Abstract
Objective. Although the incidence of dcSSc is higher in African-American and Hispanic populations compared with European Caucasian patients, it is not clear whether there are differences in subsequent disease course. Also, the potential impact of gender on the disease course of dcSSc is not well defined. Our objective was to assess the course of modified Rodnan skin score (MRSS), HAQ-disability index (HAQ-DI) and forced vital capacity per cent (FVC%) predicted between men vs women and three ethnic groups with dcSSc participating in three randomized clinical trials (RCTs).
Method. Data from RCTs (n = 495) were pooled and analysed. Baseline characteristics were compared in men vs women and among ethnic groups. A linear mixed effects model was used to assess the predictors of MRSS, HAQ-DI and FVC%. The primary independent variables were time-in-study and its interaction with gender and ethnicity. The models were adjusted for other covariates that were significant at baseline between gender and ethnicity analyses.
Results. Men had lower HAQI-DI scores compared with women (P < 0.05). Among the three ethnic groups, Caucasians were older, African-Americans had lower FVC% predicted and Hispanics had greater tender joint counts (P < 0.05). The course of MRSS, HAQ-DI and FVC% predicted during the study period was not significantly different between gender and three ethnicities. Time-in-study was an independent predictor of improvement in MRSS and HAQ-DI.
Conclusion. Our analysis explores the influence of gender and ethnicity on disease course in RCTs. These findings are relevant to issues of future trial design.
Keywords: Scleroderma, Randomized clinical trial, Clinical trials, Systemic sclerosis, Gender, Race, Ethnicity, Diffuse cutaneous systemic sclerosis
Introduction
SSc is a generalized disorder that affects the skin and internal organs, including gastrointestinal tract, lung, heart and kidney. Genetics and environmental exposures are thought to play a role in the susceptibility to SSc [1]. In a published SSc cohort, African-American (AA) patients had a younger age of disease onset, higher probability of having diffuse SSc and higher mortality [2]. Data on the impact of gender on disease expression and survival were, however, less clear. One study showed that males had a greater risk of death due to their SSc [3], whereas another epidemiological study showed the opposite [4]. In addition, it is not clear whether ethnicity and gender influence the course of the disease with respect to skin thickness, functional disability or lung function once a patient develops dcSSc. These disease features are common measures of disease outcome in clinical trials—thus gender and ethnicity can be viewed as confounding influences on interpretability of clinical trials.
In the present study, we examined the baseline differences and course of modified Rodnan skin score (MRSS), HAQ-disability index (HAQ-DI) and forced vital capacity per cent (FVC%) predicted between men vs women and among three different ethnic groups (European Caucasian, AA and Hispanic) in three double-blind, randomized control trials (RCTs) of patients with dcSSc.
Patients and methods
RCTs
We utilized data from the following three double-blind RCTs in patients with dcSSc.
d-Penicillamine
This trial was a prospective, controlled study examining the efficacy of high-dose vs low-dose d-penicillamine (d-Pen). One hundred and thirty-four patients with dcSSc who had disease duration <18 months from the onset of the first non-RP SSc manifestation participated in the study and 68 completed the study [5].
Recombinant human relaxin
Two hundred and thirty-one subjects with dcSSc were enrolled in this open clinical trial evaluating the safety, efficacy and dose–response effect of continuous subcutaneously infused recombinant human relaxin and 195 completed the study. All participants had SSc disease duration of ≤5 years since the onset of the first sign or symptom of SSc other than RP [6].
Oral bovine type I collagen
This trial randomized 168 dcSSc patients with baseline disease duration of up to 10 years to receive 12 months of oral bovine type I collagen (500 µg/day) or placebo and 139 completed the study [7].
Statistical analysis
For the purpose of the present study, data from the three RCTs were pooled and analysed irrespective of treatment assignments because none of the active therapies differed significantly from the placebo (or control) group in the primary and secondary outcomes.
