Abstract
Background
Current estimates of the costs of cancer care in the United States are based on data from 2003 and earlier. However, incidence, survival, and practice patterns have been changing for the majority of cancers.
Methods
Cancer prevalence was estimated and projected by phase of care (initial year following diagnosis, continuing, and last year of life) and tumor site for 13 cancers in men and 16 cancers in women through 2020. Cancer prevalence was calculated from cancer incidence and survival models estimated from Surveillance, Epidemiology, and End Results (SEER) Program data. Annualized net costs were estimated from recent SEER–Medicare linkage data, which included claims through 2006 among beneficiaries aged 65 years and older with a cancer diagnosis. Control subjects without cancer were identified from a 5% random sample of all Medicare beneficiaries residing in the SEER areas to adjust for expenditures not related to cancer. All cost estimates were adjusted to 2010 dollars. Different scenarios for assumptions about future trends in incidence, survival, and cost were assessed with sensitivity analysis.
Results
Assuming constant incidence, survival, and cost, we projected 13.8 and 18.1 million cancer survivors in 2010 and 2020, respectively, with associated costs of cancer care of 124.57 and 157.77 billion 2010 US dollars. This 27% increase in medical costs reflects US population changes only. The largest increases were in the continuing phase of care for prostate cancer (42%) and female breast cancer (32%). Projections of current trends in incidence (declining) and survival (increasing) had small effects on 2020 estimates. However, if costs of care increase annually by 2% in the initial and last year of life phases of care, the total cost in 2020 is projected to be $173 billion, which represents a 39% increase from 2010.
Conclusions
The national cost of cancer care is substantial and expected to increase because of population changes alone. Our findings have implications for policy makers in planning and allocation of resources.
CONTEXTS AND CAVEATS
Prior knowledge
The costs of cancer care are expected to rise with increased cancer incidence in an aging population, along with advances in diagnostic technology and novel targeted treatments.
Study design
Data on incidence and survival from the Surveillance, Epidemiology, and End Results (SEER) database linked to Medicare records were used to estimate the costs at initial, continuing, and final phases of cancer care for 13 cancers in men and 16 in women. Different models of incidence, survival, and cost increases were used to project total cost of care through 2020 in the United States.
Contribution
Changes in the US population alone are projected to result in a cost increase of 27% by 2020. However, if costs in the initial and final phases of care increase by 2% annually, the total cost of care in 2020 is projected to be $173 billion an increase of 39% from 2010.
Implications
Expanding costs of cancer care due to increases in an aging population are inevitable, but the costs of new treatments and diagnostic technologies could potentially be managed to ensure access to quality care for all patients.
Limitations
The estimates of cancer prevalence were based on data from SEER-9 areas, which do not cover the entire United States. The estimates were also based on first tumor diagnosed and may not be applicable to patients with multiple tumors. The presence of other diseases in addition to cancer was not included in the analysis.
From the Editors
Rapid scientific progress in oncology during the 1990s led to new tools for diagnosis and the development of novel targeted therapies. During the same period, incidence declined and survival improved for many cancers (1). The average cost of treating the most common cancers increased as well (2). With more expensive targeted treatments adopted as standards of care, the costs of cancer care are expected to escalate more rapidly in the near future. The US Bureau of Census projects that the population aged 65 years and older is expected to increase from 40 million in 2009 to 70 million in 2030 (3). Because cancer incidence is highest in the elderly, the impact of these population changes on cancer prevalence may exceed the impact of declining cancer incidence rates for some cancers. As a result, both the number of cancer survivors and cancer expenditures are likely to increase in the future.
Previous estimates of national expenditures for cancer care have mostly been based on older data for incidence, patterns of care, and survival and on inflation adjusted with general medical care inflation adjusters (4). For example, the estimate of the direct costs of “neoplasms” of $99 billion in 2009 is based on broadly defined self-reported disease categories from 1995 and inflated to 2009 dollars (5). This national estimate does not reflect current cancer incidence, patterns of care, and survival. Furthermore, escalation in the costs of cancer chemotherapy has been greater than general medical care inflation (4,6), suggesting that the use of general medical care inflation adjusters might underestimate the cost of cancer care.
The purpose of this study was to estimate and project the national medical cost of cancer care through the year 2020 separately for 13 cancers in men and 16 cancers in women using the most recent available US population projections and cancer incidence, survival, and cost of care data. We used methods developed specifically for estimating and projecting cancer prevalence by phase of care, including the initial phase following diagnosis, the last year of life phase, and the continuing phase between the initial and last year of life phases (7). We projected the costs of cancer care through the year 2020 using US population projections and scenarios with varying assumptions about trends in incidence, survival, and cost. To our knowledge, the estimated national cost of cancer care has not been previously projected dynamically. These data may be particularly useful for policy makers in understanding and anticipating the future burden of cancer care in the United States
Methods
Overview
Estimates and projections of the medical cost of cancer care through the year 2020 were calculated by combining cancer prevalence with average annual costs of cancer care by phase of care and tumor site for 13 cancers in men and 16 cancers in women. We also estimated and projected cost for all cancer sites combined. Cancer incidence, survival, practice patterns, and costs of care have changed in the past decades (1,2), but the degree to which these changes will continue in the future is unclear. Because of the uncertainty surrounding future trends in incidence, survival, and costs of care, we evaluated multiple scenarios with varying assumptions, including 1) constant current incidence, survival, and cost, the base case scenario; 2) recent incidence trends only; 3) recent survival trends only; 4) recent incidence and survival trends; and 5) recent incidence, survival, and cost trends. In all of the sensitivity analysis scenarios, we assumed a dynamic population increase as projected by the US Bureau of the Census and used the most recently available data to estimate incidence, survival, and cost of cancer care.
Data Sources
Incidence, survival, all-cause mortality, and population data from 1975 through 2005 were obtained from the Surveillance, Epidemiology, and End Results (SEER) program. The SEER registries collected every occurrence of a primary incident cancer, month and year of diagnosis, cancer site, stage, histology, and vital status, with cause of death for patients who died in geographically defined areas. Cancer incidence and survival were obtained by cancer site, sex, and year and age at diagnosis.
Population projections were obtained from the National Interim Projections of the US population from 2006 through 2020 from the US Census Bureau Web site (3). These population projections are based on assumptions about future births, deaths, and international migration. Other cause mortality in the years 2006–2020 were obtained from the Berkeley Mortality cohort life tables (8). These life table projections incorporate observed trends in life expectancy in the past century. Life tables and related documentation are available at http://www.demog.berkeley.edu/∼bmd/states.html.
The cost of cancer care was estimated from Medicare claims data linked to the SEER data (SEER–Medicare) among beneficiaries aged 65 years and older with a cancer diagnosis (9). Control subjects without cancer were identified from a 5% random sample of all Medicare beneficiaries residing in SEER areas. A more detailed description of the linked SEER–Medicare data is available at http://healthservices.cancer.gov/seermedicare/.
Phase of Care Definitions
We defined phase of care using three distinct clinically relevant periods or phases, including the initial period following diagnosis, the last year of life, and the period in between the initial and last year of life. The initial period was defined as the first 12 months following diagnosis, the last year of life was the final 12 months of life, and the continuing phase included all the months in-between. However, not all cancer patients contribute months of observation to all phases of care. For patients who survived less than 24 months after diagnosis, months of survival were assigned to the last year of life phase, and the remaining months of observation were allocated to the initial phase, with no allocation to the continuing phase of care. For patients who survived less than 12 months after diagnosis, those months between diagnosis and death were allocated to the last year of life.
