Abstract
Background. The specificity and duration of circulating human antibody-secreting cells (ASCs) after vaccination have been well described, but characteristics of ASCs during acute respiratory infections have not been well studied.
Methods. Circulating antigen-specific ASCs were measured at 3 time points (enrollment, days 10–16, and days 22–45) in 40 adults during respiratory syncytial virus (RSV) infection.
Results. Of the 40 patients, 36 (90%) had detectable circulating RSV F protein-specific ASCs within 11 days after illness onset. The magnitude of the RSV-specific ASCs was 1–1500 spots per 10 6peripheral blood mononuclear cells (mean frequency [± standard deviation], 200 ± 256 spots per 106 peripheral blood mononuclear cells). ASCs were detected on day 8–16 and day 22–45 after symptom onset in 78% and 48% of subjects, respectively. Subjects shedding virus for >10 days were more likely to have a positive response to ASC enzyme-linked immunospot assay at the late time point than those shedding for ⩽10 days (8 of 12 subjects vs 2 of 11 subjects; P = .02).
Conclusions. The kinetics of ASC circulation during acute mucosal viral infections was more prolonged than that we had observed after a single intramuscular injection with inactivated influenza vaccine in a study reported elsewhere. The association between the duration of virus shedding and the persistence of detectable viral-specific ASCs suggests that ongoing antigen persistence induces a prolonged temporal pattern of ASC generation.
Respiratory syncytial virus (RSV) is a leading cause of severe respiratory tract disease in infants [1] and the elderly [2]. Approximately 70% of all infants are infected with RSV in their first year of life, with the remainder infected the following year [3]. Despite the lack of evidence that strain variation is clinically significant and that RSV-neutralizing antibody regularly develops after infection, repeated infections are common throughout life, indicating that immunity is brief and incomplete [4]. However, results of studies in animals and in humans suggest that immune correlates of relative protection include memory T and B cells and neutralizing serum and mucosal antibody [5–8]. The most compelling support for the role of serum antibody in protection is derived from clinical trials in which a humanized neutralizing monoclonal antibody (palivizumab; MedImmune) administered prophylactically to high-risk infants reduced disease severity [9]. In adults, the level of RSV-specific serum antibody has been correlated with both protection from infection and severity of disease [7, 10]. However, after infection in adults, the antibody titer rapidly increases and then quickly returns to baseline levels within 16 months [11]. Interestingly, we have consistently observed that serum antibody responses in older adults are greater than those in younger adults, although the responses in older adults are also relatively brief in duration [10].
After immune stimulation, antibody-secreting cells (ASCs) are generated in the secondary lymphoid structures and transit through the blood to their final destination in bone marrow, spleen, or target tissues, such as the respiratory tract [12]. Many of these ASCs eventually undergo apoptosis and are referred to as short-lived plasmablasts, whereas a fraction survive to become long-lived plasma cells responsible for sustaining protective antibody levels after infection. Unfortunately, characterizing these cells in the bone marrow, spleen, and lungs is very difficult in humans. However, the period of time when these ASCs migrate through the blood during and after infection provides an opportune window to study some of the characteristics of cells that secrete antibodies after vaccination and infection [13]. For example, we and others have found that ∼90% of detectable ASCs after administration of a protein-based vaccine are specific to the immunizing antigens and can be identified as early as 4 days after vaccination, with a sharp peak on days 5–8 and disappearance by day 15 [13–20]. It is presumed that ASCs would also migrate though the blood during acute mucosal respiratory viral infection. However, unlike the effects of parenteral immunization with an inactivated protein vaccine, the specificity, magnitude, heterogeneity, and kinetics of the human ASCs during acute infection are virtually unexplored. Interrogating the ASC population in the blood during acute infections may prove useful in understanding the biology of the cells that ultimately compose the long-lived antibody compartment. Therefore, we sought to explore the B cell effector response in adults by analyzing the appearance of ASCs in the blood of RSV-infected adults.
Methods
Subjects and illness evaluation. Two groups of adults, ⩾21 years of age, were evaluated during the winter of 2007–2008 in Rochester, New York, as part of an ongoing study of the pathogenesis of RSV infection in elderly persons. The first group included community-dwelling persons and those with underlying cardiac and/or pulmonary disease who were prospectively enrolled in the fall of 2007, when demographic and medical data were recorded and a preseason serum sample was obtained. Subjects were followed up throughout the ensuing winter season (15 November through 15 April) for the development of acute respiratory tract illness (nasal congestion, sore throat, new or worsening cough, wheezing, or dyspnea, with or without fever). The second group included persons with acute respiratory tract illness who were hospitalized at Rochester General Hospital or outpatients who were evaluated by study personnel during the same period. The presence of an active immunosuppressive condition was an exclusion criterion for both groups; these conditions included human immunodeficiency virus infection, active chemotherapy, hematological malignancy, or treatment with immunosuppressive medications, including >20 mg prednisone within the past 14 days. However, treatment with glucocorticosteroids for the acute illness was not an exclusion criterion.
