Abstract
Children with leukocyte adhesion deficiency type 1 (LAD-1) and dogs with canine LAD (CLAD) develop life-threatening bacterial infections due to mutations in the leukocyte integrin CD18. Here, we compared the human phosphoglycerate kinase (hPGK) promoter to the murine stem cell virus (MSCV) promoter/enhancer in a self-inactivating HIV-1–derived lentiviral vector to treat animals with CLAD. Four CLAD dogs were infused with CD34+ cells transduced with the hPGK vector, and two CLAD dogs received MSCV vector–transduced CD34+ cells. Infusions were preceded by a nonmyeloablative dose of 200 cGy total body irradiation. Comparable numbers of transduced cells were infused in each group of animals. Only one of four CLAD animals treated with the hPGK-cCD18 vector had reversal of CLAD, whereas both MSCV-cCD18 vector–treated dogs had reversal of the phenotype. Correction of CLAD depends both upon the percentage of CD18+ myeloid cells and the level of expression of CD18 on individual myeloid cells. In this regard, the hPGK promoter directed low levels of expression of CD18 on neutrophils compared to the MSCV promoter, likely contributing to the suboptimal clinical outcome with the hPGK vector.
In this study, Hunter and colleagues compare self-inactivating lentiviral vectors expressing canine CD18 driven by two different promoters—the human phosphoglycerate kinase-1 (hPGK) promoter and the murine stem-cell virus (MSCV) long terminal repeat promoter/enhancer—in hematopoietic stem-cell gene therapy for canine leukocyte-adhesion deficiency (CLAD). They demonstrate that reversal of the CLAD phenotype can be achieved using a self-inactivating lentiviral vector containing the hPGK promoter; however, the MSCV promoter/enhancer provides a more consistent therapeutic effect, most likely due to higher levels of expression of CD18 on the surface of myeloid cells.
Introduction
Recent gene therapy trials with gammaretroviral (RV) vectors incorporating viral promoter/enhancers have demonstrated the risk of insertional mutagenesis with these vectors, thus emphasizing the need for alternative vectors and promoter/enhancer elements (Ott et al., 2006; Hacein-Bey-Abina et al., 2008). Third-generation human immunodeficiency virus (HIV)-derived lentiviral (LV) vectors possess several distinct advantages over RV vectors: LV vectors transduce hematopoietic stem cells at a higher efficiency than RV vectors since LV vectors do not require mitosis for nuclear entry; LV vectors have a more favorable integration profile when compared to RV vectors, thus reducing potential genotoxic effects; self-inactivating (SIN) modifications in LV vectors, generated by deletions in the long terminal repeat (LTR), reduce the potential for activation of nearby genes (Lewis and Emerman 1994; Cavazzana-Calvo et al., 2000; Aiuti et al., 2002; Wu et al., 2003; Montini et al., 2006).
Several in vitro studies have demonstrated decreased genotoxicity using cellular promoters in place of viral promoters in LV vectors (Zychlinski et al., 2008; Modlich et al., 2009). However, the most efficacious cellular promoter has not been identified. Selection of the optimal promoter in a LV vector to treat a particular disease would ideally be determined by testing the vector in a disease-specific, large-animal model of the target disease. We have used canine leukocyte adhesion deficiency (CLAD) as a disease-specific animal model in which to test new vector systems for hematopoietic stem cell gene therapy for LAD-1 (Bauer et al., 2006, 2008). Dogs with CLAD and children with LAD-1 display the same phenotype of life-threatening bacterial infections, and both diseases are due to mutations in the leukocyte integrin CD18 gene (Kishimoto et al., 1987; Trowald-Wigh et al., 2000).
In this study we compared self-inactivating (SIN) LV vectors expressing canine CD18 (cCD18) driven by two different promoters—the human phosphoglycerate kinase-1 (hPGK) promoter and the murine stem cell virus (MSCV) LTR promoter/enhancer—in hematopoietic stem cell gene therapy for CLAD. One of four hPGK vector–treated dogs and both MSCV vector–treated dogs had reversal of the CLAD phenotype. Both vectors resulted in a low percentage of CD18+ neutrophils; however, the MSCV promoter directed higher levels of CD18 expression on individual myeloid cells compared to the hPGK promoter despite equivalent copy numbers of vector. These results indicate that reversal of the CLAD phenotype can be achieved using a SIN LV vector containing the hPGK promoter; however, the MSCV promoter/enhancer provides a more consistent therapeutic effect, most likely due to higher levels of expression of CD18 on the surface of myeloid cells.
