Abstract
The therapeutic application of recombinant retroviruses and other integrating gene transfer vectors has been limited by problems of vector expression and vector-mediated genotoxicity. These problems arise in large part from the interactions between vector sequences and the genomic environment surrounding sites of integration. Strides have been made in overcoming both of these problems through the modification of deleterious vector sequences, the inclusion of better enhancers and promoters, and the use of alternative virus systems. However, these modifications often add other restrictions on vector design, which in turn can further limit therapeutic applications. As an alternative, several groups have been investigating a class of DNA regulatory elements known as chromatin insulators. These elements provide a means of blocking the interaction between an integrating vector and the target cell genome in a manner that is independent of the vector transgene, regulatory elements, or virus of origin. This review outlines the background, rationale, and evidence for using chromatin insulators to improve the expression and safety of gene transfer vectors. Also reviewed are topological factors that constrain the use of insulators in integrating gene transfer vectors, alternative sources of insulators, and the role of chromatin insulators as one of several components for optimal vector design.
Chromatin insulators are a class of DNA regulatory elements. They provide a means of blocking the interaction between an integrating vector and the target-cell genome in a manner that is independent of the vector transgene, regulatory elements, or virus of origin. In this review, Dr. David Emery outlines the background, rationale, and evidence for using chromatin insulators to improve the expression and safety of gene-transfer vectors.
Vector–Genome Interactions
Two types of interactions
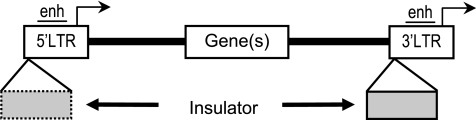
It is now widely recognized that integrating gene transfer vectors, such as those based in recombinant retroviruses, other transposable elements, and even plasmids, integrate semirandomly throughout the target cell genome (e.g., Berry et al., 2006). Although the details of integration site preferences vary between vectors of different origins, the resulting patterns inevitably subject the incoming vector sequences to a wide array of genomic environments. As diagrammed in Fig. 1, this property of vector integration has two major ramifications for vector performance. First, the genomic sequences and/or overall chromatin structures flanking the sites of vector integration can influence the level and pattern of vector expression, a phenomenon referred to as chromosomal position effects. This typically manifests as overt vector silencing or as position effect variegation, wherein expression levels are highly variable between, and even within, the progeny of transduced cell clones (Emery et al., 2003; Ellis, 2005). Second, vector sequences can influence the expression of cellular genes flanking the sites of vector integration, a phenomenon referred to as insertional mutagenesis. This typically manifests as the disruption or dysregulation of cellular gene expression, and in the most extreme cases can lead to clonal expansion and even oncogenic transformation of individual cell clones, a phenomenon referred to collectively as vector-mediated genotoxicity (Baum et al., 2006a; Nienhuis et al., 2006).
FIG. 1.
Ramifications of semi-random vector integration. Gene transfer vectors integrate in semi-random locations throughout the target cell genome (Berry et al., 2006). This has two major ramifications for vector performance. As shown on the left, the chromatin surrounding integrated vectors can influence vector expression, often leading to vector silencing and position effect variegation (a phenomenon referred to generically as chromosomal position effects). As shown on the right, vector sequences can also influence the expression of the genes surrounding the site of vector integration, leading to a risk of insertional mutagenesis and even proto-oncogene activation (a phenomenon referred to generically as vector-mediated genotoxicity).
Silencing chromosomal position effects
The impact of chromosomal position effects on integrating gene transfer vectors most frequently manifests as partial or complete loss of transgene expression, often occurring over time or in association with differentiation (Emery et al., 2003; Ellis, 2005; Mok et al., 2007). This phenomenon is not unique to retroviral vectors, since such silencing position effects have been reported in settings ranging from transposable elements in Drosophila to transgenic mice. This propensity for transgene silencing presumably reflects, at least in part, the ability of heterochromatin to easily spread in the genomes of higher eukaryotes (Gaszner and Felsenfeld, 2006; Raab and Kamakaka, 2010), resulting in the silencing of vector proviruses that integrate within or near heterochromatin, or in genomic loci that become heterochromatinized during differentiation. In the case of retroviral vectors, the level and clonal frequency of transgene expression can be influenced by specific elements of the residual viral sequences, most notably elements of the viral long-terminal repeats (LTRs) (Challita et al., 1995; Emery et al., 2003; Ellis, 2005) or bacterial reporter gene sequences (Artelt et al., 1991). The expression profile of retroviral vectors can also be influenced by the origin of the parental retrovirus, the use of exogenous enhancers and promoters, and the inclusion of RNA-stability elements. However, there are still many examples where the expression of otherwise optimized vectors based on gammaretroviruses and lentiviruses are subject to chromosomal position effects.
The influence of silencing chromosomal position effects on vector expression is often obscured by experimental parameters. For example, the use of high vector copy numbers makes it difficult to assess both the frequency and the level of expression for individual provirus. Likewise, the use of selection strategies can result in a bias for clones containing provirus at integration sites that are favorable for expression (Aker et al., 2006), resulting in an underestimation of the frequency of vector silencing. The impact of position effects are most readily apparent upon analysis of cell clones, derived in the absence of selection, that contain single vector copies (e.g., Rivella et al., 2000; Aker et al., 2007; Arumugam et al., 2009b). Unfortunately, most gene transfer research involves the analysis of vector expression in polyclonal cell cultures, or settings of in vivo reconstitution, where it is difficult to compare the frequency of vector transfer to the frequency of vector expression. At a minimum, this requires a direct comparison between the frequency of cells expressing the vector transgene to the vector copy number determined by quantitative Southern blotting or PCR-based methods (e.g., Rivella et al., 2000; Emery et al., 2000; Ramezani et al., 2003; Aker et al., 2007). In settings where the average number of vector copies is well below 1 per cell, this approach provides a relatively good first approximation of the frequency of transduced cells. However, at higher vector copy numbers, additional methods must be used to either directly assess the fraction of cells containing vector provirus, or to use statistical methods such as the Poisson distribution to estimate the fraction of cells containing vector provirus (Emery et al., 2000). It is also important to note that position effects generally manifest most strongly during shifts in heterochromatinization (such as in the erythroid lineage [Emery et al., 2002]) or after in vivo differentiation (as in the case of neural cells [Jakobsson et al., 2004] or hematopoietic cells [Ramezani et al., 2006]).
Vector-mediated genotoxicity
Although maintaining transgene expression is important to the success of clinical gene therapy, problems of vector-mediated genotoxicity have taken center stage. As reviewed extensively elsewhere (Baum et al., 2006a; Nienhuis et al., 2006), examples of retroviral vector-mediated genotoxicity, including clonal outgrowth and overt malignant transformation, have been documented in both animal models and clinical trials. This hazard has typically been attributed to the ability of vector enhancers to activate proto-oncogenes, such as genes involved in cell growth or differentiation, resulting in the expansion of rare cells bearing these vector integration sites. However, the potential for vector promoters to transcribe flanking cellular genes, or other vector sequences to interfere with the proper processing of primary cellular transcripts, can also be problematic (Zaiss et al., 2002; Cavazzana-Calvo et al., 2010). Likewise, the vector transgene and/or disease pathology can also play a role in the transformation process, such as in cases in which the vector transgene provides a selective growth advantage (e.g., Shou et al., 2006).
Several approaches have been proposed for decreasing the rate of retroviral vector-mediated genotoxicity (Baum et al., 2006b; Nienhuis et al., 2006; Chang and Sadelain, 2007). These include the use of vectors based on lentiviruses or other viruses that are less likely to integrate near gene promoters (Montini et al., 2006), the use of self-inactivating (SIN) vectors that delete potent enhancers and promoters in the viral long-terminal repeats (Modlich et al., 2006), the use of lineage-restricted enhancers and promoters (Chang and Sadelain, 2007; Zychlinski et al., 2008; Arumugam et al., 2009a), and improvements to the 3′ RNA processing signals (Zaiss et al., 2002). However, each of these approaches has potential drawbacks. For example, switching to a purportedly less toxic vector system, such as lentiviruses, may in some cases require the complete reengineering of vectors that already enjoy a strong track record in clinical trials. This is especially troublesome because the clinical track record with lentiviral vectors is exceedingly sparse, with at least one apparent case of vector-associated clonal expansion (Cavazzana-Calvo et al., 2010). Likewise, the use of SIN vector designs necessitates abandoning the 5′ LTR as a means of transcribing vector transgenes, a conventional engineering design that has been used in both preclinical and clinical applications since the advent of retroviral vectors. The use of lineage-restricted expression is not desirable in applications where robust, pan-cellular transgene expression is needed, and the use of enhanced 3′ RNA processing signals may, in some settings, reduce vector titers.
Need for an alternative means of reducing vector–genome interactions
Clearly, overt vector gene silencing and vector-mediated genotoxicity can be minimized through the judicious choice of vector sequences and transcriptional regulatory elements. However, these choices have limits. Strong enhancers can increase the likelihood of vector expression, but can also potentially increase vector genotoxicity. The use of attenuated or tissue-specific enhancers can reduce vector genotoxicity, but can also make it harder to achieve adequate levels of vector expression. In addition, there are very few clinical safety or efficacy data involving the use of alternative vector systems, such as lentiviruses or simian foamy viruses, which demonstrate they are safer and more effective. Thus, there is a clear need for other methods of reducing vector–genome interactions that are applicable to a broad range of vector types. The following sections describe the growing body of evidence showing that a class of DNA regulatory elements known as chromatin insulators can mitigate both vector silencing due to chromosomal position effects and vector-mediated genotoxicity.
