Abstract
Background. Most deaths in the 1918 influenza pandemic were caused by secondary bacterial pneumonia. Methods. We performed a systematic review and reanalysis of studies of bacterial vaccine efficacy (VE) in preventing pneumonia and mortality among patients with influenza during the 1918 pandemic.
Results.A meta-analysis of 6 civilian studies of mixed killed bacterial vaccines containing pneumococci identified significant heterogeneity among studies and estimated VE at 34% (95% confidence interval [CI], 19%-47%) in preventing pneumonia and 42% (95% CI, 18%-59%) in reducing case fatality rates among patients with influenza, using random-effects models. Using fixed-effect models, the pooled VE from 3 military studies was 59% (95% CI, 43%-70%) for pneumonia and 70% (95% CI, 50%-82%) for case fatality. Military studies showed less heterogeneity and may provide more accurate results than civilian studies, given the potential biases in the included studies. Findings of 1 military study using hemolytic streptococci also suggested that there was significant protection.
Conclusions.Despite significant methodological problems, the systematic biases in these studies do not exclude the possibilities that whole-cell inactivated pneumococcal vaccines may confer cross-protection to multiple pneumococcal serotypes and that bacterial vaccines may play a role in preventing influenza-associated pneumonia.
The 1918 influenza pandemic caused an estimated 20–100 million deaths worldwide [1]. There is growing epidemiologic, clinical, and pathologic evidence that the majority of deaths in this pandemic resulted directly from secondary bacterial pneumonia [2–5]. In the 1918 pandemic, Streptococcus pneumoniae was the predominant organism isolated from antemortem cultures from normally sterile sites in patients with influenza-associated pneumonia, followed by hemolytic streptococci, presumably representing Streptococcus pyogenes [4, 5].
The etiology of influenza was unknown at the time of the 1918 pandemic. Many contemporaneous investigators erroneously believed that bacteria, in particular Bacillus influenzae (Pfeiffer's bacillus, now known as Haemophilus influenzae) was the cause of influenza [6].
It was also generally believed, however, that most 1918 pandemic influenza deaths resulted from secondary bacterial pneumonia following primary influenza infections of whatever cause [2]. In attempts to prevent the primary disease of influenza, to reduce pneumonia and mortality, and to investigate the etiology of influenza, many bacterial vaccines were produced, tested, and administered during the 1918 pandemic. Here we review studies of whole-cell bacterial vaccines administered to healthy subjects during the 1918 pandemic to examine their efficacy in preventing influenza-associated pneumonia and mortality.
An important concern about such a review is that the scientific quality of 1918 vaccine studies was low by today's standards, owing to such methodological flaws as lack of subject randomization. Moreover, whereas most vaccinations were given during the declining phase of the pandemic (fall-winter 1918–1919), the incidences of influenza, influenza-associated pneumonia, and deaths in vaccinated individuals were usually compared with the same outcomes in unvaccinated individuals from the beginning of the epidemic [6, 7], introducing unequal observation periods more favorable to vaccinated individuals. In addition, vaccinated individuals might come from select populations with reduced exposure or susceptibility to influenza because they had not had influenza between the appearance of the pandemic and the start of vaccination. Not fully appreciating such potential design flaws, investigators studying bacterial vaccines often believed that they had demonstrated a reduction in the incidence of influenza, which is not consistent with our understanding of influenza etiology.
We reasoned that any true effect of bacterial vaccines on influenza disease might more plausibly result from reduced attack rates of secondary bacterial pneumonias and consequent reduced case fatality rates among patients with influenza. To examine this possibility while addressing methodological flaws of the original studies, we reanalyzed published data, asking whether vaccinated patients who developed influenza had lower attack rates of pneumonia or lower case fatality rates than unvaccinated patients with influenza. This approach should diminish bias caused by unequal observation periods, because these measures were less likely to be influenced by changing influenza incidence during the progress of the pandemic. In addition, the attack rate of pneumonia and case fatality rates among patients with influenza seems to be higher in the later phase of influenza epidemics [8–10]. Therefore, this approach may result in more conservative estimates of vaccine efficacy (VE), because the vaccinated individuals were more likely to be from the later phase of the 1918 pandemic.
Methods
Search strategy and criteria. In an effort to obtain all relevant publications reporting bacterial vaccine studies in the 1918 pandemic, a literature search was performed on the Journal Storage (JSTOR) database using the search terms “ influenza or flu,” “ vaccine or vaccination or inoculation” and “ year: 1918 to 1920” in the full text, without language restriction. We also manually searched 2 bibliographic sources— Index Medicus and Index-Catalogue of the Library of the Surgeon-General's Office— for relevant articles in any language between 1918 and 1920. In addition, we examined all articles from an archive at the National Institute of Allergy and Infectious Diseases, National Institutes of Health (http://www3.niaid.nih.gov/topics/Flu/1918/bibliography.htm) [3]. The archive was originally developed to identify publications containing information on influenza pathology and bacteriology in the 1918 pandemic but was expanded to contain other topics. We examined all retrieved articles to identify additional articles.