Summary statistics and P-values for group differences on key characteristics at baseline are presented in Tables 1 and 2. To compare gender groups, the Student’s t-test and Wilcoxon rank sum test were used for continuous variables, while the chi-square and Fisher’s exact tests were used for categorical variables. To compare ethnicity groups, analysis of variance and the Kruskal–Wallis test were used for continuous variables, while the chi-square test was used for dichotomous variables. These omnibus tests were followed up by pairwise comparisons of the Caucasian, AA and Hispanic groups using the Tukey–Kramer method for variables meeting the normality assumption, and the Wilcoxon rank-sum test (with significance level adjusted by the Bonferroni correction) for variables not normally distributed. For dichotomous variables with significant omnibus tests, pairwise comparisons were made using logistic regression combined with post-estimation tests of coefficients. Estimated creatinine clearance was calculated using a modification of diet in renal disease formula that adjusts for age, gender and ethnicity (AA) [8]. Due to physiological differences in the values of haematocrit [9] and creatine phosphokinase (CPK), we also assessed the percentage of patients who had values greater than the upper limit of normal (ULN). In addition, because AAs have lower lung function compared with Caucasians, the American Thoracic Society has suggested using a scaling factor of 12% to adjust predicted values for FVC% and a factor of 8% to adjust for diffusion lung capacity for carbon monoxide (DLCO)% [10]. Since two of three RCTs were performed in the last decade when the FVC% and DLCO% predicted were not adjusted for ethnicity, we applied an adjustment of 12% for FVC% and 8% for DLCO% as an exploratory analysis.
Table 1.
Baseline characteristics of study patients, overall group and stratified by gender
| All |
Men |
Women |
||||
|---|---|---|---|---|---|---|
| n | Mean (s.d.) | n | Mean (s.d.) | n | Mean (s.d.) | |
| Age, years | 495 | 47.47 (11.84) | 92 | 47.57 (12.79) | 403 | 47.45 (11.63) |
| Ethnicitya | ||||||
| Caucasian, n (%) | 352 | 71.1 | 71 | 77.2 | 281 | 69.7 |
| AA, n (%) | 79 | 16.0 | 9 | 9.8 | 70 | 17.4 |
| Hispanic, n (%) | 47 | 9.5 | 9 | 9.8 | 38 | 9.4 |
| Other, n (%) | 17 | 3.4 | 3 | 3.2 | 14 | 3.4 |
| Women | 403 | 81.4 | ||||
| Men | 92 | 18.6 | ||||
| Disease duration, months | 495 | 26.99 (24.73) | 92 | 26.53 (26.91) | 403 | 27.1 (24.23) |
| BMI, kg/m2 | 439 | 26.62 (5.81) | 82 | 27.59 (6.59) | 357 | 26.39 (5.6) |
| MRSS, 0–51 | 495 | 25.29 (7.91) | 92 | 24.91 (8.47) | 403 | 25.38 (7.78) |
| Tender joint count, 0–8b | 330 | 1.34 (2.1) | 59 | 1.19 (2.02) | 271 | 1.37 (2.12) |
| Swollen joint count, 0–8b | 330 | 0.91 (1.52) | 59 | 1.07 (1.72) | 271 | 0.87 (1.48) |
| Physician global, 0–100c | 355 | 46.78 (21.64) | 62 | 44.85 (22.35) | 293 | 47.19 (21.5) |
| Patient global, 0–100c | 360 | 45.19 (26.92) | 62 | 44.2 (27.68) | 298 | 45.39 (26.81) |
| Diffusing capacity % predicted | 488 | 70.45 (20.54) | 91 | 69.97 (21.31) | 397 | 70.56 (20.38) |
| FVC% predicted | 493 | 84.64 (16.88) | 92 | 86.03 (16.91) | 401 | 84.32 (16.88) |
| Cutaneous ulcersb | 188 | 0.81 (1.85) | 36 | 0.78 (1.53) | 152 | 0.82 (1.92) |
| HAQ-DI, 0–3 | 492 | 1.19 (0.69) | 91 | 0.99 (0.65)* | 401 | 1.24 (0.7) |
| Creatinine clearance, ml/min | 489 | 107.46 | 92 | 108.62 | 397 | 107.19 |
| S. creatinine, mg/dl | 491 | 0.76 (0.28) | 92 | 0.92 (0.33)* | 397 | 0.73 (0.25) |
| Creatinine, ULN, n (%) | 11 | 2.3 | 3 | 3.3 | 8 | 2.0 |
| S. haematocrit, n (%) | 491 | 38.82 (3.77) | 92 | 40.96 (3.89)* | 397 | 38.32 (3.58) |
| Haematocrit, ULNd, n (%) | 54 | 11.0 | 5 | 5.4 | 49 | 12.3 |
| S. creatine phosphokinase, U/l | 487 | 119.04 (197.63) | 90 | 149.13 (171.1)* | 395 | 111.69 (203) |
| Creatine phosphokinase, ULN,dn (%) | 57 | 11.8 | 9 | 10.0 | 48 | 12.2 |
| SF-36 PCS | 354 | 33.9 (10.74) | 59 | 35.56 | 295 | 33.59 (10.56) |
| SF-36 MCS | 354 | 49.47 (9.92) | 59 | 51.01 | 295 | 49.16 (10.09) |
aEthnicity is expressed in percentage. bNot available in collagen data set. cNot available in d-Pen set. dHaematocrit ULN = 42.1% (women), 47.0% (men); creatine phosphokinase ULN = 182 U/l (women), 335 U/l (men). *P < 0.05 between men vs women. SF-36: short form-36; PCS: physical component summary; MCS: mental component summary.
