Abstract
Background
The treatment of patients with advanced cancer with multiple comorbid illnesses is complex. Although an increasing number of such patients are being referred to hospice, the comorbidity burden of this patient population is largely unknown but has implications for the complexity of care provided by hospices. This study reports the comorbidity burden in a national sample of hospice users with cancer and estimates the effect of higher comorbidity on health care use and site of death.
Methods
Cross-sectional study using Surveillance, Epidemiology and End Results-Medicare data for hospice users who died of cancer in 2002 (N = 27,166). We measured comorbidity burden using the Charlson comorbidity index and used multivariable generalized estimating equations to estimate the association between comorbidity burden and the following outcomes: emergency department and intensive care unit (ICU) admission, hospitalization, hospice disenrollment, and hospital death.
Results
Patients with cancer who used hospice had an average Charlson comorbidity index value of 1.24, including 18.8% who suffered from comorbid dementia. In analyses adjusted for patient demographics, site of primary cancer, and number of days with hospice, higher comorbidity burden was associated with higher likelihood of emergency department admission (odds ratio [OR] = 1.69, 95% confidence interval [CI] 1.52, 1.87), ICU admission (OR = 3.28, 95% CI 2.45, 4.38), inpatient hospitalization (OR = 2.14, 95% CI 1.90, 2.42), hospice disenrollment (OR = 1.41, 95% CI 1.29, 1.56) and hospital death (OR = 2.51, 95% CI 2.08, 3.02).
Conclusion
These findings underscore the complexity of the hospice patient population and highlight a potential need to risk adjust the per diem hospice reimbursement rates to account for increased resource requirements for hospices serving patients with higher comorbidity burden.
Introduction
With the aging of the U.S. population, increases in longevity, and advances in the management of chronic illness, an increasing number of oncologists are treating patients with advanced cancer with multiple comorbid illnesses. Caring for such patients is complex. Multiple interacting disease processes (e.g., cognitive impairment from dementia; dyspnea resulting from obstructive lung cancer, heart failure and chronic obstructive pulmonary disease; pain from cancer, osteoarthritis; neuropathy from diabetes; and chemotherapy), drug–drug interactions, and functional debility all present considerable challenges to high-quality medical care for patients with cancer with comorbid illness. It is not surprising, therefore, that studies of patients with multiple serious illnesses demonstrate a high prevalence of pain and symptom distress,1–7 high prevalence of caregiver burden,8–10 communication problems between patients, families, and physicians about goals of care and medical decisions,11,12 prognostic difficulty,13 and poor transition management14 particularly in comparison to patients with advanced cancer without comorbidities.
Although comorbid illness may complicate the care of patients throughout the disease trajectory, little is known about the prevalence of comorbid conditions for patients who are receiving hospice care and the potential challenges faced by hospices in caring for patients with comorbid illness. Hospices' focus on the assessment and management of pain and other symptoms at the end of life, communication with patients about their preferences and goals of care, and focus on keeping patients at home may be complicated by the presence of multiple chronic diseases that are associated with acute exacerbations often amenable to restorative treatments. Patients with comorbid dementia may be particularly challenging given their cognitive impairment and accompanying behavioral disorders (e.g., depression, psychosis, aggression, or apathy)15,16 that complicate effective communication and symptom assessment. Furthermore, although hospices are charged with providing comprehensive care to patients near the end of life, patients with cancer and multiple comorbid illnesses often require ongoing care from specialists (e.g., cardiologists, geriatricians, geriatric psychiatrists), which poses additional billing and care coordination complexities to the hospice program.
We sought to estimate the comorbidity burden of a national sample of hospice users with a primary diagnosis of cancer. We estimate the association between comorbidity burden and the following outcomes: emergency department admission, intensive care unit (ICU) admission, inpatient hospitalization, disenrollment from hospice, and hospital death. In addition, given the known challenges in caring for individuals with dementia,17–19 we separately estimate the prevalence of comorbid dementia in the sample and the association between comorbid dementia and the previously listed outcomes.