Materials and Methods
Construction of LV vectors
The pRRLSIN.cPPT.hPGK-EGFP transfer vector is a SIN third-generation LV containing the human phosphoglycerate kinase-1 (hPGK) promoter (Naldini et al., 1996). The SIN LV vector pRRLSIN.cPPT.MSCV-cCD18.WPRE was constructed by polymerase chain reaction (PCR) amplification and subcloning of the MSCV promoter/enhancer (same sequence as ΔφMSCV-cCD18; Bauer et al., 2008) as a XhoI/AgeI fragment into the pRRLSIN.cPPT.hPGK-EGFP.WPRE in place of the hPGK promoter. Primers for the MSCV promoter: 5′MSCV-XhoI ATCCGTCTCGAGTGTAGGTTTGGCAAGCTAGC and 3′MSCV-AgeI TTATAAACCGGTCGGGCGACGCAGTCTATCGG. Both SIN LV vectors containing the hPGK promoter or the MSCV promoter/enhancer driving expression of canine CD18 cDNA (cCD18) (Kijas et al., 1999) were constructed by subcloning canine CD18 cDNA as a BamHI/SalI fragment in place of EGFP.
Vector production and titration
LV vector was produced using a four-plasmid transient transfection protocol as previously described (Nelson et al., 2010). The vectors were titered by Southern blot analysis as previously described (Zhao et al., 2009). The p24 assay was performed using the Lenti-X p24 Rapid Titer Kit (Clontech) according to the manufacturer's instructions. Titer (infectious units [IFU]/ml) was determined based on the following estimations found in the user manual: 1 ng of p24 is equivalent to 1.25 × 107 LPs (LV particles), for a typical lentivirus vector there is 1 IFU for every 100–1000 LPs, therefore a supernatant titer of 107 IFU/ml is approximately 80–800 ng p24/ml.
Transduction of CLAD CD34+ cells
Transduction of CLAD CD34+ cells was performed on RetroNectin-coated (TaKaRa), non–tissue culture–treated 24-well plates in X-VIVO-15/1% human serum albumin with 5 μg/ml of protamine sulfate, and a cytokine cocktail consisting of 50 ng/ml each of canine interleukin-6, canine stem cell factor, human Flt3-L, human thrombopoietin, and human granulocyte colony-stimulating factor. Cells were analyzed by flow cytometry for CD18 expression at 5 and 14 days post-transduction. For long-term growth, cells were cultured in StemSpan (STEMCELL Technology)/10% fetal bovine serum and the cytokine cocktail mentioned above until analysis by flow cytometry for CD18 expression and real-time quantitative PCR for vector copy number at day 14.
Animals
Dogs were housed in National Institutes of Health (NIH) facilities in Bethesda, MD, and Poolesville, MD, in accordance with NIH guidelines. These facilities are approved by the American Association for Accreditation of Laboratory Animal Care and Use Committees (ACUC) of the National Cancer Institute, NIH, Bethesda, MD. The studies were performed in accordance with the principles outlined in the Guide for Laboratory Animals Facilities and Care of the National Academy of Sciences, National Research Council.
Bone marrow harvest and CD34+ isolation
Bone marrow was collected and CD34+ cells were isolated as previously described (Bauer et al., 2008; Nelson et al., 2010).
Transplantation of autologous hematopoietic stem cells
One day prior to transplant, autologous CLAD CD34+ cells were thawed, resuspended in X-VIVO 15/1% human serum albumin, and transduced overnight as described for the in vitro studies. On the day prior to infusion all dogs received a single, nonmyeloablative dose of 200 cGy total body irradiation (TBI) delivered from a 60Co source. The following morning the CD34+ cells were harvested, washed, and resuspended in a solution of PlasmaLyte A and 1% heat-inactivated autologous serum, and infused intravenously over 15 min.
Clinical monitoring of CLAD animals
All CLAD animals were monitored daily by physical exam and temperature. CLAD dogs were treated prophylactically with oral amoxicillin/potassium clavulanate as described (Bauer et al., 2006). Treatment with intravenous antibiotics was used when needed. White blood cell (WBC) and differentials were performed commercially (Antec Diagnostics).
Flow cytometry for CD18+ levels following infusion
To assess the percentages of circulating CD18+ leukocytes after infusion, peripheral blood leukocytes were collected monthly from each recipient and analyzed for CD18+ surface expression (Bauer et al., 2006, 2008). Leukocyte subsets including neutrophils, monocytes, T cells, and B cells were determined using subset-specific antibodies as described (Bauer et al., 2008).
Lymphocyte proliferation assay
Proliferation assays were performed as described by Bauer et al. (2008).
Vector copy number by real-time quantitative PCR
For in vitro and in vivo experiments, genomic DNA was analyzed for vector copy number by real-time quantitative PCR on an Applied Biosystems Real-Time PCR and StepOne Plus system as previously described (Kim et al., 2009). N-ras was used as an endogenous control for all experiments. 5′ primer for N-ras: AACCTGTCTGTTGGATATACTGGATA. 3′ primer for N-ras: CGCCTGTCCTCATGTATTGGT. VIC/TAMRA TaqMan probe (Applied Biosystems): TCTCATGGCACTGTACTCTTCTTGTCCAGCT. The primers for the HIV LV vectors: 5′ ACTTGAAAGCGAAAGGGAAAC. 3′ CACCCATCTCTCTCCTTCTAGCC. FAM/NFQ-MGB probe: AGCTCTCTCGACGCAGGACTCGGC. Cycling conditions: 95°C for 20 sec, 40 cycles: 95°C for 1 sec and 60°C for 20 sec. Twenty nanograms of genomic DNA was used for each reaction.