Ill subjects underwent directed history and lung examination and were screened for the presence of RSV infection by reverse-transcription polymerase chain reaction (RT-PCR) analysis of nasal swab and sputum samples. If subjects were found to be infected with RSV, nasal swab and sputum samples were collected daily for the first 7 days and then every other day until 2 consecutive negative samples were obtained. At the initial illness evaluation, blood samples were collected from all RSV-infected subjects for antibody ASC enzyme-linked immunospot assay (ELISPOT), and in most subjects additional samples were obtained at intermediate and late time points (day 10–16 of illness and after 22–45 days). Convalescent-stage serum samples were also obtained for serologic testing at the late time point. All subjects provided signed informed consent, and the University of Rochester Research Subject Review Board and the Rochester General Hospital Clinical Investigation Committee approved the study.
RT-PCR. The initial nasal swabs and sputum samples were screened for the presence of RSV RNA by RT-PCR, using a nonquantitative multiplex group A and B RSV-specific RT-PCR assay, as described elsewhere [21, 22]. Viral load, recorded as log10 plaque forming units (PFUs) per milliliter, was determined for all respiratory secretion samples from RSV-infected subjects, using a quantitative RSV group-specific RT-PCR assay, as reported elsewhere [23]. RT-PCR assays for influenza A and B virus were also performed on samples from selected individuals, according to published methods [2].
Serologic testing. An enzyme immunoassay (EIA) for serum immunoglobulin G (IgG) antibody to purified RSV F (fusion) protein was performed, according to published methods [4]. A microneutralization assay for group A and group B RSV was used to determine neutralizing titers in serum samples, as described elsewhere [7]. An assay for serum IgG antibody to influenza A and B virus was performed in samples from selected individuals, using methods published elsewhere [2].
Peripheral blood mononuclear cell isolation. Peripheral blood mononuclear cells (PBMCs) were isolated within 4 h of sampling with sodium heparin sulfate tubes. Tubes were immediately inverted 8–10 times and centrifuged at 1500 g for 30 min at 20°C, and the buffy coat layer was transferred to a 50-mL tube. The cells were washed 3 times and centrifuged at 300 g for 10 min at 4°C, and viability was assessed by trypan blue exclusion. A portion of the cells were used immediately in the ASC ELISPOT, and the remainder were preserved in liquid nitrogen.
ASC ELISPOT. The frequency of RSV antigen-specific ASCs was measured by ELISPOT, as described elsewhere [24]. Briefly, 96-well ELISPOT plates (Maips 4510; Millipore) were coated overnight at 4°C in a humidified chamber, with the following antigens diluted in sterile phosphate-buffered saline (PBS): affinity-purified RSV F protein of A2 strain (a group A virus; 2 μg/mL) [25], trivalent influenza virus vaccine (6 μg/ mL; Sanofi Pasteur), tetanus toxoid (1 lime flocculation unit per milliliter; Cylex), and anti-human IgG (5μ g/mL; Jackson Immunoresearch). Bovine serum albumin (MP Biomedicals), 2% in sterile PBS, was used as a control antigen to determine background ASC frequencies. Plates were blocked with Roswell Park Memorial Institute medium containing 8% fetal bovine serum for 2 h and incubated at 37°C for 18–20 h with 300,000, 100,000, or 33,333 PBMCs, each dilution in triplicate. For total IgG plates, 100,000, 33,000, and 11,000 PBMCs per well were added. These dilutions had been determined to provide the optimal density of readable spots in the ELISPOT.
After incubation, cells were aspirated, and plates were washed with PBS and 0.1% Tween. Bound antibodies were detected with alkaline phosphatase-conjugated anti-human IgG antibody (1 μg/mL; Jackson Immunoresearch) for 2 h and developed with a Vector Blue Alkaline Phosphatase Substrate Kit III (Vector Laboratories). Spots in each of the antigen-coated wells were counted using a CTL ImmunoSpot reader (Cellular Technology), and background spot numbers from bovine serum albumin-coated wells were subtracted to calculate the number of antigen-specific spots. In the development of the ACS assay, we found that wells with 20–250 spots provide the most accurate results, and we included data whenever possible from the triplicate wells at the dilution that yielded 20–250 spots [13]. Antigen-specific IgG ASC responses were considered positive if they were >4 standard deviations above the mean for each antigen, which was calculated using results from 28 previously evaluated control subjects who were asymptomatic at the time of blood collection. Positive values for the antigens were >13, >22, and 18 spots per 106 PBMCs for RSV F, trivalent influenza virus, and tetanus toxoid, respectively.
Statistical analysis. Normally distributed continuous variables were compared with the Student t test and dichotomous data with the χ2 or Fisher exact test. Correlations were calculated using the Pearson correlation coefficient. Differences were considered significant at P ⩽ .05.