Methods
Sample and data
Our sample, which is described elsewhere,20 includes all patients in the linked Surveillance, Epidemiology and End Results (SEER)-Medicare database21 who died of cancer in 2002 and received hospice care in the 6 months prior to death. A primary diagnosis of cancer was identified as one with an International Classification of Diseases, 9th revision, clinical modification (ICD-9-CM) code22 between 140 and 239. The Mount Sinai School of Medicine Institutional Review Board approved this Study.
Outcome measures
We used Medicare claims to identify the following binary outcomes for each individual from time of hospice enrollment until death: emergency department use, ICU use, inpatient hospitalization, hospice disenrollment, and hospital death. Patients were identified as having disenrolled from hospice if the date of their last hospice claim was before the date of their death, consistent with existing studies of hospice disenrollment.20,23,24
Independent variables
Patient demographic characteristics included in our analyses were age at death (<70, 70–77, 78–84, ≥85), reported race/ethnicity (white non-Hispanic, white Hispanic, black, and other), gender, marital status at the time of entry into the SEER cancer registry (married or not married), and census region.
Patient clinical characteristics were Charlson comorbidity index, site of primary cancer (lung, colon, prostate, breast, pancreatic, bladder, lymph, stomach, ovarian, kidney, uterine, and other), and log-transformed number of days from hospice enrollment to death. We measured patient comorbidity by calculating the Charlson comorbidity index25,26 using the comorbidity weights and methodology suggested by the National Cancer Institute27 for use with the SEER-Medicare data. The Charlson comorbidity index predicts the 1-year mortality for a patient who may have a range of comorbid conditions.25 It has been shown25 that with each increased level of the comorbidity index, there is a stepwise increase in the cumulative mortality attributable to comorbid disease. The Charlson comorbidity index as modified for use with administrative databases has also been shown to be associated with mortality.28 Each comorbid condition is assigned a score of 1, 2, 3, or 6 depending on the risk of dying associated with the condition.25 The scores are then summed to yield a total index value which predicts mortality. To identify comorbid conditions, for each patient, we reviewed all Medicare inpatient hospital, inpatient and outpatient physician, and hospice claims for the year prior to death. We then categorized Charlson comorbidity index as 2 or greater (i.e., high comorbidity) compared with patients with a Charlson index of 0 or 1 (i.e., low comorbidity). We chose to define high comorbidity as a Charlson comorbidity index value of 2 or more because it has been shown to identify a more complex group of patients with an increased risk of death.29
Previously described30,31 dementia-related ICD-9-CM codes were used to identify patients with dementia: 331.0 to 331.2, 331.7, 290.0, 290.1, 290.10 to 290.13, 290.20, 290.21, 290.3, 290.40 to 290.43, 294.0, 294.1, 294.8, and 797.
Statistical analysis
We summarized patient demographic and clinical characteristics using standard descriptive statistics. We compared the unadjusted rates of emergency department use, ICU use, inpatient hospitalization, hospice disenrollment and hospital death for patients with high comorbidity versus low comorbidity and with dementia versus without dementia using χ2 tests.
We used multivariable logistic generalized estimating equations (GEE)32 to estimate the associations between comorbidity burden, as a categorical variable and as a continuous variable, and the following outcomes: emergency department use, ICU use, inpatient hospitalization, hospice disenrollment, and hospital death. Multivariate models were adjusted for patient demographic (age at death, race, gender, marital status, and region) and clinical characteristics (site of primary cancer and log-transformed number of days from hospice enrollment to death). We repeated the analyses using dementia as the independent variable. All tests accounted for the clustering of patient observations within hospices.
Results
Characteristics of the sample
Our analysis includes 27,166 patients who died with a primary diagnosis of cancer in 2002 and who used hospice (Table 1). The average patient age at death was 78, and 86% were white non-Hispanic. Females comprised 52% of the patient sample, and 51% of patients were married. The most prevalent site of cancer was lung (26.8% of patients), followed by colon (11.8%). For the 23.7% of patients with site of cancer categorized as “other,” the most prevalent sites were miscellaneous (4.9%), liver (1.7%), and brain (1.6%).