Selection criteria and data extraction. Original reports of prophylactic administration of bacterial vaccines to humans during the autumn 1918 or winter 1918–1919 pandemic waves were eligible for inclusion. We then searched for studies in which case fatality rates or attack rates of pneumonia among both vaccinated and unvaccinated patients with influenza could be determined. Vaccinated patients with influenza were defined as patients with clinically diagnosed influenza who had received ⩾1 dose of a bacterial vaccine at any time before the onset of influenza. We excluded reports that did not provide exact denominators (numbers of vaccinated and unvaccinated patients with influenza) or in which 1 or both of the vaccine exposure denominators was <10. When multiple publications reported results from the same study population, only results from the most recent publication were included. Because these early articles did not provide much detail, we assessed the quality of study using 4 criteria: (1) whether vaccinees were randomized, (2) whether vaccination was completed before the occurrence of the first patients with influenza in the facility, (3) whether the vaccinated and unvaccinated groups were from the same population, and (4) whether the bacterial vaccine given was not reported to reduce the incidence of influenza among vaccinated subjects compared with unvaccinated subjects.
Statistical methods. For each included study, we calculated unadjusted risk ratios (RRs) comparing case fatality rates and pneumonia attack rates in vaccinated and unvaccinated patients with influenza, with 95% confidence intervals (CIs). When no pneumonia cases or deaths were recorded for a study group, a value of 0.5 was assigned. VE was calculated as 1 -RR.
We stratified the studies according to the vaccine formula and study population (civilian or military). Meta-analysis was performed on studies of bacterial vaccines containing pneumococci. An estimate of heterogeneity across studies was assessed using Q and I2 statistics; heterogeneity was considered significant at P< .10 (Q statistic) or I2 > 50% [11]. When significant heterogeneity was found, pooled RR estimates and 95% CIs were derived using a random-effects model; otherwise, a fixed-effects model with Mantel-Haenszel weighting was used. Publication bias was assessed by using funnel plots [11]. To explore the sensitivity of the meta-analysis results, we (1) determined whether the results were strongly influenced by excluding each included study one at a time and (2) used the “ trim-and-fill” method to adjust for potential publication bias [12]. Analyses were performed using free MIX software (version 1.7) [13, 14].
Results
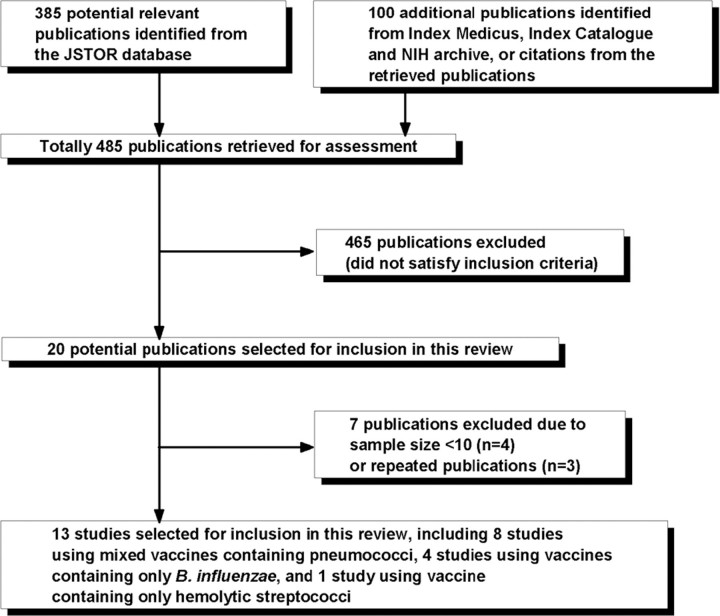
Study selection. We identified and retrieved full texts of 485 publications for assessment. Figure 1 summarizes the study selection process; 13 studies were included in the final analysis.
Characteristics and quality of included studies. Information on the vaccines in the 13 studies is shown in Table 1. Eight studies reported mixed inactivated vaccines containing multiple serotypes of S. pneumoniae in addition to other bacteria, such as B. influenzae , hemolytic streptococci, or Staphylococcus aureus [15, 16, 18–23] . Four studies used a vaccine containing multiple strains of B. influenzae [24, 25, 27, 28], and the remaining study used a vaccine containing multiple strains of hemolytic streptococci [29]. The strains of bacteria used in the vaccines were usually obtained from patients during local influenza epidemics. The vaccines were whole-cell bacterial vaccines inactivated by heat, tricresol, or chloroform. The amounts of each organism and the inoculation schedules differed between studies.