Estimates and Projections of Cancer Prevalence
Cancer patients were classified by the site of their first diagnosis between 1975 and 2005 into one of the following 17 cancer sites: bladder, brain and other nervous system, female breast, cervix, colorectal, corpus uteri, esophagus, head and neck, kidney and renal pelvis, leukemia, lymphoma, lung, melanoma of the skin, ovary, pancreas, prostate and stomach. Nonmalignant cervical cancer and benign brain and other nervous system tumors were excluded; other nonmalignant tumors were included. All cancer sites combined were modeled as a single site and included only the first primary tumor between 1975 and 2005.
Cancer prevalence was estimated using a statistical method that projects prevalence from cancer incidence, cancer survival, and mortality for other causes of death (ie, prevalence incidence approach model [PIAMOD]) (7,10). Prevalence was estimated separately by tumor site for 13 cancer sites in men and 16 cancer sites in women. To estimate the prevalence of the remaining cancer sites, we calculated the difference between the prevalence of all cancer sites combined and the sum of the site-specific prevalence separately for men and women. Because PIAMOD can only provide results for closed age classes, and populations are reported with an age class of 85 years or older, prevalence for this age class was obtained by applying prevalence proportions of the 80–84 year age class to the population aged 85 years or older. For further details on the PIAMOD method, refer to Mariotto et al (7).
Cancer prevalence for years beyond the last year of data, 2005, was projected assuming different scenarios of future incidence and survival trends. In all of the sensitivity analysis scenarios, we assumed dynamic population trends, as estimated by the US Bureau of Census. We evaluated five prevalence scenarios: 1) constant current incidence, survival, and cost, the base case scenario; 2) recent incidence trends only; 3) recent survival trends only; 4) recent incidence and survival trends; and 5) recent incidence, survival, and cost trends.
The base case scenario assumes that future incidence and survival rates are constant and were based on rates from the most recent data period, 2003–2005, for each cancer site. For the incidence trends scenario, we projected the tumor and site-specific incidence trends estimated in years 1996–2005 into the future for years 2006–2020. In mathematical terms, the estimated annual percent change was calculated for each cancer site and sex by fitting a regression line to the natural logarithm of the age-adjusted rates I in years 1996 through 2005, ln(I)= α + β y, where α and β were coefficients to be estimated and y is calendar year. The annual percent change was calculated as 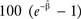 where
where 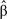 is the estimate of β. Let I(2003–2005,a) be the average incidence rate at age a at years 2003–2005. For each single age a, the SEER incidence rates were projected to years y = 2006, … , 2020 by applying the estimate annual percent change to the baseline incidence rate, that is
is the estimate of β. Let I(2003–2005,a) be the average incidence rate at age a at years 2003–2005. For each single age a, the SEER incidence rates were projected to years y = 2006, … , 2020 by applying the estimate annual percent change to the baseline incidence rate, that is 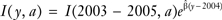 The national number of incident cancer patients by age and year were calculated by multiplying the SEER age and year incidence rates by the respective US populations for each tumor site and sex.
The national number of incident cancer patients by age and year were calculated by multiplying the SEER age and year incidence rates by the respective US populations for each tumor site and sex.
For the survival trends scenario, linear survival trends were estimated by fitting a parametric mixture cure survival model to the SEER data by tumor site, sex, and age group (11). This type of model assumes that a hypothetical fraction of the patients will not die of cancer and will experience the same mortality risk as the general population, whereas the complementary fraction will die of cancer, and their survival time follows a Weibull distribution (12). The cumulative probability of surviving t years from diagnosis, for people diagnosed at year y and at the age class a is given by
where ca is the cure fraction, the proportion of patients who at diagnosis will eventually not die of their cancer, λa and γa are, respectively, the scale and shape parameters of the Weibull survival distribution of patients not cured, and exp(δa) represents the relative risk (RR) of cancer death for being diagnosed 1 year later than an arbitrary reference year y0 (eg, 1985). Survival parameters were estimated for each cancer site, sex, and age group (0–44, 45–54, 55–64, 65–74, and 75–84 years). The use of a parametric survival model allows survival extrapolation beyond the range of the empirical data. The constant survival trend scenario assumes future survival as equal to the most recent year of data 2005, whereas the linear trend scenario extrapolates survival using the period trend parameter δa. The survival model and the prevalence method are described in greater detail elsewhere (7). The incidence and survival trends scenario combined both recent incidence and recent survival trends.
Estimates and Projections of the Cost of Cancer Care
We updated previously published direct medical cost estimates by phase of care (13) using the most recently available linked SEER–Medicare data from all registries (SEER-17), which included cancer patients diagnosed through the year 2005. We used all Medicare claims files and standard methods of estimating mean annualized net costs of care with these data (13,14) as described below. Patients with a cancer diagnosis in SEER between 1975 and 2005 and aged 65 years and older during 2001–2006, the observation period, were selected for the cohort. Cancer patients with a prior cancer diagnosis or identified through a death certificate or autopsy were excluded. Months of observation in which patients received coverage through managed care or were covered through fee-for-service and without both Medicare part A and part B were excluded because these data would not completely capture costs of care received during this period. Months of observation and costs were assigned to the initial, continuing, and last year of life phases of care. A total of 390 683 cancer patients were included in the initial phase of care, 926 793 in the continuing phase of care, and 475 750 in the last year of life phase of care. Cancer patients in the last year of life were also classified by cause of death on the death certificate as cancer vs all other causes.
Noncancer control subjects were randomly assigned a “pseudo-diagnosis date” that corresponded to the date of diagnosis of one of the pool of cancer patients. Control subjects were frequency matched to cancer patients by sex, 5-year age group (65–69, 70–74, 75–79, and ≥80 years), and SEER area strata in up to a 5:1 ratio. For the initial and continuing phases of care, costs for cancer patients were compared with costs for “continuing control subjects” that included data from the pseudo-diagnosis date through 1 year before the date of death. To reflect costs associated with cancer care in the last year of life, cancer patients who died of cancer were matched to continuing control subjects, whereas cancer patients who died of other causes (eg, accident) were matched to control subjects in their last year of life. As with cancer patients, average monthly estimates of cost of care were calculated for each phase of care for control subjects. A total of 1 648 138 control subjects were included in the initial phase of care, 2 141 794 in the continuing phase of care, and 1 662 486 in the last year of life phase of care.
We used payments to reflect costs and estimated Medicare part A (inpatient services) and part B (outpatient services) separately. Monthly costs were estimated for cancer patients and control subjects by phase of care. Net monthly medical costs were calculated as differences in costs for cancer patients and control subjects by tumor site and phase of care. Mean annualized net costs were calculated by multiplying the net monthly costs by 12 months by tumor site and phase of care. We added adjustments for patient deductibles and coinsurance expenses separately for Medicare parts A and B. The Medicare Prospective Payment System adjuster and the Medicare Economic Index were used to adjust for inflation in Medicare part A and part B, respectively. The Medical Geographic Adjustment Factor and the Geographic Practice Cost Index were used to adjust for cost difference across different SEER locations for part A and part B costs, respectively (15).
Patterns of care have been reported to be more aggressive for younger cancer patients compared with elderly cancer patients in many health-care settings (16). In addition, because the prevalence of comorbid conditions and levels of medical care increase with age, health-care spending also typically increases with age (17). To extrapolate net cost estimates from the patient population aged 65 years and older to the population aged 65 years and younger, we used ratios of the relationship between costs in the elderly to those in younger patients in the initial and last year of life phases of care from published studies conducted in managed care settings (18). We used ratios of 1.2 and 1.5 to adjust the annual net medical cost for those younger than 65 years treated in the initial and last year of life, respectively. Costs in the continuing phase of care were assumed to be the same. All costs are reported in 2010 US dollars. National estimates of costs of care were calculated by combining the mean net costs of cancer care with US prevalence estimates by age, sex, and cancer site.
