Abstract
The present study was designed to test the hypothesis that hypoxia inducible factor (HIF)-1α mediates profibrotic effects of angiotensin (ANG) II and to determine whether HIF prolyl-hydroxylase, the enzyme that promotes the degradation of HIF-1α, is involved in the profibrotic action of ANG II. In cultured renal medullary interstitial cells, ANG II (10−6 M) treatment for 20 hours remarkably increased HIF-1α levels, which was accompanied by the significant upregulation of collagen I/III and tissue inhibitor of metalloproteinases (TIMP)-1. HIF-1α siRNA decreased HIF-1α levels and completely blocked the effects of ANG II on collagen I/III and TIMP-1. HIF-1α siRNA also abolished ANG II-induced elevation of proliferating cell nuclear antigen, a marker of cell proliferation, and vimentin, a marker of cell transdifferentiation. HIF-2α siRNA did not affect the action of ANG II on collagen I/III and TIMP-1. Overexpression of PHD2 transgene, the predominant renal HIF prolyl-hydroxylase, attenuated ANG II-induced profibrotic action and silencing of PHD2 gene enhanced ANG II-induced profibrotic action. Removal of H2O2 eliminated ANG II-induced profibrotic effects. Two week ANG II infusion (150 ng/Kg/min) increased the expression of HIF-1α and α-smooth muscle actin in the renal medullary interstitial cells in vivo. Our data suggest that HIF-1α mediates ANG II-induced profibrotic effects through activation of cell transdifferentiation and that redox regulation of PHD plays a critical role in ANG II-induced activation of HIF-1α and consequent cell proliferation, transdifferentiation and abnormal extracellular matrix metabolism in renal cells.
Keywords: Metalloproteinases, free radicals, HIF prolyl-hydroxylase, end-stage renal disease
Interstitial fibrosis is correlated with the progression of chronic renal diseases and has been proposed as a final common pathway to end-stage renal diseases (ESRD) (1–3). Hypoxia inducible factor (HIF)-1α has been recently associated with the progression of chronic renal injuries (2, 4–6). Although up-regulation of HIF-1α has been shown to be protective in acute ischemic injury (7–9), long-term activation of HIF-1α in chronic renal diseases is implicated to be pathogenic (2–3, 5–6, 8, 10–12). HIF-1α has been reported to be up-regulated in chronic renal diseases (2, 4–5, 8, 13). It has also been demonstrated that HIF-1α stimulates collagen accumulation by activation of fibrogenic factors, such as plasminogen activator inhibitor (PAI) and tissue inhibitor of metalloproteinase (TIMP) (13–16). Angiotensin II (ANG II) is a major pathogenic factor producing renal fibrosis in chronic renal injury ((3–4, 17–19). Meanwhile, it has been shown that ANG II stimulates HIF-1α accumulation (20–21). However, the contribution of HIF-1α to ANG II-induced profibrotic action has not been evidenced. In addition, the role of HIF prolyl-hydroxylases, the enzymes that promote the degradation of HIF-1α (22–24), in the regulation of fibrogenesis has not been investigated. A recent study has shown that ANG II inhibits HIF prolyl-hydroxylases activity and increase HIF-1α level (25), indicating a possible role of HIF prolyl-hydroxylases in ANG II-induced profibrotic action. HIF prolyl-hydroxylases are present in the kidneys and regulate HIF-1α levels (7, 26–28). Three HIF prolyl-hydroxylases including prolyl-hydroxylase domain-containing proteins 1, 2, and 3 (PHD1, 2, and 3) have recently been identified (22–23, 29) and PHD2 is the primary PHD in the kidneys (7, 26–28). The present study was designed to test the hypothesis that HIF-1α accumulation by PHD inhibition is a critical mediator in the profibrotic action of ANG II using renal interstitial cells, one of the important cell types involved in progression of chronic renal diseases (30–32).
We first utilized HIF-1α small interference RNA (siRNA) to silence the gene expression of HIF-1α and evaluated the contributing role of HIF-1α in ANG II-induced increases in collagen I/III and TIMP-1 in cultured renal medullary interstitial cells (RMICs). We then transfected the vectors expressing rat full length PHD2 or rat PHD2 siRNA into the cells to determine whether PHD2 was involved in ANG II-induced profibrotic action. To our knowledge, the present study provides the first evidence suggesting that PHD/HIF-1α-mediated gene regulation importantly participates in ANG II-induced profibrotic effects in renal cells.
Results
HIF-1α siRNA blocked ANG II-induced increases in collagen I/III, TIMP-1, proliferating cell nuclear antigen (PCNA) and vimentin
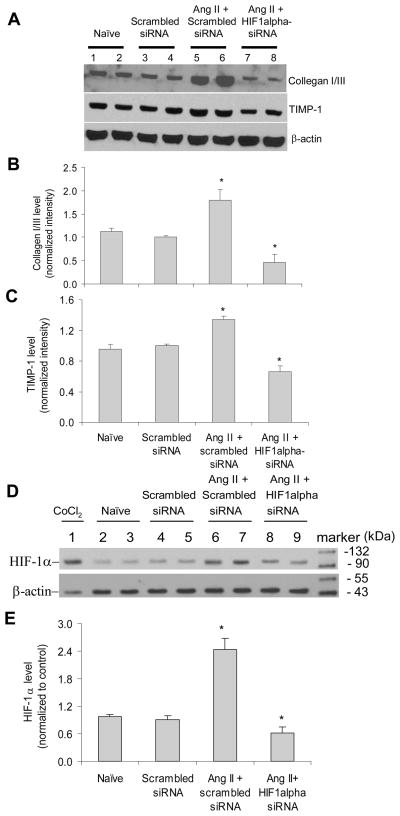
Our result showed that in RMICs ANG II induced the accumulation in collagen I/III and TIMP-1 protein levels, which is consistent with previous reports (18, 33–34). To determine the role of HIF-1α in ANG II-induced increases in these fibrogenesis-associated factors, we examined whether gene silencing of HIF-1α would block these profibrotic effects of ANG II. As shown in Figure 1, in the cells transfected with HIF-1α siRNA, ANG II-induced increases in collagen I/III and TIMP-1 were abolished, suggesting that ANG II-induced stimulatory effects on collagen I/III and TIMP-1 are through the activation of HIF-1α. Figure 1D and E confirmed the accumulation of HIF-1α induced by ANG II, which was abolished by HIF-1α siRNA. The concentration of ANG II (10−6 M) used in the present study was a concentration that induced the maximal activation of HIF-α based on the preliminary experiments (Figure 2 in online supplement). This high concentration of ANG II allowed us to determine the inhibitory effect of HIF-1α siRNA on ANG II-induced activation of fibrogenic factors under the maximal stimulation.
Figure 1. Effect of HIF-1α siRNA on ANG II-induced increases in collagen I/III and TIMP-1 in RMICs by Western blot analysis.
Panel A and D: representative ECL gel documents of Western blot analyses depicting the protein levels of collagen I/III, TIMP-1 and HIF-1α. Panel B, C and E: summarized intensities of collagen I/III, TIMP-1 and HIF-1α blots normalized to control. *P < 0.05 vs. all other groups (n=6). In panel D, the sample from CoCl2-treated cells was used as a positive control. A blot image containing full size markers for panel D was presented in figure 1 in online supplement to further illustrate the location of HIF-1α blots.
Because both hypoxia and activation of ANG II have been implicated in chronic renal injury (4), we determined whether ANG II and hypoxia synergistically stabilized HIF-1α. Our results demonstrated that hypoxia alone exhibited a stronger effect on HIF-1α levels than ANG II alone. However, ANG II + hypoxia did not show significantly further effects on HIF-1α accumulation compared with hypoxia alone (figure 3, online supplement). These data indicate that ANG II and hypoxia may share the same pathway, probably by inhibition of PHD activity, in stabilizing HIF-1α.