Table 2.
Baseline characteristics of study patients, by ethnicity
| Variables | Caucasian |
AA |
Hispanic |
||||
|---|---|---|---|---|---|---|---|
| n | Mean (s.d.) | n | Mean (s.d.) | n | Mean (s.d.) | P-value | |
| Gender: women, n (%) | 281 | 79.8 | 70 | 88.6 | 38 | 80.9 | 0.347 |
| Age, years | 352 | 49.39 (11.5)*,** | 79 | 42.36 (11.62) | 47 | 42.91 (11.44) | <0.0001 |
| Disease duration, months | 352 | 26.72 (24.07) | 79 | 28.81 (27.34) | 47 | 27.33 (26.94) | 0.8175 |
| BMI, kg/m2 | 311 | 26.32 (5.35)* | 72 | 28.53 (7.52) | 40 | 26.29 (5.73) | 0.0461 |
| MRSS, 0–51 | 352 | 25.78 (8.05) | 79 | 24.44 (8.41) | 47 | 23.96 (5.9) | 0.1317 |
| Tender joint count, 0–8a | 236 | 1.09 (1.85)** | 52 | 1.17 (1.85) | 33 | 2.73 (2.72) | 0.0011 |
| Swollen joint count, 0–8a | 236 | 0.94 (1.85) | 52 | 0.62 (1.33) | 33 | 1.21 (1.58) | 0.1755 |
| Physician global, 0–100b | 256 | 46.68 (22.26) | 53 | 51.99 (20.72) | 35 | 44.25 (17.44) | 0.0356 |
| Patient global, 0–100b | 260 | 43.98 (26.77)** | 53 | 42.97 (26.62)*** | 36 | 58.92 (26.52) | 0.0232 |
| Diffusing capacity % predicted | 350 | 71.4 (20.38)* | 76 | 62.35 (19.76)*** | 45 | 73.84 (21.13) | 0.0005 |
| Corrected diffusing capacity % predictedc | 350 | 71.4 (20.38) | 76 | 67.34 (21.34) | 45 | 73.84 (21.13) | 0.0713 |
| FVC% predicted | 350 | 86.78 (16.24)* | 79 | 74.81 (17.88)*** | 47 | 85.2 (14.3) | 0.0001 |
| Corrected FVC% predictedc | 350 | 86.78 (16.24) | 79 | 83.79 (20.03) | 47 | 85.2 (14.3) | 0.2383 |
| Cutaneous ulcersa | 131 | 0.82 (1.72) | 32 | 0.41 (0.87) | 18 | 1.72 (3.49) | 0.3154 |
| HAQ-DI, 0–3 | 349 | 1.2 (0.67) | 79 | 1.12 (0.73) | 47 | 1.29 (0.81) | 0.4885 |
| Creatinine clearance, ml/min | 348 | 102.82 | 79 | 118.4 | 45 | 124.3 | 0.0002 |
| S. creatinine, mg/dl | 348 | 0.77 (0.27)** | 79 | 0.8 (0.34)*** | 45 | 0.66 (0.22) | 0.0097 |
| Creatinine, ULN, n (%) | 6 | 1.7 | 4 | 5.1 | 1 | 2.2 | 0.206 |
| S. haematocrit, n (%) | 348 | 38.91 (3.8) | 79 | 37.85 (4.04) | 45 | 39.35 (3.07) | 0.0418 |
| S. creatine phosphokinase, U/l | 345 | 110.11 (200)* | 79 | 168.7 (214.1)*** | 44 | 114.5 (173.9) | <0.0001 |
| SF-36 PCS | 255 | 33.95 (10.73) | 52 | 33.67 (11.14) | 36 | 33.43 (11.41) | 0.8474 |
| SF-36 MCS | 255 | 49.72 (9.71) | 52 | 49.8 (10.53) | 36 | 45.99 (10.13) | 0.0463 |
P-values are adjusted for multiple comparisons at α = 0.05 level using Bonferroni and Tukey corrections. aNot available in collagen data set. bNot available in d-Pen data set; cFVC% adjusted = 12%, DLCO% adjusted = 8% for AA. *P-value significant at overall 0.05 level between Caucasian and AA. **P-value significant at overall 0.05 level between Caucasian and Hispanic. ***P-value significant at overall 0.05 level between AA and Hispanic.