Results
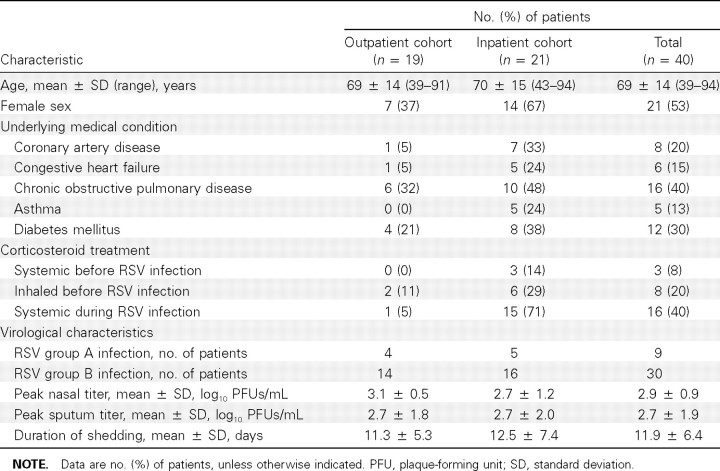
RSV illnesses. Forty subjects with acute RSV respiratory illnesses were identified by RT-PCR during the winter of 2007- 2008. The demographic, clinical, and virological characteristics of these subjects and their illnesses are shown in Table 1. RSV infection was identified in 20 outpatients, 1 of whom was hospitalized. An additional 20 subjects were identified as having RSV infection when they were hospitalized with an acute respiratory illness. Thus, 19 subjects were treated as outpatients and 21 as inpatients. The mean age of RSV-infected subjects was 69 years (range, 39–94 years). Female subjects slightly outnumbered male subjects, and a high percentage of subjects had underlying medical conditions, primarily cardiopulmonary disease and diabetes mellitus.
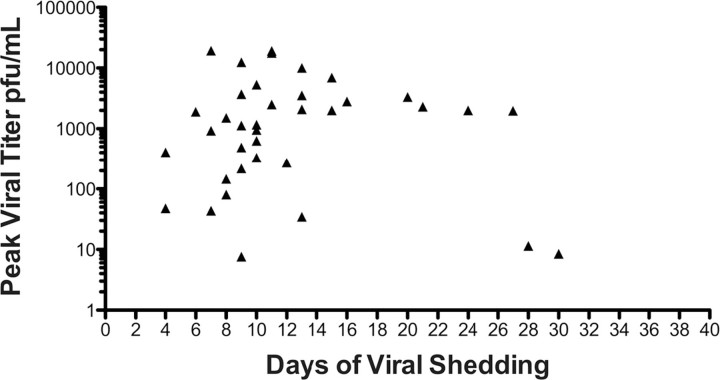
Isolates from 9 RSV infections were identified as group A RSV and 30 as group B RSV; 1 isolate was not typed. The mean peak viral load (± standard deviation [SD]) in nasal swab and sputum samples, determined by quantitative RT-PCR, was 2.9 ± 0.9 and 2.7 ± 1.9 log10 PFUs/mL, respectively. The mean (± SD) duration of virus shedding in nasal secretion samples was 11.9 ± 6.4 days (range, 4–30 days). Peak viral titers from the nasopharyngeal swab samples did not correlate with duration of viral shedding (Figure 1). There were no significant differences between the outpatient and hospitalized groups with regard to infecting RSV strain or shedding parameters. As expected, hospitalized subjects had more severe illness, as evidenced by more frequent signs and symptoms of lower respiratory illness, greater supplemental oxygen requirements, and more treatment with systemic corticosteroids during the illness (15 of 21 subjects vs 1 of 19 subjects; P! .001). Four hospitalized subjects were admitted to the intensive care unit.
Figure 1.
Respiratory syncytial virus shedding patterns for the 40 subjects, with peak viral titer in nasal secretion samples on the y-axis and days of viral shedding on the x-axis. PFU, plaque-forming unit.