In the base case scenario, we assumed that recent cancer costs remained constant through the year 2020. In addition to evaluating scenarios with varying assumptions about incidence and survival, in the cost trends scenario, we also evaluated different assumptions about costs. For example, the introduction of more effective, but dramatically more expensive treatments (6,19), will likely affect cancer costs in the future. However, the patterns of diffusion of these therapies are unknown. To reflect this uncertainty, we evaluated three cost trends scenarios: 1) overall cost increase of 2% annually, 2) recent trends in which costs increased by 2% annually in the initial and last year of life phases, and 3) escalating costs in which costs increased by 5% annually in the initial and last year of life phases.
Results
Recent and Projected Incidence
For most of the cancer sites, incidence has been decreasing, and we estimated a negative annual percent change (Table 1) during the period 1996–2005. The largest decreases in men were for lung, stomach, and colorectal cancers, respectively, −2.72, −2.24, and −2.22 annual percent change in age-adjusted rates. More dramatic decreases were observed for women for ovarian and cervical cancer, −4.71 and −3.95, respectively, annual percent change in age-adjusted rates. Incidence of kidney cancer and melanoma has been increasing in both men and women, and incidence of lymphoma and brain cancer has been increasing in women (Table 1). Among the five major cancer sites, the largest decreases in incidence were observed for lung and colorectal cancers in men, −2.72 and −2.22, respectively, annual percent change in age-adjusted incidence rates (Figure 1; Similar figures for more cancer sites are available at http://costprojections.cancer.gov.).
Table 1.
Incidence and survival trends used in the incidence and survival trend scenario*
| Site | APC in age-adjusted incidence rates | RR of cancer death for being diagnosed one year later† | ||
| Women | Men | Women | Men | |
| All sites | −0.46 | −0.68 | 0.982 | 0.959 |
| Bladder | −1.28 | −1.36 | 0.982 | 0.974 |
| Brain | 0.18 | −0.89 | 0.987 | 0.987 |
| Breast | −1.05 | — | 0.935 | — |
| Cervix | −3.95 | — | 0.997 | — |
| Colorectal | −1.78 | −2.22 | 0.978 | 0.974 |
| Esophagus | −1.57 | 0.00 | 0.980 | 0.971 |
| Head and neck | −1.59 | −1.66 | 0.995 | 0.993 |
| Kidney | 2.36 | 1.83 | 0.974 | 0.977 |
| Leukemia | −0.64 | −0.69 | 0.993 | 0.988 |
| Lung | −0.81 | −2.72 | 0.994 | 0.994 |
| Lymphoma | 0.19 | −0.63 | 0.978 | 0.977 |
| Melanoma | 3.09 | 2.09 | 0.915 | 0.905 |
| Ovary | −4.71 | — | 0.976 | — |
| Pancreas | −0.24 | 0.02 | 0.988 | 0.989 |
| Prostate | — | −0.70 | — | 0.889 |
| Stomach | −1.75 | −2.24 | 0.985 | 0.988 |
| Uterus | −0.88 | — | 0.991 | — |
Incidence trends were modeled using estimated annual percent change (APC) in age-adjusted cancer incidence rates 1996–2005. Survival trends were modeled for men and women diagnosed in age groups 0–44, 45–54, 55–64, and 75–84 years. RR = relative risk.
Trends were summarized using estimated relative risk of cancer death for patients diagnosed in a given year compared with patients diagnosed in the previous year. Results were similar by age groups, estimates are presented for the 65–74 age group.
Figure 1.
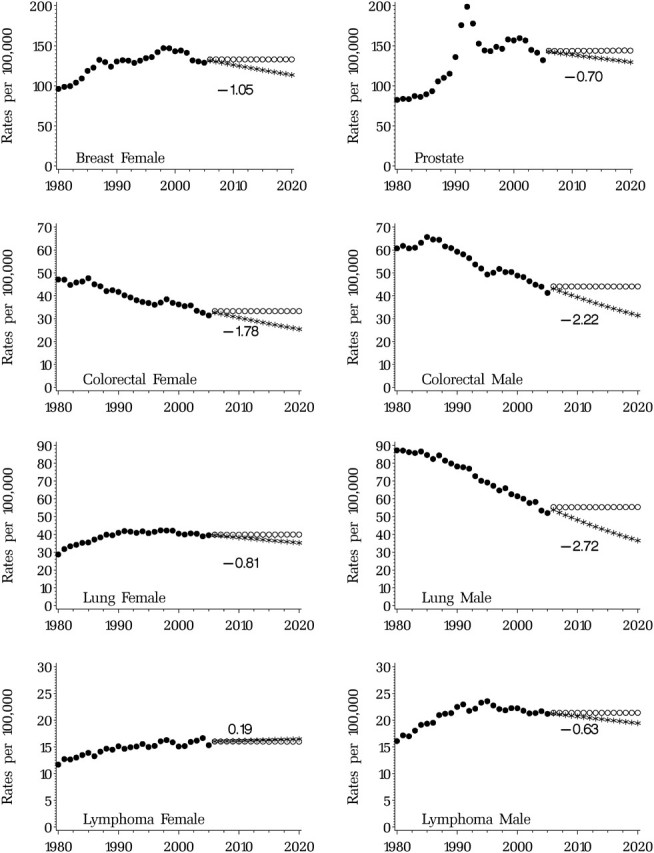
Observed age-adjusted incidence (solid circles) and projected age-adjusted incidence under the assumption of future constant incidence (open circles) and continuing incidence trend (asterisks). The number represents the estimated annual percent change from 1996 through 2005.
Recent and Projected Survival
Survival has been improving for almost all cancer sites. Survival trends are summarized by the estimated relative risk, which represents the risk of dying of cancer for patients diagnosed in a given year compared with patients diagnosed in the previous year. The largest improvements in survival were for prostate cancer in men, where the risk of dying of cancer death for patients diagnosed in a given year compared with patients diagnosed in the previous year was 89% smaller (ie, RR = 0.89), followed by melanoma (RR = 0.91 in men and RR = 0.92 in women), and female breast cancer (RR = 0.94) (Table 1). For bladder, cervix, and uterus, a flat or slight decline (younger age groups) in survival trend was estimated (data not shown). The observed and modeled 5-, 10-, and 15-year relative survival trends for the major cancer sites for people diagnosed between the ages of 65 and 74 years showed the largest increases in survival for prostate cancer in men and breast cancer in women (Figure 2).
Figure 2.