Table 1.
Characteristics of Patients
| Total N = 27,166 | % | |
|---|---|---|
| Age at death | ||
| <70 years | 3223 | 11.9% |
| 70 to 77 years | 9391 | 34.6% |
| 78 to 84 years | 8597 | 31.6% |
| 85 years and older | 5955 | 21.9% |
| Race | ||
| White non-Hispanic | 23,290 | 85.7% |
| White Hispanic | 1101 | 4.1% |
| Black | 1965 | 7.2% |
| Other | 757 | 2.8% |
| Unknown | 53 | 0.2% |
| Gender | ||
| Female | 14,191 | 52.2% |
| Male | 12,975 | 47.8% |
| Marital status | ||
| Single | 12,849 | 49.3% |
| Married | 13,240 | 50.7% |
| Site of primary cancer | ||
| Lung | 7271 | 26.8% |
| Colon | 3209 | 11.8% |
| Prostate | 2201 | 8.1% |
| Breast | 1841 | 6.8% |
| Pancreatic | 1819 | 6.7% |
| Bladder | 927 | 3.4% |
| Lymph | 869 | 3.2% |
| Stomach | 769 | 2.8% |
| Ovarian | 674 | 2.5% |
| Kidney | 648 | 2.4% |
| Uterine | 508 | 1.9% |
| Other | 6430 | 23.7% |
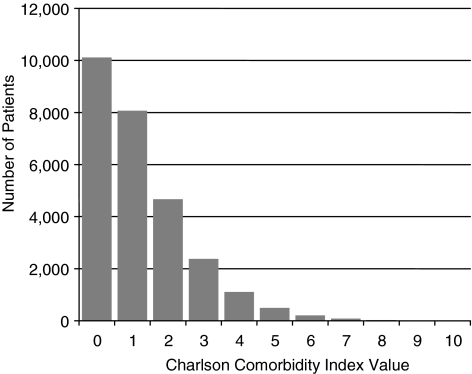
The mean Charlson comorbidity index value was 1.24 (standard deviation 1.38) and the range was 0 to 10. A comorbidity index value of 2 or higher (i.e., high comorbidity) indicated that a patient was in the top 33% of the distribution of Charlson comorbidity index values in our sample. Figure 1 shows the distribution of Charlson comorbidity index values for the sample of hospice users with cancer.
FIG. 1.
Variability in Charlson comorbidity index value for patients with cancer receiving hospice care (N=27,166).
The prevalence of comorbid dementia was 18.8% and other prevalent comorbid conditions were as follows: chronic pulmonary disease (43.3%), diabetes (30.6%), congestive heart failure (30.2%), and cerebrovascular disease (25.3%).
The health care utilization for our sample of patients with cancer was as follows: 7.2% were admitted to the emergency department, 0.9% were admitted to the ICU, 5.9% were hospitalized as an inpatient, 10.0% were disenrolled from hospice, and 1.5% died in the hospital. Health care utilization and hospital death were related: of those patients admitted to the emergency department, 15.4% experienced a hospital death; of those patients admitted to the ICU, 35.3% experienced a hospital death; and of those patients hospitalized as an inpatient, 22.4% experienced a hospital death. Patients who are hospitalized may continue to receive hospice care if they remain eligible for the Medicare Hospice Benefit and if the hospital has a contract with the hospice agency. The association between hospice disenrollment and health care utilization has been previously reported.23
Comorbidity burden and health care utilization
In unadjusted analyses, patients with high comorbidity (i.e., 2 or more comorbid conditions) were more frequently admitted to the emergency department (8.3% versus 6.6%, p < 0.001), admitted to the ICU (1.5% versus 0.6%, p < 0.001), hospitalized as an inpatient (7.6% versus 5.0%, p < 0.001) and more frequently died in the hospital (2.4% versus 1.1%, p < 0.001; Table 2).