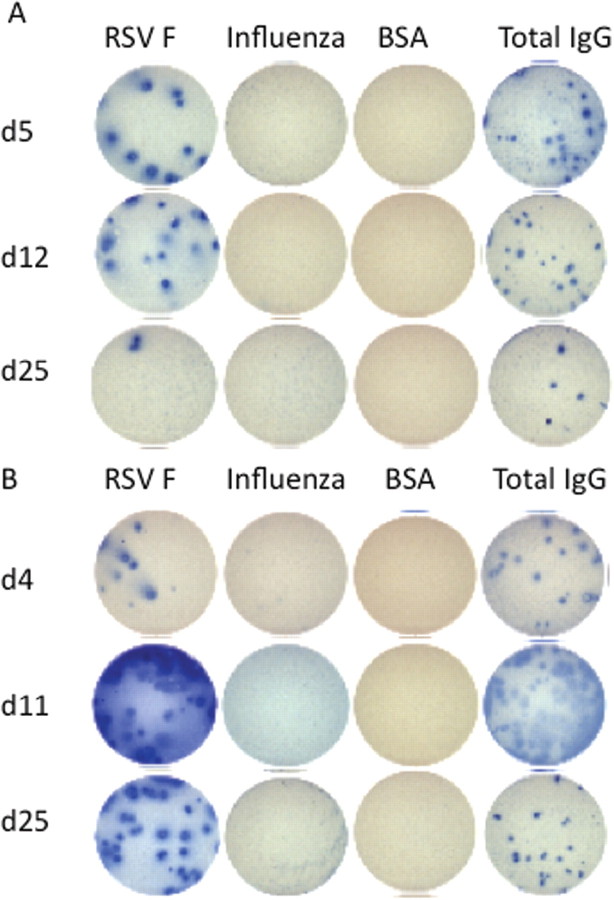
ASC response to infection. All RSV-infected subjects had an initial blood sample available for ASC ELISPOT, 14 subjects had a sample collected at an intermediate time point (day 10- 16), and 23 had a sample collected at a late time point (day 22–45). Overall, 27 subjects had 2 samples and 10 subjects had 3 samples evaluated by ASC ELISPOT. Figures 2 A and 2 B illustrate a typical RSV F-specific ASC response in 2 RSV-infected subjects, both of whom had negative responses for influenza A and B virus.
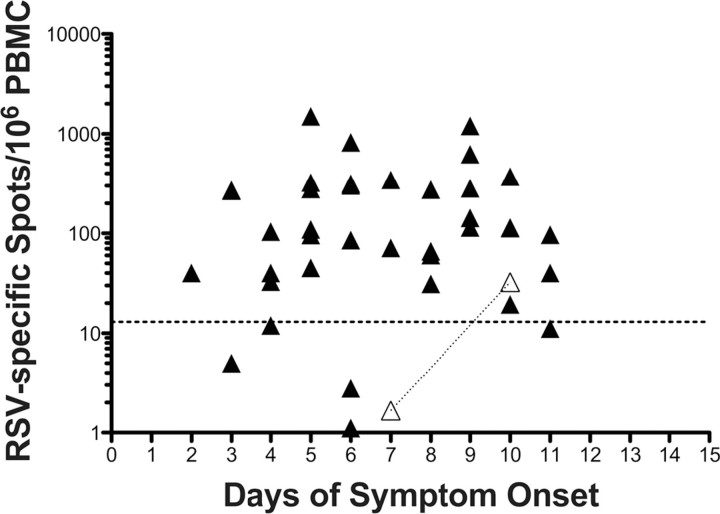
Of the 40 subjects, 35 (88%) had samples that were positive by RSV-specific ASC ELISPOT at initial evaluation, with a mean frequency (± SD) of 200 ± 256 spots per 106 PBMCs (range, 1–1500 spots per 10 6PBMCs) (Figure 3). These initial samples were collected at a mean (± SD) of 6.7 ± 2.5 days after symptom onset (range, 3–11 days). In 4 of the 5 subjects with an initially negative result of the RSV F-specific ASC assay, results remained negative in subsequent blood samples, whereas 1 subject with a negative result on day 7 of illness was found to have a positive assay on day 10 (33 spots per 10 6PBMCs). Thus, 36 (90%) of 40 subjects had detectable circulating RSV F-specific ASCs within 11 days after illness onset. Of the 36 ASC-positive subjects, 9 had a second blood sample collected at the late time point 8–16 days after symptom onset (mean [± SD], 11.6 ± 2.1 days), with a mean (± SD) of 150 ± 238 spots per 106 PBMCs (range, 1–695 spots per 106 PBMCs); samples from 7 (78%) of these 9 subjects remained positive. Twenty-three of the 36 ASC-positive subjects had a blood sample available at the late time point after symptom onset (mean [± SD], 29 ± 5 days; range, 22–45 days), with a mean (± SD) of 81 ± 246 spots per 10 6PBMCs (range, 0–1191 spots per 10 6PBMCs). Of these 23 subjects, 11 (48%) still tested positive by RSV F-specific ASC ELISPOT.
Figure 3.
Numbers of antibody-secreting cells (ASCs) specific to respiratory syncytial virus (RSV) in blood samples of 40 patients with acute infection confirmed by reverse-transcription polymerase chain reaction, according to number of days after symptom onset. Each filled symbol represents a single patient. Open triangles represent 2 time points for a single patient who had a negative RSV-specific ASC response on day 7 and a positive response on day 10. The horizontal dotted line represents the threshold for a positive RSV-specific ASC response (>13 spots per 106 peripheral blood mononuclear cells [PBMCs]).
