Abstract
Introduction:
Waterpipe tobacco smoking is increasing worldwide and is believed by many users to be less harmful and addictive than cigarette smoking. In fact, waterpipe tobacco and cigarette smoke contain many of the same chemicals, and users are exposed to the dependence-producing drug nicotine as well as other smoke toxicants. The subjective effect profile of these 2 tobacco use methods has not been compared directly, though this information is relevant to understanding the risk of dependence development.
Methods:
Fifty-four participants who reported waterpipe and cigarette smoking completed 2, 45-min, counter-balanced sessions in which they completed a waterpipe use episode (mean smoking time = 43.3 min) or a cigarette (mean = 6.1 min). Outcome measures included plasma nicotine, carboxyhemoglobin (COHb), and subjective effects, including those relevant to predicting dependence potential.
Results:
Mean (±SEM) peak plasma nicotine concentration did not differ by session (waterpipe = 9.8 ± 1.0 ng/ml; cigarette = 9.4 ± 1.0 ng/ml). Mean peak COHb concentration differed significantly (waterpipe = 4.5% ± 0.3%; cigarette = 1.2% ± 0.1%). Subjective effect changes for waterpipe and cigarette were comparable in magnitude but often longer lived for waterpipe.
Conclusions:
Relative to a cigarette, waterpipe tobacco smoking was associated with similar peak nicotine exposure, 3.75-fold greater COHb, and 56-fold greater inhaled smoke volume. Waterpipe and cigarette influenced many of the same subjective effect measures. These findings are consistent with the conclusion that waterpipe tobacco smoking presents substantial risk of dependence, disease, and death, and they can be incorporated into prevention interventions that might help deter more adolescents and young adults from experimenting with an almost certainly lethal method of tobacco use.
Introduction
Waterpipe tobacco smoking involves using charcoal to heat sweetened and flavored tobacco and then inhaling the resulting smoke after it has been drawn through water (Cobb, Ward, Maziak, Shihadeh, & Eissenberg, 2010; Knishkowy & Amitai, 2005; Maziak, Ward, Soweid, & Eissenberg, 2004). This form of tobacco use is popular among young adults and adolescents worldwide (e.g., Combrink et al., 2010; Dugas, Tremblay, Low, Cournoyer, & O’Loughlin, 2010; Jackson & Aveyard, 2008; Pärna, Usin, & Ringmets, 2008). This popularity may be due, at least in part, to the perception that, relative to cigarette smoking, waterpipe tobacco smoking is less harmful (Aljarrah, Ababneh, & Al-Delaimy, 2009; Combrink et al., 2010; Jackson & Aveyard, 2008; Jamil, Elsouhag, Hiller, Arnetz, & Arnetz, 2010; Smith-Simone, Maziak, Ward, & Eissenberg, 2008). Contrary to this perception, recent data indicate that waterpipe tobacco smoking involves significant delivery of toxicants, such as nicotine (Neergaard, Singh, Job, & Montgomery, 2007; Salameh, Bacha, & Waked, 2009) and carbon monoxide (CO; El-Nachef & Hammond, 2008; Maziak et al., 2009). In a recent preliminary report of a direct comparison of the toxicant exposure associated with a single cigarette smoking episode and a single 45-min waterpipe tobacco smoking episode, we observed that waterpipe tobacco smoking led to equivalent nicotine and three times the CO exposure (Eissenberg & Shihadeh, 2009). The fact that waterpipe tobacco smoking exposes users to these and other smoke toxicants may explain why this form of tobacco use has been associated with adverse health effects, including cardiovascular disease and cancer (Akl et al., 2010; World Health Organization, 2005).
There is very little information regarding the subjective effects associated with waterpipe tobacco smoking. Certain subjective effects are relevant to understanding a drug’s abuse liability: the likelihood that the drug will be used recreationally as opposed to therapeutically (Carter & Griffiths, 2009; Carter et al., 2009; Jasinski & Henningfield, 1989). Abuse liability, in turn, is related to the development of dependence when recreational use transitions to uncontrolled use that continues despite cessation attempts and knowledge of adverse consequences (Carter & Griffiths, 2009). Waterpipe tobacco smoking is clearly recreational and may have significant abuse liability. However, many waterpipe tobacco smokers report their perception that this form of tobacco use poses less risk of dependence/addiction than cigarette smoking (Eissenberg, Ward, Smith-Simone, & Maziak, 2008; Primack et al., 2008; Smith-Simone et al., 2008).
Two studies address the risk of tobacco/nicotine dependence in waterpipe tobacco smokers (Maziak et al., 2009; Salameh et al., 2009). The first relates waterpipe dependence scale scores with objective measures of tobacco exposure and concludes that scale factors resemble those observed in dependent cigarette smokers (Salameh, Waked, & Aoun, 2008; Salameh et al., 2009). The second notes that, in 61 waterpipe tobacco smokers, subjective effects commonly associated with tobacco abstinence in cigarette smokers (i.e., urges to smoke, restlessness, craving) were suppressed following a single waterpipe tobacco use episode (Maziak et al., 2009). Both reports highlight the value of measuring subjective effects when attempting to understand if waterpipe tobacco smoking results in tobacco/nicotine dependence, but neither report directly compares the subjective effect profile of waterpipe tobacco smoking with that produced by a form of tobacco use known to support dependence: cigarette smoking. The dependence potential of cigarettes is obviously great, and the subjective effects of cigarettes that are associated with their dependence potential have been well characterized. Determining the extent to which waterpipe tobacco smoking produces similar subjective effects is important in assessing the dependence potential of this tobacco use form. Similarly, the relatively rapid uptake of nicotine following cigarette smoke inhalation appears to be an important determinant of the high dependence potential of cigarettes (Benowitz, 2008). Comparing nicotine absorption profiles of waterpipe tobacco and cigarette smoking would also be important in assessing the dependence potential of waterpipe smoke. Thus, this study, an elaboration and extension of our previous report (Eissenberg & Shihadeh, 2009), compares the subjective effect profile of waterpipe tobacco and cigarette smoking.
Methods
Participants
Fifty-nine participants recruited in 2008–2009 from the Richmond VA community (a medium-sized metropolitan area) provided informed consent and attended at least one session in this Virginia Commonwealth University Institutional Review Board-approved study. One was discontinued for low blood pressure during a session and four withdrew voluntarily. The remaining 54 participants (36 men, 17 non-White) were healthy, aged 18–50 years (M = 21.2 years, SD = 2.3), reported smoking tobacco using a waterpipe at least two times per month (M = 5.7, SD = 4.4) for the past six months (M = 1.8 years, SD = 1.2), and reported smoking at least 5 cigarettes/week (M = 9.8/day, SD = 6.4) for the past month. Exclusion criteria included a history of chronic health problems or psychiatric conditions, low or high blood pressure, regular use of prescription medication (other than vitamins or birth control), and current pregnancy or breast feeding. In addition, past month use of other tobacco products and past month use (via self-report or positive urine test) of cocaine, opioids, benzodiazepines, and methamphetamine was exclusionary. Individuals who reported using marijuana more than five days or alcohol more than twenty days in the past thirty days were also excluded. Any participant who reported a current intent to quit smoking was excluded and referred to a smoking cessation provider.