The characteristics of the 13 studies are shown in Table 2. Eight studies were from civilian and 3 from military populations. Cadham reported military and civilian data separately [15]. In the study by Minaker and Irvine, vaccinated individuals were mainly from the military, and unvaccinated individuals mainly from the civilian population [21]; this study was regarded as a civilian study in our analyses.
None were double-blinded randomized trials. The studies by McCoy et al and Hinton and Kane were considered the highest quality, because vaccinated individuals were assigned randomly and vaccinations were completed before outbreaks appeared in the facilities where they were performed [20, 25]. The study reported by Minaker and Irvine was lowest in quality, because vaccinated and unvaccinated persons were from different populations [21]. For the rest of the studies, vaccinated and unvaccinated subjects were from the same military or civilian populations, but it was not possible to fully evaluate their comparability, owing to insufficient information on potential confounders, such as age, sex, and health status, as well as on how vaccinated individuals were chosen.
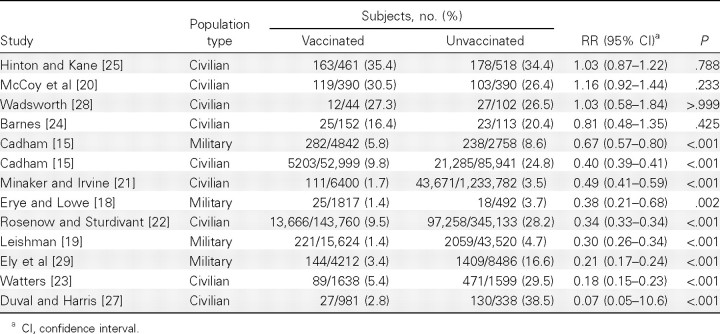
Table 3 shows the incidence of influenza among vaccinated and unvaccinated subjects, as reported in the original analyses of all the studies except the Cherry study, which also used patients with influenza as the denominator in the analysis [16]. The incidence of influenza among unvaccinated subjects varied from 3.5% to 38.5%, probably reflecting differences in case identification, among other factors. According to the US house-to-house survey, ∼28% of the population suffered an influenza attack during the 1918 pandemic [30]. Three studies with an influenza incidence of <10% probably used hospital admission records for case identification, whereas those with an incidence close to 28% may have included outpatients with influenza.
Except for 2 studies with random allocation [20, 25] and 1 small study [28], the included studies found a lower incidence of influenza in vaccinated subjects, presumably because vaccination usually began after the epidemic had occurred. We reanalyzed the original data including only patients with diagnosed influenza in the denominators to compare attack rates of influenza-associated pneumonia and case fatality among vaccinated and unvaccinated patients with influenza.
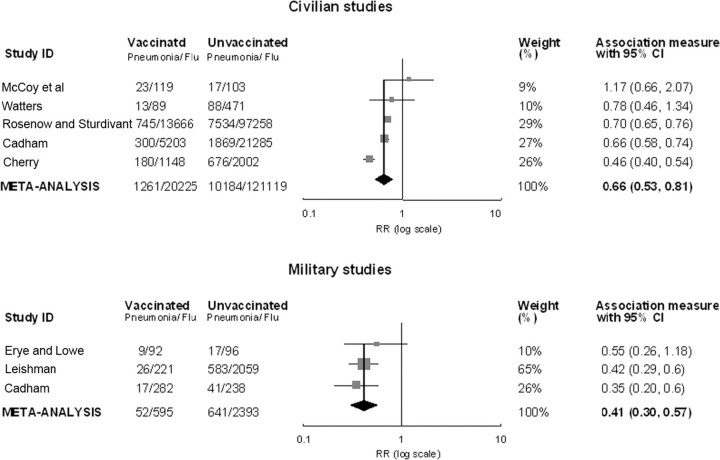
Effect of mixed bacterial vaccines containing pneumococci on the attack rate of pneumonia. RR estimates for the comparison of pneumonia attack rates between vaccinated and unvaccinated patients with influenza ranged from 0.46 to 1.17 in for a VE of 34% (95% CI, 19%-47%). Combined RRs were 5 civilian studies (Figure 2). Three of the 5 studies showed changed most by excluding the Cherry study [16]; after exclusignificant vaccine protection against pneumonia, but findings sion of that study, no heterogeneity was indicated, and the of the highest-quality study, by McCoy et al, suggested no pro-pooled VE with a fixed-effects model was 31% (95% CI, 26%- tective effect [20]. There was heterogeneity among the civilian 35%). The funnel plot suggested potential publication bias, and studies (P< .001 for Q statistic; I2 = 86.23%). The random-the trim-and-fill adjusted VE was 40% (95% CI, 26%-51%) effects estimate of pooled RRs was 0.66 (95% CI, 0.53–0.81), for the civilian studies.