There was a weak association between duration of virus shedding and a positive ASC assay at the late time point. Using the median value of 10 days for virus shedding to dichotomize those subjects with a late ASC time point, we found that subjects shedding virus for >10 days were more likely to have a positive ASC ELISPOT response at the late time point than those shedding for >10 days (8 of 12 subjects vs 2 of 11 subjects; P = .02) (Figure 4). Although the mean duration of virus shedding was slightly longer in the 10 subjects who still tested positive by ASC assay at the late time point (14.6 vs 12.4 days), this difference was not significant.
Figure 4.
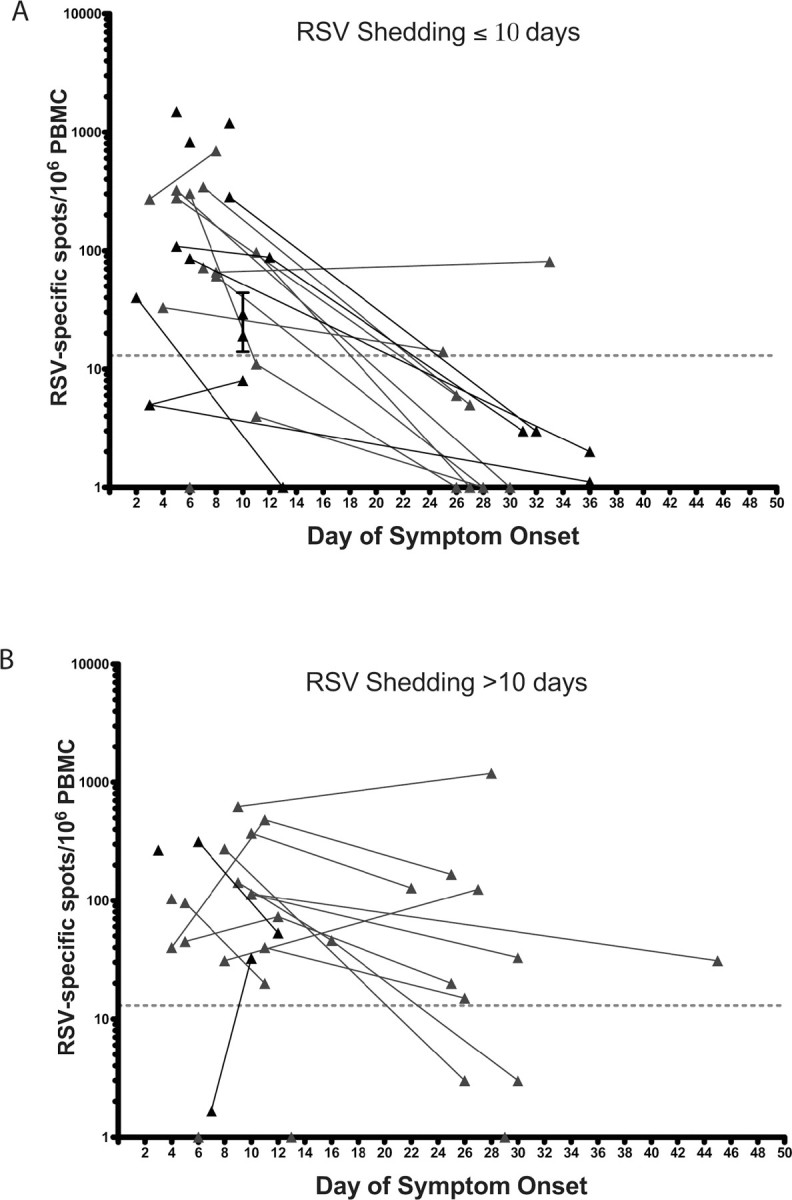
Respiratory syncytial virus (RSV)-specific antibody-secreting cell (ASC) frequencies in blood samples from the 40 patients with acute RSV infection, by days after symptom onset. A, Patients shedding virus for ⩽10 days. B, Patients shedding virus for >10 days. The dotted line represents the threshold for a positive RSV-specific ASC response (>13 spots per 106 peripheral blood mononuclear cells [PBMCs]).
There was no correlation between the number of RSV F- specific ASCs in the initial blood sample and peak virus load in nasal secretion samples, RSV strain designation, duration of viral shedding, subject age, or requirement for hospitalization. All 9 subjects with group A RSV infection and 25 of 30 with group B RSV infection had a positive ASC assay in the initial sample; this difference was not significantly different (P = .3) and was consistent with the high degree of antigenic conservation of the F protein between RSV strains [4]. Additionally, there was no correlation between the number of RSV F-specific ASCs in the initial blood sample and the magnitude of the serum IgG response to RSV F protein by EIA, nor to the neutralizing antibody response to either RSV group A or group B. However, subjects with detectable ASCs at the late time point had 2-fold higher convalescent-stage RSV F IgG titers than those without detectable ASCs (mean titer [± SD], 18.6 ± 0.7 vs 17.5 ± 1.3; Pp .02), although there was no difference in fold increase during infection.