Results
Construction and titering of LV vectors expressing canine CD18
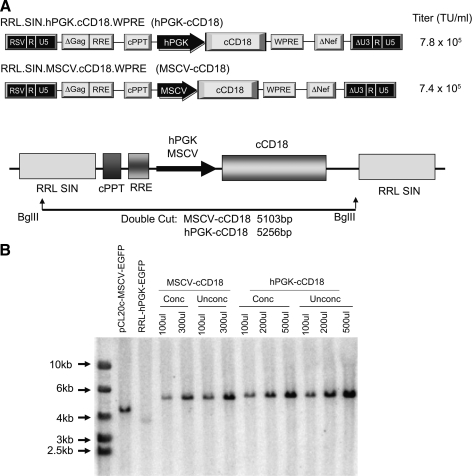
SIN LV vectors expressing canine CD18 cDNA from either the hPGK promoter or the MSCV promoter/enhancer were constructed in the LV vector backbone pRRLSIN.cPPT.PGK-EGFP.WPRE (Addgene plasmid: 12252) (Fig. 1A) (Lim et al., 1989; Hawley et al., 1996). Vector-conditioned media (VCM) was prepared by co-transfection of the LV plasmid along with the three viral components (see Materials and Methods) (Naldini et al., 1996; Dull et al., 1998). The hPGK and MSCV vectors were titered by Southern blot analysis as previously described (Zhao et al., 2009). Unconcentrated vector titers were comparable in both vectors, ranging from 7.4 × 105 to 7.8 × 105 transducing units/ml (Fig. 1A and B). Vector-conditioned media was concentrated by ultracentrifugation to 300 × prior to transduction of CLAD CD34+ cells. To measure infectivity of our LV vector stock preparations we performed p24 assays. For RRL-hPGK-cCD18 there was 32.1 ng p24/ml which corresponded to an infectious titer of 4 × 105 to 4 × 106 IFU/ml. For RRL-MSCV-cCD18 there was 21.4 ng p24/ml, which corresponded to an infectious titer of 2.7 × 105 to 2.7 × 106 IFU/ml. For both vector stocks, the infectious titer obtained by the p24 assay was consistent with the titer obtained by Southern blot (Fig. 1A and B). These vector stocks are representative of the LV vectors used in the in vivo experiments.
FIG. 1.
Schematic of the lentiviral (LV) vectors and transduction of canine leukocyte adhesion deficiency (CLAD) CD34+ cells. (A) pRRLSIN.cPPT.hPGK-cCD18.WPRE was generated by replacing EGFP with the canine CD18 cDNA. pRRLSIN.cPPT.MSCV-cCD18.WPRE was generated by replacing the human phosphoglycerate kinase (hPGK) promoter with the murine stem cell virus (MSCV) promoter/enhancer. (B) Titers of unconcentrated vector stocks were determined by Southern blot analysis as described in the Material and Methods.
Transduction of CLAD CD34+ cells with LV vectors
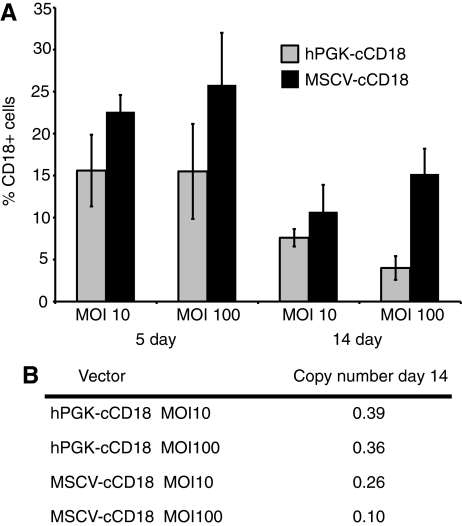
In vitro transduction experiments were performed prior to the in vivo experiments to determine the transduction efficiency of the hPGK and MSCV LV vectors. CLAD CD34+ cells were transduced with VCM at a multiplicity of infection (MOI) of 10 or 100 for 18 hr. After the overnight transduction, the cells were then cultured in fresh media for an additional 4 or 14 days before analysis by flow cytometry (Fig. 2A). The percentage of transduced cells with the MSCV LV vector reached a maximum of 25% at day 5, while the hPGK LV vector reached a maximum of 15% at 5 days. With both vectors the percentage of CD18+ cells at day 14 was approximately one-half of that observed at day 5 (Fig. 2A). Of note, the mean fluorescence intensity (MFI) of vector-transduced cells at day 14 was 1119 for MSCV and 846 for hPGK. To determine whether the lower percentage of CD18+ cells at day 14 with the hPGK vector compared to the MSCV vector might be due to the differences in vector copy number in the transduced CD34+ cells. Real-time quantitative PCR was used to determine vector copy number from cultures of transduced CLAD CD34+ cells at day 14 (Fig. 2B). The vector copy numbers were higher in the hPGK vector–transduced cells (0.39) than in the MSCV vector–transduced cells (0.26), indicating that the vector copy number did not explain the lower percentage of CD18+ cells with the hPGK vector (Fig. 2B). Also, the lower copy number found in cells transduced at the higher MOI is most likely due to higher toxicity resulting from adding larger volumes of concentrated VCM.