Chromatin Insulators
Two types of insulators
As reviewed extensively elsewhere (West et al., 2002; Emery et al., 2003; Gaszner and Felsenfeld, 2006; Raab and Kamakaka, 2010), chromatin insulators are naturally occurring DNA elements that help form functional boundaries between adjacent chromatin domains. They have been reported in species as diverse as yeast and man and play a key role in genomic architecture and gene regulation. There are two basic classes of chromatin insulators: enhancer-blocking insulators and barrier insulators. As diagrammed in Fig. 2, enhancer-blocking insulators prevent enhancer-mediated transcriptional activation of adjoining regions that are heterochromatic or otherwise transcriptionally quiescent. In contrast, barrier insulators block the encroachment of silencing heterochromatin into adjoining regions of open chromatin that are transcriptionally permissive. The sequences that mediate these activities appear to be physically separable and mechanistically distinct. Unlike more conventional cis-regulatory elements, stereotypic chromatin insulators do not exhibit inherent transcriptional enhancing or repressing activities on their own. As such, they allow other cis-regulatory elements to function without interference from, or action upon, the surrounding chromatin. These properties make chromatin insulators ideal elements for reducing the interaction between gene transfer vectors and the target cell genome. Also unlike more conventional cis-regulatory elements, chromatin insulators typically must be physically located between promoters and either enhancers or heterchromatin in order to provide their protective function. This unique topological consideration is key to the successful use of chromatin insulators in gene transfer vectors.
FIG. 2.
Chromatin insulator functions. There are two classes of chromatin insulator functions. As shown on the left, some chromatin insulators exhibit barrier activity, which serves to physically block the encroachment of heterochromatin or other sources of gene silencing from adjoining regions. As shown on the right, some chromatin insulators exhibit enhancer-blocking activity, which serves to block the activating effects of enhancers on adjoining regions. Rare chromatin insulators such as the cHS4 element exhibit both activities.
Enhancer-blocking insulators
Of the insulator elements identified to date, most are of the enhancer-blocking variety (e.g., West et al., 2002; Raab and Kamakaka, 2010). In vertebrates, the function of these elements is mediated through the zinc-finger DNA-binding factor CTCF (Gaszner and Felsenfeld, 2006; Phillips and Corces, 2009). In most models, these elements are thought to function through physical loop structures that are established through CTCF-mediated interactions between adjacent insulator elements or through CTCF-mediated tethering of the chromatin fiber to structural elements within the nucleus (e.g., Ling et al., 2006; Guelen et al., 2008; Rubio et al., 2008; Hou et al., 2010). Such structures presumably form functionally distinct domains, either by sequestering these domains into discrete compartments of the nucleus, or by physically blocking the tracking of enhancers along the DNA axis. These mechanistic considerations help drive two key concepts that are critical for the rational use of enhancer-blocking insulators in gene transfer vectors. First, an insulator must be physically located between the vector enhancer and cellular gene promoter in order to functionally separate the two. Second, it is likely that these elements work best when used in pairs to physically bracket the element to be insulated. This arrangement allows for the availability of pairing partners that can serve to partition the vector expression cassette from the surrounding genome, whether through the formation of a closed loop or by other proposed mechanisms.
Barrier insulators
In contrast to the enhancer-blocking insulators, far fewer barrier insulators have been reported in the literature, and much less is known about the mechanisms of their function (e.g., Gaszner and Felsenfeld, 2006; Raab and Kamakaka, 2010). In the context of higher eukaryotes these elements appear to function at least in part by blocking the self-perpetuating spread of heterochromatin mediated by methylation of lysine 9 on histone 3 (H3K9) and the subsequent recruitment of heterochromatin protein 1 and additional histone methyltransferases (Gaszner and Felsenfeld, 2006). This is typically achieved by the recruitment of a protein complex that modifies the proximal histones so as to maintain an open, transcriptionally permissible state, possibly associated with specific histone variants (Jin et al., 2009). This involves in part the recruitment of histone acetyltransferases that competitively acetylate the H3K9 moiety, thereby preventing the methylation of this site that otherwise precedes the cascade of heterchromatinization. As such, it appears that barrier insulators may function in an autonomous and independent fashion at the level of the local chromatin, rather then in a coordinate fashion involving higher-order chromatin structures such as that seen with CTCF-mediated enhancer-blocking insulators (Gaszner and Felsenfeld, 2006; Phillips and Corces, 2009), although this model has also recently been questioned (Raab and Kamakaka, 2010). These mechanistic considerations again have implications for the rational use of barrier insulators in gene transfer vectors. First, as with enhancer-blocking insulators, barrier insulators must be physically located between the vector expression cassette and the source of silencing heterochromatin in order to functionally separate the two. Second, and unlike enhancer-blocking insulators, there is no mechanistic reason to assume that barrier insulators should necessarily work better in pairs (e.g., require a pairing partner), except for the need to block encroachment of heterochromatin from both sides of a transgene.
The prototypic insulator cHS4
Of all the vertebrate insulators identified to date, the most well characterized is derived from DNase hypersensitive site 4 of the locus control region of the chicken β-globin gene cluster (cHS4). It is also one of the rare insulator elements that exhibit both enhancer-blocking and barrier activity, making it an ideal candidate for use in gene transfer applications. Initial studies with this element involved the use of a 1.2-kb fragment that contains the DNase hypersensitive site at the conventionally recognized 5′ end, and demonstrated its functionality by plasmid transfection in a murine erythroleukemia (MEL) cell line and transgenesis in Drosophila (Chung et al., 1993). Later studies demonstrated that these dual activities are separable and can be mapped for the most part to distinct footprints contained within a 250-bp core fragment surrounding the DNase hypersensitive site (Chung et al., 1997; West et al., 2002; Gaszner and Felsenfeld, 2006). One of these footprints (FII) is bound by CTCF and appears to account for most of the enhancer-blocking activity (Bell et al., 1999; Yao et al., 2003). This activity is associated with CTCF-mediated tethering of cHS4 to distinct subnuclear sites, presumably a key aspect of its ability to form functionally distinct domains (Yusufzai and Felsenfeld, 2004). In addition, there is some evidence that CTCF bound at cHS4 FII can directly block PolII procession from enhancer to promoter (Zhao, 2006). Most of the barrier insulator activity associated with cHS4 has been mapped to the four other footprints located on the 250-bp cHS4 core fragment. One of these footprints is bound by USF1/USF2, which serve to recruit histone-modifying enzymes (West et al., 2004). Other footprints are bound by VEZF1, which is involved in regulating DNA methylation (Dickson et al., 2010). It important to note that this 250-bp core contains much, but not all of the barrier insulator activity associated with the full-length 1.2-kb cHS4 element. Deletion studies using a gammaretroviral vector reporter system indicated that an extended 400-bp version of this core does contain all of the barrier activity of the full-length cHS4 element (Aker et al., 2007), and that the activity of this extended core is associated with specific binding by poly(ADP-ribose) polymerase 1 (PARP-1) (Aker et al., 2010). PARP-1 is an abundant nuclear protein that has the capacity to bind DNA through zinc finger motifs and to catalyze the addition of poly(ADP)-ribose (PAR) chains to itself and other proteins (Kraus, 2008). It has been implicated in many biochemical and gene regulation pathways, including the regulation of CTCF (Farrar et al., 2010). This in turn suggests a possible link between the enhancer-blocking and barrier activities of the cHS4 insulator. Another recent study also suggest the most distal portion of the 1.2-kb cHS4 element may also contribute to barrier insulator activity and contains a separate binding site for CTCF (Arumugam et al., 2009b).
Use of Insulators to Reduce Vector Silencing
Initial studies
Initial efforts to assess the ability of insulators to protect retroviral vectors from silencing chromosomal position effects focused on testing the prototypic insulator cHS4 in gammaretroviral vectors (Rivella et al., 2000; Emery et al., 2000). As diagrammed in Fig. 3, this involved deploying the full-length 1.2-kb cHS4 fragment in a “double-copy” configuration. In such a configuration, a fragment inserted in the proximal portion of the 3′ LTR is copied into the corresponding location of the 5′ LTR during formation of vector provirus (Hantzopoulos et al., 1989), resulting in a flanking arrangement that brackets the internal segment of the vector. The first report was based on a Moloney Murine Leukemia Virus (MoMLV) vector designed to express an inert cell surface marker (NTP) from the 5′ LTR, and transduction studies in a MEL cell line and an embryonic stem cell line (Rivella et al., 2000). Individual transduced clones were isolated in the absence of drug selection and screened to identify those harboring single vector copies. A control vector containing a neutral insert was also included. In the studies with MEL cells, inclusion of the cHS4 element increased the frequency of individual provirus that were expressed over threefold, from 21% to 74%. In contrast, no such improvement in the likelihood of vector expression was seen in the studies with embryonic stem cells. It is unclear whether this was due to a failure of the cHS4 element to prevent the encroachment of heterochromatin in this setting, or to viral sequences contained within the insulator bracket serving as targets of active silencing as suggested by the results of others (Challita et al., 1995).
FIG. 3.
General schema for flanking retroviral vectors with insulators. Insulators are best able to improve vector transgene expression and safety by using a flanking arrangement as shown. This is accomplished using a “double-copy“ arrangement, in which the insulator is first inserted into the proximal end of the 3′ long-terminal repeat (LTR), from which it is copied into the 5′ LTR during formation of the vector provirus (Hantzopoulos et al., 1989; Emery et al., 2000). Note that this arrangement still fails to fully bracket the enhancer and promoter in the 3′ LTR.
The second report was based on a vector derived from the silencing-resistant Murine Stem Cell Virus (MSCV) and transduction studies in mouse bone marrow cells (Emery et al., 2000). In this case, the vector contained two reporter expression cassettes: an enhanced green fluorescent protein (GFP) gene transcribed from the 5′ LTR and a neomycin phosphotransferase (Neo) gene transcribed from an internal Pgk promoter. This vector was also flanked with the 1.2-kb cHS4 fragment (in either orientation), and the frequency and level of vector expression was assessed using a combination of ex vivo bone marrow progenitor cultures and in vivo bone marrow transplantation. Flanking the vector with the cHS4 element in the same orientation as was used in the original study, such that the DNase hypersensitive site core was located at the far 5′ end, significantly increased the fraction of bone marrow cells expressing each of the reporter genes. From the in vivo studies, this included an approximately sevenfold increase in both the fraction of vector-containing white blood cells expressing LTR-GFP (from 4% to 29%) and vector-containing bone marrow progenitors expressing Pgk-Neo (11% to 73%). Somewhat surprisingly, when the orientation of the cHS4 element was reversed, there was no appreciable protection of the LTR-GFP cassette against silencing position effects compared to the uninsulated control, suggesting initially a possible orientation dependency. However, later studies showed instead that the cHS4 element was effective in both orientations (Yannaki et al., 2002), and that this apparent lack of activity more likely arose from a steric incompatibility when the cHS4 core was located too close to the LTR enhancer (Aker et al., 2007).