Study Design and Procedures
Screened, qualified, consenting participants attended the laboratory for two counter-balanced approximately 2-hr sessions that differed by product administered: waterpipe or cigarette. Before each session, 12 hr of tobacco abstinence was required, as verified with an expired-air CO concentration ≤10 ppm (e.g., Breland, Evans, Buchhalter, & Eissenberg, 2002). Once abstinence was verified, a pulmonary function test (PFT; see below) was administered and a catheter was inserted into a forearm vein. Following catheter insertion, the session began (at Time 0) with continuous physiological recording. Thirty minutes after session onset (Time +30), blood was sampled for immediate determination of carboxyhemoglobin (COHb) concentration and later determination of plasma nicotine concentration. Additionally, an expired-air sample was assessed for nitric oxide (NO) and CO concentration, and participants responded to subjective effect questionnaires.
For the waterpipe session, a waterpipe (described below) with a head loaded with 15 g of the participant’s preferred brand and flavor of waterpipe tobacco and covered in perforated foil was presented to participants. Study staff lit a single quick-lighting charcoal briquette (Three Kings, Holland), and in every session, the waterpipe hose was tipped with a new, sterile disposable mouthpiece. Additional preweighed half charcoal briquettes were available to add to the waterpipe head upon participant request. For the cigarette smoking session, an own-brand cigarette was lit by the experimenter and placed in the mouthpiece used to measure puff topography.
In each session, participants were seated comfortably and viewed a video of their choice. Participants inhaled ad libitum during both sessions, and topography was measured while the cigarette was smoked and during the 45-min waterpipe smoking period. Subjective measures were administered, and blood was sampled at 5, 15, 30, and 45 min after the onset of puffing. Expired-air CO and then NO concentrations were assessed at 50 and 60 min. A second PFT was performed following the 60-min NO measurement (at approximately 65–70 min). At the end of each session, participants were paid (total of $175 for completion of both sessions). The laboratory was ventilated with exhaust fans and ambient CO levels (measured for 41 participants) never exceeded 7 ppm during any session (M = 3.2 for the waterpipe and 0.2 for the cigarette session).
Materials
The waterpipe consisted of a chrome body (height = 43 cm) screwed into an acrylic base (height = 24 cm; volume = 1230 ml; www.myasaray.com). Water (870 ml) was poured into the base, submerging about 2.5 cm of the body’s conduit. The waterpipe head was made of fired ceramic (6-cm diameter) with five holes in the base. A circular sheet of aluminum foil (diameter = 11.5 cm; www.smoking-hookah.com) separated the charcoal from the tobacco, after the foil had been perforated with a “screen pincher” (see www.smoking-hookah.com). A sterile plastic tip (www.hookahcompany.com) was added to the mouthpiece for each session.
The waterpipe tobacco and cigarettes used in all sessions were the participants’ preferred brand and flavor. For the waterpipe tobacco, fruit flavors were most common (n = 46; e.g., mango, strawberry, and melon). Other preferred flavors included mint (n = 4), vanilla (n = 2), X on the beach (n = 1), and jasmine (n = 1). Preferred brands included Al-Fakher (United Arab Emirates; n = 7) and Starbuzz (United States; n = 5). When a participant could not name a preferred brand, Nakhla (Egypt; n = 25) was used. In some cases, participants named a preferred flavor that was unavailable (e.g., not produced by Nakhla). In these cases, other brands were used: Starbuzz (n = 14), Al-Amir (Saudi Arabia; n = 2), or Al-Fakher (n = 1). All waterpipe products were purchased from www.hookahcompany.com. Participants’ preferred cigarettes varied greatly: Marlboro Lights (n = 10), Camel Lights (n = 8), Newport (n = 5), Marlboro Lights menthol (n = 4), Marlboro Reds (n = 3), Marlboro 27 (n = 3), Marlboro Smooth (n = 3), Marlboro menthol (n = 2), Camel Turkish Gold (n = 2), Camel Turkish Silver (n = 2), Camel Crush (n = 2), Camel #9 (n = 2), Camel (n = 2), Pall Mall Lights (n = 1), Parliament (n = 1), Camel Ultra-Lights NM (n = 1), Camel Jade Light (n = 1), Camel Jade (n = 1), and Camel Signature Infused (n = 1). On average, participants’ preferred cigarettes yielded per Federal Trade Commission (FTC) method 0.9 mg nicotine (SD = 0.2), 12.5 mg tar (SD = 2.9), and 12.6 mg CO (SD = 2.6; data available for 35 participants; FTC, 2000).
Outcome Measures
Physiological Measures
Expired-air CO was assessed with a BreathCO monitor (Vitalograph, Lenexa, KS). COHb level was analyzed less than 1 min after sampling (NPT7 blood gas analyzer; Radiometer America), and 10 ml of blood was centrifuged, plasma stored at −70°C, and analyzed for nicotine concentration (limit of quantitation [LOQ] = 2.0 ng/ml; a modified version of that reported by Naidong, Shou, Chen, & Jiang, 2001; see Breland, Kleykamp, & Eissenberg, 2006 for details). Heart rate (HR) was measured every 20 s (Model 506; Criticare Systems; fitted with a reusable finger pulse oximeter sensor). Cigarette smoking has been shown to alter expired-air nitric oxide (NO) acutely (Chambers, Tunnicliffe, & Ayres, 1998; Kharitonov, Robbins, Yates, Keatings, & Barnes, 1995), and the influence of waterpipe tobacco smoking on NO is uncertain. Thus, expired-air NO concentrations were measured with the Nitric Oxide Analyzer 280i (Ionics Inst., Boulder, CO). Three satisfactory NO measurements were made for each timepoint, and the average was used. PFTs were performed with a spirometer (Vitalograph) to measure three respiratory outcomes: forced expiratory volume in 1 s (FEV1), forced expiratory vital capacity (FVC), and the FEV1/FVC ratio, where a lower ratio reflects an increase in airway obstruction (National Collaborating Centre for Chronic Conditions, 2003). Two satisfactory PFT maneuvers were recorded, and the one with best effort in terms of FEV1 was analyzed.