Figure 2.
Random-effects meta-analysis of 8 risk ratio (RR) estimates comparing attack rates of pneumonia among vaccinated and unvaccinated patients with influenza in studies of bacterial vaccines containing pneumococci, stratified by study population (civilian or military). RRs of <1 indicated that the vaccine was protective. Point estimates and 95% confidence intervals (CIs) are shown for each study and for pooled results. Data are plotted on a log base 10 scale.
RR estimates from 3 military studies ranged from 0.35 to 0.55, statistically significant in 2 studies (Figure 2). There was no heterogeneity for the military studies (P = .627 for Q statistic; I2 = 0%). The pooled VE using a fixed-effect model was 59% (95% CI, 43%-70%). After exclusion of the Cadham study, the pooled VE was 57% (95% CI, 37%-70%), and the trim-and-fill adjusted VE was 60% (95% CI, 46%-70%).
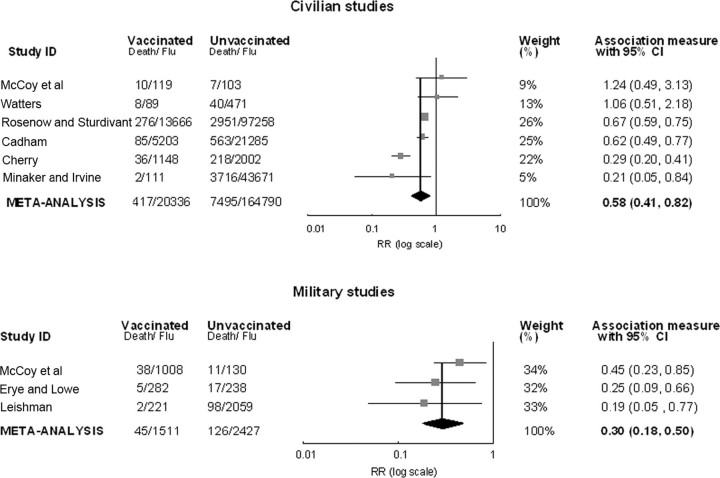
Effect of mixed bacterial vaccines containing pneumococci on case fatality rates. Four of 6 civilian studies showed a significant protective effect of the bacterial vaccines for reducing influenza case fatality rates, but the best-quality study, by McCoy et al, did not suggest any vaccine effect [20] (Figure 3). There was significant heterogeneity (P< .001 for Q statistic; I2 = 81.47%). The random-effects pooled RR among civilian studies was 0.58 (95% CI, 0.41–0.82), for a VE of 42% (95% CI, 18%-59%). After exclusion of the Cherry study [16], which had the strongest influence on meta-analysis results, no heterogeneity was indicated, and the fixed-effects VE estimate was 34% (95% CI, 27%-41%). The funnel plot did not suggest publication bias.
Figure 3.
Random-effects meta-analysis of 9 risk ratio (RR) estimates comparing case fatality rates among vaccinated and unvaccinated patients with influenza in studies of bacterial vaccines containing pneumococci, stratified by study population (civilian or military). RRs of <1 indicated that the vaccine was protective. Point estimates and 95% confidence intervals (CIs) are shown for each study and for pooled results. Data are plotted on a log base 10 scale.
RR estimates from 3 military studies ranged from 0.19 to 0.45, all statistically significant (Figure 3). There was no heterogeneity for the military studies (P = .360 for Q statistic; I2 = 2.25%). The fixed-effects estimate of VE was 70% (95% CI, 50%-82%). After exclusion of the Leishman study, the combined efficacy was 65% (95% CI, 41%-79%). The trim-andfill efficacy, adjusted for potential publication bias, was 62% (95% CI, 37%-77%).
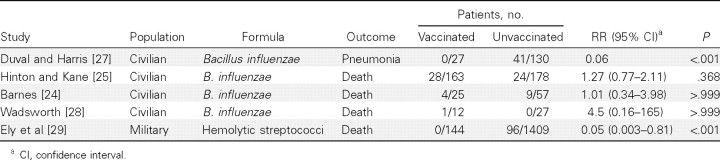
Effect of bacterial vaccines without pneumococci. Four civilian studies used bacterial vaccines containing pure B. influenzae (Table 4). The study by Hinton and Kane, with random allocation of vaccination, did not find a vaccine effect for the reduction of case fatality [25], and neither did 2 of the other studies [24, 28]. However, the remaining study, by Duval and Harris, suggested that the VE for reducing the attack rate of pneumonia was 94% (P < .001) [27]. Hemolytic streptococci were the main cause of influenza-associated pneumonia in the study by Ely et al, who reported use of a vaccine containing only this pathogen [29]; none of the 144 vaccinated patients with influenza died, and the estimated RR was 0.05 (P< .001), corresponding to a VE of 95% (95% CI, 19%–100%).
