chance (Freeman-Halton extension of the Fisher exact probability test; p < .01). As a group, the 14-year-old non-learners did not differ from the 11-year-olds in their pattern of performance across training sessions (2 × 10 interaction: F(9, 63) = 0.25, p = .98). Interestingly, the group of 14-year-old learners showed a different performance pattern during the training phase from that of adults (2 × 10 interaction: F(9, 72) = 3.46, p < .01), indicating that the learning among these adolescents, while present (slope = −6.44, t(38) = −2.28, p = .03), still was not adult-like. Thus, it appears that the influence of this training regimen on across-session performance becomes fully mature sometime after 14 years of age.
We also analyzed the results from all three trained groups using only the data from the first five, rather than from all 10, training sessions. In doing so, we focused the analyses on those training sessions across which the adults showed the greatest improvements and also eliminated the across-session worsening that was observed in the 11-year-old group. Only the adults learned over the first five sessions (slope = −4.36, t(28) = −2.44, p < .01); the 11-year-olds and the 14-year-old non-learners neither improved nor worsened (11-year-olds: slope = 8.02, t(23) = 0.99, p = .33; 14-year-old non-learners: slope = 5.90, t(18) = 0.44, p = .67). Thus, the adolescents classified as non-learners showed no improvement over the period of time during which the adults learned the most and hence were not adult-like even before they began to worsen with additional training. Further, the 14-year-olds who learned over all 10 sessions did not improve over the first five sessions (Figure 1e; slope = 0.51, t(18) = 0.09, p = .93), indicating that the learning in adolescents, when present, occurred at a slower rate than in adults.
Within-session performance
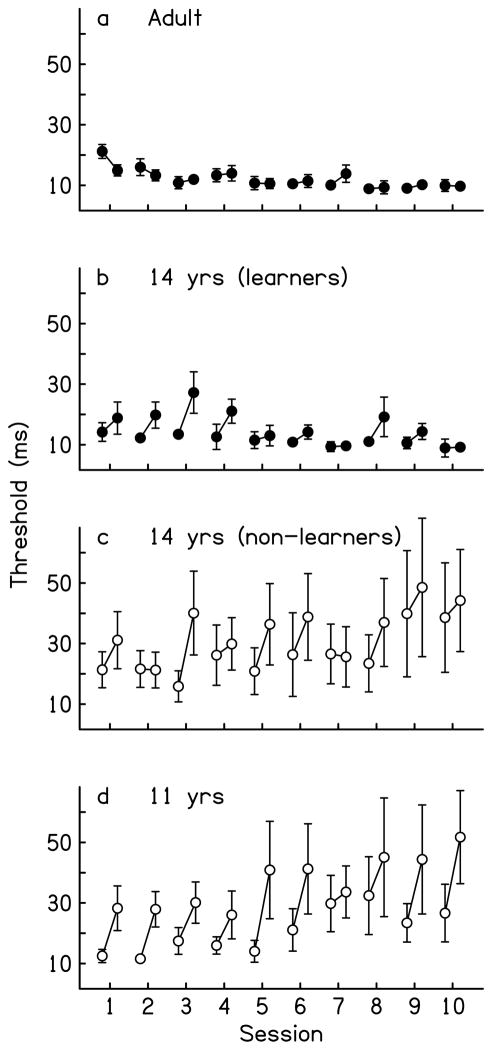
In addition to these across-session differences, there were also developmental changes in the patterns of performance within sessions. Within each session, the thresholds of the 11- and 14-year-olds increased across the multiple estimates, while those of the adults either improved or stayed relatively constant (Figure 2). To analyze within-session performance, we compared the thresholds obtained early to those obtained late in each training session separately for each group using 2 time (early: mean of first three thresholds per session vs. late: mean of last three thresholds) × 10 session ANOVAs with repeated measures on time. For the adults (Figure 2a), the influence of time differed across sessions (time × session interaction: F(9, 50) = 2.58, p = .02). The adults improved during the first session but not in any subsequent sessions, according to follow-up paired t-tests on the early versus late performance within each session (session 1: t(5) = 4.99, p < .01; sessions 2–10: all |t(5)| ≤ 2.03, all p ≥.10; see also Wright & Sabin, 2007). In contrast, for each of the three adolescent groups (Figure 2b–d), performance tended to deteriorate within sessions, as indicated by significant main effects of time and non-significant time by session interactions (main effects for time: all F(1, 40 or 1, 30) ≥ 15.38, all p < .01; time × session interactions: all F(9, 40 or 9, 30) ≤ 1.07, all p ≥.41). Direct comparisons across the groups confirmed that the patterns of within-session performance differed with age (4 group × 2 time × 10 session ANOVA; group × time interaction: F(3, 150) = 10.59, p < .01). The adult pattern differed from that of each of the three adolescent groups (2 group × 2 time interactions: all F(1, 90 or 1, 80) ≥ 13.18, p < .01). Among the adolescents, the magnitude of within-session worsening was greater for the 11-year-olds than for the 14-year-old learners (F(1, 70) = 7.08, p = .01), while that of the 14-year-old non-learners differed from neither of the other two adolescent groups (both F(1, 60 or 1, 70) ≤ 2.54, p ≥ .15). For the 14-year-old learners, the magnitude