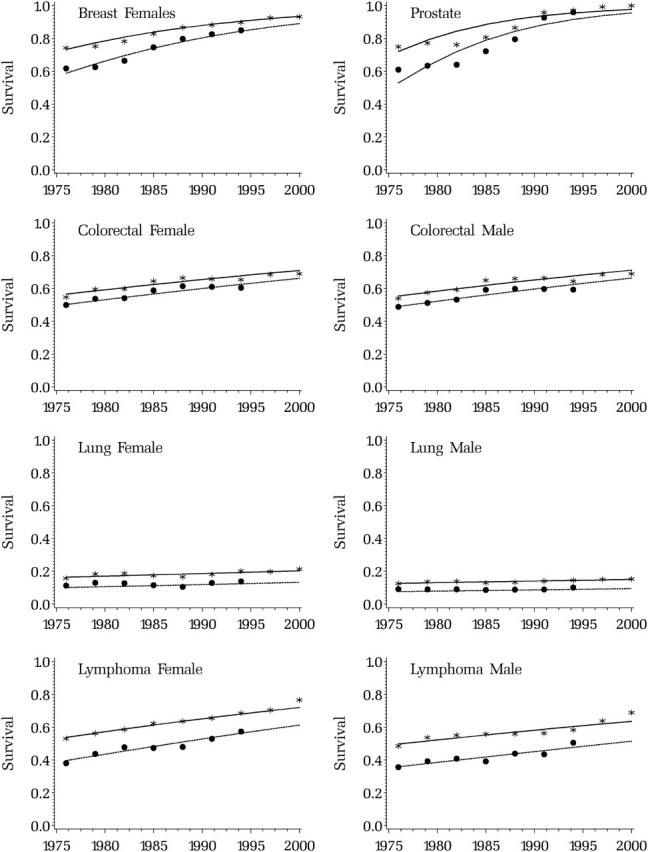
Fit of the survival model to observed data. Observed (solid circles [10-year] and asterisks [5-year]) and modeled (solid line [5-year] and dashed line [10-year]) relative survival trends for the major cancer sites for people diagnosed between the ages of 65 and 74 years.
Cancer Prevalence.
In the year 2010, we estimated that a total of 13 772 000 cancer survivors will be alive, with 42% younger than 65 years and the remaining 58% aged 65 years and older (Table 2). Under the base case scenario, holding incidence and survival constant, the number of cancer survivors in 2020 will increase by 31% to approximately 18 071 000. The largest increase between 2010 and 2020 (42%) was projected for the population aged 65 years and older (Table 2) because of the aging of the US population. The largest proportion of cancer survivors were in the continuing phase of care, representing 86% of all cancer survivors in 2010 and 2020. Female breast (3 461 000), prostate (2 311 000), colorectal (1 216 000), and melanoma (1 225 000) were the sites with the largest number of survivors in 2010 (Table 2). These sites were also projected to have the largest number of survivors in the year 2020. For most of the cancer sites, 2020 cancer prevalence projections were robust under the different incidence and survival assumptions (Table 3). For all cancers, the 2020 projections under the base case, incidence trend, survival trend, and incidence and survival trend scenarios were very similar, with approximately 18 million survivors (Table 3).
Table 2.
United States cancer prevalence estimates for 2010 and 2020*
| Site | No. of people in thousands |
||||||||
| Initial |
Continuing |
Last year |
All phases |
||||||
| <65 | ≥65 | <65 | ≥65 | <65 | ≥65 | <65 | ≥65 | All ages | |
| Bladder | |||||||||
| 2010 | 15 | 29 | 117 | 312 | 4 | 38 | 135 | 379 | 514 |
| 2020 | 16 | 39 | 123 | 399 | 4 | 47 | 144 | 485 | 629 |
| Brain and ONS | |||||||||
| 2010 | 7 | 3 | 103 | 14 | 9 | 3 | 118 | 20 | 139 |
| 2020 | 7 | 4 | 123 | 25 | 11 | 5 | 142 | 35 | 176 |
| Female breast | |||||||||
| 2010 | 141 | 92 | 1320 | 1735 | 32 | 141 | 1493 | 1068 | 3461 |
| 2020 | 148 | 120 | 1496 | 2538 | 36 | 200 | 1680 | 2858 | 4538 |
| Cervix | |||||||||
| 2010 | 7 | 2 | 140 | 119 | 5 | 9 | 152 | 130 | 281 |
| 2020 | 7 | 3 | 128 | 125 | 5 | 9 | 139 | 137 | 276 |
| Colorectal | |||||||||
| 2010 | 41 | 65 | 286 | 724 | 16 | 84 | 343 | 873 | 1216 |
| 2020 | 48 | 82 | 338 | 926 | 19 | 104 | 405 | 1112 | 1517 |
| Esophagus | |||||||||
| 2010 | 3 | 4 | 9 | 13 | 2 | 4 | 14 | 21 | 35 |
| 2020 | 3 | 5 | 11 | 22 | 2 | 6 | 17 | 33 | 50 |
| Head and neck | |||||||||
| 2010 | 14 | 10 | 110 | 122 | 7 | 18 | 132 | 150 | 283 |
| 2020 | 16 | 12 | 124 | 156 | 8 | 23 | 149 | 191 | 340 |
| Kidney | |||||||||
| 2010 | 14 | 12 | 123 | 136 | 5 | 17 | 142 | 166 | 308 |
| 2020 | 16 | 17 | 151 | 211 | 6 | 25 | 173 | 254 | 426 |
| Leukemia | |||||||||
| 2010 | 10 | 11 | 148 | 72 | 8 | 13 | 166 | 97 | 263 |
| 2020 | 10 | 14 | 182 | 105 | 10 | 19 | 202 | 138 | 340 |
| Lung | |||||||||
| 2010 | 28 | 52 | 64 | 171 | 13 | 46 | 105 | 269 | 374 |
| 2020 | 31 | 68 | 69 | 217 | 14 | 59 | 113 | 343 | 457 |
| Lymphoma | |||||||||
| 2010 | 26 | 22 | 333 | 212 | 15 | 31 | 374 | 265 | 639 |
| 2020 | 27 | 29 | 369 | 323 | 17 | 47 | 413 | 399 | 812 |
| Melanoma | |||||||||
| 2010 | 53 | 30 | 629 | 466 | 10 | 38 | 691 | 534 | 1225 |
| 2020 | 55 | 42 | 753 | 788 | 12 | 63 | 820 | 893 | 1714 |
| Ovary | |||||||||
| 2010 | 8 | 7 | 108 | 91 | 7 | 16 | 124 | 114 | 238 |
| 2020 | 8 | 9 | 108 | 127 | 8 | 22 | 125 | 158 | 282 |
| Pancreas | |||||||||
| 2010 | 5 | 9 | 7 | 3 | 3 | 3 | 15 | 16 | 30 |
| 2020 | 6 | 12 | 9 | 7 | 3 | 5 | 17 | 23 | 40 |
| Prostate | |||||||||
| 2010 | 87 | 126 | 387 | 1546 | 10 | 154 | 484 | 1827 | 2311 |
| 2020 | 108 | 175 | 510 | 2243 | 13 | 215 | 631 | 2633 | 3265 |
| Stomach | |||||||||
| 2010 | 4 | 6 | 18 | 37 | 2 | 6 | 24 | 50 | 74 |
| 2020 | 5 | 8 | 22 | 49 | 3 | 8 | 29 | 64 | 93 |
| Uterus | |||||||||
| 2010 | 20 | 16 | 156 | 360 | 3 | 31 | 179 | 407 | 586 |
| 2020 | 23 | 20 | 177 | 413 | 3 | 34 | 204 | 468 | 672 |
| All sites | |||||||||
| 2010 | 528 | 552 | 5066 | 6725 | 178 | 723 | 5772 | 8000 | 13 772 |
| 2020 | 583 | 724 | 5902 | 9645 | 209 | 1008 | 6694 | 11 377 | 18 071 |
The estimated number of individuals with a previous cancer diagnosis in the initial year after diagnosis, continuing and last year of life phases of care by age (<65 and ≥65 years), and year was estimated using a method that calculates prevalence from projected incidence and survival models (7,10). ONS = other nervous system tumors.
Table 3.