Table 2.
Comorbidity Burden and Health Care Utilization for Patients with Cancer
| Patients with high comorbiditya% | Patients with low comorbiditya% | Adjusted odds ratio | 95% CI | |
|---|---|---|---|---|
| Emergency department use | 8.3% | 6.6%b | 1.69 | (1.52, 1.87)c |
| Intensive care unit use | 1.5% | 0.6%b | 3.28 | (2.45, 4.38)c |
| Inpatient hospitalization | 7.6% | 5.0%b | 2.14 | (1.90, 2.42)c |
| Hospice disenrollment | 10.3% | 9.9% | 1.41 | (1.29, 1.56)c |
| Hospital death | 2.4% | 1.1%b | 2.51 | (2.08, 3.02)c |
High comorbidity is defined as a Charlson comorbidity index value of 2 or more.
Low comorbidity is defined as a Charlson comorbidity index value of 0 or 1.
p < 0.0001 based on a χ2 test.
p < 0.0001 for generalized estimating equations models with a gamma distribution and log link accounting for patient age race, gender, maritial status site of primary cancer, days from hospice enrollment to death and region.
CI, confidence interval.
In models adjusted for patient demographic characteristics (age at death, race, gender, marital status, and region) and clinical characteristics (site of primary cancer and log-transformed number of days from hospice enrollment to death), patients with high comorbidity burden were significantly more likely to be admitted to the emergency department (OR = 1.69, 95% CI 1.52, 1.87), admitted to the ICU (OR = 3.28, 95% CI 2.45, 4.38), hospitalized as an inpatient (OR = 2.14, 95% CI 1.90, 2.42), disenrolled from hospice (OR = 1.41, 95% CI 1.29, 1.56), and to die in the hospital (OR = 2.51, 95% CI 2.08, 3.02). In multivariable models with patients' Charlson comorbidity index values modeled as a continuous variable, higher comorbidity was significantly associated with being admitted to the emergency department (OR = 1.25, 95% CI 1.21, 1.29), admitted to the ICU (OR = 1.48, 95% CI 1.38, 1.59), hospitalized as an inpatient (OR = 1.34, 95% CI 1.29, 1.39), disenrolled from hospice (OR = 1.16, 95% CI 1.12, 1.20), and dying in the hospital (OR = 1.36, 95% CI 1.28, 1.44).
Comorbid dementia and health care use
Patients with comorbid dementia compared with those without comorbid dementia were more frequently admitted to the emergency department (9.3% versus 6.7%, p < 0.001), hospitalized as an inpatient (7.4% versus 5.5%, p < 0.001) and disenrolled from hospice (12.3% versus 9.6%, p < 0.001; Table 3).
Table 3.
Comorbid Dementia and Health Care Utilization for Patients with Cancer
| Patients with dementia % | Patients without dementia % | Adjusted odds ratio | 95% CI | |
|---|---|---|---|---|
| Emergency department use | 9.3% | 6.7%a | 1.26 | (1.12, 1.41)b |
| Intensive care unit use | 1.1% | 0.9% | 1.13 | (0.81, 1.59) |
| Inpatient hospitalization | 7.4% | 5.5%a | 1.21 | (1.05, 1.40)c |
| Hospice disenrollment | 12.3% | 9.6%a | 1.18 | (1.05, 1.32)c |
| Hospital death | 1.3% | 1.6% | 0.92 | (0.70, 1.21) |
p < 0.0001 based on a χ2 test.
p < 0.0001 for generalized estimating equations models with a gamma distribution and log link accounting for patient age, race, gender, maritial status, site of primary cancer, days from hospice enrollment to death and region.
p < 0.01 for generalized estimating equations models with a gamma distribution and log link accounting for patient age, race, gender, maritial status, site of primary cancer, days from hospice enrollment to death and region.