Table 4.
Attack Rates of Pneumonia and Case Fatality Rates among Vaccinated and Unvaccinated Patients with Influenza and Corresponding Risk Ratios (RRs) in Studies Using Vaccines Not Containing Pneumococci
Discussion
Strengths and limitations. The quality of vaccine studies in 1918–1919 was lower than that of studies conducted today, because accepted modern approaches to study design and evaluation were unknown or not well recognized in 1918. In addition, owing to the scope of the 1918 pandemic and the exigency of war, medical personnel were forced to work under a great strain, so it was difficult to obtain complete data and perform good trials at that time [15].
Misclassification of influenza or pneumonia may have occurred in the vaccine studies we examined, because diagnosis was based largely on physical examination findings and unstandardized diagnostic criteria. Vaccinated persons suffering from constitutional adverse reactions to the vaccine might be misclassified as having influenza, although these reactions usually appeared early and were of short duration [27]. Influenza cases diagnosed late in the pandemic may have reflected respiratory illness from less virulent viral infections after influenza activity decreased, potentially introducing differential misclassification, because vaccinated persons were usually from this phase of the pandemic. Chest radiographs were available at the time, but we do not know how much they were used in these studies to diagnose pneumonia. Because death is an outcome less susceptible to misclassification, analysis of case fatality should be less susceptible to bias. Except for 2 studies using random allocation, other studies failed to control for important confounders. Because of population homogeneity, better standardized diagnosis, and case identification, military studies should provide more valid estimates than civilian studies by controlling for factors that can influence pneumonia attack rates and case fatality rates, such as age, health status, and environmental exposure, as well as by reducing misclassification.
Subject self-selection was another potential problem in the 1918 vaccine studies, because vaccination was usually given voluntarily. However, the direction of potential “ volunteer bias” is difficult to determine and might differ among studies. One vaccine study conducted shortly after the pandemic found that high-risk individuals were more likely to be vaccinated [31], which would have resulted in bias toward the null. On the other hand, “ healthy vaccinee bias” is well described in observational influenza vaccine studies today and could have played a role in the 1918 studies [32]. It is unlikely that vaccination self-selection based on health status occurred in military populations, because the military population is fairly homogeneous and selected for excellent health.
Finally, although we sought to identify all existing articles, there may be studies that we did not find. However, we believe that missing reports would not have biased our results in a specific direction, because most contemporaneous vaccine studies did not use patients with influenza as the denominators in their analyses, as we did. Although we searched only reports published until the end of 1920, we believe that the time window we selected would have covered vaccine trials pertinent to the pandemic years, because clinical studies were published much more quickly than happens now. The latest study included in our analysis was published in February 1920 [19].
Despite these limitations, we believe that our method of analysis could remove biases caused by unequal observation periods and that the subgroup analysis of military studies may be less susceptible to other sources of bias. The estimated VE of bacterial vaccines containing pneumococci for preventing case fatalities in the military studies (70%) may be the most accurate figure in our analyses, because of less confounding, misclassification, and self-selection.
We hesitate to interpret findings in civilian studies, because residual biases could still be large in some civilian studies even with our method of analysis. Studies of B. influenzae provide a chance to examine this possibility, because that organism was not an important cause of secondary pneumonia in 1918 [4, 5]. Our analyses seemed to completely remove biases in the Barnes study [24]; using our method, we found no protective effect of B. influenzae vaccines, whereas the original analysis showed a significant protective effect. However, in our analysis of the study by Duval and Harris [27], the VE of this vaccine in preventing pneumonia was still estimated to be high. This study found a very high attack rate of pneumonia among unvaccinated patients with influenza (32%), suggesting that these patients were a very special population in this study and probably not a fair comparison group. This also reminds us of the limitations of observational vaccine studies; we need to be cautious in interpreting these results, because biases may not be completely removed, even with good statistical analyses.
Biologic plausibility. It has been suggested that most of the US Army training camps in ∼1918 experienced “colonization epidemics” with specific pathogenic bacteria, either pneumococci or hemolytic streptococci, resulting in a huge number of pneumonia cases caused by these 2 bacteria during epidemics of measles (winter 1917–1918) and influenza (fall 1918 and winter 1918–1919) [3, 3,3]. The effect of locally produced bacterial vaccines thus depended on the bacteria circulating locally. It is very likely that ⩾70% of military deaths were caused by secondary pneumonia, because soldiers were healthy adults unlikely to die because of deterioration of underlying medical conditions caused by influenza. In addition, 1 military study published in 1989 found a pneumococcal carriage rate of 1% among healthy men entering military service, compared with 13% among healthy recruits already in service [34], suggesting higher colonization prevalence and higher transmission of the pneumococcus in barracks.