Subjective Measures
Participants used a computer keyboard and mouse to respond to four subjective measures that have been demonstrated to be sensitive to the acute effects of cigarette smoking. The Tiffany–Drobes Questionnaire of Smoking Urges—Brief Form (QSU-Brief) consists of 10 smoking-related items and has been empirically validated (Cox, Tiffany, & Christen, 2001). Participants rated each item on a 7-point scale ranging from 0 (strongly disagree) to 6 (strongly agree). The items form two factors: Factor 1 (intention to smoke) and Factor 2 (anticipation of relief from withdrawal; Cox et al., 2001). During the waterpipe session, QSU-Brief items were modified by replacing the word “cigarette” with “waterpipe.” For the Hughes–Hatsukami Withdrawal Scale, tobacco abstinence symptoms (Hughes & Hatsukami, 1986) were used to form 11 computerized Visual Analog Scale (VAS) items (see Table 1). A word or phrase was centered above a horizontal line anchored on the left with “Not at all” and on the right with “Extremely.” Participants responded by moving a cursor to any point on the line and clicking, producing a vertical mark, which could be adjusted if necessary. The score for each scale was the distance of the vertical mark from the left anchor, expressed as a percentage of total line length (see Breland et al., 2002). During the waterpipe session, for the items “Urges to smoke a cigarette” and “Craving a cigarette/Nicotine” the word “cigarette” was replaced by the word “waterpipe.” Table 1 presents the 10 VAS items of the Direct Effects of Nicotine Scale (DEN; Evans, Blank, Sams, Weaver, & Eissenberg, 2006). Finally, the Direct Effects of Tobacco (DET) Scale, a 13 VAS item questionnaire, was adapted from previous studies of smoking’s subjective effects (e.g., Foulds et al., 1992; Pickworth, Bunker, & Henningfield, 1994). Ten of these items were taken verbatim from previous reports (Kleykamp, Jennings, Sams, Weaver, & Eissenberg, 2008; see Table 1 for abbreviated text), and three additional items were included in this study: “Did the cigarette taste bad?”, “Did the cigarette make you feel confused?”, and “Did the cigarette make you sleepy?”. During the waterpipe session, DET items were modified by replacing the word “cigarette” with “waterpipe.” Across these four subjective measures, multiple individual items can be used to indicate dimensions of dependence potential: withdrawal relief (Hughes–Hatsukami Withdrawal Scale-“Urges to smoke,” “Anxious”; QSU-Brief-Factor 2), positive physiological effects (DEN-“Lightheaded,” DEN-“Dizzy,” and DET-“Dizzy”), and drug/product liking (DET-“Satisfy,” DET-“Pleasant,” and DET-“Taste good”).
Table 1.
Statistical Analyses Results for Physiological and Subjective Measures
| Physiological measures | Session (S) | p value | Time (T) | p value | S × T | p value |
| Plasma nicotinea | 1.5 | ns | 35.9 | <.001 | 28.2 | <.001 |
| Heart rateb | 2.3 | ns | 82.2 | <.001 | 45.6 | <.001 |
| COHbc | 82.8 | <.001 | 95.8 | <.001 | 83.1 | <.001 |
| Expired-air COd | 84.4 | <.001 | 119.4 | <.001 | 89.4 | <.001 |
| Expired-air NOe | 1.7 | ns | 0.8 | ns | 0.2 | ns |
| PFT: FEV1f | 3.3 | ns | 7.3 | <.05 | 0.3 | ns |
| PFT: FVCf | 3.7 | ns | 10.3 | <.01 | 0.0 | ns |
| PFT: FEV1/FVC ratiof | 0.9 | ns | 1.3 | ns | 0.1 | ns |
| Subjective measuresg | ||||||
| QSU-Brief | ||||||
| Factor 1 | 0.8 | ns | 15.6 | <.001 | 28.6 | <.001 |
| Factor 2 | 1.7 | ns | 9.6 | <.001 | 16.2 | <.001 |
| Hughes–Hatsukami Withdrawal Scale | ||||||
| Urges to smoke a [cigarette/waterpipe] | 2.4 | ns | 10.0 | <.001 | 15.5 | <.001 |
| Irritability/frustration/anger | 5.1 | <.05 | 7.9 | <.01 | 1.9 | ns |
| Anxious | 5.8 | <.05 | 19.0 | <.001 | 1.2 | ns |
| Difficulty concentrating | 0.9 | ns | 3.1 | <.05 | 2.0 | ns |
| Restlessness | 3.0 | ns | 7.8 | <.01 | 1.5 | ns |
| Hunger | 1.1 | ns | 11.0 | <.001 | 1.8 | ns |
| Impatient | 8.6 | <.01 | 11.7 | <.001 | 2.7 | ns |
| Craving a [cigarette/waterpipe]/Nicotine | 3.0 | ns | 20.0 | <.001 | 13.3 | <.001 |
| Drowsiness | 4.2 | <.05 | 2.1 | ns | 1.5 | ns |
| Depression/feeling blue | 5.4 | <.05 | 2.3 | ns | 1.7 | ns |
| Desire for sweets | 0.0 | ns | 3.1 | <.05 | 3.1 | <.05 |
| DEN | ||||||
| Nauseous | 1.2 | ns | 0.7 | ns | 1.2 | ns |
| Dizzy | 1.2 | ns | 6.2 | <.001 | 3.4 | <.05 |
| Lightheaded | 8.7 | <.01 | 15.5 | <.001 | 7.0 | <.001 |
| Nervous | 1.5 | ns | 0.2 | ns | 0.8 | ns |
| Sweaty | 0.9 | ns | 2.5 | ns | 1.6 | ns |
| Headache | 0.0 | ns | 0.8 | ns | 0.8 | ns |
| Excessive salivation | 3.1 | ns | 0.2 | ns | 0.9 | ns |
| Heart pounding | 0.2 | ns | 1.3 | ns | 2.4 | ns |
| Confused | 2.9 | ns | 0.2 | ns | 0.9 | ns |
| Weak | 2.9 | ns | 0.2 | ns | 0.5 | ns |
| DET | ||||||
| Satisfy | 11.4 | <.01 | 248.2 | <.001 | 5.2 | <.01 |
| Pleasant | 18.0 | <.001 | 255.0 | <.001 | 4.3 | <.01 |
| Taste good | 23.2 | <.001 | 258.5 | <.001 | 8.0 | <.001 |
| Taste bad | 8.8 | <.01 | 7.7 | <.001 | 5.3 | <.01 |
| Dizzy | 2.6 | ns | 19.5 | <.001 | 1.5 | ns |
| Calm | 2.2 | ns | 85.5 | <.001 | 1.5 | ns |
| Confused | 0.0 | ns | 4.8 | <.01 | 1.4 | ns |
| Concentrate | 0.2 | ns | 37.0 | <.001 | 0.8 | ns |
| Awake | 0.0 | ns | 55.3 | <.001 | 0.6 | ns |
| Reduce hunger | 0.0 | ns | 61.2 | <.001 | 0.8 | ns |
| Sick | 3.1 | ns | 3.2 | <.05 | 3.5 | <.05 |
| Sleepy | 5.7 | <.05 | 15.8 | <.001 | 0.9 | ns |
| Right now | 4.5 | <.05 | 82.3 | <.001 | 11.7 | <.001 |
Note. DEN = Direct Effects of Nicotine Scale; DET = Direct Effects of Tobacco Scale; FVC = forced expiratory vital capacity; FEV1 = forced expiratory volume in 1 s; ns = nonsignificant; PFT = pulmonary function test; QSU-Brief = Questionnaire of Smoking Urges—Brief Form.
dfsession (S) = (1, 50), dftime (T) = (4, 200), dfS × T = (4, 200).
dfS = (1, 52), dfT = (9, 468), dfS × T = (9, 468).
dfS = (1, 52), dfT = (4, 208), dfS × T = (4, 208).
dfS = (1, 53); dfT = (2, 106), dfS × T = (2, 106).
dfS = (1, 53), dfT = (2, 106), dfS × T = (2, 106).
dfS = (1, 50), dfT = (1, 50), dfS × T = (1, 50).
dfS = (1, 53), dfT = (4, 212), dfS × T = (4, 212).