Subsequent studies with cHS4
Since these two initial reports, there have been many other reports of studies using retroviral vectors flanked with all or part of the 1.2-kb cHS4 element, including gammaretroviral vectors (e.g., Emery et al., 2002; Yannaki et al., 2002; Yao et al., 2003; Ramezani et al., 2006; Nishino et al., 2006; Aker et al., 2007; Li and Emery, 2008; Li et al., 2009) and lentiviral vectors (e.g., Ramezani et al., 2003; Ma et al., 2003; Puthenveetil et al., 2004; Jakobsson et al., 2004; Hino et al., 2004; Bank et al., 2005; Chang et al., 2005; Pluta et al., 2005; Evans-Galea et al., 2007; Aker et al., 2007; Arumugam et al., 2007; Vieyra and Goodell, 2007; Arumugan et al., 2009b; Urbinati et al., 2009; Hanawa et al., 2009; Zhou et al., 2010). Some of these studies focused on defining the optimal topology and size, or mechanisms of action, for this prototypic insulator (Yannaki et al., 2002; Yao et al., 2003; Ramezani et al., 2006; Aker et al., 2007; Li and Emery, 2008; Arumugan et al., 2009b; Urbinati et al., 2009; Hanawa et al., 2009). However, many of these studies simply included the cHS4 element to improve vector transgene expression for specific therapeutic applications. A number of these involved vectors for either β-globin or γ-globin designed to treat the β-chain hemoglobinopathies (Emery et al., 2002; Nishino et al., 2006; Puthenveetil et al., 2004; Bank et al., 2005; Aker et al., 2007; Arumugam et al., 2007). Some other examples include the use of cHS4 to assure expression of a CD20 transgene as part of a “suicide” gene therapy strategy for allo-reactive T cells (Van Meerten et al., 2006), to improve expression of siRNA transgenes as part of an anti-HIV gene therapy strategy (Chang et al., 2005), to reduce the inappropriate activation of a tet-inducible promoter (Pluta et al., 2005; Vieyra and Goodell, 2007), and to improve the expression, as well as safety, of a vector to treat SCID-X1 (Zhou et al., 2010). Although some of these studies were not designed to assess the effects of the cHS4 insulator directly, those that were consistently demonstrated that inclusion of the 1.2-kb version of this element increased the likelihood and/or consistency of vector transgene expression (e.g., Emery et al., 2002; Yannaki et al., 2002; Ma et al., 2003; Ramezani et al., 2003; Hino et al., 2004; Jakobsson et al., 2004; Pluta et al., 2005; Aker et al., 2007; Arumugam et al., 2007, 2009b; Evans-Galea et al., 2007; Li and Emery, 2008).
Mechanisms of cHS4 barrier insulation in retroviral vectors
There is a growing body of knowledge about the mechanisms underlying the barrier insulating activity of the cHS4 element (Gaszner and Felsenfeld, 2006). Notably, at the native locus and in a plasmid-based transgene system, this activity has been shown to be associated with a decrease in DNA methylation and a very strong peak of histone modifications indicative of open chromatin, including H3K9/K14 hyperacetylation, that decreases precipitously over a 1- to 2-kb window around the insulator core (Litt et al., 2001; Mutskov et al., 2002). Early studies demonstrated that inclusion of the cHS4 insulator in a MoMLV-based vector dramatically reduced the level of DNA methylation of the vector 5′ LTR in MEL cells, and that this reduction occurred regardless of whether or not there was active transcription arising from that LTR (Rivella et al., 2000). More recent studies confirmed and expanded these studies using an MSCV-based vector in mice transplanted with transduced marrow, showing that this decrease in methylation included most of the vector provirus (Li and Emery, 2008). In addition, these studies demonstrated that inclusion of the cHS4 insulator in a double-copy arrangement resulted in elevated levels of H3K9/K14 acetylation throughout the vector provirus, with notable peaks located over the cHS4 cores that diminished over approximately the same distance seen in the native locus. It was hypothesized that this topology may have implications for the design of retroviral vectors, in that it implies that the promoters located within the body of the vector may only benefit from the barrier insulator activity at the flanking locations, whereas promoters located near the cHS4 element may also benefit directly from locally elevated histone acetylation levels. In addition, the barrier activity of the extended 400-bp cHS4 core was also found to be dependent on the site-specific binding of the chromatin-modifying enzyme PARP-1 (Aker et al., 2010), although the mechanism by which PARP-1 contributes to barrier activity has yet to be determined.
Studies with alternative barrier insulators
Relatively little has been reported to date on the use of chromatin insulators other than cHS4 for reducing silencing chromosomal position effects directly in the context of retroviral vectors. The most promising involves an insulator element derived from the sea urchin arylsulfatase gene locus termed ArsI. Using the flanking double-copy arrangement, this element has been shown to reduce the rate of transgene silencing for both gammaretroviral and lentiviral vectors in the setting of cell lines, at levels similar to that seen with the cHS4 insulator (Hino et al., 2004; Tajima et al., 2006). As with the cHS4 element, this activity has been associated with increased histone acetylation. This element is about half the size of the full-length cHS4 element and has not been associated with enhancer-blocking insulator activity. Similar results have been obtained using an even smaller 462-bp element from the sea urchin H2A early histone gene (Sns) termed sns5 (D'Apolito et al., 2009). In this case, inclusion of the element in the double-copy position rendered a gammaretroviral reporter vector even more resistant than the cHS4 insulator to silencing position effects in an erythroid cell line model. Once again, this activity was shown to be associated with histone hyperacetylation. A portion of this element has also been shown to exhibit enhancer-blocking activity (Di Simone et al., 2001), making it a potentially ideal candidate for improving both the expression and safety of retroviral vectors. Preliminary studies with the Drosophila gypsy element, another well-described element with both enhancer-blocking and barrier insulator activity, failed to show any effects on gammaretroviral vector expression in a mouse bone marrow progenitor colony assay (Emery et al., 1999) or in a transgenic mouse model (Yao et al., 2003), demonstrating the potential of species specificity for some chromatin insulators. Finally, recent studies demonstrate that an element from the Ankyrin-1 gene promoter exhibits barrier activity with many of the same characteristics of the cHS4 insulator, although this element has not yet been tested directly in retroviral vector assays (Gallagher et al., 2010).
Manifestations and failures of cHS4 barrier insulation
Although a preponderance of the evidence indicates that cHS4 and other insulators can be helpful in reducing the impact of chromosomal position effects on integrating vector expression, both the extent and consequences of this protection are variable. As reviewed elsewhere (Yao et al., 2004; Ellis, 2005; Ramunas et al., 2007), retroviral vector silencing can manifest as both overt silencing and expression variegation. In addition, expression patterns can change over time as a result of memory and extinction mechanisms that act during differentiation (Yao et al., 2004). Various studies indicate that flanking vectors with the cHS4 insulator can help reduce all of these types of silencing. First and foremost, both the initial studies (Emery et al., 2000; Rivella et al., 2000) and numerous subsequent studies (e.g., Emery et al., 2002; Yannaki et al., 2002; Hino et al., 2004; Jakobsson et al., 2004; Aker et al., 2007; Arumugam et al., 2007), demonstrated the ability of this element to increase the frequency of vector proviruses that are expressed at both the interclonal and intraclonal levels. These included studies in a variety of hematopoietic and neural cell lineages and with both in vitro and in vivo reconstitution models that involve a high degree of differentiation. In addition, there are several reports in which the insulating capacity of the cHS4 element predominantly manifested as a decrease in the variability of vector transgene expression (e.g., Ma et al., 2003; Ramezani et al., 2003; Pluta et al., 2005; Aker et al., 2007; Arumugam et al., 2007, 2009b; Evans-Galea et al., 2007; Hanawa et al., 2009). In most cases, this manifestation was most obvious when comparing variations in the levels of vector expression, as determined by flow cytometry, for individual cell clones. Finally, under certain circumstances, flanking with the cHS4 insulator resulted in an apparent increase in the level of vector transgene expression (e.g., Emery et al., 2000, 2002; Yannaki et al., 2002; Ramezani et al., 2003; Aker et al., 2007). Because the cHS4 element has been consistently shown not to convey conventional enhancer activity, apparent increases in transgene expression likely reflect a lack of variegation silencing.
Because expression of integrating gene transfer vectors can be influenced by many factors (regulatory elements, expression cassette design, presence of viral/prokaryotic sequences, etc.), it is not surprising that inclusion of the most widely used chromatin insulator, cHS4, does not necessarily assure consistent high-level expression. For example, initial studies found that flanking a gammaretroviral reporter vector with this element only prevented silencing of an LTR-GFP cassette an estimated 29% of the time and an internal Pgk-Neo cassette an estimated 73% of the time in a mouse bone marrow transduction and transplantation model (Emery et al., 2000). As such, there appears to be an outstanding need for more efficient barrier insulators or other elements to combat vector silencing in the setting of retroviral vectors. Initial attempts based on a functional screen proved only marginally productive in this regard (Groth and Emery, 2010), leaving the door open for alternative approaches.