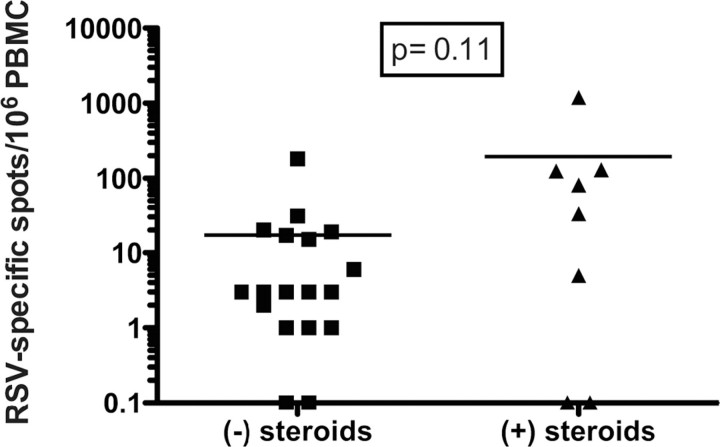
We noted that RSV-specific ASCs were detected in initial and late blood samples as frequently in those from subjects treated with systemic corticosteroid therapy during infection as in those from subjects not receiving corticosteroids (early samples, 11 of 14 subjects vs 24 of 26 subjects; P = .3; late samples, 5 of 10 subjects vs 4 of 16 subjects; P = .2). However, the group receiving systemic corticosteroids had a trend toward more RSV-specific ASCs at the 1-month time point (P = .11) (Figure 5).
Figure 5.
Enzyme-linked immunospot assay frequencies of respiratory syncytial virus (RSV)-specific antibody-secreting cells in blood samples obtained at late time points in RSV-infected patients who did (plus sign) or did not (minus sign) receive corticosteroids for acute treatment. PBMC, peripheral blood mononuclear cells.
As noted, 4 subjects did not have detectable RSV-specific ASCs at any of 3 time points. Three of these subjects (age, 43- 76 years) were hospitalized and received systemic corticosteroids. All 4 failed to mount a serum IgG F-specific response detectable by EIA. One subject, a 54-year-old woman, had a single RSV-positive nasal swab sample by RT-PCR and a negative sputum sample. Notably, in this subject, ELISPOT showed a strongly positive influenza-specific ASC response at the initial illness evaluation (818 spots per 106 PBMCs), EIA demonstrated seroresponse to influenza B virus, and RT-PCR results were positive for influenza B virus in nasal secretion samples. Thus, she probably had an influenza B virus infection and a false-positive RSV RT-PCR assay.
Two other subjects also had positive influenza-specific ASC assays during the course of their RSV infection. One shed RSV for 9 days but also tested positive for influenza B virus by RTPCR on initial evaluation and subsequently developed a >4fold IgG response to influenza B virus. This subject probably had a dual RSV-influenza B virus infection. The other subject shed RSV for 10 days, but an ELISPOT of the day 36 blood sample was positive for influenza ASCs, and EIA demonstrated a seroresponse to influenza B virus. This subject probably had sequential infections with RSV followed by influenza B virus infection during the convalescent period.
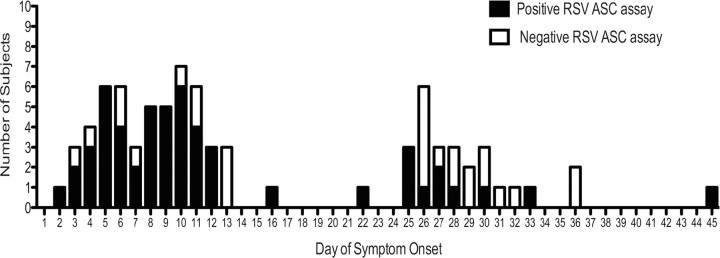
In all, 41 (84%) of the 49 blood samples collected from the 40 subjects between days 2 and 12 of illness were positive by ASC assay (Figure 6). We could not be confident of the number of positive samples between days 13 and 24, because very few blood samples were collected at these time points, but 10 (38%) of 26 samples were positive between days 25 and 45. Overall, 16 of the 36 ASC-positive subjects had detectable RSV-specific ASCs beyond the last day of viral shedding, as determined by daily RT-PCR analysis of nasal secretion samples.
Figure 6.
Graphic display of temporal pattern of positive (filled bars) and negative (open bars) respiratory syncytial virus (RSV)-specific antibody-secreting cell (ASC) assays in all blood samples from all RSV-infected subjects.