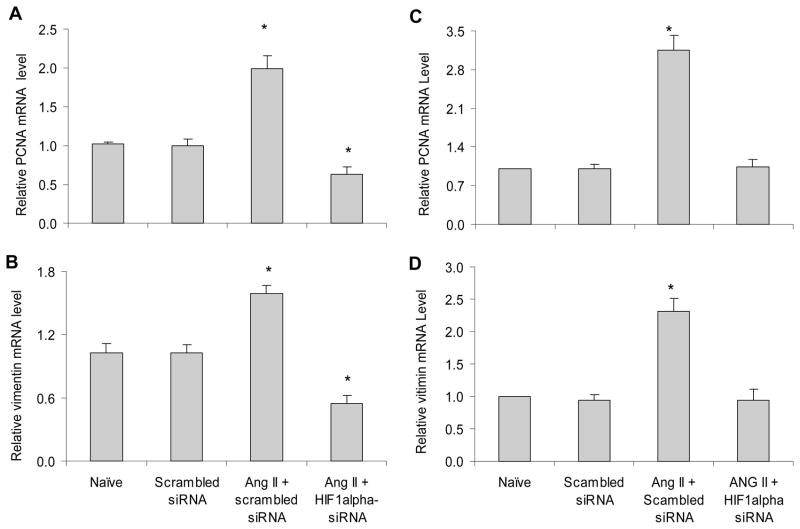
In addition, cell proliferation and transdifferentiation have been reported to participate in ANG II-induced renal tubulointerstitial injury (35–36). Therefore, we further determined the role of HIF-1α in ANG II-induced cell proliferation and transdifferentiation. Our results showed that gene silencing of HIF-1α blocked ANG II-induced increases in the transcriptions of proliferating cell nuclear antigen (PCNA), a marker of cell proliferation (37), and vimentin, a marker of cell transdifferentiation (38) (Figure 2A&B). Since ANG II has been reported to induce epithelial to mesenchymal transdifferentiation/transition (EMT) (39–40), we also detected PCNA and vimentin mRNA levels in rat renal tubular cells (NRK-52E, ATCC, Manassas, VA, USA) and demonstrated that HIF-1α siRNA similarly blocked ANG II-induced increases in PCNA and vimentin in renal epithelial cells (Figure 2C&D). These results suggest that activation of HIF-1α mediates ANG II-induced cell proliferation and transdifferentiation.
Figure 2. Effect of HIF-1α siRNA on ANG II-induced increases in PCNA and vimentin mRNA levels by Real-time RT-PCR analysis.
Panel A and B: in RMICs; Panel C and D: in tubular cells. *P < 0.05 vs. all other groups (n=6).
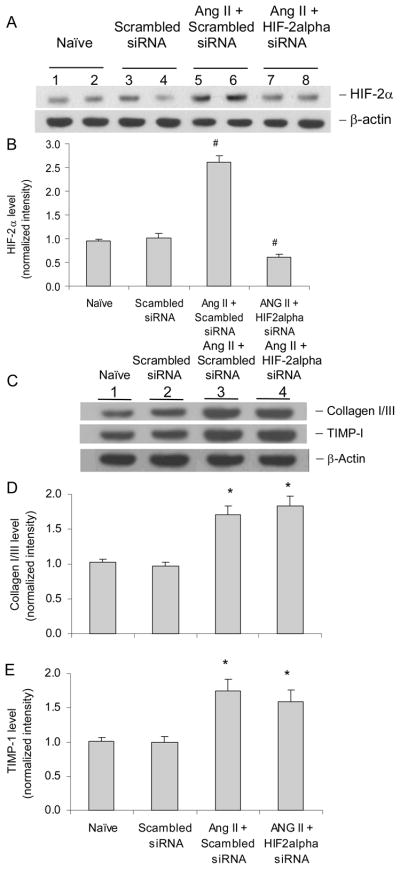
HIF-2α siRNA did not affect ANG II-induced increases in collagen I/III and TIMP-1 in RMICs
It has been shown that HIF-2α is expressed in renal interstitial cells (41–42). We determined the contribution of HIF-2α to the effects of ANG II on collagen I/III and TIMP-1 in RMICs. Our results showed that the mRNA levels of HIF-2α was about 25 times less than that of HIF-1α, and that HIF-2α siRNA, though it decreased HIF-2α levels by 76%, did not affect ANG II-induced changes in collagen I/III and TIMP-1 (figure 3). These data suggest that HIF-2α is not the primary isoform of HIF in these cells and considerably low level of HIF-2α does not significantly contribute to the profibrotic action of ANG II in RMICs in the present study.
Figure 3. Effect of HIF-2α siRNA on ANG II-induced increases in collagen I/III and TIMP-1 in RMICs by Western blot analysis.
Panel A and C: representative ECL gel documents of Western blot analyses depicting the protein levels of HIF-2α, collagen I/III and TIMP-1. Panel B, D and E: summarized intensities of HIF-2α, collagen I/III and TIMP-1 blots normalized to control. # P < 0.05 vs. all other groups. * P < 0.05 vs. control groups. (n=6)
PHD2 regulated HIF-1α, collagen I/III and TIMP-1 levels
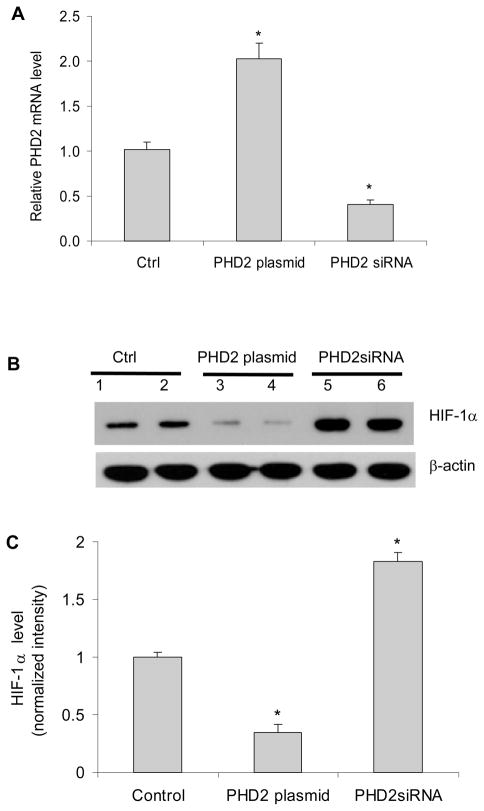
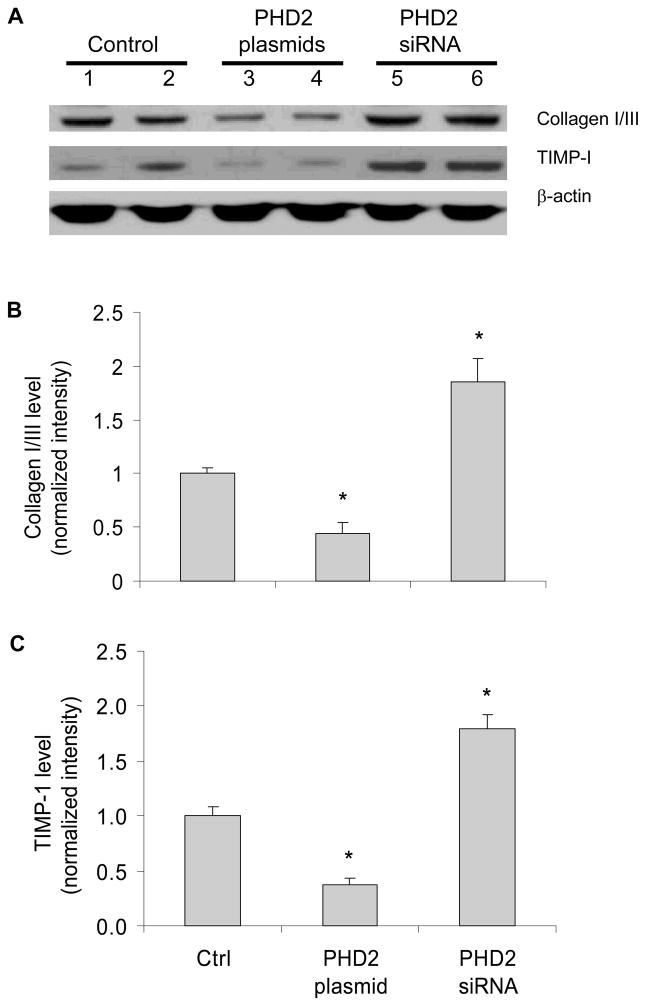
It has been well characterized that PHDs promote the degradation of HIF-1α (22). Although the results above showed that HIF-1α was involved in ANG II-induced profibrotic action, the role of PHDs this process remained to be proven. To address this issue, we first verified the overexpression or silencing of PHD2 gene by transfection of PHD2 expressing plasmid or PHD2 siRNA vectors (Figure 4A). We also confirmed that expression of PHD2 transgene decreased HIF-1α protein level and silencing of PHD2 gene increased the HIF-1α levels in our experiments (Figure 4B&C). Interestingly, overexpression of PHD2 transgene decreased collagen I/III and TIMP-1, and silencing of PHD2 gene increased collagen I/III and TIMP-1 protein levels (Figure 5). These results suggest that PHD2 regulates fibrogenesis-associated factors in RMICs. PHD2 was chosen because it is the predominant PHD in the kidneys (7, 27–28, 43) and quantitatively predominant in the cells used in the present study (figure 4 in online supplement).