of the worsening decreased over the course of training, as indicated by a significant main effect of time (early vs. late) during the first five sessions of the training phase (F(1, 15) = 10.14, p = .01) but not during the last five sessions (F(1, 15) = 3.04, p = .10). Within-session worsening was significant during both the first five and last five sessions for the other two adolescent groups (main effects for time: all F(1, 15 or1, 20) ≥ 4.84, all p ≤.04). Thus, it looks as if within-session performance with this regimen changes during adolescence and becomes fully mature sometime after 14 years of age.
Figure 2.
Within-session performance of adults, 14-year-olds, and 11-year-olds. (a–d) Mean values for the first and last three threshold estimates of each training session. Results are shown separately for the adults (a), 14-year-old learners (b), 14-year-old non-learners (c), and 11-year-olds (d). Groups that learned across sessions are represented by filled circles and those that did not learn across sessions are represented by open circles. Error bars indicate ± one standard error.
To determine whether the within-session worsening was masking across-session improvement in the adolescents, we reanalyzed the adolescent data using only the first three (early) or lowest three (best) threshold estimates per session from each listener. Just as when all of the estimates were included in the analyses, both the early and the best thresholds of the adolescent groups neither improved nor worsened across the first five sessions (all |t(18 or 23) | ≤ 0.91, all p ≥.37), while over all 10 sessions, these thresholds got worse in the 11-year-olds (early: t(48) = 2.64, p = .01, best: t(48) = 6.01, p = .04), did not change in the 14-year-old non-learners (both t(38) ≤ 1.37, p ≥.18) and improved in the 14-year-old learners (early: t(38) = −2.05, p = .05, best: t(38) = −2.39, p = .02). These results suggest that the lack of across-session improvement in the adolescent non-learners cannot be attributed solely to their within-session worsening. They also imply that the decrease in within-session worsening over the course of training in the 14-year-old learners cannot fully account for the across-session improvement in that group. We also note that, even though the groups were matched for pre-test performance, the first three (early) thresholds of the first session were actually significantly better for the 11-year-olds than the adults (t(9) = 1.39, p = .03). However, this difference does not seem to account for the lack of across-session learning in the 11-year-olds. For example, during the first session, the early thresholds of the 11-year-olds who did not learn (mean = 12.5 ms) were similar to those of the 14-year-olds who did (mean = 14.2 ms), suggesting that good performance early in Session 1 did not preclude across-session learning. Conversely, the adults who learned had early thresholds in Session 1 (mean = 21.2 ms) that were quite similar to those of the 14-year-olds who did not improve (mean = 21.3 ms), suggesting that poorer performance early in Session 1 did not assure across-session learning.
Discussion
The present results suggest that mature perceptual learning on auditory temporal-interval discrimination does not emerge until late in adolescence, even after naïve performance is adult-like. The 10-session training regimen that we employed yielded learning in the adults, but not in most of the adolescents tested. The improvement in adults accumulated across sessions and typically occurred between, rather than within, sessions. In contrast, the majority of the adolescents, all of whom started like adults, did not improve across the sessions and actually got worse within each session. Even the adolescents who improved across sessions learned more slowly than adults and showed deteriorating performance during each session. Based on these cross-sectional data, it appears that both across- and within-session performance reached maturity sometime after 14 years of age.