Insulators as one means of overcoming vector silencing
Means other than chromatin insulators exist for improving retroviral vector expression in transduced cells. For example, it is possible to compensate for modest levels of vector silencing by simply increasing the number of vector provirus per cell (e.g., Persons et al., 2003; Nishino et al., 2006). Indeed, this is probably one reason why the susceptibility of lentiviral vectors to silencing position effects was difficult to recognize (Ellis, 2005). Compensation for modest levels of vector silencing can also be achieved through the use of vector-based selection strategies such as drug selection (Emery et al., 2000) or selective expansion (Emery et al., 2005), or in cases where disease pathology provides a means of selection, such as X-SCID (Hacein-Bey-Abina et al., 2002) or β-thalassemia (Nishino et al., 2006; Cavazzana-Calvo et al., 2010). Vector silencing due to chromosomal position effects can also be overcome in part through the judicious use of enhancers and promoters (Ellis, 2005), as well as other regulatory elements that modulate chromatin structure, such as locus control regions (May et al., 2000), scaffold attachment regions (Ramezani et al., 2003), or autonomous chromatin opening elements (Zhang et al., 2007). Nevertheless, the overwhelming evidence indicates that chromatin insulators can provide one means of reducing silencing chromosomal position effects on retroviral vector expression, a means which can be broadly applied to essentially all vector designs.
Use of Insulators to Reduce Vector-Mediated Genotoxicity
Rationale for using insulators to reduce vector-mediated genotoxicity
There are two main reasons to hypothesize that chromatin insulators should be helpful for reducing retroviral vector-mediated genotoxicity. At the simplest level, the ability of barrier insulators to help reduce the rate of vector silencing can translate into the need for a lower vector “dose” necessary to achieve therapeutic efficiency (Emery et al., 2002). Although a direct relationship between vector dose and vector-mediated genotoxicity was not evident in clinical studies (Hacein-Bey-Abina et al., 2003; Ott et al., 2006), several preclinical models have clearly demonstrated a dose-dependent component of vector-mediated genotoxicity (Modlich et al., 2006; Montini et al., 2006; Li et al., 2009). However, because many documented examples of vector-mediated genotoxicity involve cellular gene activation (e.g., Modlich et al., 2006; Nienhuis et al., 2006; Hargrove 2008; Li et al., 2009), it is likely that the enhancer-blocking capacity of chromatin insulators such as cHS4 hold the most promise for abrogating this genotoxicity. Further, the deep mechanistic understanding of this particular insulator property can help inform the rational application of enhancer-blocking insulators in retroviral and other forms of integrating gene transfer vectors.
There are also reasons to suspect that chromatin insulators may prove only partially effective at reducing vector-mediated genotoxicity. For example, as recently reviewed (Baum et al., 2006a), vector-mediated transformation can also occur through mechanisms other than simple enhancer activation, such as transcriptional read-through or alternative splicing that involve vector sequences. There are also examples of vector-mediated transformation associated with the heterozygous knockout of tumor suppressor or key regulatory genes. In these cases, the inclusion of chromatin insulators would likely have little effect. There are also scenarios where inclusion of enhancer-blocking insulators could be imagined to effectively increase vector-mediated genotoxicity, either by blocking cellular enhancer–promoter interactions directly or by disrupting native insulator networks, as seen in Drosophila (Savitskaya et al., 2006). The ability of insulators such as cHS4 to functionally open chromatin could also increase the rate of cellular gene activation, although the distance over which this opening occurs is relatively limited (Litt et al., 2001; Li and Emery, 2008). Given these potential caveats, it became necessary to directly assess the ability of chromatin insulators to reduce the rate of vector-mediated genotoxicity.
Blocking dysregulation of cellular genes
In order for chromatin insulators to reduce vector-mediated genotoxicity, it is critical that they block the activation of cellular genes by vector sequences. This property has been demonstrated by two general approaches. In the first approach, recombination-mediated cassette exchange was used to artificially generate a setting in which vector sequences were located near specific cellular genes. Insulator elements were then inserted between these sequences, and the effects of the insulators on the expression of these specific cellular genes were assessed. This general approach was used to demonstrate that the cHS4 insulator can substantially reduce, but not eliminate, the LTR-mediated activation of LMO-2 in a setting designed to mimic the cases of vector-mediated oncogenesis seen in a clinical trial (Ryu et al., 2008; Hacein-Bey-Abina et al., 2003). These general findings were subsequently confirmed using other genomic locations and insulator elements (Desprat and Bouhassira, 2009). The second general approach focused on a candidate-independent, genome-wide assessment of vector integration sites. In one early study, this entailed the use of an indirect promoter-trap assay to demonstrate that cHS4 could reduce the activation of cellular genes by a vector-derived enhancer (Ryu et al., 2007). A more recent study involved the direct, genome-wide assessment of cellular gene dysregulation using panels of transduced cell clones and expression microarrays (Li et al., 2009). In these studies, flanking a gammaretroviral vector with the cHS4 insulator reduced the frequency of dysregulated cellular genes roughly sixfold.
Blocking malignant transformation
The most direct evidence that insulators can reduce the rate of retroviral vector-mediated genotoxicity comes from a series of functional studies. In the first of these, it was shown that flanking a lentiviral reporter vector with the 1.2-kb version of the cHS4 chromatin insulator reduced the emergence of dominant clones in a culture of transduced Jurkat cells (Evans-Galea et al., 2007). Subsequent studies showed that a similar protection was afforded by different versions of the cHS4 insulator or a composite cHS4/BEAD-1 insulator in a robust bone marrow transformation/replating assay (Modlich et al., 2006). This included studies with both gammaretroviral and lentiviral reporter vectors (Ramezani et al., 2008; Zychlinski et al., 2008), as well as a lentiviral vector for human β-globin (Arumugam et al., 2009b). In general these studies showed a consistent threefold or greater reduction in the transformation potential of these vectors.
In another approach, the ability of the cHS4 insulator to reduce vector-mediated malignant transformation was directly assessed using a reproducible and quantitative model based on the IL-3–dependent cell line 32D (Li et al., 2009). Based on in vitro colony formation, flanking a gammaretroviral reporter vector with the 1.2-kb version of the cHS4 insulator reduced the frequency of vector-mediated transformation of 32D cells to IL-3 independence by eightfold. Similar results were also obtained using an in vivo tumor-formation assay, in which inclusion of the insulator reduced the frequency of 32D cell tumor formation in mice sixfold. Integration site analysis revealed the presence of genes involved in cell cycle regulation, apoptosis, cytokine signaling, or oncogenic transformation within a ± 150-kb window around vector provirus in almost all transformed clones analyzed, including many examples of oncogenes present in the Retroviral Tagged Cancer Gene Database (Akagi et al., 2004). Taken together, all of these studies provide direct evidence that the cHS4 chromatin insulator can be used to reduce retroviral vector-mediated genotoxicity, including malignant transformation.
Preliminary studies with a wild-type gammaretrovirus confirm this property. In these studies, flanking a replication-competent murine leukemia virus with multiple copies of a subfragment from cHS4 reduced the rate of viral-mediated activation of oncogenes and T-cell lymphogenesis (Mitra et al., 2007). However, this protection was only partial and required multiple copies of the cHS4 subfragment that tended to delete during viral propagation. A full report of these studies should help to clarify these issues.
Insulators as one means of overcoming vector-mediated genotoxicity
There are clearly other means of reducing vector-mediated genotoxicity besides the use of chromatin insulators (as reviewed elsewhere [Baum et al., 2006a; Nienhuis et al., 2006]). This includes in part the use of a SIN vector design that deletes strong viral enhancers (Modlich et al., 2006), the use of alternative viral vectors that provide a more favorable pattern of integration (Montini et al., 2006), the improvement of polyadenylation signals to reduce transcription read-through (Higashimoto et al., 2007; Schambach et al., 2007), and the use of tissue-specific enhancers (Chang and Sadelain, 2007; Zychlinski et al., 2008; Arumugam et al., 2009a). Of these, the SIN vector design has been proven most effective at reducing vector-mediated genotoxicity (Modlich et al., 2006). This presumably reflects a decrease in the overall transcriptional activity of the vector, although the SIN design also deletes a potential PolIII transcript thought to play a role in tissue tropisms and pathogenic potential of at least some retroviruses (Abujamra et al., 2006). However, it is important to note that only chromatin insulators provide an option for reducing vector-mediated genotoxicity that can be broadly applied to essentially all vector designs.
Other Uses of Chromatin Insulators in Gene Therapy Applications
Retroviral vectors
Chromatin insulators have also been utilized in retroviral vectors for purposes other than reducing silencing chromosomal position effects or vector-mediated genotoxicity. For example, deploying the cHS4 insulator in a double-copy arrangement has also been shown to help reduce activating chromosomal position effects (Pluta et al., 2005; Vieyra and Goodell, 2007). This activity was reported in the context of a lentiviral vector containing a tet-inducible promoter and presumably reflects the ability of cHS4 element to block the activating effects of cellular enhancers located near integrated provirus. Although such activating chromosomal position effects are relatively rare, they can undermine therapeutic strategies where success is dependent on keeping a vector transgene transcriptionally silent within specific lineages or until activated pharmacologically.
Chromatin insulators have also been investigated as a means of blocking genetic interactions within retroviral vectors. Although this represents an application that is distinct from the use of insulators to prevent the cross-talk between vector and genomic sequences, it nevertheless relies on the same insulator functions. In some cases, this has involved assessing whether repressor-blocking insulators could be used to prevent the silencing effects of viral sequences located in the LTR and primer-binding site from shutting off expression of transgenes contained within the body of the vector (Modin et al., 2000; Yao et al., 2003). In one such study, where the cHS4 element or other insulators were used as a single copy immediately 3′ of the primer-binding site, no decrease in vector transgene silencing was observed (Modin et al., 2000). In contrast, a significant degree of blocking was observed when tandem copies of the cHS4 element or other insulators were used to flank the internal expression cassette (Yao et al., 2003). However, this arrangement could only be assessed in the form of plasmid-based transgenes, since tandem copies and internal flanking arrangements recombine frequently during viral replication (Rhode et al., 1987; Pathak and Temin, 1990). Taken together, these studies serve to reiterate the importance of deploying insulators in a flanking arrangement, as well as the hazards associated with duplicated sequences in retroviral vectors. More favorable results were reported when the cHS4 insulator was used as a single copy to block the transcriptional interference of one internal expression cassette on a second cassette within a lentiviral vector (Osti et al., 2006). Favorable results were also obtained when either the Sns or cHS4 insulators were used as a single copy to block the activation of internal expression cassettes by internal enhancers in either gammaretroviral or lentiviral vectors, respectively (Di Simone et al., 2001; Robert-Richard et al., 2007). These studies demonstrate that insulators can help reduce regulatory cross-talk within vectors, that they can be incorporated within the body of retroviral vectors, and that enhancer-blocking can be achieved without the use of flanking elements.