Puff Topography
To measure waterpipe topography, a differential pressure flow sensor was integrated into the waterpipe hose (Shihadeh, Antonios, & Azar, 2005). For cigarettes, participants smoked through a mouthpiece that was connected to a pressure transducer (CReSS Lab; Borgwaldt KC). In both cases, previously calibrated software converted digital signals to air flow (milliliters per second) and integrated these data to produce measures of puff volume, duration, number, and interpuff interval (IPI).
Data Analyses
Data were analyzed as in our earlier report of the nicotine, COHb, CO, HR, and topography results from the first 31 participants (Eissenberg & Shihadeh, 2009). Plasma nicotine results below the LOQ were replaced with the LOQ. HR values were averaged for 5-min periods beginning with the 5 min preceding product administration. In the event of missing data, an average of the value before and after the missing value was used (less than 0.2% of data were missing). When surrounding values were missing (e.g., due to equipment failure), the participant’s data were excluded from analysis for that measure. Accordingly, analyses for HR and COHb are based on 53 participants, and for puff topography, plasma nicotine, and PFT outcomes are based on 51 participants.
Initially, a mixed repeated measures analysis of variance (ANOVA) was performed for all outcomes where charcoal added during the waterpipe session was the between-subjects factor (yes or no; 10 participants opted to add charcoal during their waterpipe session). For plasma nicotine, CO, COHb, HR, NO, PFT, and subjective effect data, the two within-subjects factors were session (waterpipe or cigarette) and time (levels varied by measure). Topography data were averaged within each session to obtain a single value for puff volume, IPI, total puff number, and total puff volume and analyzed using a single-factor (session) within-subjects ANOVA. Of 184 main effects and interactions involving the between-subjects factor (charcoal added), only 7 were significant (p < .05). Because these few significant results may reflect Type I error rather than a real difference due to the effects of adding charcoal or the participants who chose to engage in this behavior, the analysis was repeated without the between-subjects factor, and the results from this completely within-subjects analysis are reported below. In addition, each session’s peak plasma nicotine and COHb concentration were determined, and these data were analyzed using a single-factor (session) within-subjects ANOVA. Huynh–Feldt corrections were used to account for violations of sphericity (Huynh & Feldt, 1976), and Tukey’s honestly significant difference (Keppel, 1991) was used to explore differences between means (p < .05; see Breland et al., 2006).
Results
Statistical analyses (main effects and interactions) for all measures are displayed in Table 1. The effects of greatest interest involve the interaction of session and time, indicating that the effects of smoking were depended on whether participants were using waterpipe or cigarette.
Physiological Measures
For plasma nicotine, HR, expired-air CO, and COHb, statistical analysis revealed a significant session by time interaction (see Table 1). Figure 1 (top panel) shows the mean data for plasma nicotine by session and time. For cigarette, the mean (±SEM) pre-smoking plasma nicotine concentration was 2.1 ± 0.1 ng/ml and rose significantly to 8.9 ± 1.0 ng/ml at 5 min after product administration. Mean plasma nicotine concentration decreased during subsequent timepoints in this session, though it remained significantly greater than baseline at 15 (6.3 ± 0.5 ng/ml) and 30 min (4.8 ± 0.4 ng/ml) but not at 45 min (3.9 ± 0.3 ng/ml) after product administration. For waterpipe, mean pre-smoking plasma nicotine concentration was 2.0 ± 0.01 ng/ml and increased significantly to 5.0 ± 0.7 ng/ml at 5 min and continued to increase at 15 (6.2 ± 0.6 ng/ml), 30 (7.7 ± 0.7 ng/ml), and 45 (8.3 ± 0.9 ng/ml) min after product administration. Relative to cigarette, the mean plasma nicotine concentration observed in the waterpipe session was significantly lower at 5 min and significantly higher at 30 and 45 min. For peak plasma nicotine concentration, there was no significant difference between cigarette (9.4 ± 1.0 ng/ml) and waterpipe, 9.8 ± 1.0 ng/ml; F(1, 50) = 0.2.
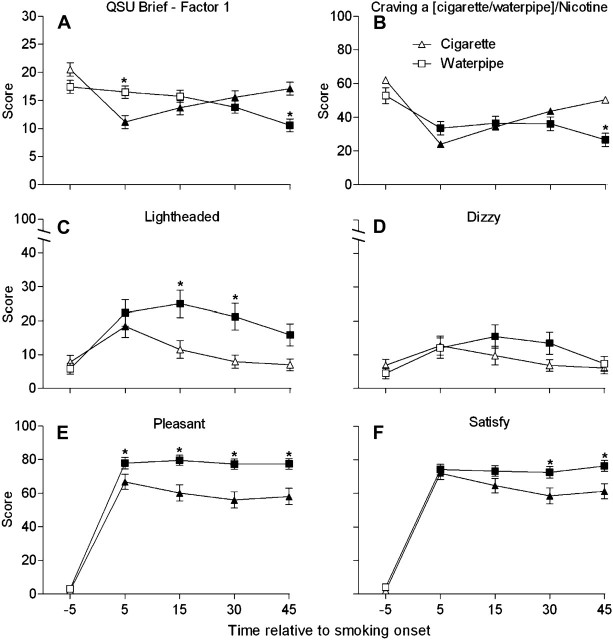
Figure 1.
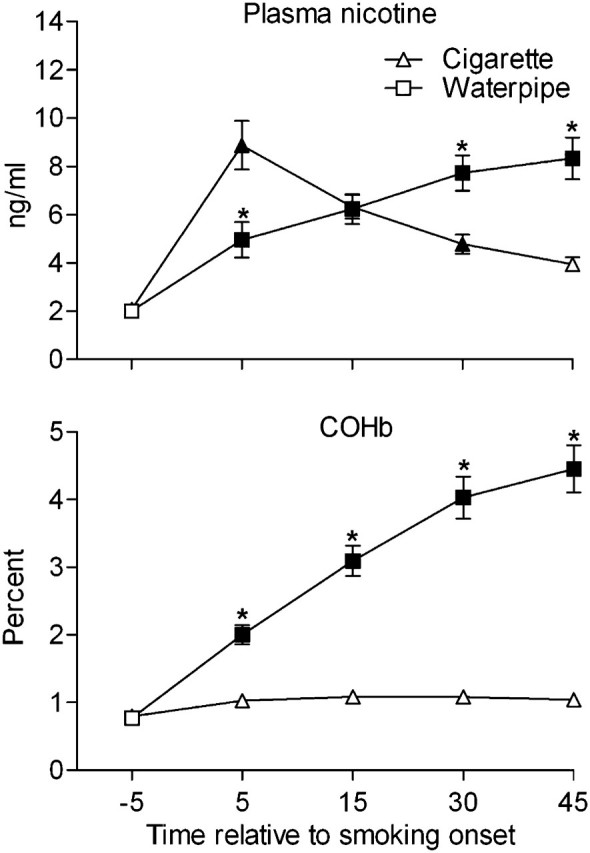
Mean data (±SEM) for plasma nicotine (top panel; n = 51) and COHb (bottom panel; n = 53). Filled symbols indicate a significant difference relative to the pre-smoking mean, and asterisks indicate a significant difference between sessions (p < .05, Tukey’s honestly significant difference).