Such a high VE might be less plausible if these whole-cell vaccines provided only type-specific protection, owing to the diversity of serotype distribution of pneumococci in 1918 [4]. Some of these vaccines included multiple pneumococcal strains known to be causing local epidemics and commonly isolated from pneumonia or fatal cases, but no systematic attempt could be made to identify and include strains beyond serotypes I-III, because the serologic tools to identify these strains were in their infancy. Findings of recent animal studies also support the possibility that whole-cell pneumococcal vaccines induce cross-protective (ie, broader than serotype-specific) immunity [35, 36]. Despite the likely diversity of hemolytic streptococcal strains in the 1918 pandemic, 1 small military study used a hemolytic streptococcal vaccine alone; if that vaccine used the dominant strain of group A streptococcus causing disease in the camp at that time, a high level of efficacy may be biologically plausible. Epidemics caused by a single M-type group A streptococcus have been shown in later military studies [37, 38]. Although it is important to consider the possibility of unappreciated biases, and the best-quality study (by McCoy et al [20]) with a small sample size suggested no vaccine effect, the data are generally consistent with a protective effect for the 2 types of bacterial vaccines designed to prevent infection with what are now accepted as the major causes of pneumonia and death in the 1918–1919 pandemic: pneumococci and hemolytic streptococci [4, 5].
Implications. This review supports the idea that although secondarily infecting bacteria played a major role in influenza-associated pneumonia and mortality in the 1918 pandemic, bacterial vaccines containing pneumococci could potentially reduce influenza-associated pneumonias and deaths in modern pandemics. Few contemporary studies have evaluated bacterial vaccinations in seasonal or pandemic influenza. In a double-blind randomized trial, a 9-valent pneumococcal conjugate vaccine given to young infants had a 45% efficacy in reducing seasonal influenza-associated pneumonia [39].
Even with the current availability of antibiotics, findings of autopsy studies using modern molecular techniques from the 2009–2010 H1N1 pandemic suggest that bacterial infections, particularly pneumococcal infections, were implicated in 29%-55% of deaths [40–42]. The current H1N1 pandemic has led to a shift in the age distributions for hospitalization, severe pneumonia, and death, from the expected elderly age groups to older children and young adults, who have been at low risk of influenza-associated complications in most other influenza pandemics and in seasonal influenza, [43, 44]. A recent study of this age group showed that the presence of pneumococcus was strongly correlated with severe disease and death (odds ratio, 126) [45], consistent with the possibility that unexplained deaths in otherwise healthy young people in 1918 could also have been due to dual infections with influenza and pneumococci.
It is a challenge to review these old vaccine studies, but we believe our method of analysis and the examination of bias have made these early data more interpretable. Although these analyses cannot provide conclusive evidence of the efficacy of whole-cell pneumococcal and group A streptococcal vaccines in preventing bacterial superinfections in patients with influenza, we believe they do support further investigation of killed bacterial vaccines in the prevention of pneumococcal pneumonia, influenza-associated pneumonia, and mortality. The 1918 VE data presented here suggest to us the possibility that cheap whole-cell pneumococcal vaccines eliciting cross-protection against multiple pneumococcal serotypes may be worthy of reconsideration.
Figure 1.
Selection of published studies of bacterial vaccines in the 1918 influenza pandemic. B. influenzae, Bacillus influenzae ; JSTOR, Journal Storage; NIH, National Institutes of Health.
Table 1.
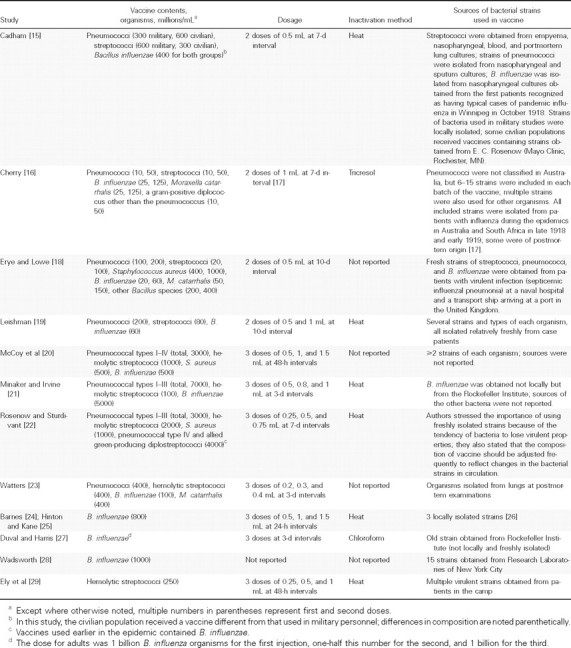
Vaccine Contents, Dosages, and Preparation Methods in the 12 Included Studies
Table 2.