Figure 4. Effect of PHD2 or PHD2 siRNA expression vectors on the levels of PHD2 and HIF-1α.
Panel A: the relative mRNA levels of PHD2 by Real-time RT-PCR analysis. Panel B: representative ECL gel documents of Western blot analyses depicting the protein levels of HIF-1α. Panel C: summarized intensities of HIF-1α blots normalized to control. *P < 0.05 vs. all other groups (n=6).
Figure 5. Effect of PHD2 or PHD2 siRNA expression vectors on the levels of collagen I/III and TIMP-1 in RMICs by Western blot analysis.
Panel A: representative ECL gel documents of Western blot analyses depicting the protein levels of collagen I/III and TIMP-1. Panel B and C: summarized intensities of collagen I/III and TIMP-1 blots normalized to control. *P < 0.05 vs. all other groups (n=6).
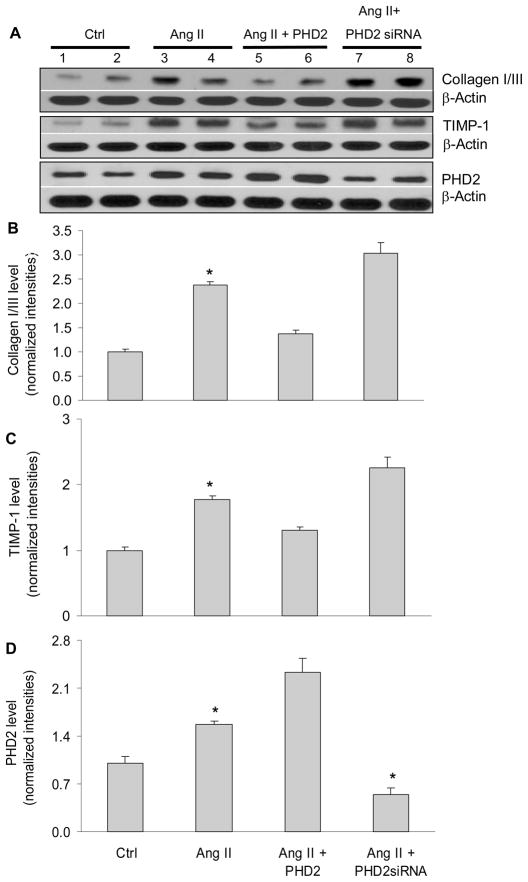
Manipulation of PHD2 gene altered ANG II-induced increases in collagen I/III and TIMP-1
The above data proved that PHD2 regulated fibrogenesis-associated factors. However, it was not clear whether PHDs participated in the physiological/pathological processes associated with these fibrogenesis-associated factors. To determine the role of PHD in the regulation of fibrogenic factors in response to pathogenic stimulation, we examined the effect of gene silencing or gene transfection of PHD2 on ANG II-induced profibrotic action. Figure 6 shows that in RMICs transfected with PHD2 plasmids, ANG II-induced increases in collagen I/III and TIMP-1 were significantly attenuated. In contrast, this ANG II-induced increases in collagen I/III and TIMP-1 were significantly enhanced when PHD2 gene was silenced. These results demonstrated that PHD2 mediated the effects of ANG II on collagen I/III and TIMP-1. Our data showed that ANG II increased the levels of PHD2, indicating that the stimulatory effects of ANG II on HIF-1α, collagen I/III and TIMP-1 were not via the down-regulation of PHD2 expression.
Figure 6. Effect of PHD2 or PHD2 siRNA expression vectors on the levels of collagen I/III, TIMP-1 and PHD2 in the presence of ANG II in RMICs by Western blot analysis.
Panel A: representative ECL gel documents of Western blot analyses depicting the protein levels of collagen I/III, TIMP-1 and PHD2. Panel B and C: summarized intensities of collagen I/III and TIMP-1 blots normalized to control. *P < 0.05 vs. all other groups (n=6).
Since PHD1 and 3 are present in renal cortical interstitial cells (44) and HIF-1α in turn regulates the levels of PHDs (44–47), we also evaluated the effects of ANG II and PHD2 siRNA on PHD1 and PHD3 levels. It was found that ANG II and PHD2 siRNA did not affect PHD3 levels, while PHD1 was marginally increased by ANG II (figure 4 in online supplement). It is known that the expression, regulation and action of three PHDs are different in specific tissues or cells (29, 44–47). These data suggest that PHD2, but not PHD1 and PHD3, is the predominant enzyme in the cells used in the present study.
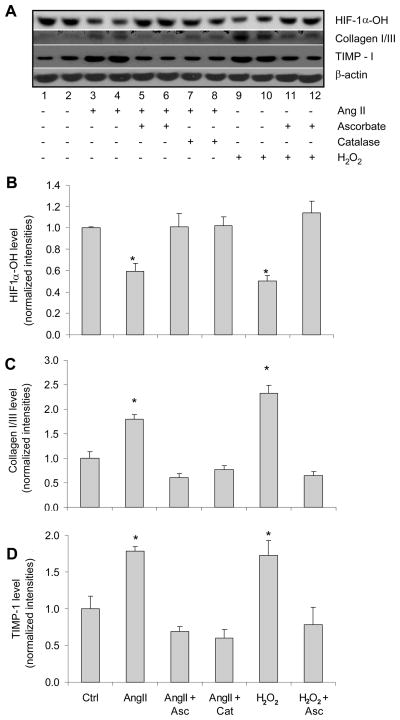
ANG II inhibited PHD activity and increased collagen I/III and TIMP-1 via stimulation of H2O2 production
The next question was that by what mechanism ANG II inhibited the action of PHD2. Since ANG II did not down-regulate the levels of PHD2 as shown in Figure 6, we investigated the effects of ANG II on PHD activities and consequent changes in collagen I/III and TIMP-1. PHDs catalyze site-specific proline hydroxylation of HIF-1α, which allows recognition by the von Hippel-Lindau tumor suppressor protein (VHL) that targets hydroxylated HIF-1α (HIF-1α-OH) for its degradation by the ubiquitin-proteasome pathway. Therefore, the levels of HIF-1α-OH represent the activity of PHDs. As illustrated in Figure 7, ANG II significantly reduced the HIF-1α-OH levels (Figure 7A, line 3 and 4), suggesting that ANG II inhibits the hydroxlylating activities of PHDs. Reduced levels of HIF-1α-OH were accompanied by increases in collagen I/III and TIMP-1, indicating that ANG II-induced profibrotic effects are through inhibition of PHD activities in RMICs.
Figure 7. Effect of ANG II or H2O2 on the levels of HIF-1α-OH, collagen I/III and TIMP-1 in the presence or absence of catalase or ascorbate by Western blot analysis.
Panel A: typical ECL gel documents of HIF-1α–OH, collagen I/III and TIMP-1 protein levels. Panel B, C and D: summarized intensities of HIF-1α-OH, collagen I/III and TIMP-1 blots normalized to control *P < 0.05 vs. all other groups except the group also marked with * (n=6).