United States cancer prevalence estimates for 2010 and projections for 2020 under different scenario assumptions*
| Site | Number of cancer survivors |
||||
| 2010 | 2020 |
||||
| Base | Base, No. (% change) | Trend incidence, No. (% change) | Trend survival, No. (% change) | Trend incidence and survival, No. (% change) | |
| Bladder | 514 000 | 629 000 (22) | 576 000 (12) | 640 000 (14) | 587 000 (14) |
| Brain | 139 000 | 176 000 (27) | 174 000 (25) | 185 000 (31) | 182 000 (31) |
| Breast | 3 461 000 | 4 538 000 (31) | 4 275 000 (24) | 4 597 000 (25) | 4 329 000 (25) |
| Cervix | 281 000 | 276 000 (−2) | 245 000 (−13) | 277 000 (−13) | 245 000 (−13) |
| Colorectal | 1 216 000 | 1 517 000 (25) | 1 327 000 (9) | 1 575 000 (13) | 1 376 000 (13) |
| Esophagus | 35 000 | 50 000 (43) | 48 000 (37) | 62 000 (71) | 60 000 (71) |
| Head and neck | 283 000 | 340 000 (20) | 308 000 (9) | 346 000 (11) | 313 000 (11) |
| Kidney | 308 000 | 426 000 (38) | 487 000 (58) | 446 000 (66) | 511 000 (66) |
| Leukemia | 263 000 | 340 000 (29) | 328 000 (25) | 356 000 (30) | 342 000 (30) |
| Lung | 374 000 | 457 000 (22) | 392 000 (5) | 481 000 (10) | 412 000 (10) |
| Lymphoma | 639 000 | 812 000 (27) | 803 000 (26) | 841 000 (30) | 831 000 (30) |
| Melanoma | 1 225 000 | 1 714 000 (40) | 1 971 000 (61) | 1 724 000 (62) | 1 983 000 (62) |
| Ovary | 238 000 | 282 000 (18) | 232 000 (−3) | 296 000 (1) | 241 000 (1) |
| Pancreas | 30 000 | 40 000 (33) | 40 000 (33) | 50 000 (67) | 50 000 (67) |
| Prostate | 2 311 000 | 3 265 000 (41) | 3 108 000 (34) | 3 291 000 (36) | 3 132 000 (36) |
| Stomach | 74 000 | 93 000 (26) | 80 000 (8) | 103 000 (19) | 88 000 (19) |
| Uterus | 586 000 | 672 000 (15) | 638 000 (9) | 667 000 (8) | 634 000 (8) |
| All sites | 13 772 000 | 18 071 000 (31) | 17 465 000 (27) | 18 878 000 (32) | 18 229 000 (32) |
Scenarios: base, incidence trend, survival trend, and incidence and survival trend. Percent change from 2010 base estimate. The 2020 estimates under the base scenario represent prevalence estimates under the assumption of flat incidence and survival trends but dynamic changes in the US population. Incidence trend and survival trend scenarios represent prevalence projections under the assumptions that survival and incidence trends will continue as observed in the last years of data. Incidence trends represent changes due to prevention and risk factor prevalence. Survival trends represent changes in early detection and treatment.
Estimates of the Medical Costs Associated With Cancer Care
The average annualized net costs of care were highest in the last year of life phase of care for patients dying from cancer for all cancer sites (Table 4). The average annualized net costs of care were more variable in the initial phase of care. Brain, pancreas, ovary, esophagus, and stomach cancers had the largest annualized initial cost, and melanoma, prostate, and breast cancers had the lowest annualized initial cost (Table 4).
Table 4.
Annualized mean net costs of care in 2010 US dollars*
| Annual costs in US 2010 dollars |
||||||||
| Age <65 |
Age ≥65 |
|||||||
| Last year of life |
Last year of life |
|||||||
| Sex and site | Initial | Continuing | Cancer death | Other cause | Initial | Continuing | Cancer death | Other cause |
| Female | ||||||||
| Bladder | 25 694 | 3710 | 118 047 | 10 005 | 21 412 | 3710 | 78 698 | 10 005 |
| Brain | 129 802 | 8803 | 211 337 | 39 893 | 108 168 | 8803 | 140 891 | 39 893 |
| Breast | 27 693 | 2207 | 94 284 | 748 | 23 078 | 2207 | 62 856 | 748 |
| Cervix | 54 209 | 1425 | 117 830 | 7949 | 45 174 | 1425 | 78 553 | 7949 |
| Colorectal | 61 593 | 3159 | 126 778 | 14 641 | 51 327 | 3159 | 84 519 | 14 641 |
| Esophagus | 95 439 | 6853 | 156 417 | 41 051 | 79 532 | 6853 | 104 278 | 41 051 |
| Head and neck | 50 376 | 4826 | 129 903 | 10 064 | 41 980 | 4826 | 86 602 | 10 064 |
| Kidney | 46 077 | 6255 | 110 765 | 24 607 | 38 397 | 6255 | 73 843 | 24 607 |
| Leukemia | 39 800 | 8537 | 195 196 | 31 517 | 33 167 | 8537 | 130 131 | 31 517 |
| Lung | 72 639 | 8130 | 138 785 | 18 897 | 60 533 | 8130 | 92 524 | 18 897 |
| Lymphoma | 69 457 | 8622 | 164 763 | 20 462 | 57 881 | 8622 | 109 842 | 20 462 |
| Melanoma | 6057 | 915 | 85 175 | 252 | 5047 | 915 | 56 784 | 252 |
| Other | 40 173 | 5859 | 95 782 | 21 721 | 40 173 | 5859 | 95 782 | 21 721 |
| Ovary | 98 788 | 8296 | 149 573 | 12 257 | 82 324 | 8296 | 99 715 | 12 257 |
| Pancreas | 112 154 | 8672 | 164 911 | 40 538 | 93 462 | 8672 | 109 941 | 40 538 |
| Stomach | 85 291 | 3977 | 155 636 | 29 172 | 71 076 | 3977 | 103 758 | 29 172 |
| Uterus | 32 129 | 1535 | 105 262 | 4437 | 26 775 | 1535 | 70 175 | 4437 |
| Male | ||||||||
| Bladder | 25 152 | 4677 | 113 659 | 8446 | 20 960 | 4677 | 75 772 | 8446 |
| Brain | 138 300 | 9434 | 201 366 | 67 914 | 115 250 | 9434 | 134 244 | 67 914 |
| Colorectal | 62 174 | 4595 | 128 507 | 15 068 | 51 812 | 4595 | 85 671 | 15 068 |
| Esophagus | 95 787 | 6450 | 155 612 | 51 035 | 79 822 | 6450 | 103 742 | 51 035 |
| Head and neck | 47 015 | 4001 | 125 493 | 9269 | 39 179 | 4001 | 83 662 | 9269 |
| Kidney | 46 048 | 6018 | 117 123 | 19 142 | 38 374 | 6018 | 78 082 | 19 142 |
| Leukemia | 43 243 | 10 249 | 199 774 | 35 941 | 36 036 | 10 249 | 133 183 | 35 941 |
| Lung | 73 062 | 7591 | 142 977 | 25 008 | 60 885 | 7591 | 95 318 | 25 008 |
| Lymphoma | 72 841 | 9337 | 174 894 | 27 200 | 60 701 | 9337 | 116 596 | 27 200 |
| Melanoma | 6524 | 1951 | 93 654 | 546 | 5437 | 1951 | 62 436 | 546 |
| Other | 41 161 | 7363 | 97 473 | 25 758 | 41 161 | 7363 | 97 473 | 25 758 |
| Pancreas | 112 911 | 11 697 | 169 673 | 47 565 | 94 092 | 11 697 | 113 115 | 47 565 |
| Prostate | 23 652 | 3201 | 93 363 | 5370 | 19 710 | 3201 | 62 242 | 5370 |
| Stomach | 94 144 | 4282 | 160 695 | 25 800 | 78 453 | 4282 | 107 130 | 25 800 |
Average annualized net costs of cancer by phase of care were estimated from claims of patients aged 65 years and older during 2001–2006 diagnosed with cancer between 1975 and 2005 in the SEER-17 areas. Control subjects without cancer were identified from a 5% random sample of all Medicare beneficiaries residing in SEER areas and their costs were compared to the costs of the cancer population to estimate the net costs of cancer care. All estimates adjusted for patient deductibles and coinsurance expenses. Adjustments for more intensive care in younger cancer patients were based on relationship between costs in populations younger than 65 and 65 and older in managed care populations. Costs adjusted to 2010 US dollars.