In models adjusted for patient demographic characteristics (age at death, race, gender, marital status, and region) and clinical characteristics (site of primary cancer and log-transformed number of days from hospice enrollment to death), patients with dementia were significantly more likely to be admitted to the emergency department (OR = 1.26, 95% CI 1.12, 1.41), hospitalized as an inpatient (OR = 1.21, 95% CI 1.05, 1.40), and disenrolled from hospice (OR = 1.18, 95% CI 1.05, 1.32). There were no significant differences between patients with and without dementia in the likelihood of ICU use or hospital death.
Comment
Our research is the first to document the comorbidity burden of a national sample of hospice users with cancer, and our findings highlight the complexity of the patient population served by hospices. One third of patients had a comorbidity index value of 2 or more and almost one fifth suffered from comorbid dementia. Patients with multiple comorbid illnesses had higher rates of hospitalizations, including admission to the emergency department and ICU, and were more likely to disenroll from hospice and to die in the hospital. These higher rates of hospitalization may be due to the fact that managing a symptom crisis is complicated for patients with multiple comorbid illnesses and hospices may not have the staff and other resources to manage such patients at home. Patients with high comorbidity were more likely than those with low comorbidity to disenroll from hospice possibly because their more complex clinical needs were not adequately addressed by the hospice. This is consistent with a recent study20 finding that smaller hospices and less experienced hospices had higher disenrollment rates than larger and more experienced hospices.
The added complexity of caring for patients with multiple comorbid illnesses is not currently reflected in the reimbursement that hospices receive from Medicare. Hospice reimbursement under the Medicare Hospice Benefit is on a per diem basis and is intended to cover the cost of care for the patient's underlying terminal diagnosis and is not risk adjusted. Care unrelated to the terminal diagnosis (e.g., treatment of comorbid conditions) may be separately billed to Medicare Part A. However, this rarely occurs. For patients who receive hospice until their death, only 11% of their total Medicare expenditures from hospice enrollment to death was for care outside of the Medicare Hospice Benefit.23 This suggests that hospices are providing comprehensive care for patients regardless of the number comorbid conditions and without significant additional reimbursement from Medicare. It may be too difficult for hospice staff to attribute the underlying cause of pain and other symptoms to a primary versus secondary diagnosis. As the number of individuals with multiple serious illnesses continues to increase, the financial burden of caring for such patients in the hospice setting under the current reimbursement structure may become increasingly difficult for hospice providers.
Our finding that approximately one-fifth of hospice users with cancer have comorbid dementia is striking. Approximately 10% of patients admitted to hospice have a primary diagnosis of dementia,33 and thus the total prevalence of dementia in the hospice patient population is upwards of 29%. Given that the complexity of caring for patients with dementia in terms of communication barriers and assessment and management of pain and other symptoms, is likely similar whether the dementia is primary or secondary, hospices are caring for a far more complex patient population than existing analyses of primary diagnoses suggests. Furthermore, although patients with comorbid dementia had only a moderate increase in the likelihood of being hospitalized, admitted to the emergency department, and disenrolled from hospice, the elevated risk is important given its potential negative impact on quality of life for patients34,35 and its impact on health care expenditures.23
A strength of this study is the large sample size, which enables us to evaluate outcomes that are relatively rare in a sample of hospice users; however, limitations of these data exist. Our sample includes individuals with cancer who used hospice and died in 2002 and thus does not evaluate the comorbidity burden of hospice users with a non-cancer primary diagnosis. However, given that approximately 40% of hospice users have a primary diagnosis of cancer,33 our analysis accurately depicts comorbidity burden for the largest single diagnostic group of hospice users. Second, our sample does not include individuals in a Medicare managed care organization, and thus our findings may not be generalizable to this group. Hospices provide comprehensive interdisciplinary care to patients near the end of life, benefiting patients and families and supporting physicians in the ongoing care of their most seriously ill patients. Patients receiving hospice care are complex, with multiple comorbid illnesses and a high prevalence of dementia. Patients with comorbid conditions experience higher rates of markers for poor-quality care and thus represent a potentially vulnerable group within the hospice patient population. This patient population may be an appropriate target for quality improvement efforts in the hospice setting.