Discussion
This is the first study (to our knowledge) describing the kinetics of ASC circulation during an acute mucosal viral infection. We found that ASCs can readily and frequently be found in the blood during acute RSV infection. The kinetics of ASCs during acute infection is quite different from that seen after immunization with inactivated influenza vaccine [13–15, 19], but this result was not unexpected. In contrast to the rapid peak of ASCs on days 5–8, followed by disappearance by day 15, after immunization with inactivated influenza vaccine, we found that RSV-infected subjects have a more prolonged window when irculating ASCs can be detected. In some cases this time frame extended beyond 1 month, and even though a limited number of time points were available, the data indicate that there is a positive correlation between duration of virus shedding and duration of ASC positivity. This suggests that persistence of antigenic stimulation induces a prolonged temporal pattern of ASC stimulation.
In our study measuring the ASC responses daily after immunization with inactivated influenza vaccine, reported elsewhere [13], we found a correlation between the maximal ASC and serum titers. In the present study, the maximal RSV F- specific ASC response did not correlate with the fold increase in serum antibody levels; however, there was a suggestion that prolonged detection of ASCs was associated with higher convalescent-stage RSV F-specific IgG titers. Although we had previously noted that older adults develop significantly greater IgG responses to RSV infection, we did not find age-related differences in ASC numbers during infection. This may be due to the small number of subjects who were <40 years of age. Our inability to find an association between numbers of ASCs and the magnitude of IgG responses to RSV F may reflect failure to collect enough tightly timed samples to identify peak ASC numbers and the precise duration of the ASC response. It may likely be that some combination of peak ASC number and response duration (ie, area under the curve) would provide the best correlation with serum immunoglobulin response during a mucosal infection.
It is interesting that glucocorticosteroid treatment did not appear to blunt ASC responses, because steroids are known to induce B and T cell lymphocyte apoptosis [26]. In fact, we found higher frequencies of ASCs specific to RSV at the late time point in corticosteroid-treated subjects. However, steroid-treated subjects tended to have more prolonged viral shedding and more severe illness (ie, they were generally hospitalized). A control group of equally ill subjects with viral shedding who did not receive steroids would be required to definitively assess the effect of steroids on ASC responses.
Our data suggest that detection of virus-specific ASCs may be a useful adjunct for diagnosis, although proving that was not the intent of this study. This finding may be especially true for adults with RSV infection, for whom serologic testing is more sensitive than RT-PCR and rapid antigen detection tests are of little value [27]. Serologic testing is impractical for patient care because it requires acute-and convalescent-stage serum samples to demonstrate an increase in serum IgG level, and immunoglobulin M (IgM) assays for RSV have been difficult to adapt for clinical use. Although it is not as sensitive as RTPCR at a given time point, the ASC cell assay may have a wider diagnostic window than that of RT-PCR, yet a shorter one than the typical persistence of serum IgM (3–6 months). Despite the limitations of infrequent sampling, we found that ASCs specific to RSV appear as early as day 2 after symptom onset and were detected in 36 of the 40 RT-PCR-positive patients (90% sensitivity) within the first 11 days of illness. In this study, we did not measure RSV-specific ASCs in patients with non-RSV viral illnesses, and thus we cannot define the specificity of this assay for diagnosis. Nevertheless, the RSV-specific ASC assay may hold some promise as an immune surrogate for diagnosis of RSV infection from a single blood sample at the time of clinical presentation or as a method to augment existing diagnostic assays for specific viral infection diagnosis. Other than the few in whom influenza B virus infection was confirmed by RT-PCR and serologic testing, none of the RSV-infected subjects had detectable influenza-specific ASCs. In addition, none of them had a positive tetanus toxoid ASC assay. Although it is not likely to replace commercial RT-PCR, this novel, relatively simple diagnostic approach could function as an adjunct to the currently available tests in a research setting.
In conclusion, we found that the kinetics of circulating ASCs during acute RSV infection is quite different from that seen after immune stimulation with inactivated vaccine. Our results indicate that this method should be useful in probing characteristics of the B cell response during a mucosal viral infection, such as identification of short-lived plasmablasts, or cells with specific homing markers for the respiratory tract or bone marrow, or for interrogating epitope-specific responses. Finally, it may also offer diagnostic potential.
Table 1.
Demographic, Clinical, and Virological Characteristics of 40 Subjects Infected with Respiratory Syncytial Virus (RSV)
Figure 2.
Response to enzyme-linked immunospot assay for circulating antibody-secreting cells during acute respiratory syncytial virus (RSV) infection in 2 patients (panels A and B)onday 4or5, 11or 12, and25 after symptom onset. Assays were specific for the following antigens: RSV F protein, influenza virus, bovine serum albumin (BSA; 300,000 peripheral blood mononuclear cells [PBMCs] per well for each), and total immunoglobulin G (IgG; 100,000 PBMCs per well). Nasal swab samples were positive for RSV by polymerase chain reaction for 16 and 20 days after symptom onset for the patient whose results are shown in panel A and the patient whose results are shown in panel B, respectively.