Under the base case scenario, the national cost of cancer care in 2010 was estimated to be $124.57 billion (Table 5). Female breast was the cancer site with the highest cost in 2010 ($16.50 billion) followed by colorectal ($14.14 billion), lymphoma ($12.14 billion), lung ($12.12 billion), and prostate ($11.85 billion). Under the base case scenario, costs in 2020 were estimated to be $157.77 billion, representing a 27% increase from 2010. Assuming constant costs, the scenario with the highest national cost estimate in 2020 was the continuing survival trend only scenario ($165.21 billion), and the lowest national cost estimate in 2020 was the continuing incidence trends only scenario ($147.57 billion). The continuing incidence trend scenario represents a smaller increase in costs from 2010, compared with the base scenario, because incidence trends have been decreasing for most of the cancer sites. The exceptions are cancer of the kidney and melanoma, for which incidence increased. If the incidence of cervical and ovarian cancers continues to decrease, care costs for these cancer sites in 2020 will remain the same or decrease compared with 2010. Under the assumption of continuing survival improvement, costs will increase compared with the base scenario. However, the impact of survival on cancer prevalence was smaller than that of incidence (Table 5). If we assume a 2% annual increase in the average costs of care in the initial and last year of life phases, the cost of cancer care is estimated to be $172.77 billion, representing a 39% increase. Costs of cancer care in 2020 were estimated to be $207 billion under the assumption of 5% increase in the costs in the initial and last year phases of care (escalating costs), representing a 66% increase from 2010.
Table 5.
National costs of cancer care in 2010 US billion dollars for 2010 and 2020 using different assumptions of future cancer incidence and survival and increases in the cost of care*
| Site | Cost in US 2010 billion dollars |
|||||||
| 2010 | 2020 |
|||||||
| Base | Base | Trend incidence | Trend survival | Trend incidence and survival | Under trend incidence and survival: cost increase |
|||
| 2% overall | 2% in initial and last year phase | 5% in initial and last year phase | ||||||
| Breast | 16.50 | 20.50 | 18.91 | 20.69 | 19.08 | 23.24 | 21.37 | 25.64 |
| Colorectal | 14.14 | 17.41 | 14.35 | 17.83 | 14.70 | 17.67 | 16.68 | 20.39 |
| Lymphoma | 12.14 | 15.26 | 15.00 | 15.71 | 15.44 | 18.66 | 17.27 | 20.69 |
| Lung | 12.12 | 14.73 | 12.14 | 15.23 | 12.53 | 15.19 | 14.73 | 18.84 |
| Prostate | 11.85 | 16.34 | 15.32 | 16.43 | 15.41 | 18.53 | 16.67 | 19.02 |
| Leukemia | 5.44 | 6.95 | 6.66 | 7.24 | 6.94 | 8.38 | 7.78 | 9.35 |
| Ovary | 5.12 | 6.03 | 4.49 | 6.27 | 4.64 | 5.64 | 5.26 | 6.42 |
| Brain | 4.47 | 5.53 | 5.38 | 5.79 | 5.62 | 6.82 | 6.51 | 8.18 |
| Bladder | 3.98 | 4.91 | 4.41 | 4.98 | 4.47 | 5.38 | 4.90 | 5.71 |
| Kidney | 3.80 | 5.12 | 6.07 | 5.30 | 6.29 | 7.56 | 6.99 | 8.30 |
| Head/Neck | 3.64 | 4.34 | 3.79 | 4.40 | 3.84 | 4.65 | 4.40 | 5.46 |
| Uterus | 2.62 | 3.05 | 2.84 | 3.04 | 2.83 | 3.42 | 3.24 | 4.00 |
| Melanoma | 2.36 | 3.16 | 3.76 | 3.18 | 3.78 | 4.60 | 4.06 | 4.58 |
| Pancreas | 2.27 | 2.83 | 2.81 | 3.16 | 3.13 | 3.80 | 3.75 | 4.92 |
| Stomach | 1.82 | 2.26 | 1.81 | 2.40 | 1.92 | 2.31 | 2.25 | 2.88 |
| Cervix | 1.55 | 1.54 | 1.20 | 1.55 | 1.21 | 1.46 | 1.39 | 1.73 |
| Esophagus | 1.33 | 1.76 | 1.70 | 2.04 | 1.97 | 2.38 | 2.32 | 2.97 |
| All sites | 124.57 | 157.77 | 147.57 | 165.21 | 154.70 | 186.69 | 172.77 | 206.59 |
The 2020 estimates under the base scenario represent prevalence estimates under the assumption of flat incidence and survival trends but dynamic changes in the US population. Incidence trend and survival trend scenarios represent prevalence projections under the assumptions that survival and incidence trends will continue as observed in the last years of data. Incidence trends represent changes due to prevention and risk factor prevalence. Survival trends represent changes in early detection and treatment. Costs scenarios were 2% annual increase in costs in all phases of care (overall) and 2% and 5% annual increase in the initial and last year phases of care.
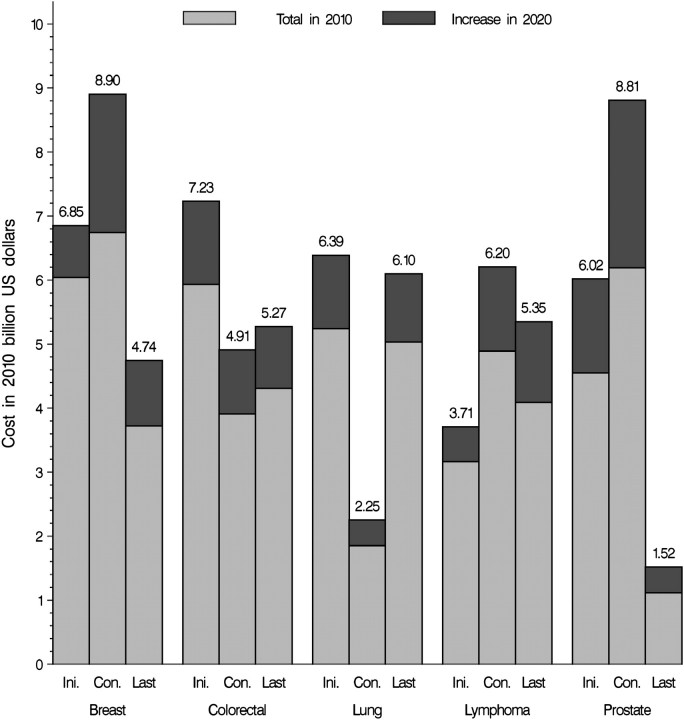
National expenditures in 2020 by phases of care for the five major cancer sites—breast, colorectal, prostate, lung, and lymphoma—were partitioned into the estimated expenditure in 2010 and the additional expenditure under the base case scenario projected for 2020 (Figure 3). Differences were due only to the aging and growth of the US population. Colorectal cancer was the site with the highest cost in the initial phase of care and lung cancer had the highest cost in the last year of life phase of care. Prostate and female breast cancers had the highest cost in the continuing phase. The highest increases in medical cost of care in 2020 were projected for female breast (32%) and prostate (42%) cancer patients in the continuing phase (Figure 3).