Acknowledgments
This work was supported grant 1R01CA116398-01A2 from the National Cancer Institute; and grant 1K99NR010495-01 from the National Institute of Nursing Research.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Whelan CT. Jin L. Meltzer D. Pain and satisfaction with pain control in hospitalized medical patients: No such thing as low risk. Arch Intern Med. 2004;164:175–180. doi: 10.1001/archinte.164.2.175. [DOI] [PubMed] [Google Scholar]
- 2.Morrison RS. Siu AL. A comparison of pain and its treatment in advanced dementia and cognitively intact patients with hip fracture. J Pain Symptom Manage. 2000;19:240–248. doi: 10.1016/s0885-3924(00)00113-5. [DOI] [PubMed] [Google Scholar]
- 3.Desbiens NA. Mueller-Rizner N. Connors AF., Jr Wenger NS. Lynn J. The symptom burden of seriously ill hospitalized patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcome and Risks of Treatment. J Pain Symptom Manage. 1999;17:248–255. doi: 10.1016/s0885-3924(98)00149-3. [DOI] [PubMed] [Google Scholar]
- 4.The SUPPORT Principal Investigators. A Controlled Trail to Improve Care for Seriously Ill Hospitalized Patients. The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 5.Teno JM. Weitzen S. Wetle T. Mor V. Persistent pain in nursing home residents. JAMA. 2001;285:2081. doi: 10.1001/jama.285.16.2081-a. [DOI] [PubMed] [Google Scholar]
- 6.Bernabei R. Gambassi G. Lapane K. Landi F. Gatsonis C. Dunlop R. Lipsitz L. Steel K. Mor V. Management of pain in elderly patients with cancer. SAGE Study Group. Systematic Assessment of Geriatric Drug Use via Epidemiology. JAMA. 1998;279:1877–1882. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- 7.Cleeland CS. Gonin R. Hatfield AK. Edmonson JH. Blum RH. Stewart JA. Pandya KJ. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 8.Desbiens NA. Mueller-Rizner N. Virnig B. Lynn J. Stress in caregivers of hospitalized oldest-old patients. J Gerontol A Biol Sci Med Sci. 2001;56:M231–235. doi: 10.1093/gerona/56.4.m231. [DOI] [PubMed] [Google Scholar]
- 9.Schulz R. Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 10.Covinsky KE. Goldman L. Cook EF. Oye R. Desbiens N. Reding D. Fulkerson W. Connors AF., Jr Lynn J. Phillips RS. The impact of serious illness on patients' families. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. JAMA. 1994;272:1839–1844. doi: 10.1001/jama.272.23.1839. [DOI] [PubMed] [Google Scholar]
- 11.Tulsky J. In: Geriatric Palliative Care. Morrison RS, editor; Meier DE, editor. New York: Oxford University Press; 2003. pp. 314–331. [Google Scholar]
- 12.Fallowfield L. Jenkins V. Communicating sad, bad, and difficult news in medicine. Lancet. 2004;363:312–319. doi: 10.1016/S0140-6736(03)15392-5. [DOI] [PubMed] [Google Scholar]
- 13.Piccirillo JF. Tierney RM. Costas I. Grove L. Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 14.Mathematica Policy Research. National Public Engagement Campaign on Chronic Illness-Physician Survey. 2001.