Footnotes
Potential conflicts of interest: F.E.-H.L. has research grants from Trellis Bioscience. A.R.F. serves on the advisory board of Quidel and has done consulting work for AstraZeneca, MedImmune, and Wyeth. I.S. has done consulting work for Genentech and Biogen. E.E.W. has consulted for AstraZeneca and Boehringer Ingelheim. A.R.F. and E.E.W. have research grants from GlaxoSmithKline and Sanofi Pasteur. J.L.H. reports no conflicts of interest.
Financial support: Trellis Bioscience (grants K23 AI 67501, UO1 AI 045969, HHSN2662005500029C, and N01-AI-500209 to F.E.-H.L.).
References
- 1.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl JMed. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, ox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 4.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 5.Graham BS. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 1996;4:290–293. doi: 10.1016/0966-842x(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 6.Graham BS, Rutigliano JA, Johnson TR. Respiratory syncytial virus immunobiology and pathogenesis. Virology. 2002;297:1–7. doi: 10.1006/viro.2002.1431. [DOI] [PubMed] [Google Scholar]
- 7.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 8.Lee FE, Walsh EE, Falsey AR, Betts RF, Treanor JJ. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res. 2004;63:191–196. doi: 10.1016/j.antiviral.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Parnes C, Guillermin J, Habersang R, et al. Palivizumab prophylaxis of respiratory syncytial virus disease in 2000–2001: results from the Palivizumab Outcomes Registry. Pediatr Pulmonol. 2003;35:484–489. doi: 10.1002/ppul.10288. [DOI] [PubMed] [Google Scholar]
- 10.Walsh EE, Falsey AR. Age related differences in humoral immune response to respiratory syncytial virus infection in adults. J Med Virol. 2004;73:295–299. doi: 10.1002/jmv.20090. [DOI] [PubMed] [Google Scholar]
- 11.Falsey AR, Singh HK, Walsh EE. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol. 2006;78:1493–1497. doi: 10.1002/jmv.20724. [DOI] [PubMed] [Google Scholar]
- 12.Radbruch A, Muehlinghaus G, Luger EO, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 13.Halliley JL, Kyu S, Kobie JJ, et al. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine. 2010;28:3582–3587. doi: 10.1016/j.vaccine.2010.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12:993–999. doi: 10.1016/0264-410x(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 15.Moldoveanu Z, Clements ML, Prince SJ, Murphy BR, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13:1006–1012. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 16.asaki S, He XS, Holmes TH, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3:2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.asaki S, Jaimes MC, Holmes TH, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81:215–228. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clutterbuck EA, Oh S, Hamaluba M, Westcar S, Beverley PC, Pollard AJ. Serotype-specific and age-dependent generation of pneumococcal polysaccharide-specific memory B-cell and antibody responses to immunization with a pneumococcal conjugate vaccine. Clin Vaccine Immunol. 2008;15:182–193. doi: 10.1128/CVI.00336-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanchard-Rohner G, Pulickal AS, Jol-van der Zijde CM, Snape MD, Pollard AJ. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood. 2009;114:4998–5002. doi: 10.1182/blood-2009-03-211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falsey AR, Formica MA, Treanor JJ, Walsh EE. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. J Clin Microbiol. 2003;41:4160–4165. doi: 10.1128/JCM.41.9.4160-4165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsey AR, Formica MA, Walsh EE. 22 . Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol. 2002;40:817–820. doi: 10.1128/JCM.40.3.817-820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan CB, Walsh EE, Peterson DR, Lee FE, Falsey AR. Risk factors for respiratory failure associated with respiratory syncytial virus infection in adults. J Infect Dis. 2009;200:1242–1246. doi: 10.1086/605948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyu SY, Kobie J, Yang H, et al. Frequencies of human influenza-specific antibody secreting cells or plasmablasts post vaccination from fresh and frozen peripheral blood mononuclear cells. J Immunol Methods. 2009;340:42–47. doi: 10.1016/j.jim.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh EE, Brandriss MW, Schlesinger JJ. Purification and characterization of the respiratory syncytial virus fusion protein. J Gen Virol. 1985;66:409–415. doi: 10.1099/0022-1317-66-3-409. [DOI] [PubMed] [Google Scholar]
- 26.Tuckermann JP, Kleiman A, McPherson KG, Reichardt HM. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit Rev Clin Lab Sci. 2005;42:71–104. doi: 10.1080/10408360590888983. [DOI] [PubMed] [Google Scholar]
- 27.Casiano-Colon AE, Hulbert BB, Mayer TK, Walsh EE, Falsey AR. Lack of sensitivity of rapid antigen tests for the diagnosis of respiratory syncytial virus infection in adults. J Clin Virol. 2003;28:169–174. doi: 10.1016/s1386-6532(03)00002-7. [DOI] [PubMed] [Google Scholar]