Other viral vectors
The use of chromatin insulators in viral vectors other than those based on retroviruses has been limited. In the setting of adenoviral vectors, the cHS4 element has proven effective at preventing the inappropriate activation of regulated or tissue-specific internal expression cassettes by viral or other vector enhancers when used as a single copy (Steinwaerder and Lieber, 2000) or in a flanking arrangement (Martin-Doque et al., 2004). The cHS4 element has also proven effective in adenoviral vectors at reducing vector transgene silencing, either by protecting against viral silencer elements or by disrupting transcriptional interference within the vector (Ye et al., 2003). The cHS4 element has also proven effective at improving the persistence of expression from helper-free vectors based on Herpes Simplex Virus in a variety of neural cell settings (e.g., Gao et al., 2007). Finally, a recent study indicates that the cHS4 insulator can prevent the activation of a flanking promoter by a vector based on the Sleeping Beauty transposon (Walisko et al., 2008), suggesting that insulators may also improve the safety profile of this and other emerging vector systems.
Effects of Insulators on Vector Titers
One of the more controversial issues surrounding the use of chromatin insulators, and in particular the cHS4 insulator, involves their impact on vector titers. This issue is complicated by several factors. First, most of the original studies involving the use of insulators in gammaretroviral or lentiviral vectors failed to comment on the effects of these elements on vector titer. Second, only a fraction of these original studies that did report on vector titers included neutral insert controls in order to account for the effects of the insulator on the overall size and complexity of the vector (Modin et al., 2000; Aker et al., 2007). Such a control is especially important for vectors that are close to exceeding the optimal packaging size capacity. Third, some studies relied on the use of duplicated copies of the insulator elements, an arrangement that is well documented to result in recombinations and deletions that can also adversely affect vector titers (Pathak and Temin, 1990). Finally, many of the studies that found the most profound effects of the cHS4 element on vector titers never made it to publication.
Initial studies with a simple and compact gammaretroviral reporter vector and several forms of the cHS4 insulator demonstrated essentially no effect or improvement on vector titers (Emery et al., 2000; Yannaki et al., 2002; Aker et al., 2007). The same was true when a 400-bp version of the cHS4 element was used to flank a large and complex lentiviral vector for human β-globin (Aker et al., 2007). Flanking a large and complex gammaretroviral vector for human γ-globin with the 1.2-kb cHS4 element decreased vector titers about threefold (Emery et al., 2002), presumably by extending the overall size of the vector beyond the optimal packaging size. In the context of gammaretroviral vectors, most groups have reported little to no effect of cHS4 on titers (Modin et al., 2000; Ramezani et al., 2006), with one exception in which a two- to sixfold reduction was observed (Van Meerten et al., 2006). For lentiviral vectors, other groups have also reported either no effect (Osti et al., 2006) or two- to threefold reductions in vector titers by addition of a 1.2-kb cHS4 element (Ramezani et al., 2003; Hino et al., 2004; Robert-Richard et al., 2007). In one case, inclusion of the 1.2-kb cHS4 element in a double-copy arrangement apparently decreased lentiviral vector titers a significant 10-fold (Jakobsson et al., 2004). This observation is similar to several unpublished findings of others and serves to illustrate the need for caution when assessing the efficacy of this particular element in gene transfer vectors. Studies involving the use of the chromatin insulators Sns and BEAD-1 in gammaretroviral vectors indicated little to no adverse effects on vector titers that could be specifically attributed to these elements (D'Apolito et al., 2009; Modin et al., 2000). Similarly, no adverse effects on lentiviral vector titers were seen with the ArsI chromatin insulator (Hino et al., 2004).
Several recent studies may shed light on this controversy, in that they demonstrate the insertion of any element into the double-copy position of the LTR can reduce the titers of lentiviral vectors, with an apparent correlation between the size of the insert and the reduction in titer (Hanawa et al., 2009; Urbinati et al., 2009; Bos et al., 2010). It appears that the reduced titers arise from inefficient processing of the viral genome following target-cell entry, with reduced reverse transcription and integration efficiency. Taken together, these studies indicate that the reduced titers reported in some studies may not necessarily be unique to the use of chromatin insulators, but rather reflect a basic property of vector biology. However, they also point to the need for identifying the minimal sequences necessary to achieve the desired barrier and/or enhancer-blocking insulator function.
Optimal Vector Design
Many elements can impact vector expression and safety
Although a preponderance of the evidence indicates that chromatin insulators can help improve the expression and safety profiles of vectors, they constitute one of many tools for addressing these issues. As expertly reviewed elsewhere (Nienhuis et al., 2006; Baum et al., 2006b), there are several elements of vector design that can influence vector expression and safety. As highlighted graphically in Fig. 4, these include (1) the virus of origin; (2) the removal of key regulatory elements from the viral LTR using a SIN design; (3) the use of less potent or tissue-specific internal enhancers and promoters; (4) the use of strong polyadenylation signals; (5) the removal of splice donors and acceptors; (6) the choice of therapeutic genes; (7) the use of chromatin insulators in a double-copy arrangement; and (8) the choice of chromatin insulator.
FIG. 4.
Optimal vector design. Several elements of design can influence the expression and safety of retroviral vectors. Examples include (1) the virus of origin; (2) the use of self-inactivating (SIN) LTRs; (3) the use of tissue-specific internal enhancers and promoters; (4) the use of strong polyadenylation signals; (5) the removal of splice donors and acceptors; (6) the choice of therapeutic genes; (7) the use of chromatin insulators in a double-copy arrangement; and (8) the choice of chromatin insulator element.
Viral vector elements
It has been suggested that the differences in integration site preferences seen between vectors of different viral origins may influence both vector expression and vector safety. However, any advantages in this regard are likely to be modest (Nienhuis et al., 2006; Chang and Sadelain, 2007). Although one study found that the degree of genotoxicity associated with a SIN lentiviral vector was less than that associated with a non-SIN gammaretroviral vector (Montini et al., 2006), more recent studies suggest that this difference was likely due to the differences in viral LTRs rather than to integration site preferences (Montini et al., 2009). Indeed, as argued elsewhere (Baum et al., 2006b; Chang and Sadelain, 2007), the era of viral vectors that rely on the LTR for transgene expression is likely coming to an end. This has been made possible in large part by the availability of later generation SIN lentiviral vectors that are high titer and genetically stable. However, it is important to appreciate that SIN vector designs can also be applied in the setting of gammaretroviral vectors (Modlich et al., 2006; Ramezani et al., 2006), and that it is gammaretroviral vectors that have a long clinical track record (Kohn et al., 2003).
The strength of the polyadenylation signal and the presence of splice donor and acceptor sequences (both of viral origin in most vector designs) can also affect vector expression and safety. Although the splice elements can interfere with the proper processing of cellular genes in which provirus have integrated, it is the polyadenylation signals that have been the focus of most attention. This is because improvements in vector transcript termination can not only improve the level of vector expression in the context of gammaretroviral and lentiviral vectors (Higashimoto et al., 2007; Schambach et al., 2007), but also reduce the rate of vector read-through into the surrounding cellular genome, reducing extension into cellular genes (Zaiss et al., 2002).
Expression cassette elements
Although SIN vector designs offer several advantages, their use nevertheless requires the addition of alternative promoters and enhancers within the body of the vector in order to achieve high-level vector expression. These elements, and in particular the enhancers, can still provide a means for activating cellular genes surrounding sites of vector integration (Hargrove et al., 2008). However, as first suggested by others (Chang and Sadelain, 2007), it has recently been shown empirically that this risk can be reduced through the use of cellular or, better yet, tissue-specific regulatory elements (Zychlinski et al., 2008; Arumugam et al., 2009a). As diagrammed in Fig. 4, expression cassettes need not be inserted in the same orientation as vector transcription. In fact, inserting the vector transgene in the reverse orientation allows that transgene to contain introns that may be necessary for high-level expression (e.g., Emery et al., 1998). However, even in this case, it is necessary to assure that the polyadenylation signal is sufficiently robust to prevent transcriptional read-through into the surrounding genome. Several aspects of the vector transgene product can also affect vector expression and safety. Most of these issues have been discussed at length elsewhere (e.g., Makrides, 2003). Indeed, much work has focused on characterizing the role of specific transgenes in vector-mediated transformation events (Baum et al., 2006a; Nienhuis et al., 2006; Shou et al., 2006; Zhou et al., 2010).
Optimal deployment of chromatin insulators
As diagrammed in Fig. 4, chromatin insulators such as cHS4 are best used in a flanking double-copy arrangement. This approach allows the insulator to be duplicated after the stage of the viral life cycle when recombination between duplicated sequences occurs (Pathak and Temin, 1990), and has proven effective in vectors based on a variety of viruses and LTR designs (e.g., Aker et al., 2007; Arumugam et al., 2009b). This flanking arrangement not only protects the vector transgenes against the encroachment of silencing heterochromatin from both sides of the integrated provirus, but also serves to protect cellular genes on both sides of the integrated vector provirus from vector sequences. In addition, current theories suggest that enhancer-blocking insulators work through the formation of closed loops (Gaszner and Felsenfeld, 2006; Phillips and Corces, 2009; Raab and Kamakaka, 2010), which in turn implies that these elements are likely to function, at least most productively, when used in pairs.