It is possible that more of the adolescents would have exhibited mature learning under different circumstances because learning patterns can differ markedly across different tasks and training regimens (for reviews, see Fine & Jacobs, 2002, visual learning; Wright & Zhang, 2009, auditory learning). However, for any given task, adolescents and adults should respond differently to the same regimen only if some aspect of learning is immature in adolescents. Conversely, if learning on a particular task is mature in adolescents, then, regardless of the training regimen, identically trained adolescents and adults should show the same learning patterns. Thus, even if the present lack of learning in adolescents is a product of the particular task and training regimen used here, the demonstration that the effectiveness of training increases with age suggests that perceptual learning can have a prolonged developmental course.
The maturation of human perceptual learning has been directly evaluated in only two previous studies, each of which employed a single training session and thus provided information about only within-session performance. In one case, an investigation of aging-related changes in visual perception, single-session learning on vernier acuity did not differ among groups of participants ranging in age from 12 to 66 years (Fahle & Daum, 1997). However, evidence of the maturation of this learning may have been obscured because the youngest age group was composed of individuals between 12 and 20 years of age, an age span similar to that over which we observed marked developmental changes. In the other case, 6- to 11-year-olds were given a single session of training on auditory frequency discrimination to determine whether they could reach the starting performance of naïve adults through practice (Halliday et al., 2008). Learning among the children was reported to be similar to that of adults who had completed the same training regimen. However, those children who had adult-like performance prior to training did not benefit from the training, suggesting a possible immaturity in learning (though parallel analyses confirming learning in the adult group alone were not reported). In addition to these limited studies of the development of perceptual learning in humans, a recent report documents the influence of multiple-session perceptual training on the detection of amplitude modulation in juvenile and adult gerbils (Sarro & Sanes, 2009). Perceptual learning in the gerbils showed similar maturational changes to those that we report in humans in this study
The present results demonstrate maturational differences in perceptual learning both within and across training sessions. Improvement over multiple sessions necessarily requires that the specific experiences that lead to improvement have been acquired and subsequently consolidated into a more stable, longer lasting form. Therefore the lack of mature across-session improvement in the adolescents indicates an immaturity in either the acquisition or consolidation of learning. Proposed requirements for the successful completion of each of these stages in adults point to several possible contributors to this immaturity.
Two apparent requirements for the successful acquisition of learning in adults are the adequate engagement of top-down processes such as attention or reward and the provision of sufficient daily training. Immaturities affecting either or both of these elements could have disrupted across-session learning in the adolescents. Top-down processes appear to play a key role in perceptual learning (Ahissar et al., 2009; Fahle, 2009; Li, Piech & Gilbert, 2004; Polley, Steinberg & Merzenich, 2006; Seitz & Dinse, 2007). Activation of these processes is thought to be necessary for the selection and sensitization of the neural substrate to be modified. Many top-down processes, including attention, reward (Geier & Luna, 2009; Spear, 2000), working memory (Luna, Garver, Urban, Lazar & Sweeney, 2004; Swanson, 1999), and executive functions (Kuhn, 2006; Welsh, Pennington & Groisser, 1991), as well as the brain regions associated with them (for a review, see Toga, Thompson & Sowell, 2006), continue to develop into adolescence or young adulthood. Thus, the particular top-down processes involved in adult learning, or the capacity of the targeted neural substrate to be influenced by these processes, may have been immature in the adolescents examined here. If so, these processes may have failed to engage, or to sustain the engagement of, the substrate to be modified, resulting in the observed lack of improvement across sessions. Acquisition also requires a sufficient number of daily training trials (Seitz & Dinse, 2007; Wright & Sabin, 2007). The stimuli encountered during these trials are thought to activate the substrate to be modified, with a critical level of activation required for successful learning. Thus, the adolescents may have needed more activation than the adults, or may have had the same activation requirement as adults but needed more trials to reach it. In either case, the adolescents would require more than the 900 daily training trials provided in the present training regimen. This number already far exceeds the number required for adults, who improve on temporal-interval discrimination with only 360 daily trials (Wright & Sabin, 2007).