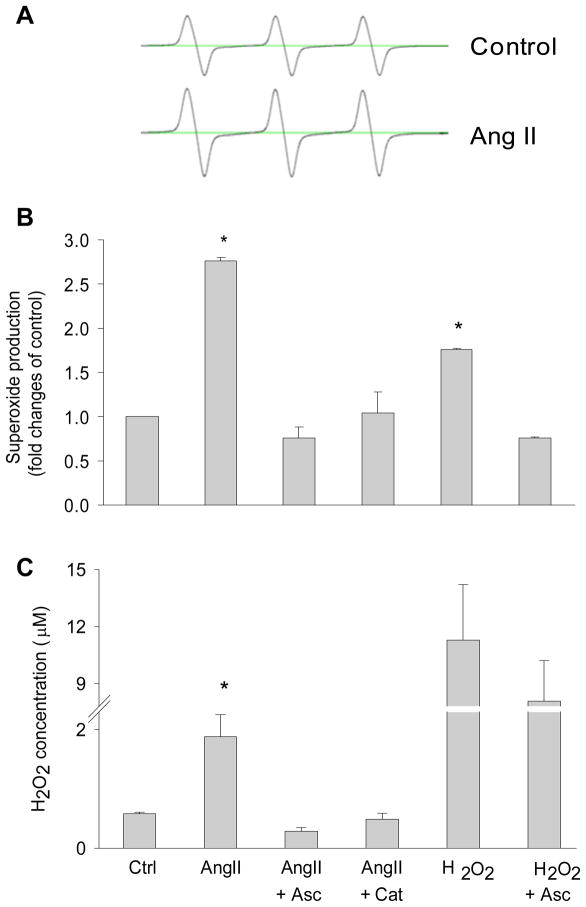
Since ANG II was shown to increase H2O2 (48–49) and H2O2 to inhibit PHD2 activity (25), we further elucidated the role of H2O2-induced PHD2 inhibition in the profibrotic action of ANG II in RMICs. These data are presented in Figure 7. In the cells treated with ascorbate or catalase to eliminate H2O2 effects or remove H2O2, ANG II-induced decreases in HIF-1α-OH were returned to the control levels (panel A, line 5–6 and 7–8). In contrast to the changes in HIF-1α-OH, ANG II-induced increases in collagen I/III and TIMP-1 were decreased to normal levels by these anti-oxidative treatments. These results demonstrated that increased H2O2 production by ANG II inhibited PHD activities and consequently promoted the activation of fibrogenic factors. In additional experiments, we also tested the effects of exogenous H2O2 and found that exogenous H2O2 exhibited similar inhibitory effects on HIF-1α-OH levels and stimulatory effects on collagen I/III and TIMP-1 as ANG II did (panel A, line 9–10). These results further indicated that the actions of ANG II were through stimulation of H2O2 production. ANG II-induced oxidative stress was confirmed by detecting the increases in superoxide production (Figure 8A and B) and H2O2 levels (Figure 8C) in ANG II-treated cells. Overall, these data showed that ANG II increased TIMP-1 and induced the accumulation of collagen I/III via inhibition of PHD activity by stimulating the production of H2O2.
Figure 8. Effects of ANG II, catalase and ascorbate on the levels of superoxide and H2O2.
A: representative ESR spectrograph of O2·− trapped by CMH in control and ANG II-treated cells. B: summarized data showing O2·− production in cells treated with different reagents. C: summarized data showing H2O2 levels in culture media from cells treated with different reagents. * P < 0.05 vs. all other groups. (n=6). Asc = ascorbate, Cat = catalase.
Infusion of ANG II stimulated the expression of HIF-1α and a-smooth muscle actin (SMA) in RMICs in vivo in the kidneys
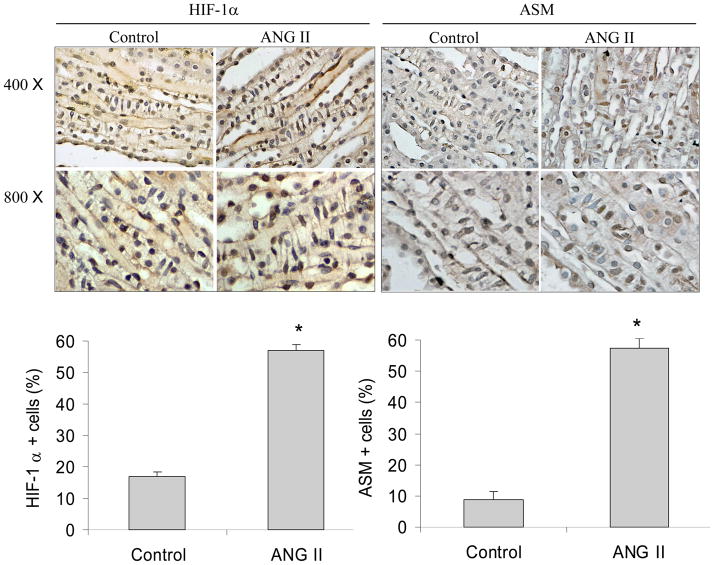
Infusion of ANG II (150 ng/Kg/min) for two weeks significantly increased the number of HIF-1α- and SMA-positive RMICs in the kidneys by immunohistochemical analysis (Figure 9). SMA is also a molecular hallmark of fibroblast activation during fibroblast to myofibroblast transition (50–52). These data demonstrate that ANG II-stimulated activation of HIF-1α and transdifferentiation in RMICs also occurs in the kidneys in vivo.
Figure 9. Immunohistochemical staining of HIF-1α and SMA in the renal inner medulla.
Top panels: Representative photomicrographs. Brown color indicates positive staining. Bottom panels: Percentage quantitation of positive cells. * P<0.05 vs. control (n=5). RMICs were identified by their unique morphological features of a ladder-like arrangement with the long axis of the cells perpendicular to the long axis of the papilla.
Discussion
The present study showed that gene silencing of HIF-1α blocked ANG II-induced increases in TIMP-1 and collagen I/III, as well as PCNA and vimentin. It was also demonstrated that PHD2 mediated the effects of ANG II on HIF-1α, collagen I/III and TIMP-1, and that ANG II induced collagen I/III and TIMP-1 via stimulating the production of H2O2 that inhibited PHD activity. These results suggest that ANG II-induced profibrotic action may be mediated by activation of HIF-1α due to redox inhibition of PHD activity.
In the present study, we used the increases of TIMP-1 and collagen as indicators of ANG II-induced profibrotic action. To our knowledge, the findings from the present study provide the first evidence that ANG II-induced activation of fibrogenesis-associated genes is mediated by activation of HIF-1α. The present study did not show the significant contribution of HIF-2α in ANG II-induced profibrotic action in RMICs. The reason for a very low level of HIF-2α is probably that HIF-2α-expressing cells are mainly located in the cortex and outer strip of outer medulla (41–42), while the RMICs used in the current study are isolated from the inner medulla. The cells used in the current study seem not characterized similar to the interstitial cells located in the cortex with respect to HIF-2α expression. However, it has been reported that the medullary interstitial fibrotic injuries are similar to or more profound than cortical interstitial fibrotic injuries in chronic renal diseases (53–54). Therefore, the medullary interstitial cells used in the present study may present a model of cells involved in renal medullary interstitial damage.
Another interesting finding in the present study is that HIF-1α may also mediate ANG II-induced cell transdifferentiation. Cell transdifferencitation, including epithelial mesenchymal transition (EMT), importantly contributes to the development of renal fibrosis in chronic renal diseases (55–58). During fibrogenesis renal resident fibroblasts are activated to transform/transdifferentiate into myofibroblasts, which are primary cell resources to produce extracellular matrix (59–61). Increased expression of vimentin has been shown to be the indicator of transition from fibroblasts to myofibroblasts (31, 52). Although both HIF-1α (5, 16, 62) and ANG II (39–40) have been shown to promote cell transdifferencitation, the interaction between HIF-1α and ANG II in the process of cell transdifferencitation remains unclear. Our results demonstrate that HIF-1α may participate in ANG II-induced transdifferentiation of renal cells. These data suggest that HIF-1α pathway may be involved in the relatively early stage of ANG II-induced profibrotic actions, which may represent a novel mechanism linking HIF-1α-regulated gene transcription to ANG II-induced profibrotic action.