FIG. 2.
Transduction of CLAD CD34+ cells. (A) CLAD CD34+ cells were transduced for 18 hr with the hPGK-cCD18 or the MSCV-cCD18 vectors at a multiplicity of infection (MOI) of 10 or 100. Flow cytometry of transduced cells was performed at 5 and 14 days. (B) Genomic DNA was extracted from bulk cultures of transduced cells and the copy number was determined by real-time quantitative polymerase chain reaction (PCR).
Treatment of CLAD animals with hematopoietic stem cell gene therapy
To test whether infusion of autologous CLAD CD34+ cells transduced with the two CD18 vectors results in a sufficient percentage of CD18+ leukocytes to reverse the CLAD phenotype, CD34+ bone marrow cells were harvested from CLAD pups, transduced in an overnight exposure to VCM at an approximate MOI of 10, and infused into CLAD recipients following a single, nonmyeloablative dose of 200 cGy TBI 1 day preceding the infusion (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/hum). We have used this conditioning regimen successfully in previous gene therapy studies (Bauer et al., 2006, 2008). Four CLAD dogs were treated with the hPGK-cCD18 vector, and two CLAD dogs were treated with the MSCV-cCD18 vector. Because untreated CLAD animals succumb to infection by 6 months of age, all CLAD pups were treated by 9 weeks of age (Trowald-Wigh et al., 2000). The ex vivo transduction efficiency ranged from 5.1% to 16.5% at day 5, resulting in a range in the number of transduced cells, however, comparable numbers of total CD18+ cells/kg were infused into the two groups of animals (Table 1).
Table 1.
Comparison of Cell Doses and Outcome with Lentiviral Vectors, hPGK-cCD18, or MSCV-cCD18 Transduced CD34+ Cells Infused into CLAD Dogs
| Dog | Infused CD34+cells/kg | % CD18+ | Transduced cells/kg infused | Outcome (weeks) |
|---|---|---|---|---|
| PGK1 | 10.5 × 106 | 16.5 | 1.7 × 106 | Death (10) |
| PGK2 | 8.6 × 106 | 12.0 | 1.0 × 106 | Death (9.4) |
| PGK3 | 11.0 × 106 | 5.1 | 0.6 × 106 | Death (10.6) |
| PGK4 | 5.4 × 106 | 14.0 | 0.8 × 106 | Alive (52) |
| MSCV1 | 12.3 × 106 | 10.3 | 1.3 × 106 | Alive (52) |
| MSCV2 | 13.0 × 106 | 6.0 | 0.8 × 106 | Alive (52) |
Flow cytometry of CD18+ leukocytes in peripheral blood following treatment
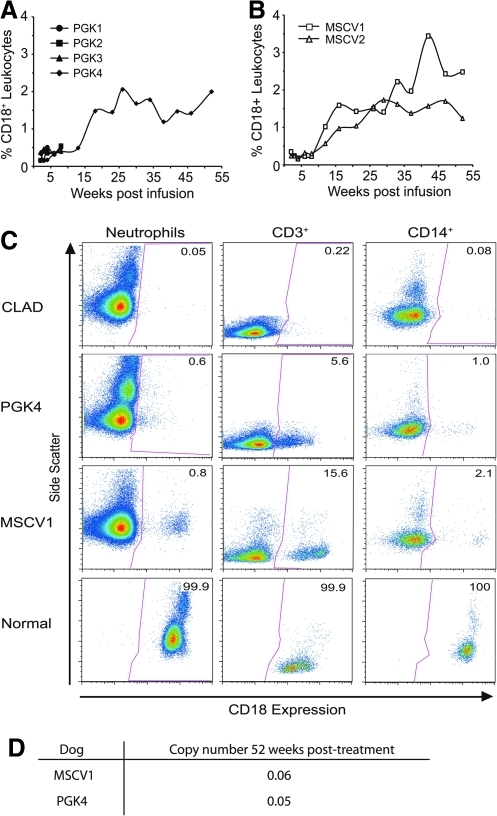
To determine the percentage of CD18+ leukocytes in the peripheral blood of the treated dogs, leukocyte subsets were analyzed at weekly, biweekly, or monthly intervals following treatment. The percentage of CD18+ leukocytes in the peripheral blood remained less than 0.5% for the first 15 weeks in all four dogs treated with the hPGK vector. Three of these four dogs were euthanized due to severe infection from CLAD during this time (Fig. 3A). In the surviving hPGK-cCD18 treated CLAD animal, and in both MSCV-vector treated dogs, the percentage of CD18+ leukocytes gradually increased over time, reaching a level of greater than 1% by 15 weeks following infusion (Fig. 3A and B). CD18+ cells were present in all leukocyte subsets in both the hPGK and MSCV vector–treated dogs; however, the percentage of CD18+ neutrophils remained less than 1% in all dogs at all time points (Fig. 3C). In contrast, the percentage of CD18+ CD3+ T-lymphocytes was 5.6% in dog PGK4 and 15.6% in dog MSCV1 (Fig. 3C).