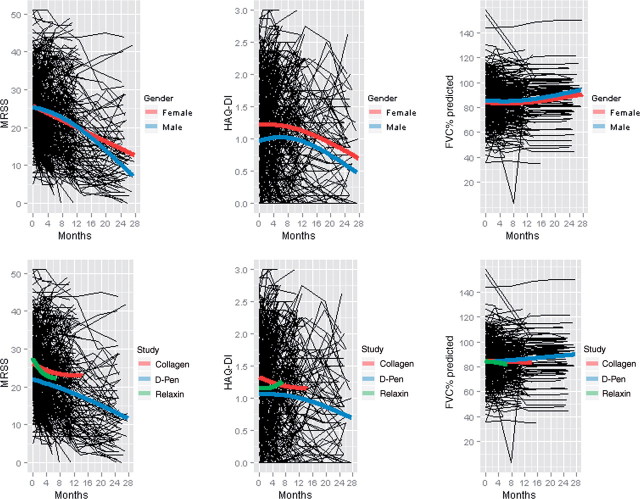
MRSS, HAQ-DI and FVC% predicted were obtained during four follow-up visits in the relaxin and collagen studies, while the d-Pen study included a fifth visit. Visits were conducted at the following intervals during the course of each study: d-Pen: baseline, 6, 12, 18 and 24 months; relaxin: baseline, 1, 3 and 6 months; and collagen: baseline, 4, 8 and 12 months. To illustrate trends in MRSS, HAQ-DI and FVC% predicted over time, profile plots were generated (Fig. 1), with natural spline smoother lines overlain for each of the gender, ethnicity and study categories. Although some nonlinearity can be discerned in the smoother lines, the trends are consistently monotonic, and for ease of interpretation it was decided that the trends could be satisfactorily modelled using a linear model.
Fig. 1.
Profile plots for MRSS, HAQ-DI and FVC% predicted, stratified by gender and type of RCT.
MRSS, HAQ-DI and FVC% predicted were each modelled as a continuous variable using a linear mixed effects model to account for within-subject correlation of the outcome variables across multiple visits. To allow for the rate of change of MRSS, HAQ-DI and FVC% predicted to differ between gender groups, gender was interacted with time-in-study (expressed in months after baseline of the visit), while for ethnicity models dummy variables for ethnicity were interacted with time-in-study of the visit. Possible confounding effects were accounted for in the models by interacting covariate variables with time-in-study. All models were adjusted for disease duration (based on its influence on MRSS [11]) and specific RCT (on the basis of profile plots). For HAQ-DI, we adjusted for baseline skin score, as functional disability is influenced by MRSS [11]. We also included covariates that were significantly different at baseline between gender groups and ethnic groups. Gender models were adjusted for baseline scores of HAQ-DI, serum creatinine, CPK and haematocrit, while ethnicity models were adjusted for age, baseline DLCO% and baseline FVC%. Both random intercept and random intercept and coefficient models with the same fixed effects were examined, with likelihood ratio tests being employed to determine the most appropriate for each model.
Under this model the P-value of a time-in-study/gender interaction term or time-in-study/ethnicity interaction term suggests whether there is a significant difference between the rate of change of the reference group (Caucasian for ethnicity models and female for gender models) and non-reference group (AA and Hispanic for ethnicity models and male for gender models). The sign and magnitude of the coefficient of the interaction term indicates whether the rate of change of the non-reference group is greater or less than the reference group and its magnitude, all other factors being equal.
We also evaluated whether specific gender–ethnicity combinations behaved differently from the rest of the population in the course of MRSS, HAQ-DI and FVC% over time. This entailed introducing three-way interaction terms between gender, ethnicity and time-in-study into the above-described models.
Results
Baseline characteristics
Pooled data from all three studies showed that 81.4% of patients were women, 71.1% of patients were Caucasian, 16% of patients were AA, 9.5% of patients were Hispanic and 3.4% of patients belonged to the other category (1.4% were Asian and 2% were of unknown ethnicity). The mean (s.d.) age of participants was 47.5 (11.8) years, disease duration was 27 (24.7) months and MRSS was 25.3 (7.91) U (Table 1). The average follow-up time in three RCTs was 9.4 (7.4) months. There were no differences in disease duration between men vs women and among three ethnic groups.