It is important to note that the within-session worsening observed in the adolescents does not necessarily indicate a failure in acquisition. The performance of both the 11- and 14-year-olds deteriorated within the training sessions, while that of the adults improved during the first session and stayed relatively constant within each of the subsequent sessions. However, these developmental differences in within-session behavior cannot account for the lack of across-session improvement in the adolescents. Even among adults, improvement during the first session is not a requirement for improvement across sessions. Adults who practiced the present trained task for only 360 trials (rather than 900 trials) per session showed no learning within the first session but nevertheless improved across sessions (Wright & Sabin, 2007). The 14-year-old learners also learned across sessions without any improvement within the first or any other session. Further, across-session improvement can occur despite within-session worsening. Perceptual deterioration within a single training session, accompanied by across-session learning, has been reported in adults (Mednick, Nakayama, Cantero, Atienza, Levin, Pathak & Stickgold, 2002; Mednick, Arman & Boynton, 2005; Mednick, Drummond, Arman & Boynton, 2008) and was also observed in multiple sessions here in the 14-year-old learners. In the cases where within-session worsening was reported in adults, it was attributed to excessive neural stimulation after the results of control experiments indicated minimal contributions of waning attention, dwindling motivation, or overall fatigue (Mednick et al., 2002; Mednick et al., 2008). Overstimulation also may have caused the worsening in the adolescents here. If so, these adolescents were much more susceptible to overstimulation than the adults, because the adults showed no deterioration with the same number of trials. The possibility also remains that inattention, poor motivation, and fatigue, which apparently play a minimal role in within-session worsening in adults, do contribute to this behavior in adolescents. However, this alternative seems inconsistent with the decline in within-session worsening across the multiple training sessions in the 14-year-old learners, because the negative influence of these general factors is likely to increase rather than decrease across training sessions.
Regardless of the exact cause of within-session worsening in the adolescents, the lack of such worsening in the adults indicates that the performance of the adolescents during the period of training was immature. The observation of learning across sessions both when there was within-session worsening (as in the 14-year-old learners) and when there was either improvement or no change within sessions (as in the adults) indicates that some of the processes engaged during a period of training need not be fully mature, and may even be unnecessary for, the acquisition that enables across-session learning. Thus, it could be that the adolescents required more daily stimulation than we provided in order to improve across sessions but less to prevent within-session worsening.
In the event that acquisition was successful in the adolescents, their lack of across-session learning would indicate a failure in consolidation. During consolidation, the experiences obtained during acquisition are transferred into a more permanent form through a cascade of molecular and systemic processes (see Dudai, 2004, for a review). Immaturities in any one of these processes could have precluded consolidation of temporal-interval discrimination learning under any circumstances. Barring that, these immaturities could have altered the requirements for these processes to occur. In adults, two apparent requirements for consolidation are sufficient time or sleep between sessions and the absence of interfering events. There is recent evidence from outside the perceptual learning literature that the exact nature of these requirements may change with development. A period of rest between training and testing yielded improvements in performance – a behavioral manifestation of consolidation – in both children and adults on a motor sequence task (children aged 6 to 8 years) (Wilhelm, Diekelmann & Born, 2008) and a test of implicit learning of grammaticality (children aged 7 to 11 years) (Fischer, Wilhelm & Born, 2007). However, only adults benefited from a period of sleep between the training and testing sessions. Likewise, an event that interfered with between-session improvements in motor-sequence performance in 17-year-olds did not do so in 9- and 12-year-olds (Dorfberger, Adi-Japha & Karni, 2007). Assuming that immaturity did not prevent consolidation entirely, the adolescents who failed to learn across sessions here may have needed a different amount of time or sleep between sessions or been more rather than less (as in the motor learning study) sensitive to intervening events than were the adults. It is also possible that the requirements for consolidation were the same for the adolescents and adults, but the adolescents failed to meet them because they slept less than the adults or engaged in activities that would have interfered with consolidation at any age. These potential explanations apply most clearly to the first five training sessions, over which the performance of the adults improved but that of the adolescents stayed relatively constant. The worsening that occurred in the 11-year-olds over the subsequent training sessions may have a different source. An obvious possibility is that this across-session worsening resulted from waning attention or motivation. However, it could also have been a consequence of cumulative overstimulation, which would itself be a form of consolidation.