FIG. 3.
Flow cytometry analysis of CD18+ cells in peripheral blood of CLAD dogs following infusion of transduced CD34+ cells. (A) Levels of CD18+ peripheral blood leukocytes following treatment with the hPGK-cCD18 vector–treated dogs. (B) Levels of CD18+ leukocytes following treatment for the MSCV-cCD18 vector–treated dogs. (C) Dot plots of CD18+ peripheral blood leukocytes (PBL) subsets: neutrophils, CD3+ T lymphocytes, and CD14+ monocytes in the LV vector–treated CLAD dogs and a normal control are shown. Data points are shown at 52 weeks following infusion. CD18+ expression (x-axis) is plotted versus side scatter (y-axis). The percentage of CD18+ cells is indicated in the upper right-hand corner of each box. (D) Genomic DNA was extracted from peripheral blood leukocytes 52 weeks after gene therapy and the vector copy number was determined by real-time quantitative PCR.
Vector copy number for vector-treated dogs at 1 year after gene therapy
Genomic DNA was extracted from peripheral blood leukocytes (PBL) of the MSCV1 and PGK4 dogs at 1 year post-treatment, and real-time quantitative PCR was used to determine the vector copy number for each dog. The vector copy number was 0.05 for PGK4 and 0.06 for MSCV1 (Fig. 3D). The percentage of CD18+ cells for PGK4 and MSCV1 in the PBL was 2% and 3%, respectively, at the same time point, indicating that the MSCV vector–treated animals did not have higher copy numbers than the PGK vector–treated animals (Fig. 3A and B).
CD18 expression per cell in peripheral blood leukocytes at 1 year post-treatment
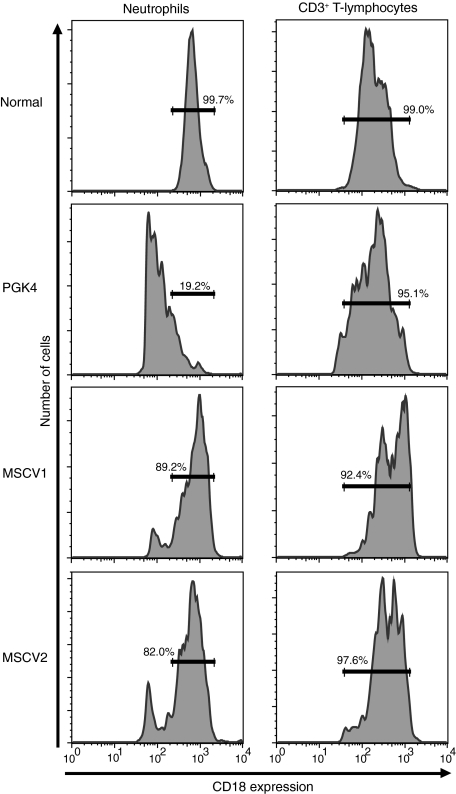
When the MFI for CD18 on neutrophils of the vector-treated dogs was compared to the level of CD18 on normal canine neutrophils, 89.2% and 82.0% of the CD18+ neutrophils from the MSCV-treated dogs had normal levels of CD18, whereas only 19.2% of the CD18+ neutrophils from the hPGK vector–treated dog (PGK4) displayed normal levels of CD18 (Fig. 4). In contrast, 95.1% of CD18+ CD3+ T lymphocytes expressed normal levels of CD18 in the hPGK vector–treated dog. This percentage was similar to that observed in both MSCV vector–treated dogs where 92.4% and 97.6% of the CD18+ CD3+ T-lymphocytes expressed normal levels of CD18 (Fig. 4).
FIG. 4.
Comparison of CD18 expression on neutrophils and CD3+ T-lymphocytes in LV vector–treated dogs following treatment. Percentage of CD18+ neutrophils and CD18+ CD3+ T lymphocytes expressing normal levels of CD18 compared to a normal control for the hPGK-cCD18 and MSCV-cCD18 vector–treated dogs. The numbers indicate the percentage of cells expressing normal levels of CD18 per cell.
The expression of CD18 per cell on the neutrophils, CD14+ monocytes, and CD3+ T lymphocytes in the MSCV-cCD18 treated dogs compared to the hPGK-cCD18–treated dog were as follows: neutrophils (MFI 868 vs. 173), CD14+ monocytes (MFI 1962 vs. 751), and CD3+ T lymphocytes (MFI 661 vs. 270). This effect can be visualized in the pseudo-color plots in Fig. 3C.