Despite its advantages, it is important to note that the double-copy flanking arrangement places most of the 3′ LTR outside of the insulator “bracket,” rendering it free to interact with genomic sequences (Fig. 4). Based on this topology, it might be expected that, in the context of a non-SIN vector, the deployment of an insulator in this setting would only decrease vector genotoxicity by half. However, one recent study demonstrated that deploying the cHS4 insulator in a gammaretroviral reporter vector with intact LTRs using a double-copy arrangement resulted in a six- to eightfold reduction in genotoxicity (Li et al., 2009). Others have shown that the degree of vector-mediated genotoxicity is related to the overall transcriptional activity of a vector, rather than the number or nature of vector-specific enhancers (Montini et al., 2006), and that reducing the number of LTR enhancers in a gammaretroviral vector from two to one using a SIN design can reduce vector-mediated genotoxicity well in excess of twofold (Modlich et al., 2006). These results suggest that flanking with the cHS4 element reduces the rate of vector-mediated genotoxicity more than would be predicted based solely on topological considerations, effectively reducing the overall transcriptional activity of the insulated vector in a manner similar to that achieved using a SIN vector design.
Optimal version of the cHS4 insulator
Although the 1.2-kb version of the cHS4 insulators has consistently proven effective at reducing vector transgene silencing, the size of this fragment can push some vectors beyond the limits of efficient packaging, and the excess sequences can interfere with vector titer or stability. Early studies with plasmid-based expression systems indicated that much of the cHS4 insulator activity could be mapped to a smaller 250-bp core, and that two copies of this core were as effective as the full-length element (Chung et al., 1997; Recillas-Targa et al., 2002). Based on these findings, several groups turned instead to this smaller core fragment for the insulation of retroviral vectors (Yao et al., 2003; Bank et al., 2005; Chang et al., 2005; Ramezani et al., 2006; Van Meerten et al., 2006; Aker et al., 2007). However, recent studies indicate this may be a poor choice. For example, careful structural analysis revealed that tandem copies of the 250-bp cHS4 fragment (or other subfragments of the cHS4 insulator), recombined at a very high frequency (Yao et al., 2003; Ramezani et al., 2006; Aker et al., 2007; Mitra et al., 2007; Cavazzana-Calvo et al., 2010). At best, this results in deletion to a single copy (Ramezani et al., 2006; Cavazzana-Calvo et al., 2010), and at worst, this can interfere with the transfer of intact provirus (Yao et al., 2003). This is not a unique property of the cHS4 element, but a basic property of retroviral vectors (Rhode et al., 1987; Pathak and Temin, 1990; Emery et al., 1998), that severely limits the inclusion of more than one copy of an insulator element except by the double-copy arrangement. Further, in the subset of studies in which the activity of this 250-bp cHS4 element was directly assessed, it was found to provide either less protection than the full-length fragment (Ramezani et al., 2006; Van Meetten et al., 2006; Arumugan et al., 2009b; Hanawa et al., 2009), or under some circumstances, no protection at all (Aker et al., 2007).
In contrast to the disappointing results with the 250-bp cHS4 core, one recent study found that a slightly larger fragment, one that includes an additional 150 bp adjacent to this minimal core, conveys the same high degree of barrier insulating activity associated with the full length 1.2-kb cHS4 fragment (Aker et al., 2007). The activity of this 400-bp cHS4 fragment was confirmed in the setting of a gammaretroviral reporter vector expressed in primary mouse bone marrow progenitor colonies, and in the setting of a lentiviral vector expressed in erythroid MEL cell clones derived in the absence of selection. The latter results are especially impressive because this vector contained extended sequences from the β-globin locus control region that confer a high degree of position-independent expression. Subsequent biochemical analysis revealed that this extended core sequence is bound by the chromatin-remodeling enzyme poly(ADP-ribose) polymerase-1 (PARP-1), and that binding of PARP-1 is essential for the barrier activity of this extended core (Aker et al., 2010). Other recent studies also point to sequences at the far 3′ end of the full-length 1.2-kb cHS4 fragment that apparently also contributes to the barrier activity of this insulator (Arumugam et al., 2009b).
A question remains as to whether truncated versions of the cHS4 element can also provide the same degree of protection against vector-mediated genotoxicity afforded by the full-length 1.2-kb version. Initial studies indicated that a single copy of the smaller 250-bp cHS4 core does not provide the same degree of enhancer-blocking activity as the full-length 1.2-kb version in plasmid-based assays (Bell et al., 1999). Studies in a cellular gene activation assay indicated that two copies of the 250-bp cHS4 core element functioned as well as the 1.2-kb cHS4 fragment at preventing viral LTR-mediated activation (Ryu et al., 2008), although the two copies are not stable in gammaretroviral or lentiviral vectors as discussed above. In one study, the 400-bp version of the cHS4 element did reduce, but not eliminate, the vector-mediated activation of a cellular gene (Zhou et al., 2010). Future studies are needed to directly address this question in functional genotoxicity assays.
Summary
Research over the past decade has demonstrated the ability of chromatin insulators to improve both the expression and the safety of integrating vectors based on recombinant gammaretroviruses and lentiviruses, and to a lesser extent other vector systems. These studies have also uncovered key topological considerations for how to best deploy chromatin insulators for this purpose, leading to improved vector designs. Advances have been made towards defining the optimal form of the most widely used insulator cHS4, as well as towards identifying other candidate insulators. Of all the elements of vector design identified to date, chromatin insulators constitute the most versatile means of improving vector performance and safety. They have been successfully applied to both non-SIN and SIN vectors, as well to vectors with transgenes transcribed from the viral LTR, internal ubiquitously active promoters, and internal tissue-specific promoters. They do not require significant modifications to existing vector designs, and are likely to complement virtually all other means of improving vector expression and safety. As such, chromatin insulators should be considered an important tool for improving the performance and safety of viral vectors.
Acknowledgments
The author wishes to thank the following individuals for helpful comments and careful reading of the manuscripts: Amy Groth, Anton A. Krumm, C. Anthony Blau, and George Stamatoyannopoulos. Preparation of this review was supported in part by NIH grants HL53750 and HL75713.
References
- Abujamra A.L. Spanjaard R.A. Akinsheye I., et al. Leukemia virus long terminal repeat activates NFkappaB pathway by a TLR3-dependent mechanism. Virology. 2006;345:390–403. doi: 10.1016/j.virol.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi K. Suzuki T. Stephens R.M., et al. RTCGD: retroviral tagged cancer gene database. Nucleic Acids Res. 2004;32:D523–527. doi: 10.1093/nar/gkh013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker M. Bomsztyk K. Emery D.W. Poly (ADP-ribose) polymerase-1 (PARP-1) contributes to the barrier activity function of a vertebrate chromatin insulator. J. Biol. Chem. 2010;285:37589–37597. doi: 10.1074/jbc.M110.174532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker M. Tubb J. Groth A.C., et al. Extended core sequences from the cHS4 insulator are necessary for protecting retroviral vectors from silencing position effects. Hum. Gene Ther. 2007;18:333–343. doi: 10.1089/hum.2007.021. [DOI] [PubMed] [Google Scholar]
- Aker M. Tubb J. Miller D.G., et al. Integration bias of gammaretrovirus vectors following transduction and growth of primary mouse hematopoietic progenitor cells with and without selection. Mol. Ther. 2006;14:226–235. doi: 10.1016/j.ymthe.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Artelt P. Grannemann R. Stocking C., et al. The prokaryotic neomycin-resistance-encoding gene acts as a transcriptional silencer in eukaryotic cells. Gene. 1991;99:249–254. doi: 10.1016/0378-1119(91)90134-w. [DOI] [PubMed] [Google Scholar]
- Arumugam P.I. Higashimoto T. Urbinati F., et al. Genotoxic potential of lineage-specific lentivirus vectors carrying the beta-globin locus control region. Mol. Ther. 2009b;17:1929–1937. doi: 10.1038/mt.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam P.I. Scholes J. Perelman N., et al. Improved human beta-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator element. Mol. Ther. 2007;15:1863–1871. doi: 10.1038/sj.mt.6300259. [DOI] [PubMed] [Google Scholar]
- Arumugam P.I. Urbinati F. Velu C.S., et al. The 3′ region of the chicken hypersensitive site-4 insulator has properties similar to its core and is required for full insulator activity. PLoS ONE. 2009a;4:e6995. doi: 10.1371/journal.pone.0006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank A. Dorazio R. Leboulch P. A phase I/II clinical trial of beta-globin gene therapy for beta-thalassemia. Ann. N. Y. Acad. Sci. 2005;1054:308–316. doi: 10.1196/annals.1345.007. [DOI] [PubMed] [Google Scholar]
- Baum C. Kustikova O. Modlich U., et al. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum. Gene Ther. 2006a;17:252–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- Baum C. Schambach A. Bohne J. Galla M. Retrovirus vectors: toward the plentivirus? Mol. Ther. 2006b;13:1050–1063. doi: 10.1016/j.ymthe.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Bell A.C. West A.G. Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387–396. doi: 10.1016/s0092-8674(00)81967-4. [DOI] [PubMed] [Google Scholar]