The present data add to a growing body of evidence that perceptual development, once thought to be complete early in life, continues well into adolescence. While most studies of perceptual development focus on infants, young children, and school-aged populations, there are a few recent reports that this development continues into adolescence on some tasks (Alain, Theunissen, Chevalier, Batty & Taylor, 2003; Hartley, Wright, Hogan & Moore, 2000; Knoblauch, Vital-Durand & Barbur, 2000; Kovacs, Kozma, Feher & Benedek, 1999; Skoczenski & Norcia, 2002; Wightman, Kistler & Brungart, 2006). The current results build on these reports, which focus on naïve perceptual performance, by demonstrating that perceptual learning can also change during adolescence. They further imply that the processes underlying naïve performance may mature prior to those underlying perceptual learning. Taken together, these data suggest that perceptual learning reaches maturity late in adolescence, that this prolonged developmental course may arise from immaturity in consolidation as well as in acquisition, that adult-like starting performance is not necessarily a predictor of mature learning, and that the most successful perceptual training regimens will differ between adolescents and adults.
Acknowledgments
We thank N. Marrone, A. Sabin, Y. Zhang and two anonymous reviewers for helpful comments on previous drafts of this manuscript, the Office of Catholic Schools in Chicago for their assistance with listener recruitment, and M. Fitzgerald for his help with data collection from the adults. This work was supported by the National Institutes of Health (1F31DC08250 to JJH, 5R01DC004452 to BAW).
References
- Ahissar M, Nahum M, Nelken I, Hochstein S. Reverse hierarchies and sensory learning. Philosophical Transactions of the Royal Society London, B Biological Sciences. 2009;364 (1515):285–299. doi: 10.1098/rstb.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C, Theunissen EL, Chevalier H, Batty M, Taylor MJ. Developmental changes in distinguishing concurrent auditory objects. Brain Research, Cognitive Brain Research. 2003;16 (2):210–218. doi: 10.1016/s0926-6410(02)00275-6. [DOI] [PubMed] [Google Scholar]
- Banai K, Ortiz JA, Oppenheimer JD, Wright BA. Learning two things at once: different constraints on the acquisition and consolidation of perceptual learning. Neuroscience. 2010;165 (2):436–444. doi: 10.1016/j.neuroscience.2009.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Karni A, Sagi D. A link between perceptual learning, adaptation and sleep. Vision Research. 2006;46 (23):4071–4074. doi: 10.1016/j.visres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Dorfberger S, Adi-Japha E, Karni A. Reduced susceptibility to interference in the consolidation of motor memory before adolescence. PLoS One. 2007;2 (2):e240. doi: 10.1371/journal.pone.0000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annual Review of Psychology. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Edwards VT, Giaschi DE, Low P, Edgell D. Sensory and nonsensory influences on children's performance of dichotic pitch perception tasks. Journal of the Acoustical Society of America. 2005;117 (5):3157–3164. doi: 10.1121/1.1861599. [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning and sensorimotor flexibility: cortical plasticity under attentional control? Philosophical Transactions of the Royal Society London, B Biological Sciences. 2009;364 (1515):313–319. doi: 10.1098/rstb.2008.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M, Daum I. Visual learning and memory as functions of age. Neuropsychologia. 1997;35 (12):1583–1589. doi: 10.1016/s0028-3932(97)00069-9. [DOI] [PubMed] [Google Scholar]
- Fine I, Jacobs RA. Comparing perceptual learning tasks: a review. Journal of Vision. 2002;2 (2):190–203. doi: 10.1167/2.2.5. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wilhelm I, Born J. Developmental differences in sleep’s role for implicit off-line learning: comparing children with adults. Journal of Cognitive Neuroscience. 2007;19 (2):214–227. doi: 10.1162/jocn.2007.19.2.214. [DOI] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacology Biochemistry and Behavior, Impulsivity and Frontal Lobes: Roles in Psychopathology and Addiction. 2009;93 (3):212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday LF, Taylor JL, Edmondson-Jones AM, Moore DR. Frequency discrimination learning in children. Journal of the Acoustical Society of America. 2008;123 (6):4393–4402. doi: 10.1121/1.2890749. [DOI] [PubMed] [Google Scholar]