HIF-1α regulates many target genes and thereby plays an important role in many physiological processes. Therefore, there may be a concern that blockade of HIF-1α-mediated gene expression may also prevent some important physiological responses and impair normal cell functions. Because there is an over activation of HIF-1α after ANG II treatment or in chronic renal diseases, inhibition of HIF-1α under these pathological conditions should aim at counteracting excessive HIF-1α activity and eliminating its pathological impact, thereby restoring normal physiological regulation. Therefore, inhibition of HIF-1α could be a useful strategy to reduce the fibrogenesis by resetting such excessive HIF-1α activity to the normal level in a variety of pathological conditions.
PHDs have been reported to regulate the target genes of HIF-1α via their actions degrading HIF-1α (63–66). Given the critical role of HIF-1α in fibrogenesis, PHDs may also be regulators of fibrogenesis. In deed, our results demonstrated that PHD2 regulates the levels of TIMP-1 and collagen in renal cells, which may reveal a novel pathway that modulates the fibrotic process. Although PHDs work as oxygen sensors to regulate the destruction of HIF-1α by promoting the oxygen-dependent proline hydroxylation of HIF-1α (22–24), recent evidences have clearly shown the oxygen-independent regulation of HIF-1α and PHDs (67–70). Most importantly, ANG II has also been shown to inhibit PHD activity and up-regulate HIF-1α levels (25). The present study proved the hypothesis that PHD participate in the regulation of fibrogenic factors and is involved in ANG II-induced profibrotic action. Overexpression of PHD2 transgenes overcame ANG II-induced profibrotic effects, suggesting that PHD may be used as an anti-fibrotic factor under different pathological situations, such as activation of ANG II in chronic renal diseases. In this respect, targeting PHD to regulate HIF-1α and its target genes is emerging as a novel therapeutic strategy in a variety of disease conditions such as tumor (64) and post-ischemic organ injuries (63, 71–73). The findings from the present study that PHDs participate in the regulation of fibrogenic factors under control condition and after ANG II treatment may stimulate the development of intervention associated with PHD/HIF pathway to retard the progression of chronic renal diseases.
Notably, our results showed that ANG II increased PHD2 levels. This ANG II-induced increase in PHD2 levels is probably caused by a feedback mechanism due to increase in HIF-1α level, because PHD2 is one of HIF-1α target genes and activation of HIF-1α up-regulates PHD2 levels (74). The present study found that ANG II stimulated HIF-1α activation and increases in collagen I/III and TIMP-1 through inhibition of PHD activity rather than down-regulation of the PHD expression. In addition, we showed that ANG II inhibited PHD activity via stimulating H2O2 production. These data demonstrate, for the first time, that ANG II stimulates fibrogenic factors via activation of H2O2 production and that H2O2 promotes fibrotic process via inhibition of PHD activity. Although redox signaling has been indicated in chronic renal injury, detailed mechanisms remain to be clarified. Our data suggest that redox regulation of PHD activity and thereafter manipulation of fibrogenic factors may represent an important mechanism mediating chronic renal injury by oxidant stress.
The present study focused on the role of HIF-1α/PHD pathway in ANG II-induced activation of fibrogenic factors using cultured RMICs. Our finding that ANG II stimulated HIF-1α and SMA in RMICs in vivo further suggests that HIF-1α/PHD pathway may be involved in ANG II-induced chronic renal injury. The exact significance of HIF-1α/PHD pathway in chronic renal injury needs to be further clarified using chronic renal disease models associated with ANG II activation. In this regard, there were controversial reports on the role of HIF-1α in different chronic renal disease models. A recent study demonstrated that stable expression of HIF-1α in tubular epithelial cells promoted interstitial fibrosis in 5/6 nephretomy mice (12), while a previous report showed that inhibition of PHD to upregulate HIF-1α protected the kidneys from damage in 5/6 nephrectomy rats (75). In addition, it was reported that genetic ablation of renal epithelial HIF-1α inhibited the development of renal tubulointerstitial fibrosis in unilateral ureteral obstruction rats (16), while upregulation of HIF-1α by cobalt, an inhibitor of PHD activity, was shown to ameliorate renal injury in an obese, hypertensive type 2 diabetes rat model (76). These discrepancies might be attributed to the differences in disease models, disease stages and approaches manipulating HIF-1α. Apparently, more detailed investigations are required regarding the role of HIF-1α/PHD pathway in the chronic renal diseases.
In summary, the present study demonstrated that ANG II stimulated H2O2 production, which inhibited PHD activity and thereby up-regulated HIF-1α levels, and consequently activated TIMP-1, resulting in collagen I/III accumulation in RIMC cells. It is concluded that PHD2 as a novel redox sensitive enzyme is critical to the regulation of HIF-1α levels when renal interstitial cells were exposed to ANG II. Such PHD-mediated regulation of HIF-1α level and activity could be one of the important early mechanisms inducing transdifferentiaion and promoting the upregulation of fibrogenic genes in renal cells under profibrotic stimulations.
Materials and Methods
Animal
Experiments were performed in male Sprague-Dawley rats (Harlan, Madison, WI), weighing 250–300 g. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Isolation and culture of rat renal medullary interstitial cells (RMICs)
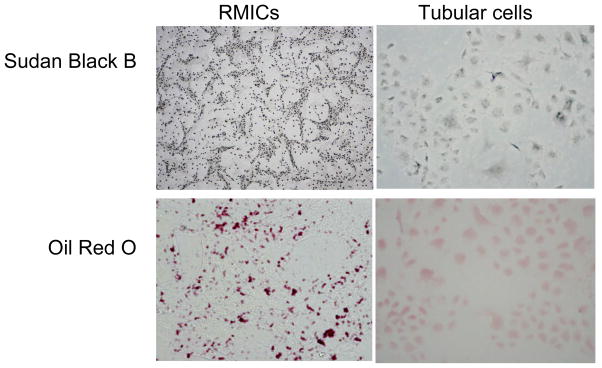
RMICs were isolated and cultured as described previously (43). Briefly, the renal inner medulla from inbred male Sprague-Dawley rats was finely minced, and the tissue suspension was injected subcutaneously into a recipient rat (from the same litter). Four days after injection, the firm yellow nodules located at the site of injections were dissected, removed, minced, and trypsinized, then washed and centrifuged to obtain a cell pellet. The cell suspension was transferred to plastic tissue culture flasks and then incubated with modified culture medium. These cells formed a confluent monolayer in 18–21 days. The cells of 7–8thpassage were used for experiments. The identity of these cells was confirmed by rich lipid granules within the cells stained with Sudan Black B and Oil Red O (Figure 10), which are characteristics of these cells (77–79).
Figure 10. Sudan Black B and Oil Red O staining in RMIC and tubular cells.
The cell shapes were apparently different between these two types of cells. There were numerous positive staining vesicles in RMICs. In contrast, there were few such positive staining vesicles observed in tubular cells. (200 ×)
Transfection of HIF-1α and HIF-2α siRNA
Transfection of siRNA was performed using the siLentFect lipid reagent (Bio-Rad) according to the manufacturer’s instructions. For a 10 cm dish, 200 pmoles of siRNA was used. After 5 hour incubation in transfection reagent, the cells were then switched to normal medium for additional 16 hours and ready for experiment. Sequence of HIF-1α siRNA: sense, GGA AAG AGA CUC AUA GAA A; antisense, UUU CUA UGA CUC UCU UUC C (Sigma-Aldrich). Sequence of HIF-2α siRNA: sense, GCA GAU GGA UAA CUU GUA C; antisense, GUA CAA GUU AUC CAU CUG C (Applied Biosystems). A scrambled small RNA (QIAGEN), which was confirmed as non-silencing double-stranded RNA, was used as control for siRNA experiments.