For HR, significant increases relative to the pre-smoking mean (68.4 ± 1.4 beats per minute [bpm]) were observed in the cigarette session at 5 (80.8 ± 1.5 bpm), 10 (85.0 ± 1.5 bpm), 15 (77.3 ± 1.4 bpm), 20 (75.9 ± 1.3 bpm), and 35 min (73.6 ± 1.2 bpm) after product administration. During the waterpipe session, significant increases relative to the pre-smoking mean (68.2 ± 1.1 bpm) were observed at each 5-min interval during smoking: HR increased to 72.7 ± 1.1 bpm at 5, 79.1 ± 1.6 bpm at 10, 77.6 ± 1.4 bpm at 15, 78.7 ± 1.3 bpm at 20, 76.0 ± 1.4 bpm at 25, 76.1 ± 1.4 bpm at 30, 78.4 ± 1.3 bpm at 35, 75.5 ± 1.4 bpm at 40, and 75.9 ± 1.3 bpm at 45 min after product administration. Compared with the cigarette, mean HR during the waterpipe session was significantly lower at 5 and 10 min and significantly higher at 25, 30, 35, 40, and 45 min after product administration.
For expired-air CO, mean pre-smoking values did not differ for cigarette and waterpipe (collapsed across condition = 4.9 ± 0.4 ppm). For cigarette, mean expired-air CO concentration increased to 7.4 ± 0.5 ppm at 50 and 7.1 ± 0.5 ppm at 60 min (nonsignificant [ns]), and for waterpipe, mean CO concentration increased significantly to 32.9 ± 2.7 ppm at 50 and 31.1 ± 2.6 ppm at 60 min after smoking. Relative to cigarette, the mean CO concentration observed for waterpipe was significantly greater at 50 and 60 min after product administration.
Mean COHb data are shown in Figure 1 (bottom panel). Mean pre-smoking concentration of COHb did not differ for cigarette and waterpipe sessions (collapsed across condition = 0.8% ± 0.1%). For cigarette, mean COHb concentration increased during the session, but these values were not significantly different from the pre-smoking mean: 1.0% ± 0.1% at 5, 1.1% ± 0.1% at 15, 1.1% ± 0.1% at 30, and 1.0% ± 0.1% at 45 min after product administration. For waterpipe, a significant increase relative to pre-smoking was observed at all post-smoking timepoints: 2.0% ± 0.1% at 5, 3.1% ± 0.2% at 15, 4.0% ± 0.3% at 30, and 4.5% ± 0.3% at 45 min. All of these waterpipe-associated increases were significantly greater than those observed during the cigarette session. Mean peak COHb concentration for cigarette was 1.2% ± 0.1% and for waterpipe was 4.5% ± 0.3%, F(1, 52) = 84.7, p < .001.
Table 1 shows that there were no significant main effects or interactions for expired-air NO concentration and the FEV1/FVC ratio, though main effects of time were observed for FEV1 and FVC. Mean NO concentration during the cigarette session at baseline was 22.6 (2.4), 22.6 (2.3) at 50 min, and 23.2 (2.3) at 60 min. Mean waterpipe NO concentration at baseline was 26.5 (4.7), 26.1 (4.5) at 50 min, and 26.7 (4.4) at 60 min. Collapsed across session, mean pre-smoking FEV1 and FVC values were 3.6 and 4.0, respectively, while post-smoking values were 3.5 and 3.9, respectively.
Subjective Measures
Questionnaire of Smoking Urges—Brief Form
Significant session by time interactions was observed for both factors of the QSU-Brief, and Figure 2A displays data for Factor 1 (intention to smoke), the factor with the higher session by time F value. For cigarette, the mean pre-smoking score was 20.5 ± 1.2 and scores decreased significantly at 5 min after the beginning of smoking to 11.2 ± 1.2. During the remainder of the cigarette session, when participants were no longer smoking (the mean time spent smoking was 6.1 min), the mean Factor 1 score gradually increased but remained significantly lower than the mean pre-smoking score at 15 (13.7 ± 1.2), 30 (15.6 ± 1.2), and 45 min (17.1 ± 1.1). For waterpipe, relative to the mean pre-smoking score of 17.4 ± 1.1, mean Factor 1 scores did not differ significantly at 5 (16.5 ± 1.1) or 15 min (15.7 ± 1.1), though they differed significantly at 30 (13.8 ± 1.1) and 45 min (10.6 ± 1.1). Mean Factor 1 score in the cigarette condition was significantly lower than waterpipe at 5 min, though the situation was reversed at 45 min. This pattern of results was virtually identical for Factor 2.
Figure 2.
Mean data (±SEM; n = 54) for Factor 1 of the Questionnaire of Smoking Urges—Brief Form (A), “Craving a [cigarette/waterpipe]/Nicotine” from the Hughes–Hatsukami Withdrawal Scale (B), “Lightheaded” from the Direct Effects of Nicotine Scale (DEN) (C), “Dizzy” from the DEN (D), “Was the [cigarette/waterpipe] pleasant?” from the DET (E), and “Was the [cigarette/waterpipe] satisfying?” from the DET (F). Filled symbols indicate a significant difference relative to the pre-smoking mean, and asterisks indicate a significant difference between sessions (p < .05, Tukey’s honestly significant difference).
Hughes–Hatsukami Withdrawal Scale
Significant session by time interactions were observed for three items on the Hughes–Hatsukami Withdrawal Scale: “Urges to smoke a [cigarette/waterpipe],” “Craving a [cigarette/waterpipe]/Nicotine,” and “Desire for sweets” (see Table 1). Figure 2B shows the results for “Craving a [cigarette/waterpipe]/Nicotine.” Mean score during the cigarette session decreased significantly relative to the mean pre-smoking score (62.0 ± 4.8) at 5 (24.1 ± 3.4), 15 (34.4 ± 4.4), and 30 min (43.6 ± 4.5). During the waterpipe session, significant decreases relative to the pre-smoking mean (52.9 ± 4.7) were observed at 5 (33.6 ± 3.9), 15 (36.4 ± 4.1), 30 (36.1 ± 4.1), and 45 min (26.6 ± 4.0). Between sessions, mean “craving” score was significantly lower during the waterpipe session relative to cigarette at 45 min after product administration. A similar pattern was observed for “Urges to smoke a [cigarette/waterpipe].”
For “Desire for sweets,” in the cigarette session, mean score decreased significantly relative to the pre-smoking mean (22.8 ± 3.9) at 5 (13.6 ± 3.2) and at 15 min (14.0 ± 3.4) after product administration. No significant changes within the waterpipe session or across sessions were observed for this item.
Direct Effects of Nicotine Scale
Significant session by time interactions was observed for “Dizzy” and “Lightheaded” (see Table 1). Figure 2C displays data for “Lightheaded.” During the cigarette session, relative to the mean pre-smoking score (7.8 ± 2.0), mean score increased significantly at 5 min (18.4 ± 3.3) only. During the waterpipe session, relative to the mean pre-smoking score (5.8 ± 1.6), significant differences were observed at 5 (22.3 ± 3.8), 15 (25.0 ± 4.1), 30 (21.3 ± 3.9), and 45 min (15.8 ± 3.2). Between sessions, waterpipe mean score was significantly greater than that observed during the cigarette session at 15 and 30 min after product administration.