Gender
On average, men compared with women had a higher serum creatinine (0.92 vs 0.73 mg/dl), serum haematocrit (40.96 vs 38.32%) and CPK (149.13 vs 111.69 U/l), but lower HAQI-DI scores (0.99 vs 1.24, P < 0.05 for all; Table 1). After accounting for physiological differences in the laboratory values, the proportion of patients with CPK and haematocrit > ULN were similar in men vs women (Table 1). In addition, there were no differences in the estimated creatinine clearance (108.62 vs 107.19 ml/min).
Ethnicity
On average, Caucasian patients (49.4 years) were older than AA (42.4 years) and Hispanic patients (42.9 years, P < 0.05). AA patients had a higher mean BMI compared with the other two groups; this reached statistical significance for comparison between AA and Caucasian patients (28.5 vs 26.32 kg/m2; Table 2). In addition, AA patients had significantly higher mean CPK levels and significantly lower DLCO% predicted and FVC% predicted compared with the other two groups (P < 0.05). However, after adjusting for FVC% and DLCO%, there were no statistical differences in the three groups (Table 2). Hispanic patients had more joint tenderness (Caucasian: 1.09; AA: 1.17; Hispanic: 2.73; P < 0.05), poorer global assessment (Caucasian: 43.98; AA: 42.97; Hispanic: 58.92) of disease severity and higher creatinine clearance (Caucasian: 102.82; AA: 118.4; Hispanic: 124.3) than Caucasian and AA patients (P < 0.05). No significant differences were noted in the swollen joint and physician global assessments (P > 0.05). The Hispanic patients had poorer mental health assessments by the SF-36 MCS and their MCS scores were 0.4 s.d. below the US general population, whereas Caucasian and AA patients were the same as the US general population. No differences were noted in the SF-36 physical component summary (PCS) between the three ethnic groups.
Trend over time
MRSS
Table 3 provides the unadjusted regression coefficient, s.e. and P-value of the time-by-gender interaction term in the gender model. No statistically significant differences were found between gender groups (P = 0.330). Table 4 provides the time-by-ethnic group interaction terms in the ethnicity model. No statistically significant differences were found in comparing the AA, Hispanic and other ethnic groups compared with the Caucasian group (P = 0.071, 0.333 and 0.961). The only significant predictor of decline in MRSS was late disease duration (>48 months, P = 0.001 in the ethnicity model and 0.016 in the gender model).
Table 3.
Course of MRSS, HAQ-DI and FVC% predicted during the study, by gender
| Outcome variable | Covariate | No. of observations | No. of subjects | Coefficient | s.e.a | P-value |
|---|---|---|---|---|---|---|
| MRSSb | (Male) × (time-in-study) | 1773 | 483 | 0.097 | 0.099 | 0.330 |
| HAQ-DIa | (Male) × (time-in-study) | 1780 | 483 | 0.003 | 0.006 | 0.586 |
| FVC%b | (Male) × (time-in-study) | 1280 | 483 | −0.131 | 0.136 | 0.335 |
aWe also included baseline MRSS and its interaction with time (months) for HAQ-DI models. bCovariates included gender, disease duration category, RCT (collagen, relaxin or d-Pen), baseline HAQ-DI, baseline serum creatinine, baseline haematocrit, baseline CPK and their interaction with time (months).
Table 4.
Course of MRSS, HAQ-DI and FVC% predicted during the study, by ethnicity
| Outcome variable | No. of observations | No. of subjects | Coefficient | s.e. | P-value |
|---|---|---|---|---|---|
| MRSSa | 1777 | 477 | |||
| (AA) × time | −0.197 | 0.109 | 0.071 | ||
| (Hispanic) × time | −0.135 | 0.140 | 0.333 | ||
| (Other) × time | 0.009 | 0.195 | 0.961 | ||
| HAQ-DIb | 1751 | 475 | |||
| (AA) × time | 0.006 | 0.006 | 0.303 | ||
| (Hispanic) × time | −0.002 | 0.008 | 0.778 | ||
| (Other) × time | 0.002 | 0.010 | 0.881 | ||
| FVC%c | 1294 | 488 | |||
| (AA) × time | −0.130 | 0.140 | 0.350 | ||
| (Hispanic) × time | 0.282 | 0.191 | 0.141 | ||
| (Other) × time | −0.176 | 0.247 | 0.476 |
aCovariates included disease duration category, RCT (collagen, relaxin or d-Pen), baseline DLCO%, baseline FVC%, baseline CPK and their interaction with time (months). bCovariates included race, disease duration category, RCT (collagen, relaxin or d-Pen), baseline DLCO%, baseline FVC%, baseline CPK, baseline MRSS and their interaction with time (months). cCovariates included race disease duration category, RCT (collagen, relaxin or d-Pen), baseline DLCO% and baseline CPK and their interaction with time (months).