Figure 3.
Estimates of the national expenditures for cancer care in 2010 (light gray areas) and the estimated increase in cost in 2020 (dark gray areas) because of the aging and growth of the US population under assumptions of constant incidence survival and cost for the major cancer sites. Costs in 2010 billion US dollars by phase of care: initial year after diagnosis (Ini.) continuing care (Con.) and last year of life (Last).
Discussion
We used the most recently available cancer incidence, survival, and medical cost of care data in the United States to estimate and project the national costs of cancer care through the year 2020. In our base case model using constant cancer incidence, survival, and cost of care, we estimated that the national costs of cancer care in 2010 will be approximately $124.57 billion. We projected national costs to increase to $157.77 billion in 2020 under the base case scenario (constant incidence, survival, and cost), a 27% increase. Because we used dynamic assumptions of aging and growing of the US population (3) for all projections, this increase in costs over time in the base case scenario reflects growth and aging in the population only. The largest increase in cost projected for 2020 was in the continuing care phase for female breast and prostate cancers (Figure 3). This increase in the number of breast and prostate cancer survivors has important implications for the demand for medical oncologists (20), as well as the interaction between primary care and oncology for coordination of surveillance care. Our findings will be particularly useful for policy makers for planning and allocation of resources.
We also evaluated a variety of sensitivity analysis scenarios reflecting different assumptions about future trends in incidence, survival, and costs of care. Projections using different assumptions of survival and incidence trends were robust and show that changes in incidence and/or survival have a smaller impact on estimates compared with the aging and growth of the US population. The 2020 predicted costs of cancer care under the assumptions of 1) continuing trends (decreasing incidence and increasing survival) and 2) constant incidence and survival were very similar, 154.70 and 157.77 billion US 2010 dollars, respectively. These estimates represent increases of 27% and 24%, respectively, in cost compared with 2010. In both of these scenarios, we assumed that currently developed cancer control technologies and their current costs will continue as in the past. It is likely that new tools for diagnosis, treatment, and follow-up of cancer patients will be developed and will be more expensive. Assuming recent incidence and survival trends, a 2% increase in annual costs of care in the initial and last year of life phases will result in a 39% increase in costs over the 10 years and a cost estimate of $173 billion in 2020. With expected increases in use of targeted chemotherapies, increases in the cost of a course of treatment are expected to escalate more rapidly. A 5% increase in the annual costs of care in the initial and last year of life phases yields a projected $207 billion in 2020, a 66% increase from 2010. However, trends in costs associated with the use of targeted chemotherapies might be mitigated somewhat through the use of genomic based prognostic markers.
Our estimates of the national cost of cancer care in the year 2010 are higher than those reported elsewhere (5), even after accounting for differences in the base year used for inflation adjustment. Important differences include our use of the most recent incidence, survival, and cost of care data, identification of cancer patients from registry rather than self-report, use of dynamic population estimates and projections, and detailed methods for estimating cancer prevalence. In particular, our cost estimates were based on Medicare claims through the year 2006, reflecting the use of targeted therapies in this population. In addition, we used a phase of care framework to measure the trajectory of cancer care from diagnosis to death to classify cancer survivors and estimate the cost of care for distinct periods. Costs of care for cancer patients who die of their disease follows a “U-shaped” curve, with the highest costs in the initial phase following diagnosis and the phase before death, and the lowest costs in the period in-between, the continuing phase. This approach not only provides more detailed information of the costs of cancer care but also allows for projections and provides more accurate estimates, especially for less common cancers.
Our estimates for 2010 were substantially higher than a recent study (21) of national expenditures for cancer treatment in 2001–2005, which used data on Medical Expenditure Panel Survey (MEPS) respondents who reported being treated for cancer. Importantly, population-based surveys such as the MEPS may underidentify respondents with less common cancers or cancers with short survival following diagnosis (eg, lung, brain, gastric, and pancreatic). Individuals who are ill may also be less likely to respond but may be more likely to receive higher levels of medical care. In addition, as shown here and elsewhere (13,18,22), costs in the continuing phase of care are higher for cancer survivors compared with similar individuals without cancer. However, cancer survivors no longer receiving active cancer treatment in the continuing phase could not be identified as having cancer in these surveys. As a result, estimates from surveys, particularly those that estimate “treated prevalence,” are likely to understate national cancer expenditures.
There were limitations to our analysis. Our estimates of cancer prevalence were based on cancer incidence and survival from the SEER-9 areas, which do not cover the entire United States. The SEER areas had lower incidence rates than most other states and have been found to have higher socioeconomic status, greater urban population, and more specialty care than the rest of the US population. In addition, because people can be diagnosed with multiple tumors, cancer prevalence and costs estimates that are based on first tumor diagnosed per person may be underestimates. Our estimates for cancer are not directly comparable to those for other diseases, in part because other diseases do not have the high-quality, comprehensive, population-based disease registries that can be linked to health insurance data to provide information from diagnosis to death. In addition, we do not explicitly control for the presence of diseases other than cancer. If the prevalence of other diseases is the same in cancer patients and control subjects, the net difference is associated only with cancer. However, if the prevalence of other diseases is higher in cancer patients than in control subjects, the net difference reflects costs in cancer patients including those associated with other diseases. Evaluating methods for allocating disease-specific health-care costs is an ongoing area of research (23).
We made a number of assumptions to develop our national cost estimates. We assumed that costs associated with cancer care in Medicare fee-for-service and managed care settings are the same. Because managed care plans have not traditionally been required to submit claims or encounter data for services received by their Medicare enrollees, we necessarily excluded managed care beneficiaries from the sample used to develop our cost estimates. To date, no studies have compared the costs of care in Medicare fee-for-service and managed care settings, although a study comparing costs of care for younger colorectal cancer patients in a health maintenance organization and a preferred provider organization reported small but not statistically significant differences (24). Furthermore, because Medicare provides coverage for almost all of those over the age of 65 years and the linkage of SEER and Medicare claims represents approximately 26% of the US population, the linked SEER–Medicare data are the most comprehensive longitudinal data available for estimating the cost of cancer care in the elderly.
We also made assumptions about the relationship between the costs of cancer care in younger populations and the elderly. In populations younger than 65 years, health insurance is predominantly employer based, with many distinct and separate insurance programs. Comprehensive, longitudinal, population-based insurance data with detailed information about patients and cancer diagnosis (ie, linkage to cancer registries) are generally not available for the population younger than 65 years outside of managed care settings. Because of this lack of comprehensive data for the population under the age of 65 years, we adjusted the SEER–Medicare cost estimates for patients aged 65 years and older by ratios of 1.2 and 1.5 to reflect more aggressive cancer care received by younger cancer patients in the initial and last year of life phases, respectively. These ratios were based on published studies comparing the costs for patients older and younger than 65 years in managed care settings from the early 1990s (18).