- Berry C. Hannenhalli S. Leipzig J. Bushman F.D. Selection of target sites for mobile DNA elements in the human genome. PLoS Comput. Biol. 2006;2:e157. doi: 10.1371/journal.pcbi.0020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos T.J. de Bruyne E. van Lint S., et al. Large double copy vectors are functional but show size-dependent decline in transduction efficiency. J. Biotechnol. 2010;150:37–40. doi: 10.1016/j.jbiotec.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M. Payen E. Negre O., et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challita P.M. Skelton D. El-Khoueiry A., et al. Multiple modifications in cis elements of the long terminal repeat of retroviral vectors lead to increased expression and decreased DNA methylation in embryonic carcinoma cells. J. Virol. 1995;69:748–755. doi: 10.1128/jvi.69.2.748-755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A.H. Sadelain M. The genetic engineering of hematopoietic stem cells: the rise of lentiviral vectors, the conundrum of the LTR, and the promise of lineage-restricted vectors. Mol. Ther. 2007;15:445–456. doi: 10.1038/sj.mt.6300060. [DOI] [PubMed] [Google Scholar]
- Chang L.J. Liu X. He J. Lentiviral siRNAs targeting multiple highly conserved RNA sequences of human immunodeficiency virus type 1. Gene Ther. 2005;12:1133–1144. doi: 10.1038/sj.gt.3302509. [DOI] [PubMed] [Google Scholar]
- Chung J.H. Bell A.C. Felsenfeld G. Characterization of the chicken beta-globin insulator. Proc. Natl. Acad. Sci. USA. 1997;94:575–580. doi: 10.1073/pnas.94.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J.H. Whiteley M. Felsenfeld G. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effects in Drosophila. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- D'Apolito D. Baiamonte E. Bagliesi M., et al. The sea urchin sns5 insulator protects retroviral vectors from chromosomal position effects by maintaining active chromatin structure. Mol. Ther. 2009;17:1434–1441. doi: 10.1038/mt.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprat R. Bouhassira E.E. Gene specificity of suppression of transgene-mediated insertional transcriptional activation by the chicken HS4 insulator. PLoS. 2009;4:e5956. doi: 10.1371/journal.pone.0005956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson J. Gowher H. Strogantsev R., et al. VEZF1 elements mediate protection from DNA methylation. PLoS Genet. 2010;6:e1000804. doi: 10.1371/journal.pgen.1000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simone P. Di Leonardo A. Costanzo G., et al. The sea urchin sns insulator blocks CMV enhancer following integration in human cells. Biochem. Biophys. Res. Commun. 2001;284:987–992. doi: 10.1006/bbrc.2001.5082. [DOI] [PubMed] [Google Scholar]
- Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum. Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- Emery D.W. Aker M. Stamatoyannopoulos G. Chromatin insulators and position effects. In: Makrides S.C., editor. Gene Transfer and Expression in Mammalian Cells. EIC Laboratories, Inc; Norwood, MA: 2003. pp. 381–395. [Google Scholar]
- Emery D.W. Chen H. Li Q. Stamatoyannopoulos G. Development of a condensed locus control region cassette and testing in retrovirus vectors for A gamma-globin. Blood Cells Mol. Dis. 1998;24:322–339. doi: 10.1006/bcmd.1998.0200. [DOI] [PubMed] [Google Scholar]
- Emery D.W. Tubb J. Nishino Y., et al. Selection with a regulated cell growth switch increases the likelihood of expression for a linked gamma-globin gene. Blood Cells Mol. Dis. 2005;34:235–247. doi: 10.1016/j.bcmd.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Emery D.W. Yannaki E. Bell A., et al. Core elements from Drosophila gypsy and chicken beta-globin HS4 do not insulate retrovirus vectors from position effects. Blood. 1999;94(suppl. 1):177a. [Google Scholar]
- Emery D.W. Yannaki E. Tubb J. Stamatoyannopoulos G. A chromatin insulator protects retrovirus vectors from position effects. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9150–9155. doi: 10.1073/pnas.160159597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery D.W. Yannaki E. Tubb J., et al. Development of virus vectors for gene therapy of β chain hemoglobinopathies: flanking with a chromatin insulator reduces γ-globin gene silencing in vivo. Blood. 2002;100:2012–2021. doi: 10.1182/blood-2002-01-0219. [DOI] [PubMed] [Google Scholar]
- Evans-Galea M.V. Wielgosz M.M. Hanawa H., et al. Suppression of clonal dominance in cultured human lymphoid cells by addition of the cHS4 insulator to a lentiviral vector. Mol. Ther. 2007;15:801–809. doi: 10.1038/sj.mt.6300103. [DOI] [PubMed] [Google Scholar]
- Farrar D. Rai S. Chernukhin I., et al. Mutational analysis of the poly(ADP-ribosyl)ation sites of the transcription factor CTCF provides an insight into the mechanism of its regulation by poly(ADP-ribosyl)ation. Mol. Cell. Biol. 2010;30:1199–1216. doi: 10.1128/MCB.00827-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P.G. Steiner L.A. Liem R.I., et al. Mutation of a barrier insulator in the human ankyrin-1 gene is associated with hereditary spherocytosis. J. Clin. Invest. 2010;120:4453–3365. doi: 10.1172/JCI42240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q. Sun M. Wang X. Geller A.I. Isolation of an enhancer from the rat tyrosine hydroxylase promoter that supports long-term, neuronal-specific expression from a neurofilament promoter, in a helper virus-free HSV-1 vector system. Brain Res. 2007;1130:1–16. doi: 10.1016/j.brainres.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaszner M. Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat. Rev. Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- Groth A.C. Emery D.W. A functional screen for regulatory elements that improve retroviral vector gene expression. Blood Cells Mol. Dis. 2010;45:343–350. doi: 10.1016/j.bcmd.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelen L. Pagie L. Brasset E., et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–951. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Le Deist F. Carlier F., et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N. Engl. J. Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. von Kalle C. Schmidt M., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hanawa H. Yamamoto M. Zhao H., et al. Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus HS4 insulator element. Mol. Ther. 2009;17:667–674. doi: 10.1038/mt.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantzopoulos P.A. Sullenger B.A. Ungers G. Gilboa E. Improved gene expression upon transfer of the adenosine deaminase minigene outside the transcriptional unit of a retroviral vector. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3519–3523. doi: 10.1073/pnas.86.10.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove P.W. Kepes S. Hanawa H., et al. Globin lentiviral vector insertions can perturb the expression of endogenous genes in beta-thalassemic hematopoietic cells. Mol. Ther. 2008;16:525–533. doi: 10.1038/sj.mt.6300394. [DOI] [PubMed] [Google Scholar]
- Higashimoto T. Urbinati F. Perumbeti A., et al. The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther. 2007;14:1298–1304. doi: 10.1038/sj.gt.3302979. [DOI] [PubMed] [Google Scholar]
- Hino S. Fan J. Taguwa S., et al. Sea urchin insulator protects lentiviral vector from silencing by maintaining active chromatin structure. Gene Ther. 2004;11:819–828. doi: 10.1038/sj.gt.3302227. [DOI] [PubMed] [Google Scholar]
- Hou C. Dale R. Dean A. Cell type specificity of chromatin organization mediated by CTCF and cohesin. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3651–3656. doi: 10.1073/pnas.0912087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson J. Rosenqvist N. Thompson L., et al. Dynamics of transgene expression in a neural stem cell line transduced with lentiviral vectors incorporating the cHS4 insulator. Exp. Cell Res. 2004;298:611–623. doi: 10.1016/j.yexcr.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Jin C. Zhang C. Wei G., et al. H3.3/H2A.Z double variant-containing nucleosides are ‘nucleosome-free regions' of active promoters and other regulatory regions. Nat. Genet. 2009;41:941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn D.B. Sadelain M. Dunbar C., et al. American Society of Gene Therapy (ASGT) ad hoc subcommittee on retroviral-mediated gene transfer to hematopoietic stem cells. Mol. Ther. 2003;8:180–187. doi: 10.1016/s1525-0016(03)00212-0. [DOI] [PubMed] [Google Scholar]
- Kraus W.L. Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr. Opin. Cell Biol. 2008;20:294–302. doi: 10.1016/j.ceb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.L. Emery D.W. The cHS4 chromatin insulator reduces gammaretroviral vector silencing by epigenetic modifications of integrated provirus. Gene Ther. 2008;15:49–53. doi: 10.1038/sj.gt.3303009. [DOI] [PubMed] [Google Scholar]
- Li C.L. Xiong D. Stamatoyannopoulos G. Emery D.W. Genomic and functional assays demonstrate reduced gammaretroviral vector genotoxicity associated with use of the cHS4 chromatin insulator. Mol. Ther. 2009;17:716–724. doi: 10.1038/mt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J.Q. Li T. Hu J.F., et al. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science. 2006;312:269–272. doi: 10.1126/science.1123191. [DOI] [PubMed] [Google Scholar]
- Litt M.D. Simpson M. Gaszner M., et al. Correlation between histone lysine methylation and developmental changes at the chicken beta-globin locus. Science. 2001;293:2453–2455. doi: 10.1126/science.1064413. [DOI] [PubMed] [Google Scholar]
- Ma Y. Ramezani A. Lewis R., et al. High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors. Stem Cells. 2003;21:111–117. doi: 10.1634/stemcells.21-1-111. [DOI] [PubMed] [Google Scholar]
- Makrides S.C., editor. Gene Transfer and Expression in Mammalian Cells. EIC Laboratories, Inc; Norwood, MA: 2003. [Google Scholar]
- Martin-Duque P. Jezzard S. Kaftansis L. Vassaux G. Direct comparison of the insulating properties of two genetic elements in an adenoviral vector containing two different expression cassettes. Hum. Gene Ther. 2004;15:995–1002. doi: 10.1089/hum.2004.15.995. [DOI] [PubMed] [Google Scholar]
- May C. Rivella S. Callegari J., et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406:82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- Mitra K. Su M. Lenz J. Multiple tandem insulators may reduce retroviral insertional activation of oncogenes and lymphomagenesis. Mol. Ther. 2007;15(suppl. 1):S28. [Google Scholar]
- Modin C. Pedersen F.S. Duch M. Lack of shielding of primer binding site silencer-mediated repression of an internal promoter in a retrovirus vector by the putative insulators scs, BEAD-1, and HS4. J. Virol. 2000;74:11697–11707. doi: 10.1128/jvi.74.24.11697-11707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlich U. Bohne J. Schmidt M., et al. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108:2545–2553. doi: 10.1182/blood-2005-08-024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok H.P. Javed S. Lever A. Stable gene expression occurs from a minority of integrated HIV-1-based vectors: transcriptional silencing is present in the majority. Gene Ther. 2007;14:741–751. doi: 10.1038/sj.gt.3302923. [DOI] [PubMed] [Google Scholar]