Scores for “Dizzy” tended to follow a similar time course for both sessions (see Figure 2D). During the cigarette session, relative to the mean pre-smoking score (6.9 ± 1.7), scores peaked at 5 (12.7 ± 2.8) min after product administration and decreased over the next three timepoints (all ns). During the waterpipe session, relative to the pre-smoking score (4.5 ± 1.6), mean score was increased significantly at 15 (15.5 ± 3.5) and 30 min (13.5 ± 3.3) after product administration and decreased during the last timepoint (ns). There were no between session differences for this item.
Direct Effects of Tobacco Scale.
A significant session by time interaction was observed for six DET items (see Table 1). Figure 2E presents the responses to “Was the [cigarette/waterpipe] pleasant?”. For cigarette, mean score for the “pleasant” item was increased significantly relative to the pre-smoking mean (2.1 ± 1.2) at 5 (66.7 ± 4.5), 15 (60.1 ± 4.8), 30 (56.0 ± 4.8), and 45 min (58.1 ± 4.8). Similarly, for waterpipe, mean score was increased significantly relative to the pre-smoking mean (3.1 ± 1.5) at 5 (77.9 ± 3.4), 15 (79.5 ± 3.1), 30 (77.3 ± 3.1), and 45 min (77.4 ± 3.2). Between sessions, waterpipe mean “pleasant” score was significantly greater than cigarette at 5, 15, 30, and 45 min after product administration.
Similarly, for “Was the [cigarette/waterpipe] satisfying?” (see Figure 2F), for the cigarette session, mean score increased significantly relative to the pre-smoking mean (2.0 ± 1.2) at 5 min (71.9 ± 3.6) and remained high at 15 (64.6 ± 4.3), 30 (58.5 ± 4.7), and 45 min (61.2 ± 4.4). Likewise, waterpipe mean score was increased relative to the pre-smoking mean (4.1 ± 2.2) at 5 min (74.1 ± 3.1) and remained high at 15 (73.2 ± 3.2), 30 (72.5 ± 3.5), and 45 (76.3 ± 3.2). Between sessions, waterpipe mean score was significantly greater than mean cigarette score at 30 and 45 min after product administration. The identical pattern of results was observed for “Did the [cigarette/waterpipe] taste good?”.
For the item assessing “Did the [cigarette/waterpipe] taste bad?”, mean score during the cigarette session increased significantly relative to the pre-smoking mean (1.9 ± 1.2) at 5 (13.0 ± 2.9), 15 (14.1 ± 3.1), 30 (13.9 ± 3.0), and 45 min (15.1 ± 3.4). Mean scores during the waterpipe session never differed significantly from the pre-smoking mean (3.2 ± 1.5). Between sessions, mean score during the cigarette session was greater than waterpipe at 15, 30, and 45 min after product administration. In contrast, for “Did the [cigarette/waterpipe] make you sick?”, no significant differences were observed during the cigarette session, and for waterpipe, relative to the pre-smoking mean (3.0 ± 1.5), significant differences were observed at 30 (10.5 ± 2.7) and 45 min (9.8 ± 2.6) after product administration only. There were no significant differences between sessions for this item.
For the item, “Would you like another [cigarette/waterpipe] RIGHT NOW?”, mean cigarette scores increased significantly relative to the pre-smoking mean (3.8 ± 1.7) at 5 (48.6 ± 4.7), 15 (53.2 ± 4.8), 30 (54.8 ± 5.0), and 45 min (61.3 ± 4.6). Waterpipe scores increased significantly relative to the pre-smoking mean (3.7 ± 1.9) at 5 min (54.8 ± 4.5) and then decreased gradually during the next three timepoints at 15 (52.4 ± 4.7), 30 (44.8 ± 4.3), and 45 min (34.9 ± 4.4) while remaining significantly higher than the pre-smoking mean score. Between sessions, mean cigarette score was significantly higher at 45 min after product administration compared with waterpipe at that time.
Overall, subjective measures for which a significant condition by time interaction was not observed but for which a significant main effect of time was observed generally showed a pattern of results that was virtually identical to the measures displayed in Figure 2.
Puff Topography
Significant differences between sessions were observed across all puff topography measures. Mean cigarette puff number (15.8 ± 0.8) was significantly less than waterpipe, 84.9 ± 6.4; F(1, 50) = 130.3, p < .001. Mean cigarette average puff volume (71.2 ± 3.8 ml) was significantly less than waterpipe, 833.5 ± 65.7 ml; F(1, 50) = 139.7, p < .001, and mean cigarette total puff volume (1.1 ± 0.1 L) was significantly less than waterpipe, 61.6 ± 5.0 l; F(1, 50) = 146.7, p < .001. Mean cigarette IPI (24.4 ± 1.5 s) was significantly shorter than that for waterpipe, 35.4 ± 2.7 s; F(1, 50) = 21.3, p < .001).
Discussion
In our previous report with 31 participants (Eissenberg & Shihadeh, 2009), we noted that, relative to a single cigarette, a single waterpipe tobacco smoking episode was associated with similar peak plasma nicotine concentration and also that waterpipe tobacco smoking elevated peak COHb levels 3-fold and was associated with a 48-fold greater amount of smoke inhaled. The results reported here, with 54 participants (including those original 31), are virtually identical and extend our previous findings by allowing a comparison of the subjective effect profile of cigarette and waterpipe tobacco smoking. That is, we observed similar peak plasma nicotine concentrations for cigarette and waterpipe and also observed that waterpipe produced a 3.75-fold greater elevation in peak COHb levels and was associated with a 56-fold greater amount of smoke inhaled. These results are also consistent with several other reports (Bacha, Salameh, & Waked, 2007; El-Nachef & Hammond, 2008; Maziak et al., 2009; Salameh et al., 2009). Thus, there is now overwhelming evidence that, like cigarette smoking, waterpipe tobacco smoking involves considerable nicotine and CO exposure. Moreover, every study that has included measures of puff topography makes clear that even a single waterpipe tobacco smoking episode involves inhalation of many liters of smoke and that smoke is known to contain numerous other toxicants (e.g., Saleh & Shihadeh, 2008; Shihadeh & Saleh, 2005). In addition, results obtained from this study support findings that waterpipe tobacco smoking presents a risk to nonsmokers via secondhand smoke exposure (see Daher et al., 2010; Monn, Kindler, Meile, & Brandli, 2007); mean ambient CO was significantly higher during the waterpipe session than during the cigarette session, waterpipe mean = 3.2 vs. cigarette mean = 0.2 ppm; t(122) = 22.7, p < .001). There is every reason to expect, therefore, that future large-scale epidemiologic studies of waterpipe tobacco smoking will demonstrate that, like cigarette smoking, this method of tobacco use presents a substantial risk of dependence, disability, and death.
With regard to subjective effects, the results reported here suggest that cigarette and waterpipe tobacco smoking can produce similar subjective effect profiles. For example, for both cigarette and waterpipe, significant suppression of tobacco abstinence symptomology was observed (see Figure 2A and B). In addition, increased ratings of nicotine-related side effects (“Dizzy” and “Lightheaded”) occurred in both sessions (see Figure 2C and D). Both cigarette and waterpipe tobacco smoking were associated with pleasurable subjective effects, which included rapid increases following product administration in ratings of “pleasant,” “taste good,” and “satisfy” (see Figure 2E and F). The similarity of subjective responses observed for cigarette and waterpipe tobacco smoking, in conjunction with the similarity in nicotine exposure, suggests a similar risk of abuse potential and subsequent dependence of these two methods of tobacco use.