HAQ-DI and FVC% predicted
No statistically significant difference were found between gender groups for HAQ-DI and FVC% (P-values: HAQ = 0.586, FVC% = 0.335; Table 3). Similarly, no significant differences were found in comparing the AA, Hispanic and other ethnic groups with the Caucasian group (P-values: HAQ = 0.288, 0.768 and 0.874; FVC% = 0.350, 0.141, 0.476; Table 4).
In analyses of gender–ethnicity interaction, no gender–ethnicity combinations were found to have statistically significant differing rates of change in MRSS, HAQ-DI and FVC% predicted compared with the rest of the population.
Discussion
We studied the course of MRSS, HAQ-DI and FVC% predicted in three large RCTs of dcSSc. Our analysis suggests that the course of skin thickness, functional disability and lung function are similar between genders and among ethnicities, even though there are several baseline differences between men and women and among the three ethnic groups. We found that, on average, men had higher CPK, haematocrit and serum creatinine that are physiological in nature. We also found that women have higher functional disability. The reason for greater functional disability in women at the entry into the RCTs is unclear, but could be related to differences in pain perception and in pain threshold. Wise et al. [12] assessed different aspects of pain in healthy men and women and found that women had a lower pain tolerance and pain threshold, and higher global pain unpleasantness compared with men (P < 0.05). In RA, women also report a greater functional disability compared with men with similar disease duration [13]. These and other studies [14] demonstrate that biological or cultural factors may play a role in the perception of pain by gender.
Despite similar disease duration among the three ethnic groups, AAs had greater impairment in lung function and higher CPK levels; Hispanics had more severe musculoskeletal signs and symptoms; and Caucasians were older when entering the RCTs. In an analysis from a single SSc centre, Nietert et al. [15] demonstrated that AAs had lower FVC% predicted (73.7 vs 82.2%, P < 0.01) and DLCO% (60.5 vs 70.4%, P < 0.05) compared with Caucasian patients. The mean disease duration in their cohort was longer in Caucasian vs AA patients (7.8 vs 4.2 years, P < 0.05). Moreover, in another cohort study, McNearney et al. [16] noted that AA patients had lower FVC% predicted (75%) compared with Caucasian patients (88.2%, P < 0.002) and lower DLCO% (61.3%) compared with Caucasian (72.2%, P < 0.018) and Hispanic patients (76.8%, P < 0.004). This suggests that AAs have greater lung impairment compared with Caucasians and Hispanics. Another potential reason may be that AAs have physiologically lower lung function compared with Caucasians and Hispanics after adjusting for age and height. It has been postulated that this discrepancy may be due to the fact that AAs have a smaller trunk:leg ratio than do Caucasians [10]. In our pooled data set, we applied the recommended adjustment of 12% for FVC% predicted and 8% for DLCO% and found that the statistical differences were no longer significant. Future studies assessing differences in lung function should be cognizant of these physiological differences and adjust for it.
Our data also show that Hispanic patients had poorer patient global assessment (meaning more severe self-perceived disease), more tender joints and poorer SF-36 MCS scores compared with other ethnic groups, but there were no significant differences in swollen joint count or physician global assessments. It is speculated that, given the differences between patient- and physician-derived measures, Hispanics may have perceived their illness as more severe than AAs or Caucasians. In a prospective observational study among three US ethnic groups comparing clinical and immunological features of patients with SSc, Reveille et al. [17] also showed that Hispanics had a lower SF-36 MCS (44.5) compared with Caucasian (47.9) and AA (47.5) patients. Incidentally, all three ethnic groups had significantly poorer SF-36 physical summary scores—1.4 s.d. below the US general population.