- Hartley DEH, Wright BA, Hogan SC, Moore DR. Age-related improvements in auditory backward and simultaneous masking in 6- to 10-year-old children. Journal of Speech, Language and Hearing Research. 2000;43:1402–1415. doi: 10.1044/jslhr.4306.1402. [DOI] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. Temporal specificity of perceptual learning in an auditory discrimination task. Learning & Memory. 2003;10 (2):141–147. doi: 10.1101/lm.55503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch K, Vital-Durand F, Barbur JL. Variation of chromatic sensitivity across the life span. Vision Research. 2000;41:23–36. doi: 10.1016/s0042-6989(00)00205-4. [DOI] [PubMed] [Google Scholar]
- Kovacs I, Kozma P, Feher A, Benedek G. Late maturation of visual spatial integration in humans. Proceedings of the National Academy of Sciences, USA. 1999;96 (21):12204–12209. doi: 10.1073/pnas.96.21.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. Do cognitive changes accompany developments in the adolescent brain? Perspectives on Psychological Science. 2006;1 (1):59–67. doi: 10.1111/j.1745-6924.2006.t01-2-.x. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America. 1971;49:467–477. [PubMed] [Google Scholar]
- Li W, Piech V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nature Neuroscience. 2004;7 (6):651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75 (5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Arman AC, Boynton GM. The time course and specificity of perceptual deterioration. Proceedings of the National Academy of Sciences, USA. 2005;102 (10):3881–3885. doi: 10.1073/pnas.0407866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, Drummond SP, Arman AC, Boynton GM. Perceptual deterioration is reflected in the neural response: fMRI study of nappers and non-nappers. Perception. 2008;37 (7):1086–1097. doi: 10.1068/p5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, Nakayama K, Cantero JL, Atienza M, Levin AA, Pathak N, Stickgold R. The restorative effect of naps on perceptual deterioration. Nature Neuroscience. 2002;5 (7):677–681. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nature Neuroscience. 2003;6 (7):697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Jenkins WM, Johnston P, Schreiner C, Miller SL, Tallal P. Temporal processing deficits of language-learning impaired children ameliorated by training. Science. 1996;271 (5245):77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. Journal of Neuroscience. 2006;26 (18):4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro E, Sanes D. Auditory learning in juvenile and adult animals. Abstracts of the Proceedings of the 32nd Annual Mid-Winter Meeting of the Association for Research in Otolaryngology. (A).2009. [Google Scholar]
- Seitz AR, Dinse HR. A common framework for perceptual learning. Current Opinion in Neurobiology. 2007;17 (2):148–153. doi: 10.1016/j.conb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Skoczenski AM, Norcia AM. Late maturation of visual hyperacuity. Psychological Science. 2002;13 (6):537–541. doi: 10.1111/1467-9280.00494. [DOI] [PubMed] [Google Scholar]
- Soderquist DR, Moore ML. Effect of training on frequency discrimination in primary school children. Journal of Auditory Research. 1970;10 (3):185–192. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24 (4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Swanson HL. What develops in working memory? A life span perspective. Developmental Psychology. 1999;35 (4):986–1000. doi: 10.1037//0012-1649.35.4.986. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29 (3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF, Groisser DB. A normative-developmental study of executive function: a window on prefrontal function in children. Developmental Neuropsychology. 1991;7 (2):131–149. [Google Scholar]
- Wightman F, Kistler D, Brungart D. Informational masking of speech in children: auditory-visual integration. Journal of the Acoustical Society of America. 2006;119 (6):3940–3949. doi: 10.1121/1.2195121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Diekelmann S, Born J. Sleep in children improves memory performance on declarative but not procedural tasks. Learning & Memory. 2008;15 (5):373–377. doi: 10.1101/lm.803708. [DOI] [PubMed] [Google Scholar]
- Wright BA, Buonomano DV, Mahncke HW, Merzenich MM. Learning and generalization of auditory temporal-interval discrimination in humans. Journal of Neuroscience. 1997;17:3956–3963. doi: 10.1523/JNEUROSCI.17-10-03956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BA, Sabin AT. Perceptual learning: how much daily training is enough? Experimental Brain Research. 2007;180 (4):727–736. doi: 10.1007/s00221-007-0898-z. [DOI] [PubMed] [Google Scholar]
- Wright BA, Zhang Y. Insights into human auditory processing gained from perceptual learning. In: Gazzangiga MS, editor. The cognitive neurosciences IV. Cambridge, MA: MIT Press; 2009. pp. 353–366. [Google Scholar]