For several subjective items, the pattern of responding over time differed across tobacco use method. For example, during the cigarette session measures of tobacco abstinence effects showed an immediate decline followed by a slow increase toward baseline levels—a pattern consistent with the actual time spent smoking the single cigarette (i.e., on average, the cigarette was completed in the first 6.1 min of the session and no further smoking was permitted). In contrast, during the waterpipe session, the suppression of tobacco abstinence effects was sometimes more gradual (see Figure 2A) and continued for the duration of the session—again, a pattern consistent with the actual time spent smoking the waterpipe (M = 43.3 min). Similar patterns of mean scores over time were observed on at least some measures of nicotine-related effects as well as several items assessing the direct effects of tobacco (see Figure 2C–F). Importantly, this pattern of results on a variety of subjective measures is consistent with the pattern observed for plasma nicotine concentration shown in Figure 1: We observed a rapid rise and gradual decline for cigarette and a slower but continuous rise for waterpipe. Thus, tobacco-delivered nicotine may underlie the subjective profile for cigarette and waterpipe, a hypothesis that is supported by our observation that HR increases closely tracked plasma nicotine concentration (also reported elsewhere, see Eissenberg & Shihadeh, 2009; Shafagoj & Mohammed, 2002; Shafagoj, Mohammed, & Hadidi, 2002). However, the absence of placebo (i.e., nicotine free) cigarette and waterpipe conditions in this study, an important study limitation, does not permit a more definitive conclusion.
Other study limitations include the population from which we sampled as well as the laboratory environment in which solitary waterpipe tobacco smoking was required. Participants in this study had used a waterpipe to smoke tobacco for a mean of 1.8 years and reported that, on average, they smoked tobacco in a waterpipe 5.7 times per month. Different toxicant exposure and subjective effect profiles might be expected with participants who have a more extensive use history. The laboratory setting necessarily differs from a typical waterpipe tobacco–smoking environment, though furnishings and activities (e.g., reclining chairs, participant-selected movies) were chosen to approximate a natural setting. One potentially critical factor that was not like the natural setting often reported was the solitary waterpipe smoking required in this study. Convenience samples of U.S. waterpipe tobacco smokers suggest that over 90% share a waterpipe when they smoke (Smith-Simone et al., 2008; Ward et al., 2007), and the influence of sharing a waterpipe on toxicant exposure has not yet been assessed.
In conclusion, this study bolsters previous evidence that waterpipe tobacco smoking is associated with exposure to the same toxicants (nicotine; CO) and produces some of the same effects (e.g., HR increase, tobacco abstinence symptom suppression) as does cigarette smoking. The large volume of toxicant-laden smoke that is inhaled during a waterpipe tobacco–smoking episode suggests reason for strong public health concern, and the magnitude and duration of some subjective effects are consistent with a method of drug delivery with considerable abuse potential and that supports dependence development. Taken together, these results should be used to address misperceptions regarding the toxicant exposure and risk for tobacco/nicotine dependence associated with waterpipe tobacco smoking. Results such as these can be incorporated into prevention interventions that might help deter more adolescents and young adults from experimenting with an almost certainly lethal method of tobacco smoking.
Funding
This work was supported by United States Public Health Service grants R01CA120142, R01DA024876, and F31DA028102.
Declaration of Interests
None declared.
Acknowledgments
Portions of this work were presented at the 15th and 16th annual meeting of the Society for Nicotine and Tobacco Research. We would like to thank Barbara Kilgalen and Janet Austin for their assistance in data collection and data management.
References
- Akl EA, Gaddam S, Gunukula SK, Honeine R, Jaoude PA, Irani J. The effects of waterpipe tobacco smoking on health outcomes: A systematic review. International Journal of Epidemiology. 2010;39:834–857. doi: 10.1093/ije/dyq002. doi:10.1093/ije/dyq002. [DOI] [PubMed] [Google Scholar]
- Aljarrah K, Ababneh ZQ, Al-Delaimy WK. Perceptions of hookah smoking harmfulness: Predictors and characteristics among current hookah users. Tobacco Induced Diseases. 2009;5:16. doi: 10.1186/1617-9625-5-16. doi:10.1186/1617-9625-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacha ZA, Salameh P, Waked M. Saliva cotinine and exhaled carbon monoxide levels in natural environment waterpipe smokers. Inhalation Toxicology. 2007;19:771–777. doi: 10.1080/08958370701401699. doi:10.1080/08958370701401699. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clinical Pharmacology & Therapeutics. 2008;83:531–541. doi: 10.1038/clpt.2008.3. doi:10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Breland AB, Evans SE, Buchhalter AR, Eissenberg T. Acute effects of advance: A potential reduced exposure product for smokers. Tobacco Control. 2002;11:376–378. doi: 10.1136/tc.11.4.376. doi:10.1136/tc.11.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8:727–738. doi: 10.1080/14622200600789585. doi:10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug & Alcohol Dependence. 2009;105S:S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. doi:10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami DK. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiology Biomarkers & Prevention. 2009;18:3241–3261. doi: 10.1158/1055-9965.EPI-09-0948. doi:10.1158/1055-9965.EPI-09-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers DC, Tunnicliffe WS, Ayres JG. Acute inhalation of cigarette smoke increases lower respiratory tract nitric oxide concentrations. Thorax. 1998;53:677–679. doi: 10.1136/thx.53.8.677. doi:10.1136/thx.53.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb C, Ward KD, Maziak W, Shihadeh AL, Eissenberg T. Waterpipe tobacco smoking: An emerging health crisis in the united states. American Journal of Health Behavior. 2010;34:275–285. doi: 10.5993/ajhb.34.3.3. Retrieved from http://www.ajhb.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combrink A, Irwin N, Laudin G, Naidoo K, Plagerson S, Mathee A. High prevalence of hookah smoking among secondary school students in a disadvantaged community in Johannesburg. South African Medical Journal. 2010;100:297–299. doi: 10.7196/samj.3965. Retrieved from http://www.samj.org.za/index.php/samj. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. doi:10.1080/14622200124218. [DOI] [PubMed] [Google Scholar]