We also found that Caucasian patients were older (49.4 years) than Hispanic (42.9 years) and AA (42.4 years) patients (P < 0.05). Mayes et al. [18] also showed that the average age at diagnosis was significantly higher for Caucasian patients than for AA patients (48.1 vs 41.0 years; P < 0.001). In addition, in a prospective cohort study among three US ethnic groups, Reveille and coworkers [17] showed that Caucasian patients were older than AA and Hispanic patients (51.4 vs 46.8 and 48.5 years; P > 0.05) at diagnosis.
Although previous studies have comprehensively assessed the baseline differences in gender and different ethnic groups, data are scarce regarding the course of MRSS, HAQ-DI and FVC% predicted once a patient develops dcSSc. In our study, skin scores improved in both gender groups and the three ethnic groups over time, but differences in the rate of change of MRSS were not statistically different among gender and ethnic groups. The improvement in MRSS after entering RCTs has been previously reported by ourselves and others [19]. In addition, our RCTs did not show any differences in the course of lung function or functional disability among the gender and ethnic groups over time. Only one other study has assessed the course of lung function in Caucasian vs AA patients. Kuwana et al. [20] assessed patients with anti-topisomerase antibody and found that lung function declined more rapidly in AA patients (FVC% predicted = 3.4%, and DLCO% predicted = 5.1%) compared with Caucasian patients (FVC% predicted = 0.96%, DLCO% predicted = 0.48%) per year. A previous observational study by Steen et al. [21] demonstrated that the major loss of FVC% occurred within the first 4–6 years of SSc: patients who developed severe restrictive disease (FVC% ≤ 50% of predicted) lost 32% of their remaining FVC% each year for the first 2 years, 12% of remaining FVC for each of the next 2 years and 3% of remaining FVC% for each of the following 2 years. However, the Scleroderma Lung Study, a placebo-controlled study, showed an average decline of 2.9% (8.2%) in the placebo group and there were no significant differences in the rate of decline in FVC% in three disease duration groups (0–2, 2–4 and >4 years) [22]. The inherent differences in the observational cohorts vs clinical trials likely explain these discrepancies. The above-noted differences in the ethnic groups may be explained by biological/genetic, geographical, socio-economic and psychological factors (14, 16, 17, 19).
Our study has several strengths. First, our data came from a large population of dcSSc patients with early disease and similar disease duration who were followed regularly per protocol. Second, we pooled data from three large RCTs from several major US scleroderma centers for the analysis. Third, our analysis is the only study to comprehensively assess protocol outcomes of dcSSc patients over time.
We have some limitations in the present study. First, our analysis is post hoc rather than based on a priori hypotheses. Second, our results are part of well-controlled RCTs of dcSSc and may not be applicable to general practice. Also, the follow-up of these patients ranged from 6 to 24 months, with a mean (s.d.) follow-up of 9.4 (7.4) months. Therefore, these findings should be interpreted with caution in clinical practice. However, the findings would be applicable for clinical trials of dcSSc. Third, the ethnicity was self-identified in all RCTs and we did not gather data on income, educational level, or family support which may be significant confounders in the ethnicity comparisons. In a prospective cohort study, Reveille and colleagues showed that AA and Hispanic patients had lower socio-economic status, such as mean education (AA: 10.8 years, Hispanic: 12.6 years and Caucasian: 13.7 years) and mean monthly income (AA: $1919, Hispanic: $2307 and Caucasian: $3757) compared with Caucasian patients [18]. Fourth, we did not measure serum antibodies in the dcSSc patients. Therefore, the present study is unable to examine the role of antibodies as a possible explanation for gender and ethnicity differences.
In conclusion, we confirm the previously reported observations regarding baseline differences in gender and ethnicity. In addition, we show that gender and ethnicity have no impact on the course of disease in patients with dcSSc participating in RCTs.

Disclosure statement: D.K. is supported by a National Institutes of Health Award (NIAMS K23 AR053858-04) and a New Investigator Grant from the Scleroderma Foundation. P.P.K. is supported by a Ruth L. Kirschstein National Research Service Award (NRSA), Institutional Research Training Grant (NIAMS 1 T32 AR053463) and American College of Rheumatology Research and Education Foundation Clinical Investigator Fellowship Award 2009–11. D.E.F. has received grants/research support, honoraria, consultancies from Actelion and Gilead, and is a member of speaker’s bureau for Actelion. M.D.M. has received honoraria, is a member of a speakers bureau and has received grants/research support from Actelion Pharma, United Therapeutics and Gilead. All other authors have declared no conflicts of interest.