A more recent study (25) of treated cancer survivors from the MEPS reported that overall costs among patients younger than 65 years are on average 35% higher than patients of all ages, which is roughly consistent with our estimate of 20% and 50% higher costs for younger patients in the initial and terminal phases, respectively. Because the estimates from the MEPS represented “treated prevalence” and were not reported by phase of care, and the cost data from Health Maintenance Organizations (HMOs) are considerably more detailed and reliable than MEPS data, we relied on the HMO data for our ratios.
Another implication of using the ratios of 1.2 and 1.5 to estimate cost in younger populations is that we assumed that the younger population would have access to care similar to that of the elderly population, and, as for the elderly, we assumed that estimates from the fee-for-service setting are consistent with those from settings with other types of insurance. Although approximately 11% of cancer survivors younger than 65 years are uninsured (25), diagnosis of cancer confers Medicaid eligibility in many states. Finally, because most cancer prevalence is among those 65 years and older, for whom our data are strongest, limitations associated with our assumptions about cost estimates for the younger age group have a smaller impact in the overall cost estimate.
Because it is difficult to anticipate future developments of cancer control technologies and their impact on survival and incidence trends, we produced future prevalence and cost estimates based on projections of trends in incidence, survival, and costs. These projections were developed separately for each sex and cancer site using reasonable assumptions of future incidence and survival trends based on historical cancer incidence and survival data. In addition, changes in survival and incidence have a reduced impact on prevalence because prevalence includes both people newly diagnosed and those diagnosed more than 1 year ago. The latter represents the vast majority of prevalence cases for most cancer sites. Projections based on these hypothetical scenarios provide a sensitivity analysis of estimates and useful information to future planning and resource allocation.
To investigate the impact of specific cancer control strategies on cancer survivorship and to estimate the societal return on investments in cancer research, more complex modeling approaches are necessary. A cooperative agreement funded by the National Cancer Institute, the Cancer Intervention Surveillance Modeling Network (http://cisnet.cancer.gov/), uses microsimulation models to investigate the impact of interventions (ie, primary prevention, screening, and treatment) on population-based cohorts of patients with breast, colorectal, prostate, lung, and esophageal cancers. These microsimulation models require as inputs direct estimates of population use, efficacy, sensitivity, and specificity of new interventions, such as screening and treatment, and can produce estimates of survival and incidence that reflect the usage patterns of the assumed interventions. Although these types of projections are undoubtedly more reliable than the projections reported in this article, they each require a substantial research effort and, therefore, can only be done for a very limited number of cancer sites and specific interventions.
Rising health-care costs represent a central challenge for both the federal government and the private sector. The estimates and projections reported in this article may be particularly useful for policy makers for understanding the future burden of cancer care and for prioritizing future resources on cancer research, treatment, and prevention.
References
- 1.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al. SEER Cancer Statistics Review, 1975-2005. Bethesda, MD: National Cancer Institute; 2007. http://seer.cancer.gov/csr/1975_2005. Accessed July 2008. [Google Scholar]
- 2.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100(12):888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S.Census Bureau, Population Division. Interim Projections Consistent With Census 2000. (released March 2004). Washington D.C.: U.S. Census Bureau, Population Division; 2008. http://www.census.gov/population/www/projections/usinterimproj/. Accessed July 2008. [Google Scholar]
- 4.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 5.Fact Book Fiscal Year 2008 From the National Heart, Lung and Blood Institute (NHLBI) of the National Institute of Health. 2009. http://www.nhlbi.nih.gov/about/factbook/FactBookFinal.pdf. Accessed July 2009. [Google Scholar]
- 6.Schrag D. The price tag on progress—chemotherapy for colorectal cancer. N Engl J Med. 2004;351(4):317–319. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 7.Mariotto AB, Yabroff KR, Feuer EJ, et al. Projecting the number of patients with colorectal carcinoma by phases of care in the US: 2000-2020. Cancer Causes Control. 2006;17(10):1215–1226. doi: 10.1007/s10552-006-0072-0. [DOI] [PubMed] [Google Scholar]
- 8.Berkeley Mortality Database. Data downloaded on November 2007. Berkeley, CA:: University of California; 2009. http://www.demog.berkeley.edu/∼bmd/. Accessed July 2009. [Google Scholar]
- 9.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 suppl):IV–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 10.Verdecchia A, De Angelis G, Capocaccia R. Estimation and projections of cancer prevalence from cancer registry data. Stat Med. 2002;21(22):3511–3526. doi: 10.1002/sim.1304. [DOI] [PubMed] [Google Scholar]
- 11.De Angelis R. Mixture models for cancer survival analysis: application to population-based data with covariates. Stat Med. 1999;18(4):441–454. doi: 10.1002/(sici)1097-0258(19990228)18:4<441::aid-sim23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Johnson NL, Kotz S. Distribution in Statistics: Continuous Univariate Distributions 1. New York, NY: John Wiley & Sons; 1970. [Google Scholar]
- 13.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 14.Brown M, Riley GF, Potosky AL, Etzioni RD. Obtaining long-term disease-specific costs of care: application to Medicare enrollees diagnosed with colorectal cancer. Med Care. 1999;43(7):637–639. doi: 10.1097/00005650-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Yabroff KR, Warren JL, Schrag D, et al. Comparison of approaches for estimating incidence costs of care for colorectal cancer patients. Med Care. 2009;47(7 suppl 1):S56–S63. doi: 10.1097/MLR.0b013e3181a4f482. [DOI] [PubMed] [Google Scholar]
- 16.Smith TJ, Penberthy L, Desch CE, et al. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer. 1995;13(3):235–252. doi: 10.1016/0169-5002(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 17.The Henry J Kaiser Family Foundation. Health Care Costs: Key Information on Health Care Costs and Their Impact. Mento Park, CA: 2009. www.kff.org/insurance/upload/7670_02.pdf. Accessed August 2010. [Google Scholar]
- 18.Fireman BH, Quesenberry CP, Somkin CP, et al. Cost of care for cancer in a health maintenance organization. Health Care Financ Rev. 1997;18(4):51–76. [PMC free article] [PubMed] [Google Scholar]
- 19.Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27(23):3868–3874. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 20.Warren JL, Mariotto AB, Meekins A, et al. Current and future utilization of services from medical oncologists. J Clin Oncol. 2008;26(19):3242–3247. doi: 10.1200/JCO.2007.14.6357. [DOI] [PubMed] [Google Scholar]
- 21.Tangka FK, Trogdon JG, Richardson LC, et al. Cancer treatment cost in the United States: has the burden shifted over time? Cancer. 2010;116(14):3477–3484. doi: 10.1002/cncr.25150. [DOI] [PubMed] [Google Scholar]
- 22.Taplin SH, Barlow W, Urban N, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87(6):417–426. doi: 10.1093/jnci/87.6.417. [DOI] [PubMed] [Google Scholar]
- 23.Rosen AB, Cutler DM. Challenges in building disease-based national health accounts. Med Care. 2009;47(7 suppl 1):S7–S13. doi: 10.1097/MLR.0b013e3181a23e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerrigan M, Howlader N, Mandelson MT, et al. Costs and survival of patients with colorectal cancer in a health maintenance organization and a preferred provider organization. Med Care. 2005;43(10):1043–1048. doi: 10.1097/01.mlr.0000178213.76463.cb. [DOI] [PubMed] [Google Scholar]
- 25.Thorpe KE, Howard D. Health insurance and spending among cancer patients. Health Aff (Millwood). 2003;(Suppl Web Exclusives):W3–98. doi: 10.1377/hlthaff.w3.189. [DOI] [PubMed] [Google Scholar]