Transfection of plasmids expressing rat PHD2 or rat PHD2 siRNA into the cells
Plasmids transfections were performed using lipids (DOTAP/DOPE, Avanti Polar Lipids, Inc.) according to the manufacturer’s instructions. In brief, 5 μg of DNA was mixed with lipids solution at a ratio of 1:10 (DNA:lipid, w/w) in serum free culture medium (5 ml for a 10 cm dish). Cells were incubated with this transfection medium for 5 hours and switched to normal medium for another 16 hours. The cells were then ready for experiment. In preliminary experiments, almost all cells were positive after transfected with luciferase plasmids when detected by bioluminescent imaging (IVIS200, Caliper Life Sciences, Inc), demonstrating a high transfection efficiency (Data not shown). Sequences used for producing rat PHD2 siRNA: sense, GTG TGA CAT GTA TAT ATT A; antisense, TAA TAT ATA CAT GTC ACA C (QIAGEN). The DNA sequence was constructed into pRNAT-CMV3.2 vector (Genscript) to generate plasmids that express rat PHD2 siRNA. Plasmids encoding full-length rat PHD2 were generous gifts from Dr. Frank S. Lee, University of Pennsylvania. The expression and function of rat PHD2 protein by this plasmid has been validated by Dr. Lee (80–81) and by us (43). Our preliminary data showed effective gene knock down or gene over-expression of PHD2 by these plasmids in cultured RMICs. Luciferase plasmids (Promega) were used as control for PHD2 and PHD2 siRNA expression vectors transfection experiments.
Cell treatment and experimental groups
After siRNA or plasmids transfection, the cells were switched to serum-free medium containing 10−6 M of ANG II. After ANG II treatment for 20 hours, the cells were harvested for protein and RNA isolation as described below. Some of the cells were treated with H2O2 (5 × 10−5 M), ascorbate (10−4 M) or catalase (1000 U/mL) as indicated in the experimental groups in results. The concentrations of H2O2 and ANG II used in the present study did not cause detectable cell damage as measured by lactate dehydrogenase (LDH) activity (assay kit, Cayman chemical, Ann Arbor, MI) (figure 5 in online supplements).
Preparation of nuclear extracts and cytosolic protein, Western blot analyses for protein levels of HIF-1α, HIF-2α, TIMP-1, collagen I/III and PHD2
Nuclear protein was prepared as we described previously (43). Cytosolic protein and nuclear protein were collected separately. The cytosolic protein was used for Western blot analyses of TIMP-1, collagen I/III and PHD2. The reason for detecting collagen I/III is that the subtype of collagen is tissue/cell specific and collagen I/III is the one expressed in renal interstitial cells. The nuclear fraction was used for Western blot analyses of HIF-1α and HIF-2α. Western blot analyses were performed as described previously (43). The intensity of the blots was determined using an imaging analysis program (ImageJ, free download from http://rsbweb.nih.gov/ij/). Primary antibodies used in the present study included anti-rat HIF-1α and HIF-2α (monoclonal, Novus Biologicals, 1:300 dilution), hydroxylated HIF-1α (HIF-1α-OH, rabbit polyclonal, Novus Biologicals, 1:500), PHD2 (rabbit polyclonal, Novus Biologicals, 1:300), TIMP-1 (monoclonal, R&D systems, 1:1000), and collagen I/III (rabbit polyclonal, Calbiochem, 1:300). For the details of this and the following methods, see the Expanded Materials and Methods section in online supplement.
RNA extraction and quantitative RT-PCR analysis of the mRNA levels of PNCA, Vimentin, and PHD
Total RNA was extracted using TRIzol solution (Life Technologies, Inc. Rockville MD) and then reverse-transcribed (RT) (cDNA Synthesis Kit, Bio-Rad, Hercules, CA). The RT products were amplified using a TaqMan Gene Expression Assays kit (Applied Biosystems). A kit for detecting the levels of 18S ribosomal RNA was used as an endogenous control. The relative gene expressions were calculated in accordance with the ΔΔCt method. Relative mRNA levels were expressed by the values of 2−ΔΔCt.
Superoxide (O2 ·−)detection by electronic spin resonance (ESR)
The measurement of O2·− by ESR was performed according to the methods in our previous studies (82–83).
Fluorescence spectrometric assay of H2O2 concentrations
Amplex red is a fluorogenic substrate with very low background fluorescence;it reacts with H2O2 with a 1:1 stoichiometry to produce highly fluorescent resorufin (84). Fluorescence spectrometric assay of H2O2 levels in culture medium was performedusing an Amplex red kit (Molecular Probes, Eugene, OR) as we described previously (85).
Infusion of ANG II and immunohistochemical analysis of HIF-1α and α-smooth muscle actin (SMA) expression in RMICs in the kidneys
Rats were infused with ANG II (Sigma-Aldrich, 150 ng/kg/min) for two weeks using ALZET mini-osmotic pumps (Model 2002) implanted intraperitoneally. At the end of infusion, kidneys were removed, cut longitudinally, fixed in 10% neutral buffered formalin and then processed for immunostaining as we described before (43) using antibody against rat HIF-1α (1:200) and SMA (rabbit polyclonal, Abcam, 1:200). Expressions of HIF-1α and SMA in RMICs were evaluated by counting RMICs in 10 microscopic fields (400 ×, around 100 cells) and then the percentages of positive staining RMICs were calculated. RMICs were identified by their unique morphological features, i.e. ladder-like arrangement with the long axis of the cells perpendicular to the long axis of the papilla (86–87).
Statistics
Data are presented as means ± SE. The significance of differences in mean values within and between multiple groups was evaluated using an ANOVA followed by a Duncan’s multiple range test. Student’s t-test was used to evaluate statistical significance of differences between two groups. P < 0.05was considered statistically significant.
Supplementary Material
Acknowledgments
Sources of support: National Institutes of Health Grants HL89563 and DK54927.
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript that was accepted for publication in Kidney International. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The International Society of Nephrology. The final copyedited article, which is the version of record, can be found at Kidney International.
Disclosure
All authors have no financial interest in the information contained in the manuscript.
Supplementary information is available at Kidney International’s website.