CD18+ lymphocyte proliferation
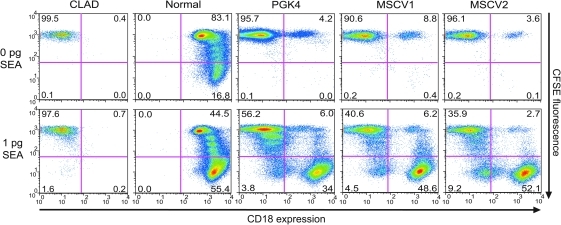
In all three surviving dogs the levels of CD18+ lymphocytes in the peripheral blood were consistently higher than the CD18+ neutrophil levels. To determine whether the higher percentage of CD18+ lymphocytes in the peripheral blood in these dogs resulted from a selective proliferative advantage of the CD18+ cells, we used an in vitro proliferation assay in which lymphocyte-enriched mononuclear cells were stimulated with staphylococcal enterotoxin A (SEA) mitogen (Bauer et al., 2008). CD18− T lymphocytes from the treated CLAD animals proliferated poorly in comparison with the CD18+ T lymphocytes (Fig. 5). The relative increase in CD3+ T lymphocytes in the surviving dogs is also consistent with our previously published data demonstrating a selective proliferation of CD18+ T lymphocytes in response to mitogens in both allotransplant- and gene therapy–treated CLAD dogs (Bauer et al., 2005, 2006, 2008). This proliferation is a normal, antigen-driven process rather than a pathologic process.
FIG. 5.
Lymphocyte proliferation assay of PBL from hPGK-cCD18 and MSCV-cCD18–treated dogs. CD18 expression (x-axis) and CFSE fluorescence (y-axis) in cells from a CLAD, normal, hPGK vector–treated (PGK4), and the two MSCV vector–treated dogs. The percentage of CD18− and CD18+ cells are indicated in the lower left and right quadrants, respectively.
Clinical outcome
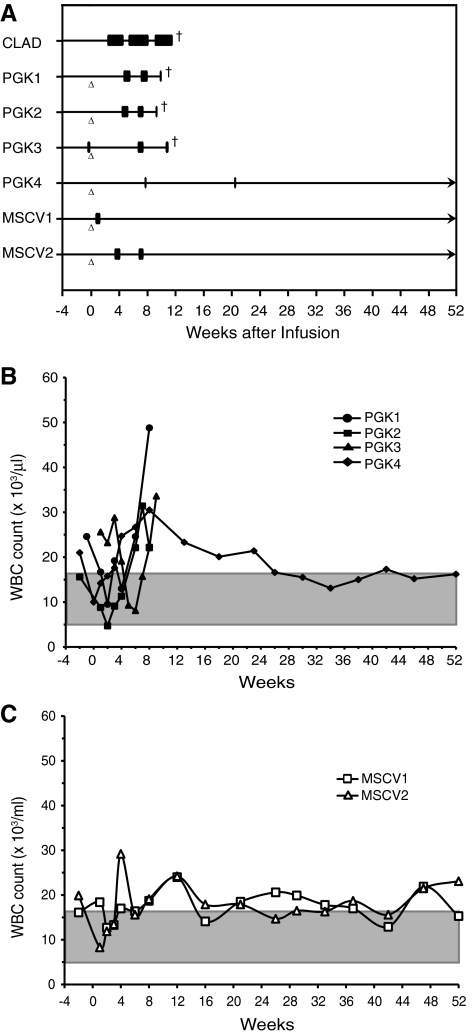
Only one of four hPGK-cCD18–treated animals (PGK4) survived 52 weeks following infusion, whereas both of the MSCV-cCD18–treated animals survived 52 weeks following treatment (Fig. 6A). The three CLAD dogs treated with the hPGK-cCD18 vector that failed to survive had very low levels of CD18+ neutrophils (<0.5%) and low expression of CD18 per neutrophil. These three dogs experienced multiple infectious episodes due to CLAD and were euthanized by 11 weeks following treatment according to humane endpoints. Of note, although the two MSCV vector–treated dogs had similar levels of CD18+ neutrophils as the three hPGK vector–treated dogs when they were euthanized, the higher levels of expression of CD18 per cell likely contributed to their survival.
FIG. 6.
Clinical course of the PGK and MSCV vector–treated dogs. (A) Individual dogs are listed on the y-axis and the time from birth appears on the x-axis. The horizontal lines represent the life of each animal. Triangles indicate the time of infusion. Boxes represent episodes of fever requiring parenteral antibiotics. Crosses indicate death. The clinical course is shown prior to and following the infusion of their autologous transduced CD34+ cells. (B) White blood cell (WBC) counts in the hPGK-cCD18 vector–treated dogs before and up to 52 weeks following treatment. Time is shown on the x-axis and WBC count on the y-axis, the shaded area represents normal WBC range. (C) WBC counts in the MSCV-cCD18 vector–treated dogs before and up to 52 weeks following treatment. Time is shown on the x-axis and WBC count on the y-axis.