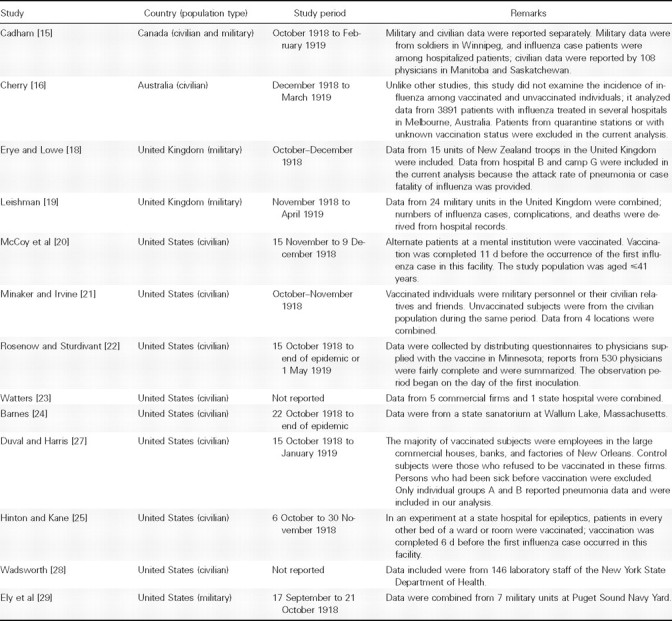
Characteristics of the 12 Included Studies
Table 3.
Incidence of Influenza among Vaccinated and Unvaccinated Subjects, with Estimated Risk Ratio (RR)
Footnotes
Potential conflicts of interest: K.P.K. has received consultant fees and research support from Pfizer Vaccines and GlaxoSmithKline but received no funding from to examine their efficacy in preventing influenza-asindustry for this analysis. All other authors report no potential conflicts of interest.
Presented in part: 7th International Symposium on Pneumococci and Pneumococcal Disease, Tel Aviv, Israel, 14–18 March 2010 (oral presentation).
Financial support: none reported.
References
- 1.Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “ Spanish” influenza pandemic. Bull Hist Med. 2002;76:105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 2.Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918–19 influenza pandemic. Emerg Infect Dis. 2008;14:1193–1199. doi: 10.3201/eid1408.071313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klugman KP, Chien YW, Madhi SA. Pneumococcal pneumonia and influenza: a deadly combination. Vaccine. 2009;27(suppl 3):9–14. doi: 10.1016/j.vaccine.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Chien YW, Klugman KP, Morens DM. Bacterial pathogens and death during the 1918 influenza pandemic. N Engl J Med. 2009;361:2582–2583. doi: 10.1056/NEJMc0908216. [DOI] [PubMed] [Google Scholar]
- 6.Eyler JM. The fog of research: influenza vaccine trials during the 1918–19 pandemic. J Hist Med Allied Sci. 2009;64:401–428. doi: 10.1093/jhmas/jrp013. [DOI] [PubMed] [Google Scholar]
- 7.McCoy GW. Status of prophylactic vaccination against influenza. JAMA. 1919;73:401–404. [Google Scholar]
- 8.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6:303–312. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elyer JM. The state of science, microbiology, and vaccines circa 1918. Public Health Rep. 2010;125(suppl 3):27–35. doi: 10.1177/00333549101250S306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Atlanta, GA: Centers for Disease Control and Prevention; 2010. 2009–2010 influenza season: week 14 ending April 10, 20 FluView: a weekly influenza surveillance report prepared by the Influenza Division. [Google Scholar]
- 11.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.0.2. http://www.cochrane-handbook.org. Updated September 2009. Accessed 20 March 2010.