References
- 1.Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Tubulointerstitial damage and progression of renal failure. Kidney Int. 2005;68:S82–S86. doi: 10.1111/j.1523-1755.2005.09915.x. [DOI] [PubMed] [Google Scholar]
- 2.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell MP. Renal tubulointerstitial fibrosis. New thoughts on its development and progression. Postgrad Med. 2000;108:159–162. 165, 171–152. doi: 10.3810/pgm.2000.07.1155. [DOI] [PubMed] [Google Scholar]
- 4.Nangaku M, Fujita T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens Res. 2008;31:175–184. doi: 10.1291/hypres.31.175. [DOI] [PubMed] [Google Scholar]
- 5.Higgins DF, Kimura K, Iwano M, et al. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7:1128–1132. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haase VH. Pathophysiological Consequences of HIF Activation. Annals of the New York Academy of Sciences. 2009;1177:57–65. doi: 10.1111/j.1749-6632.2009.05030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberger C, Rosen S, Shina A, et al. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant. 2008;23:3472–3478. doi: 10.1093/ndt/gfn276. [DOI] [PubMed] [Google Scholar]
- 8.Nangaku M, Eckardt KU. Hypoxia and the HIF system in kidney disease. J Mol Med. 2007;85:1325–1330. doi: 10.1007/s00109-007-0278-y. [DOI] [PubMed] [Google Scholar]
- 9.Hill P, Shukla D, Tran MGB, et al. Inhibition of Hypoxia Inducible Factor Hydroxylases Protects Against Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2008;19:39–46. doi: 10.1681/ASN.2006090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004;13:279–284. doi: 10.1097/00041552-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Klahr S, Morrissey J. Progression of chronic renal disease. Am J Kidney Dis. 2003;41:S3–7. doi: 10.1053/ajkd.2003.50074. [DOI] [PubMed] [Google Scholar]
- 12.Kimura K, Iwano M, Higgins DF, et al. Stable expression of HIF-1{alpha} in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295:F1023–1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 14.Norman JT, Orphanides C, Garcia P, et al. Hypoxia-induced changes in extracellular matrix metabolism in renal cells. Exp Nephrol. 1999;7:463–469. doi: 10.1159/000020625. [DOI] [PubMed] [Google Scholar]
- 15.Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 16.Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Ortega M, Ruperez M, Esteban V, et al. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006;21:16–20. doi: 10.1093/ndt/gfi265. [DOI] [PubMed] [Google Scholar]
- 18.Yoshiji H, Kuriyama S, Yoshii J, et al. Angiotensin-II induces the tissue inhibitor of metalloproteinases-1 through the protein kinase-C signaling pathway in rat liver fibrosis development. Hepatology Research. 2003;27:51–56. doi: 10.1016/s1386-6346(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Wang J, Zhou F, et al. STAT proteins mediate angiotensin II-induced production of TIMP-1 in human proximal tubular epithelial cells. Kidney Int. 2003;64:459–467. doi: 10.1046/j.1523-1755.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen TH, Wang JF, Chan P, et al. Angiotensin II stimulates hypoxia-inducible factor 1alpha accumulation in glomerular mesangial cells. Ann N Y Acad Sci. 2005;1042:286–293. doi: 10.1196/annals.1338.051. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Lopez E, Lopez AF, Esteban V, et al. Angiotensin II Regulates Vascular Endothelial Growth Factor via Hypoxia-Inducible Factor-1alpha Induction and Redox Mechanisms in the Kidney. Antioxidants & Redox Signaling. 2005;7:1275–1284. doi: 10.1089/ars.2005.7.1275. [DOI] [PubMed] [Google Scholar]
- 22.Bruick RK, McKnight SL. A Conserved Family of Prolyl-4-Hydroxylases That Modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 23.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 24.Jaakkola P, Mole DR, Tian Y-M, et al. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 25.Page EL, Chan DA, Giaccia AJ, et al. Hypoxia-inducible Factor-1{alpha} Stabilization in Nonhypoxic Conditions: Role of Oxidation and Intracellular Ascorbate Depletion. Mol Biol Cell. 2008;19:86–94. doi: 10.1091/mbc.E07-06-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, Yi F, Sundy CM, et al. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol. 2007;292:F207–216. doi: 10.1152/ajprenal.00457.2005. [DOI] [PubMed] [Google Scholar]
- 27.Takeda K, Cowan A, Fong G-H. Essential Role for Prolyl Hydroxylase Domain Protein 2 in Oxygen Homeostasis of the Adult Vascular System. Circulation. 2007;116:774–781. doi: 10.1161/CIRCULATIONAHA.107.701516. [DOI] [PubMed] [Google Scholar]
- 28.Takeda K, Aguila HL, Parikh NS, et al. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–3235. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 30.Muller G, Markovic-Lipkovski J, Frank J, et al. The role of interstitial cells in the progression of renal diseases. J Am Soc Nephrol. 1992;2:S198–205. doi: 10.1681/ASN.V210s198. [DOI] [PubMed] [Google Scholar]
- 31.Pozdzik AA, Salmon IJ, Debelle FD, et al. Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int. 2008;73:595–607. doi: 10.1038/sj.ki.5002714. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 33.Castoldi G, di Gioia CRT, Pieruzzi F, et al. ANG II increases TIMP-1 expression in rat aortic smooth muscle cells in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H635–643. doi: 10.1152/ajpheart.00986.2001. [DOI] [PubMed] [Google Scholar]
- 34.Lijnen P, Papparella I, Petrov V, et al. Angiotensin II-stimulated collagen production in cardiac fibroblasts is mediated by reactive oxygen species. Journal of Hypertension. 2006;24:757–766. doi: 10.1097/01.hjh.0000217860.04994.54. [DOI] [PubMed] [Google Scholar]
- 35.Raizada V, Skipper B, Luo W, et al. Intracardiac and intrarenal renin-angiotensin systems: mechanisms of cardiovascular and renal effects. J Investig Med. 2007;55:341–359. doi: 10.2310/6650.2007.00020. [DOI] [PubMed] [Google Scholar]
- 36.Daniel C. Blocking of angiotensin II is more than blocking of transforming growth factor-beta. Kidney Int. 2008;74:551–553. doi: 10.1038/ki.2008.290. [DOI] [PubMed] [Google Scholar]
- 37.Rastaldi MP, Ferrario F, Giardino L, et al. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002;62:137–146. doi: 10.1046/j.1523-1755.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 38.Manotham K, Tanaka T, Matsumoto M, et al. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int. 2004;65:871–880. doi: 10.1111/j.1523-1755.2004.00461.x. [DOI] [PubMed] [Google Scholar]
- 39.Lan HY. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens. 2003;12:25–29. doi: 10.1097/00041552-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Carvajal G, Rodriguez-Vita J, Rodrigues-Diez R, et al. Angiotensin II activates the Smad pathway during epithelial mesenchymal transdifferentiation. Kidney Int. 2008;74:585–595. doi: 10.1038/ki.2008.213. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberger C, Mandriota S, Jurgensen JS, et al. Expression of Hypoxia-Inducible Factor-1{alpha} and -2{alpha} in Hypoxic and Ischemic Rat Kidneys. J Am Soc Nephrol. 2002;13:1721–1732. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- 42.Paliege A, Rosenberger C, Bondke A, et al. Hypoxia-inducible factor-2[alpha]-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int. 2010;77:312–318. doi: 10.1038/ki.2009.460. [DOI] [PubMed] [Google Scholar]
- 43.Li N, Yi F, Sundy CM, et al. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol. 2007;292:F207–216. doi: 10.1152/ajprenal.00457.2005. [DOI] [PubMed] [Google Scholar]
- 44.Schodel J, Klanke B, Weidemann A, et al. HIF-prolyl hydroxylases in the rat kidney: physiologic expression patterns and regulation in acute kidney injury. Am J Pathol. 2009;174:1663–1674. doi: 10.2353/ajpath.2009.080687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marxsen JH, Stengel P, Doege K, et al. Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases. Biochem J. 2004;381:761–767. doi: 10.1042/BJ20040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minamishima YA, Moslehi J, Padera RF, et al. A Feedback Loop Involving the Phd3 Prolyl Hydroxylase Tunes the Mammalian Hypoxic Response In Vivo. Mol Cell Biol. 2009;29:5729–5741. doi: 10.1128/MCB.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Appelhoff RJ, Tian Y-M, Raval RR, et al. Differential Function of the Prolyl Hydroxylases PHD1, PHD2, and PHD3 in the Regulation of Hypoxia-inducible Factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 48.Chen X-L, Tummala PE, Olbrych MT, et al. Angiotensin II Induces Monocyte Chemoattractant Protein-1 Gene Expression in Rat Vascular Smooth Muscle Cells. Circ Res. 1998;83:952–959. doi: 10.1161/01.res.83.9.952. [DOI] [PubMed] [Google Scholar]