The WBC level in the peripheral blood provides a surrogate marker for CLAD; untreated CLAD animals have a marked elevation in peripheral blood leukocytes (Trowald-Wigh et al., 2000). The WBC level remained elevated in the three hPGK-cCD18 vector–treated dogs that failed to survive beyond 10 weeks, whereas the WBC returned to near normal levels in the surviving hPGK and MSCV vector–treated dogs (Fig. 6B and C).
Discussion
We compared two SIN LV vectors driving canine CD18—one with the hPGK promoter and one with the MSCV LTR promoter/enhancer—for their ability to generate sufficient levels of CD18+ neutrophils to reverse the phenotype in CLAD. Both vectors transduced similar numbers of CLAD CD34+ cells; however, the MSCV promoter resulted in considerably higher expression of CD18 per neutrophil. Only one of four dogs treated with the hPGK-cCD18 vector had reversal of CLAD, whereas both MSCV-cCD18 dogs had reversal of CLAD. Although the hPGK-cCD18 vector is capable of reversing the phenotype, the MSCV-cCD18 vector provides a more consistent therapeutic effect, most likely due to higher levels of CD18 expression on neutrophils.
In human gene therapy protocols involving ex vivo transduction of hematopoietic stem cells, none have utilized homologous, large-animal, disease models prior to the initiation of human studies, and instead have relied on murine knockout models when applicable. Marking studies in large animals such as dogs or nonhuman primates have been invaluable for optimization of transduction conditions and testing of new vectors, and these studies have significant value for the study of insertional mutagenesis. However, these studies do not address the role of therapeutic genes for particular diseases. The current studies were designed to address this gap in our knowledge by testing two SIN LV vectors in the CLAD model.
In previous studies using a foamy viral (FV) vector with an internal MSCV promoter driving canine CD18 expression (ΔφMSCV-cCD18), we demonstrated that an overnight exposure to the vector in the presence of cytokines resulted in 15% to 25% transduction levels (Bauer et al., 2008). In vivo infusion of autologous FV vector–transduced cells into CLAD animals following 200 cGy TBI resulted in 2% to 6% circulating CD18+ neutrophils 2 years following infusion with near normal levels of CD18 expression per neutrophil. The percentage of CD18+ neutrophils and the level of CD18 expression per cell resulted in correction of the CLAD phenotype.
The argument supporting the use of cellular promoters in vectors, such as the human elongation factor 1 alpha (EF1α) promoter and the PGK promoter, is that these physiological promoters have been shown to reduce the risk of genotoxicity in in vitro model systems (Zychlinski et al., 2008). SIN LV vectors with these promoters displayed a 10-fold reduced risk of insertional transformation in vitro compared to viral promoters (Modlich et al., 2009).
Two groups compared these promoters head-to-head for efficiency in transducing primary hematopoietic stem cells. Salmon and colleagues transduced cord blood CD34+ cells with a SIN LV vector with the murine PGK promoter (bp 424–920) and the human EF1α promoter driving EGFP (Salmon et al., 2000). The transduction percentages ranged from 8% to 18%, and strong expression of EGFP was observed with each vector. When the CD34+ cells were differentiated in vitro, the percentages of GFP+ cells with the PGK promoter decreased significantly compared to the EF1α promoter. Ramezani et al. (2000) compared several SIN LV vectors containing alternative promoters, including an MSCV promoter, the human EF1α promoter containing an intron, CAG (CMV immediate early enhancer combined with the chicken β-actin promoter). All vectors contained a WPRE. Vectors with the MSCV and PGK promoters were the most efficient at transducing primary human CD34+ cord blood progenitors.
There are several possible reasons for the relatively poor success with the hPGK-cCD18 LV vector in this study. The transduction levels with the CD18-containing vectors were relatively low. This problem could possibly be overcome by generating higher titer vectors or including a 24-hr prestimulation period with growth factors prior to transduction. The lower expression of CD18 on a per cell basis (lower MFI) observed with the hPGK-cCD18 vector may also have inhibited the ability of neutrophils to participate in normal adhesion, migration, and phagocytic function. LAD has been characterized as severe or moderate according to quantitative differences in expression of the leukocyte integrins on the cell surface (Anderson et al., 1985). Severity of clinical complications correlates with the degree of deficiency. Children with severe deficiency frequently experience lethal bacterial infections in the first few years of life, whereas those with moderate deficiency survive into adolescence or young adulthood (Anderson and Springer 1987). The lower MFI on neutrophils with the hPGK vector may be an intrinsic limitation with using this promoter in treating a disease due primarily to neutrophil dysfunction. Alternatively, transcriptional silencing might provide an explanation for the lower levels of CD18+ cells in the hPGK vector–treated dog as compared to the MSCV vector–treated dogs, and the lower CD18 MFI on neutrophils in the hPGK vector–treated dogs. Both MSCV1 and PGK4 vector–treated dogs had comparable numbers of CD18+ neutrophils, and very similar copy number in the peripheral blood leukocytes, but differed greatly in the level of CD18 expression on neutrophils. The hPGK vector–treated dog, PGK4, did have near normal levels of CD18 expression on CD3+ T lymphocytes, suggesting that the hPGK promoter may be useful for gene therapy of lymphoid diseases.