- 15.Lawlor B. Bhriain SN. Psychosis and behavioural symptoms of dementia: Defining the role of neuroleptic interventions. Int J Geriatr Psychiatry. 2001;16(Suppl 1):S2–6. doi: 10.1002/1099-1166(200112)16:1+<::aid-gps567>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Kverno KS. Rabins PV. Blass DM. Hicks KL. Black BS. Prevalence and treatment of neuropsychiatric symptoms in advanced dementia. J Gerontol Nurs. 2008;34:8–15. doi: 10.3928/00989134-20081201-03. quiz 16–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shuster JL., Jr Palliative care for advanced dementia. Clin Geriatr Med. 2000;16:373–386. doi: 10.1016/s0749-0690(05)70062-8. [DOI] [PubMed] [Google Scholar]
- 18.van der Steen JT. Dying with dementia: What we know after more than a decade of research. J Alzheimers Dis. 2010;22:37–55. doi: 10.3233/JAD-2010-100744. [DOI] [PubMed] [Google Scholar]
- 19.Chang E. Daly J. Johnson A. Harrison K. Easterbrook S. Bidewell J. Stewart H. Noel M. Hancock K. Challenges for professional care of advanced dementia. Int J Nurs Pract. 2009;15:41–47. doi: 10.1111/j.1440-172X.2008.01723.x. [DOI] [PubMed] [Google Scholar]
- 20.Carlson MD. Herrin J. Du Q. Epstein AJ. Cherlin E. Morrison RS. Bradley EH. Hospice characteristics and the disenrollment of patients with cancer. Health Serv Res. 2009;44:2004–2021. doi: 10.1111/j.1475-6773.2009.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute: Surveillance, Epidemiology, and End Results database. http://healthservices.cancer.gov/seermedicare/ [Apr 5;2011 ]. http://healthservices.cancer.gov/seermedicare/
- 22.US Department of Health and Human Services. The International Classification of Diseases, 9th Revision, Clinical Modification: Public Health Service. Health Care Financing Administration: DHHS Publication No. (PHS) 89-1260; Mar, 1989. [Google Scholar]
- 23.Carlson MD. Herrin J. Du Q. Epstein AJ. Barry CL. Morrison RS. Back AL. Bradley EH. Impact of hospice disenrollment on health care use and medicare expenditures for patients with cancer. J Clin Oncol. 2010;28:4371–4375. doi: 10.1200/JCO.2009.26.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor DH. Steinhauser K. Tulsky JA. Rattliff J. Van Houtven CH. Characterizing hospice discharge patterns in a nationally representative sample of the elderly, 1993–2000. Am J Hosp Palliat Care. doi: 10.1177/1049909107310136. (in press). [DOI] [PubMed] [Google Scholar]
- 25.Charlson ME. Pompei P. Ales KL. MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Klabunde CN. Potosky AL. Legler JM. Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute: SEER-Medicare: Calculation of Comorbidity Weights. http://healthservices.cancer.gov/seermedicare/program/comorbidity.html. [May;2008 ]. http://healthservices.cancer.gov/seermedicare/program/comorbidity.html
- 28.Deyo RA. Cherkin DC. Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 29.Singh B. Bhaya M. Stern J. Roland JT. Zimbler M. Rosenfeld RM. Har-El G. Lucente FE. Validation of the Charlson comorbidity index in patients with head and neck cancer: A multi-institutional study. Laryngoscope. Nov. 1997;107(11 Pt 1):1469–1475. doi: 10.1097/00005537-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Taylor DH., Jr Fillenbaum GG. Ezell ME. The accuracy of medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol. 2002;55:929–937. doi: 10.1016/s0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 31.Raji MA. Kuo YF. Freeman JL. Goodwin JS. Effect of a dementia diagnosis on survival of older patients after a diagnosis of breast, colon, or prostate cancer: implications for cancer care. Arch Intern Med. 2008;168:2033–2040. doi: 10.1001/archinte.168.18.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang KY. Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 33.National Hospice and Palliative Care Organization: NHPCO Facts and Figures. www.nhpco.org/files/public/Statistics_Research/NHPCO_facts_and_figures.pdf. 2009. [Nov;2009 ]. www.nhpco.org/files/public/Statistics_Research/NHPCO_facts_and_figures.pdf
- 34.Earle CC. Landrum MB. Souza JM. Neville BA. Weeks JC. Ayanian JZ. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright AA. Keating NL. Balboni TA. Matulonis UA. Block SD. Prigerson HG. Place of death: Correlations with quality of life of patients with cancer and predictors of bereaved caregivers' mental health. J Clin Oncol. 2010;28:4457–4464. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]