References
- 1.Krishnan E, Furst DE. Systemic sclerosis mortality in the United States: 1979–1998. Eur J Epidemiol. 2005;20:855–61. doi: 10.1007/s10654-005-2210-5. [DOI] [PubMed] [Google Scholar]
- 2.Laing TJ, Gillespie BW, Toth MB, et al. Racial differences in scleroderma among women in Michigan. Arthritis Rheum. 1997;40:734–42. doi: 10.1002/art.1780400421. [DOI] [PubMed] [Google Scholar]
- 3.Reveille JD, Fischbach M, McNearney T, et al. Systemic sclerosis in 3 US ethnic groups: a comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin Arthritis Rheum. 2001;30:332–46. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- 4.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–54. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 5.Clements PJ, Wong WK, Hurwitz EL, et al. Correlates of the disability index of the health assessment questionnaire: a measure of functional impairment in systemic sclerosis. Arthritis Rheum. 1999;42:2372–80. doi: 10.1002/1529-0131(199911)42:11<2372::AID-ANR16>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Khanna D, Clements PJ, Furst DE, et al. Recombinant human relaxin in the treatment of systemic sclerosis with diffuse cutaneous involvement: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:1102–11. doi: 10.1002/art.24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postlethwaite AE, Wong WK, Clements P, et al. A multicenter, randomized, double-blind, placebo-controlled trial of oral type I collagen treatment in patients with diffuse cutaneous systemic sclerosis: I. oral type I collagen does not improve skin in all patients, but may improve skin in late-phase disease. Arthritis Rheum. 2008;58:1810–22. doi: 10.1002/art.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller WG, Myers GL, Ashwood ER, et al. Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med. 2005;129:297–304. doi: 10.5858/2005-129-297-CMSOTA. [DOI] [PubMed] [Google Scholar]
- 9.Ryan DH. Examination of the blood. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ, Seligsohn U, editors. William’s Hematology. 6th edition. New york: McGraw-Hill; 2001. pp. 9–17. [Google Scholar]
- 10.Miller A. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1992;146:1368–9. doi: 10.1164/ajrccm/146.5_Pt_1.1368b. [DOI] [PubMed] [Google Scholar]
- 11.Khanna PP, Furst DE, Clements PJ, et al. Tendon friction rubs in early diffuse systemic sclerosis: prevalence, characteristics and longitudinal changes in a randomized controlled trial. Rheumatology. 2010;49:955–9. doi: 10.1093/rheumatology/kep464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wise EA, Price DD, Myers CD, et al. Gender role expectations of pain: relationship to experimental pain perception. Pain. 2002;96:335–42. doi: 10.1016/s0304-3959(01)00473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tengstrand B, Ahlmen M, Hafstrom I. The influence of sex on rheumatoid arthritis: a prospective study of onset and outcome after 2 years. J Rheumatol. 2004;31:214–22. [PubMed] [Google Scholar]
- 14.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–45. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 15.Nietert PJ, Mitchell HC, Bolster MB, et al. Racial variation in clinical and immunological manifestations of systemic sclerosis. J Rheumatol. 2006;33:263–8. [PubMed] [Google Scholar]
- 16.McNearney TA, Reveille JD, Fischbach M, et al. Pulmonary involvement in systemic sclerosis: associations with genetic, serologic, sociodemographic, and behavioral factors. Arthritis Rheum. 2007;57:318–26. doi: 10.1002/art.22532. [DOI] [PubMed] [Google Scholar]
- 17.Reveille JD, Fischbach M, McNearney T, et al. Systemic sclerosis in 3 US ethnic groups: a comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin Arthritis Rheum. 2001;30:332–46. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- 18.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–55. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 19.Amjadi S, Maranian P, Furst DE, et al. Course of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: analysis of three large multicenter, double-blind, randomized controlled trials. Arthritis Rheum. 2009;60:2490–8. doi: 10.1002/art.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuwana M, Kaburaki J, Arnett FC, et al. Influence of ethnic background on clinical and serologic features in patients with systemic sclerosis and anti-DNA topoisomerase I antibody. Arthritis Rheum. 1999;42:465–74. doi: 10.1002/1529-0131(199904)42:3<465::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Steen VD, Conte C, Owens GR, et al. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37:1283–9. doi: 10.1002/art.1780370903. [DOI] [PubMed] [Google Scholar]
- 22.Khanna D, Farmani N, Tseng CH, et al. Course of lung physiology in patients with scleroderma and active interstitial lung disease: results from the scleroderma lung study (SLS) Philadelphia, PA: American College of Rheumatology, 2009:605. [Google Scholar]