- Montini E. Cesana D. Schmidt M., et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotech. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Montini E. Cesana D. Schmidt M., et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutskov V.J. Farrell C.M. Wade P.A., et al. The barrier function of an insulator couples high histone acetylation levels with specific protection of promoter DNA from methylation. Genes Dev. 2002;16:1540–1554. doi: 10.1101/gad.988502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A.W. Dunbar C.E. Sorrentino B.P. Genotoxicity of retroviral integration in hematopoietic cells. Mol. Ther. 2006;13:1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Nishino T. Tubb J. Emery D.W. Partial correction of murine beta-thalassemia with a gammaretrovirus vector for human γ-globin. Blood Cells Mol. Dis. 2006;37:1–7. doi: 10.1016/j.bcmd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Osti D. Marras E. Ceriani I., et al. Comparative analysis of molecular strategies attenuating positional effects in lentiviral vectors carrying multiple genes. J. Virol. Methods. 2006;136:93–101. doi: 10.1016/j.jviromet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Ott M.G. Schmidt M. Schwarzwaelder K., et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- Pathak V.K. Temin H.M. Broad spectrum of in vivo forward mutations, hypermutations, and mutational hotspots in a retroviral shuttle vector after a single replication cycle: deletions and deletions with insertions. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6024–6028. doi: 10.1073/pnas.87.16.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons D.A. Hargrove P.W. Allay E.R., et al. The degree of phenotypic correction of murine beta-thalassemia intermedia following lentiviral-mediated transfer of a human gamma-globin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003;101:2175–2183. doi: 10.1182/blood-2002-07-2211. [DOI] [PubMed] [Google Scholar]
- Phillips J.E. Corces V.G. CTCF: Master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta K. Luce M.J. Bao L., et al. Tight control of transgene expression by lentivirus vectors containing second-generation tetracycline-responsive promoters. J. Gene Med. 2005;7:803–807. doi: 10.1002/jgm.712. [DOI] [PubMed] [Google Scholar]
- Puthenveetil G. Scholes J. Carbonell D., et al. Successful correction of the human beta-thalassemia major phenotype using a lentiviral vector. Blood. 2004;104:3445–3453. doi: 10.1182/blood-2004-04-1427. [DOI] [PubMed] [Google Scholar]
- Raab J.R. Kamakaka R.T. Insulators and promoters: closer than we think. Nat. Rev. Genet. 2010;11:439–446. doi: 10.1038/nrg2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani A. Hawley T.S. Hawley R.G. Performance- and safety-enhanced lentiviral vectors containing the human interferon-beta scaffold attachment region and the chicken beta-globin insulator. Blood. 2003;101:4717–4724. doi: 10.1182/blood-2002-09-2991. [DOI] [PubMed] [Google Scholar]
- Ramezani A. Hawley T.S. Hawley R.G. Stable gammaretroviral vector expression during embryonic stem cell-derived in vitro hematopoietic development. Mol. Ther. 2006;14:245–254. doi: 10.1016/j.ymthe.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani A. Hawley T.S. Hawley R.G. Combinatorial incorporation of enhancer-blocking components of the chicken beta-globin 5'HS4 and human T-cell receptor alpha/delta BEAD-1 insulators in self-inactivating retroviral vectors reduces their genotoxic potential. Stem Cells. 2008;26:3257–3266. doi: 10.1634/stemcells.2008-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramunas J. Montgomery H.J. Kelly L., et al. Real-time fluorescence tracking of dynamic transgene variegation in stem cells. Mol. Ther. 2007;15:810–817. doi: 10.1038/sj.mt.6300073. [DOI] [PubMed] [Google Scholar]
- Recillas-Targa F. Pikaart M.J. Burgess-Beusse B., et al. Position-effect protection and enhancer blocking by the chicken beta-globin insulator are separable activities. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6883–6888. doi: 10.1073/pnas.102179399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode B.W. Emerman M. Temin H.M. Instability of large direct repeats in retrovirus vectors. J. Virol. 1987;61:925–927. doi: 10.1128/jvi.61.3.925-927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivella S. Callegari J.A. May C., et al. The cHS4 insulator increases the probability of retroviral expression at random chromosomal integration sites. J. Virol. 2000;74:4679–4687. doi: 10.1128/jvi.74.10.4679-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Richard E. Richard E. Malik P., et al. Murine retroviral but not human cellular promoters induce in vivo erythroid-specific deregulation that can be partially prevented by insulators. Mol. Ther. 2007;15:173–182. doi: 10.1038/sj.mt.6300030. [DOI] [PubMed] [Google Scholar]
- Rubio E.D. Reiss D.J. Welcsh P.L., et al. CTCF physically links cohesin to chromatin. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8309–8314. doi: 10.1073/pnas.0801273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B.Y. Evans-Galea M.V. Gray J.T., et al. An experimental system for the evaluation of retroviral vector design to diminish the risk for proto-oncogene activation. Blood. 2008;111:1866–1875. doi: 10.1182/blood-2007-04-085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B.Y. Persons D.A. Evans-Galea M.V., et al. A chromatin insulator blocks interactions between globin regulatory elements and cellular promoters in erythroid cells. Blood Cells Mol. Dis. 2007;39:221–228. doi: 10.1016/j.bcmd.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Savitskaya E. Melnikova L. Kostuchenko M., et al. Study of long-distance functional interactions between Su(Hw) insulators that can regulate enhancer-promoter communication in Drosophila melanogaster. Mol. Cell. Biol. 2006;26:754–761. doi: 10.1128/MCB.26.3.754-761.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambach A. Galla M. Maetzig T., et al. Improving transcriptional termination of self-inactivating gamma-retroviral and lentiviral vectors. Mol. Ther. 2007;15:1167–1173. doi: 10.1038/sj.mt.6300152. [DOI] [PubMed] [Google Scholar]
- Shou Y. Ma Z. Lu T. Sorrentino B.P. Unique risk factors for insertional mutagenesis in a mouse model of XSCID gene therapy. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11730–11735. doi: 10.1073/pnas.0603635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwaerder D.S. Lieber A. Insulation from viral transcriptional regulatory elements improves inducible transgene expression from adenovirus vectors in vitro and in vivo. Gene Ther. 2000;7:556–567. doi: 10.1038/sj.gt.3301139. [DOI] [PubMed] [Google Scholar]
- Tajima S. Shinohara K. Fukumoto M., et al. Ars insulator identified in sea urchin possesses an activity to ensure the transgene expression in mouse cells. J. Biochem. 2006;139:705–714. doi: 10.1093/jb/mvj075. [DOI] [PubMed] [Google Scholar]
- Urbinati F. Arumugam P. Higashimoto T., et al. Mechanism of reduction in titers from lentivirus vectors carrying large inserts in the 3′LTR. Mol. Ther. 2009;17:1527–1536. doi: 10.1038/mt.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meerten T. Classen M.J. Hagenbeek A. Ebeling S.B. The CD20/alphaCD20 “suicide” system: novel vectors with improved safety and expression profiles and efficient elimination of CD20-transgenic T cells. Gene Ther. 2006;13:789–797. doi: 10.1038/sj.gt.3302705. [DOI] [PubMed] [Google Scholar]
- Vieyra D.S. Goodell M.A. Pluripotentiality and conditional transgene regulation in human embryonic stem cells expressing insulated tetracycline-ON transactivator. Stem Cells. 2007;25:2559–2566. doi: 10.1634/stemcells.2007-0248. [DOI] [PubMed] [Google Scholar]
- Walisko O. Schorn S. Rolfs F., et al. Transcriptional activities of the Sleeping Beauty transposon and shielding its genetic cargo with insulators. Mol. Ther. 2008;16:359–369. doi: 10.1038/sj.mt.6300366. [DOI] [PubMed] [Google Scholar]
- West A.G. Gaszner M. Felsenfeld G. Insulators: many functions, many mechanisms. Genes Dev. 2002;16:271–288. doi: 10.1101/gad.954702. [DOI] [PubMed] [Google Scholar]
- West A.G. Huang S. Gaszner M., et al. Recruitment of histone modifications by USF proteins at a vertebrate barrier element. Mol. Cell. 2004;16:453–463. doi: 10.1016/j.molcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Yannaki E. Tubb J. Aker M., et al. Topological constraints governing the use of the chicken HS4 chromatin insulator in oncoretrovirus vectors. Mol. Ther. 2002;5:589–598. doi: 10.1006/mthe.2002.0582. [DOI] [PubMed] [Google Scholar]
- Yao S. Osborne C.S. Bharadwaj R.R., et al. Retrovirus silencer blocking by the cHS4 insulator is CTCF independent. Nucleic Acids Res. 2003;31:5317–5323. doi: 10.1093/nar/gkg742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S. Sukonnik T. Kean T., et al. Retrovirus silencing, variegation, extinction, and memory are controlled by a dynamic interplay of multiple epigenetic modifications. Mol. Ther. 2004;10:27–36. doi: 10.1016/j.ymthe.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Ye X. Liang M. Meng X., et al. Insulation from viral transcriptional regulatory elements enables improvement to hepatoma-specific gene expression from adenovirus vectors. Biochem. Biophys. Res. Commun. 2003;307:759–764. doi: 10.1016/s0006-291x(03)01251-8. [DOI] [PubMed] [Google Scholar]
- Yusufzai T.M. Felsenfeld G. The 5′-HS4 chicken beta-globin insulator is a CTCF-dependent nuclear matrix-associated element. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8620–8624. doi: 10.1073/pnas.0402938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss A.K. Son S. Chang L.J. RNA 3′ readthrough of oncoretrovirus and lentivirus: implications for vector safety and efficacy. J. Virol. 2002;76:7209–7219. doi: 10.1128/JVI.76.14.7209-7219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. Thornhill S.I. Howe S.J., et al. Lentiviral vectors containing an enhancer-less ubiquitously-acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood. 2007;110:1448–1457. doi: 10.1182/blood-2006-12-060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. Dean A. An insulator blocks spreading of histone acetylation and interferes with RNA polymerase II transfer between an enhancer and gene. Nucleic Acids Res. 2006;32:4903–4919. doi: 10.1093/nar/gkh832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. Mody D. Deravin S.S., et al. A self-inactivating lentiviral vector for SCID-X1 gene therapy that does not activate LMO2 expression in human T cells. Blood. 2010;116:900–908. doi: 10.1182/blood-2009-10-250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinski D. Schambach A. Modlich U., et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol. Ther. 2008;16:718–725. doi: 10.1038/mt.2008.5. [DOI] [PubMed] [Google Scholar]