This study indicates that there is significant potential to use the MSCV promoter in clinical studies and that, although it is possible to reverse the CLAD phenotype with a SIN LV vector containing the hPGK promoter, the low expression of CD18 on neutrophils with the hPGK-cCD18 vector indicates that it may be difficult to achieve a consistent therapeutic effect in a disease requiring expression on myeloid cells, and that alternative nonviral promoters should be investigated for gene therapy of CLAD and other myeloid diseases.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We wish to thank Veena Kapoor, National Cancer Institute, for assistance with flow cytometry.
Author Disclosure Statement
The authors have declared that no conflict of interest exists.
References
- Aiuti A. Slavin S. Aker M., et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- Anderson D.C. Springer T.A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu. Rev. Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Anderson D.C. Schmalsteig F.C. Finegold M.J., et al. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J. Infect. Dis. 1985;152:668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Bauer T.R., Jr. Allen J.M. Hai M., et al. Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors. Nat. Med. 2008;14:93–97. doi: 10.1038/nm1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer T.R., Jr. Gu Y.C. Tuschong L.M., et al. Nonmyeloablative hematopoietic stem cell transplantation corrects the disease phenotype in the canine model of leukocyte adhesion deficiency. Exp. Hematol. 2005;33:706–712. doi: 10.1016/j.exphem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Bauer T.R., Jr. Hai M. Tuschong L.M., et al. Correction of the disease phenotype in canine leukocyte adhesion deficiency using ex-vivo hematopoietic stem cell gene therapy. Blood. 2006;108:1767–1769. doi: 10.1182/blood-2006-03-006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana-Calvo M. Hacein-Bey S. de Saint Basile G., et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Dull T. Zufferey R. Kelly M., et al. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Garrigue A. Wang G.P., et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R.G. Hawley T.S. Fong A.Z., et al. Thrombopoietic potential and serial repopulating ability of murine hematopoietic stem cells constitutively expressing interleukin 11. Proc. Natl. Acad. Sci. U. S. A. 1996;93:10297–302. doi: 10.1073/pnas.93.19.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijas J.M.H. Bauer T.R., Jr. Gäfvert S., et al. A missense mutation in the b-2 integrin gene (ITGB2) causes canine leukocyte adhesion deficiency. Genomics. 1999;61:101–107. doi: 10.1006/geno.1999.5948. [DOI] [PubMed] [Google Scholar]
- Kim Y.J. Kim Y.S. Larochelle A., et al. Sustained high-level polyclonal hematopoietic marking and transgene expression 4 years after autologous transplantation of rhesus macaques with SIV lentiviral vector-transduced CD34+ cells. Blood. 2009;113:5434–5443. doi: 10.1182/blood-2008-10-185199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T.K. Hollander N. Roberts T.M., et al. Heterogenous mutations in the b subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987;50:193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- Lewis P.F. Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B. Apperley J.F. Orkin S.H. Williams D.A. Long-term expression of human adenosine deaminase in mice transplanted with retrovirus-infected hematopoietic stem cells. Proc. Natl. Acad. Sci. U. S. A. 1989;86:8892–8896. doi: 10.1073/pnas.86.22.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlich U. Navarro S. Zychlinski D., et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol. Ther. 2009;17:1919–1928. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montini E. Cesana D. Schmidt M., et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Naldini L. Blomer U. Gallay P., et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Nelson E.J. Tuschong L.M. Hunter M.J., et al. Lentiviral vectors incorporating a human elongation factor 1alpha promoter for the treatment of canine leukocyte adhesion deficiency. Gene Ther. 2010;17:672–677. doi: 10.1038/gt.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M.G. Schmidt M. Schwarzwaelder K., et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- Ramezani A. Hawley T.S. Hawley R.G. Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol. Ther. 2000;2:458–469. doi: 10.1006/mthe.2000.0190. [DOI] [PubMed] [Google Scholar]
- Salmon P. Kindler V. Ducrey O., et al. High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood. 2000;96:3392–3398. [PubMed] [Google Scholar]
- Trowald-Wigh G. Ekman S. Hansson K., et al. Clinical, radiological and pathological features of 12 Irish setters with canine leucocyte adhesion deficiency. J. Small Anim. Pract. 2000;41:211–217. doi: 10.1111/j.1748-5827.2000.tb03198.x. [DOI] [PubMed] [Google Scholar]
- Wu X. Li Y. Crise B. Burgess S.M. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- Zhao H. Pestina T.I. Nasimuzzaman M., et al. Amelioration of murine beta-thalassemia through drug selection of hematopoietic stem cells transduced with a lentiviral vector encoding both gamma-globin and the MGMT drug-resistance gene. Blood. 2009;113:5747–5756. doi: 10.1182/blood-2008-10-186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinski D. Schambach A. Modlich U., et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol. Ther. 2008;16:718–725. doi: 10.1038/mt.2008.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.