- 12.Duval S. The trim and fill method. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication bias in meta-analysis: prevention, assessment and adjustments. West Sussex, United Kingdom: John Wiley&Sons; 2005. pp. 128–144. [Google Scholar]
- 13.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM. MIX: comprehensive free software for meta-analysis of causal research data. Version 1.7. http://mix-for-meta-analysis.info. Accessed 20 March 2010. [DOI] [PMC free article] [PubMed]
- 15.Cadham FT. The use of a vaccine in the recent epidemic of influenza. Lancet. 1919;193:885–886. [PMC free article] [PubMed] [Google Scholar]
- 16.Cherry TM. The value of inoculation: a statistical inquiry. In: Cumpston JHL, editor. Influenza and maritime quarantine in Australia. Vol. 18. Melbourne, Australia: Australian Quarantine Service; 1919. pp. 89–113. [Google Scholar]
- 17.Penfold WJ. Influenza vaccine and inoculation. In: Cumpston JHL, editor. Influenza and maritime quarantine in Australia. Melbourne, Australia: Australian Quarantine Service; 1919. pp. 73–88. [Google Scholar]
- 18.Erye J, Lowe C. Autumn influenza epidemic (1918) as it affected the N.Z.E.F. in the United Kingdom. Lancet. 1919;193:553–560. [Google Scholar]
- 19.Leishman WB. The results of protective inoculation against influenza in the army at home, 1918–. Lancet. 1920;195:366–368. doi: 10.1136/bmj.1.3085.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCoy GW, Murray VB, Teeter AL. The failure of a bacterial vaccine as a prophylactic against influenza. JAMA. 1918;71:1997. [Google Scholar]
- 21.Minaker AJ, Irvine RS. Prophylactic use of mixed vaccine against pandemic influenza and its complications. JAMA. 1919;72:847–850. [Google Scholar]
- 22.Rosenow EC, Sturdivant BF. Studies in influenza and pneumonia. IV. Further results of prophylactic inoculations. JAMA. 1919;73:396–401. [Google Scholar]
- 23.Watters WH. Vaccines in influenza. Boston Med Surg J. 1919;181:727–731. [Google Scholar]
- 24.Barnes HL. The prophylactic value of Leary's vaccine. JAMA. 1918;71:1899. [Google Scholar]
- 25.Hinton WA, Kane ES. Use of influenza vaccine as a prophylactic: an experimental study conducted by the Massachusetts State Department of Health. J Tennessee State Med Assn. 1918;11:442–446. [Google Scholar]
- 26.Leary T. The use of influenza vaccine in the present epidemic. Am J Public Health. 1918;8:754–755. doi: 10.2105/ajph.8.10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duval CW, Harris WH. The antigenic property of the Pfeiffer bacillus as related to its value in the prophylaxis of epidemic influenza. J Immunol. 1919;4:317–330. [Google Scholar]
- 28.Wadsworth AB. The results of preventive vaccination with suspensions of the influenza bacillus. Public Health J. 1919;10:309–314. [Google Scholar]
- 29.Ely CF, Lloyd BJ, Hitchcock CD, Nickson DH. Influenza as seen at the Puget Sound Navy Yard. JAMA. 1919;72:24–28. [Google Scholar]
- 30.Frost WH. The epidemiology of influenza. JAMA. 1919;73:313–318. [Google Scholar]
- 31.Jordan EO. Influenza studies. IV. Effect of vaccination against influenza and some other respiratory infections. J Infect Dis. 1921;28:357–366. [Google Scholar]
- 32.Jackson LA, Nelson JC, Benson P, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol. 2006;35:345–352. doi: 10.1093/ije/dyi275. [DOI] [PubMed] [Google Scholar]
- 33.MacCallum WG. Pathological studies in the recent epidemics of pneumonia. Trans South Surg Assoc. 1919;31:180–192. [Google Scholar]
- 34.Jousimies-Somer HR, avolainen S, Ylikoski JS. Comparison of the nasal bacterial floras in two groups of healthy subjects and in patients with acute maxillary sinusitis. J Clin Microbiol. 1989;27:2736–2743. doi: 10.1128/jcm.27.12.2736-2743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malley R, Lipsitch M, Stack A, et al. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun. 2001;69:4870–4873. doi: 10.1128/IAI.69.8.4870-4873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malley R, Morse SC, Leite LC, et al. Multiserotype protection of mice against pneumococcal colonization of the nasopharynx and middle ear by killed nonencapsulated cells given intranasally with a nontoxic adjuvant. Infect Immun. 2004;72:4290–4292. doi: 10.1128/IAI.72.7.4290-4292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention Outbreak of group A streptococcal pneumonia among Marine Corps Recruits: California, November 1-December 20, 2002. JAMA. 2003;289:1373–1375. [PubMed] [Google Scholar]
- 38.Brundage JF, Gunzenhauser JD, Longfield JN, et al. Epidemiology and control of acute respiratory diseases with emphasis on group A betahemolytic streptococcus: a decade of U.S. Army experience. Pediatrics. 1996;97:964–970. [PubMed] [Google Scholar]
- 39.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1): United States, May-August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–1074. [PubMed] [Google Scholar]
- 41.Mauad T, Hajjar LA, Callegari GD, et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med. 2009;181:72–79. doi: 10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]
- 42.Gill JR, Sheng ZM, Ely SF, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134:235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–679. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 44.Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 45.Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS ONE. 2009;4:8540. doi: 10.1371/journal.pone.0008540. [DOI] [PMC free article] [PubMed] [Google Scholar]