- 49.Feliers D, Gorin Y, Ghosh-Choudhury G, et al. Angiotensin II stimulation of VEGF mRNA translation requires production of reactive oxygen species. Am J Physiol Renal Physiol. 2006;290:F927–936. doi: 10.1152/ajprenal.00331.2005. [DOI] [PubMed] [Google Scholar]
- 50.Grupp C, Troche I, Klass C, et al. A novel model to study renal myofibroblast formation in vitro. Kidney Int. 2001;59:543–553. doi: 10.1046/j.1523-1755.2001.059002543.x. [DOI] [PubMed] [Google Scholar]
- 51.Hu K, Wu C, Mars WM, et al. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Invest. 2007;117:3821–3832. doi: 10.1172/JCI32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arena S, Fazzari C, Implatini A, et al. Dextranomer/hyaluronic Acid copolymer implant for vesicoureteral reflux: role of myofibroblast differentiation. J Urol. 2009;181:2695–2701. doi: 10.1016/j.juro.2009.02.059. [DOI] [PubMed] [Google Scholar]
- 53.Suga S, Mazzali M, Ray PE, et al. Angiotensin II type 1 receptor blockade ameliorates tubulointerstitial injury induced by chronic potassium deficiency. Kidney Int. 2002;61:951–958. doi: 10.1046/j.1523-1755.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- 54.Sun Y, Zhang J, Zhang JQ, et al. Local Angiotensin II and Transforming Growth Factor-{beta}1 in Renal Fibrosis of Rats. Hypertension. 2000;35:1078–1084. doi: 10.1161/01.hyp.35.5.1078. [DOI] [PubMed] [Google Scholar]
- 55.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med. 2004;82:175–181. doi: 10.1007/s00109-003-0517-9. [DOI] [PubMed] [Google Scholar]
- 56.Burns WC, Kantharidis P, Thomas MC. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs. 2007;185:222–231. doi: 10.1159/000101323. [DOI] [PubMed] [Google Scholar]
- 57.Strutz FM. EMT and proteinuria as progression factors. Kidney Int. 2009;75:475–481. doi: 10.1038/ki.2008.425. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 59.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strutz F, Muller GA. Renal fibrosis and the origin of the renal fibroblast. Nephrol Dial Transplant. 2006;21:3368–3370. doi: 10.1093/ndt/gfl199. [DOI] [PubMed] [Google Scholar]
- 61.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo Y, He DL, Ning L, et al. Hypoxia-inducible factor-1alpha induces the epithelial-mesenchymal transition of human prostatecancer cells. Chin Med J (Engl) 2006;119:713–718. [PubMed] [Google Scholar]
- 63.Ockaili R, Natarajan R, Salloum F, et al. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542–548. doi: 10.1152/ajpheart.00089.2005. [DOI] [PubMed] [Google Scholar]
- 64.Erez N, Milyavsky M, Eilam R, et al. Expression of prolyl-hydroxylase-1 (PHD1/EGLN2) suppresses hypoxia inducible factor-1alpha activation and inhibits tumor growth. Cancer Res. 2003;63:8777–8783. [PubMed] [Google Scholar]
- 65.Wright G, Higgin JJ, Raines RT, et al. Activation of the Prolyl Hydroxylase Oxygen-sensor Results in Induction of GLUT1, Heme Oxygenase-1, and Nitric-oxide Synthase Proteins and Confers Protection from Metabolic Inhibition to Cardiomyocytes. J Biol Chem. 2003;278:20235–20239. doi: 10.1074/jbc.M301391200. [DOI] [PubMed] [Google Scholar]
- 66.Wu S, Nishiyama N, Kano MR, et al. Enhancement of Angiogenesis Through Stabilization of Hypoxia-inducible Factor-1 by Silencing Prolyl Hydroxylase Domain-2 Gene. Mol Ther. 2008;16:1227–1234. doi: 10.1038/mt.2008.90. [DOI] [PubMed] [Google Scholar]
- 67.Callapina M, Zhou J, Schnitzer S, et al. Nitric oxide reverses desferrioxamine- and hypoxia-evoked HIF-1[alpha] accumulation--Implications for prolyl hydroxylase activity and iron. Experimental Cell Research. 2005;306:274–284. doi: 10.1016/j.yexcr.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Baek JH, Mahon PC, Oh J, et al. OS-9 Interacts with Hypoxia-Inducible Factor 1[alpha] and Prolyl Hydroxylases to Promote Oxygen-Dependent Degradation of HIF-1[alpha] Molecular Cell. 2005;17:503–512. doi: 10.1016/j.molcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 69.McMahon S, Charbonneau M, Grandmont S, et al. Transforming Growth Factor beta1 Induces Hypoxia-inducible Factor-1 Stabilization through Selective Inhibition of PHD2 Expression. J Biol Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 70.Lu H, Dalgard CL, Mohyeldin A, et al. Reversible Inactivation of HIF-1 Prolyl Hydroxylases Allows Cell Metabolism to Control Basal HIF-1. J Biol Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 71.Bernhardt WM, Campean V, Kany S, et al. Preconditional Activation of Hypoxia-Inducible Factors Ameliorates Ischemic Acute Renal Failure. J Am Soc Nephrol. 2006;17:1970–1978. doi: 10.1681/ASN.2005121302. [DOI] [PubMed] [Google Scholar]
- 72.Natarajan R, Salloum FN, Fisher BJ, et al. Hypoxia Inducible Factor-1 Activation by Prolyl 4-Hydroxylase-2 Gene Silencing Attenuates Myocardial Ischemia Reperfusion Injury. Circ Res. 2006;98:133–140. doi: 10.1161/01.RES.0000197816.63513.27. [DOI] [PubMed] [Google Scholar]
- 73.Ratan RR, Siddiq A, Aminova L, et al. Translation of Ischemic Preconditioning to the Patient: Prolyl Hydroxylase Inhibition and Hypoxia Inducible Factor-1 as Novel Targets for Stroke Therapy. Stroke. 2004;35:2687–2689. doi: 10.1161/01.STR.0000143216.85349.9e. [DOI] [PubMed] [Google Scholar]
- 74.D’Angelo G, Duplan E, Boyer N, et al. Hypoxia Up-regulates Prolyl Hydroxylase Activity: A FEEDBACK MECHANSIM THAT LIMITS HIF-1 RESPONSES DURING REOXYGENATION. J Biol Chem. 2003;278:38183–38187. doi: 10.1074/jbc.M302244200. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka T, Kojima I, Ohse T, et al. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest. 2005;85:1292–1307. doi: 10.1038/labinvest.3700328. [DOI] [PubMed] [Google Scholar]
- 76.Ohtomo S, Nangaku M, Izuhara Y, et al. Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol Dial Transplant. 2008;23:1166–1172. doi: 10.1093/ndt/gfm715. [DOI] [PubMed] [Google Scholar]
- 77.Muirhead EE, Rightsel WA, Pitcock JA, et al. Isolation and culture of juxtaglomerular and renomedullary interstitial cells. Methods Enzymol. 1990;191:152–167. doi: 10.1016/0076-6879(90)91013-v. [DOI] [PubMed] [Google Scholar]
- 78.Burger-Kentischer A, Muller E, Marz J, et al. Hypertonicity-induced accumulation of organic osmolytes in papillary interstitial cells. Kidney Int. 1999;55:1417–1425. doi: 10.1046/j.1523-1755.1999.00382.x. [DOI] [PubMed] [Google Scholar]
- 79.Moeckel GW, Zhang L, Fogo AB, et al. COX2 activity promotes organic osmolyte accumulation and adaptation of renal medullary interstitial cells to hypertonic stress. J Biol Chem. 2003;278:19352–19357. doi: 10.1074/jbc.M302209200. [DOI] [PubMed] [Google Scholar]
- 80.Huang J, Zhao Q, Mooney SM, et al. Sequence determinants in hypoxia-inducible factor-1alpha for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002;277:39792–39800. doi: 10.1074/jbc.M206955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Percy MJ, Zhao Q, Flores A, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–659. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang AY, Yi F, Jin S, et al. Acid Sphingomyelinase and Its Redox Amplification in Formation of Lipid Raft Redox Signaling Platforms in Endothelial Cells. Antioxidants & Redox Signaling. 2007;9:817–828. doi: 10.1089/ars.2007.1509. [DOI] [PubMed] [Google Scholar]
- 83.Zhang G, Zhang F, Muh R, et al. Autocrine/paracrine pattern of superoxide production through NAD(P)H oxidase in coronary arterial myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H483–495. doi: 10.1152/ajpheart.00632.2006. [DOI] [PubMed] [Google Scholar]
- 84.Mohanty JG, Jaffe JS, Schulman ES, et al. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods. 1997;202:133–141. doi: 10.1016/s0022-1759(96)00244-x. [DOI] [PubMed] [Google Scholar]
- 85.Li N, Zhang G, Yi F-X, et al. ACTIVATION OF NAD(P)H OXIDASE BY OUTWARD MOVEMENTS OF H+ IONS IN RENAL MEDULLARY THICK ASCENDING LIMB OF HENLE. Am J Physiol Renal Physiol. 2005:00416.02004. doi: 10.1152/ajprenal.00416.2004. [DOI] [PubMed] [Google Scholar]
- 86.Lemley KV, Kriz W. Anatomy of the renal interstitium. Kidney Int. 1991;39:370–381. doi: 10.1038/ki.1991.49. [DOI] [PubMed] [Google Scholar]
- 87.Kneen MM, Harkin DG, Walker LL, et al. Imaging of renal medullary interstitial cells in situ by confocal fluorescence microscopy. Anat Embryol (Berl) 1999;200:117–121. doi: 10.1007/s004290050265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.