- Daher N, Saleh R, Jaroudi E, Sheheitli H, Badr T, Sepetdjian E, et al. Comparison of carcinogen, carbon monoxide, and ultrafine particle emissions from narghile waterpipe and cigarette smoking: Sidestream smoke measurements and assessment of second-hand smoke emission factors. Atmospheric Environment. 2010;44:8–14. doi: 10.1016/j.atmosenv.2009.10.004. doi:10.1016/j.atmosenv.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas E, Tremblay M, Low NC, Cournoyer D, O’Loughlin J. Water-pipe smoking among north American youths. Pediatrics. 2010;125:1184–1189. doi: 10.1542/peds.2009-2335. doi:10.1542/peds.2009-2335. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Shihadeh A. Waterpipe and cigarette smoking: A direct comparison of toxicant effects. American Journal of Preventive Medicine. 2009;37:518–523. doi: 10.1016/j.amepre.2009.07.014. doi:10.1016/j.amepre.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg T, Ward KD, Smith-Simone S, Maziak W. Waterpipe tobacco smoking on a U.S. college campus: Prevalence and predictors. Journal of Adolescent Health. 2008;42:526–529. doi: 10.1016/j.jadohealth.2007.10.004. doi:10.1016/j.jadohealth.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nachef WN, Hammond SK. Exhaled carbon monoxide with waterpipe use in US students. Journal of the American Medical Association. 2008;299:36–38. doi: 10.1001/jama.2007.6. Retrieved from http://jama.ama-assn.org/ [DOI] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptoms suppression: Nicotine dose and smokers’ gender. Experimental & Clinical Psychopharmacology. 2006;14:121–135. doi: 10.1037/1064-1297.14.2.121. doi:10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Trade Commission. “Tar,” nicotine, and carbon monoxide of the smoke of 1284 varieties of domestic cigarettes for the year 1998. 2000. Retrieved from http://www.ftc.gov/reports/tobacco/1998tar&nicotinereport.pdf. [Google Scholar]
- Foulds J, Stapleton J, Feyerabend C, Vesey C, Jarvis M, Russell MAH. Effects of transdermal nicotine patches on cigarette smoking: A double blind crossover study. Psychopharmacology. 1992;106:421–427. doi: 10.1007/BF02245429. doi:10.1007/BF02245429. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. Retrieved from http://archpsyc.ama-assn.org/ [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the box correction for degrees of freedom from sample data in a randomized block and split-plot designs. Journal of Education Statistics. 1976;1:69–82. doi:10.3102/10769986001001069. [Google Scholar]
- Jackson D, Aveyard P. Waterpipe smoking in students: Prevalence, risk factors, symptoms of addiction, and smoke intake. Evidence from one British university. BMC Public Health. 2008;8:174. doi: 10.1186/1471-2458-8-174. doi:10.1186/1471-2458-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil H, Elsouhag D, Hiller S, Arnetz JE, Arnetz BB. Sociodemographic risk indicators of hookah smoking among white Americans: A pilot study. Nicotine & Tobacco Research. 2010;12:525–529. doi: 10.1093/ntr/ntq026. doi:10.1093/ntr/ntq026. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Henningfield JE. Human abuse liability assessment by measurement of subjective and physiological effects. In: Fischman MW, Mello NK, editors. Testing for abuse liability of drugs in humans [NIDA Research Monograph 92] Rockville, MD: USDHHS; 1989. Retrieved from http://archives.drugabuse.gov/pdf/monographs/92.pdf. [PubMed] [Google Scholar]
- Keppel G. Design and analysis: A researcher's handbook. Englewood Cliffs, NJ: Prentice Hall; 1991. [Google Scholar]
- Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. American Journal of Respiratory and Critical Care Medicine. 1995;152:609–612. doi: 10.1164/ajrccm.152.2.7543345. Retrieved from http://ajrccm.atsjournals.org/ [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Jennings JM, Sams C, Weaver MF, Eissenberg T. The influence of transdermal nicotine on tobacco/nicotine abstinence and the effects of a concurrently administered cigarette in women and men. Experimental & Clinical Psychopharmacology. 2008;16:99–112. doi: 10.1037/1064-1297.16.2.99. doi:10.1037/1064-1297.16.2.99. [DOI] [PubMed] [Google Scholar]
- Knishkowy B, Amitai Y. Water-pipe (narghile) smoking: An emerging health risk behavior. Pediatrics. 2005;116:e113–e119. doi: 10.1542/peds.2004-2173. doi:10.1542/peds.2004-2173. [DOI] [PubMed] [Google Scholar]
- Maziak W, Rastam S, Ibrahim I, Ward KD, Shihadeh A, Eissenberg T. CO exposure, puff topography, and subjective effects in waterpipe tobacco smokers. Nicotine & Tobacco Research. 2009;11:806–811. doi: 10.1093/ntr/ntp066. doi:10.1093/ntr/ntp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Ward KD, Soweid RA, Eissenberg T. Tobacco smoking using a waterpipe: A re-emerging strain in a global epidemic. Tobacco Control. 2004;13:327–333. doi: 10.1136/tc.2004.008169. doi:10.1136/tc.2004.008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monn C, Kindler P, Meile A, Brandli O. Ultrafine particle emissions from waterpipes. Tobacco Control. 2007;16:390–393. doi: 10.1136/tc.2007.021097. doi:10.1136/tc.2007.021097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidong W, Shou W, Chen YL, Jiang X. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. Journal of Chromatography B: Biomedical Sciences and Applications. 2001;754:387–399. doi: 10.1016/s0378-4347(01)00021-4. doi:10.1016/S0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease: National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2003;59(Suppl. 1):1–232. doi:10.1136/thx.2004.022707. [PMC free article] [PubMed] [Google Scholar]
- Neergaard J, Singh P, Job J, Montgomery S. Waterpipe smoking and nicotine exposure: A review of the current evidence. Nicotine & Tobacco Research. 2007;9:987–994. doi: 10.1080/14622200701591591. doi:10.1080/14622200701591591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärna K, Usin J, Ringmets I. Cigarette and waterpipe smoking among adolescents in Estonia: HBSC survey results, 1994-2006. BMC Public Health. 2008;8:392. doi: 10.1186/1471-2458-8-392. doi:10.1186/1471-2458-8-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Bunker EB, Henningfield JE. Transdermal nicotine: Reduction of smoking with minimal abuse liability. Psychopharmacology. 1994;115:9–14. doi: 10.1007/BF02244745. doi:10.1007/BF02244745. [DOI] [PubMed] [Google Scholar]
- Primack BA, Sidani J, Agarwal A, Shadel WG, Donny EC, Eissenberg TE. Prevalence of an association with waterpipe tobacco smoking among college students. Annals of Behavioral Medicine. 2008;36:81–86. doi: 10.1007/s12160-008-9047-6. doi:10.1007/s12160-008-9047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh P, Bacha ZA, Waked M. Saliva cotinine and exhaled carbon monoxide in real life waterpipe smokers: A post hoc analysis. Tobacco Use Insights. 2009;2:1–10. Retrieved from http://www.la-press.com/tobacco-use-insights-journal-j139. [Google Scholar]
- Salameh P, Waked M, Aoun Z. Waterpipe smoking: Construction and validation of the Lebanon waterpipe dependence scale (LWDS-11) Nicotine & Tobacco Research. 2008;10:149–158. doi: 10.1080/14622200701767753. doi:10.1080/14622200701767753. [DOI] [PubMed] [Google Scholar]
- Saleh R, Shihadeh A. Elevated toxicant yields with narghile waterpipes smoked using a plastic hose. Food and Chemical Toxicology. 2008;46:1461–1466. doi: 10.1016/j.fct.2007.12.007. doi:10.1016/j.fct.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Shafagoj YA, Mohammed FI. Levels of maximum end-expiratory carbon monoxide and certain cardiovascular parameters following hubble-bubble smoking. Saudi Medical Journal. 2002;23:953–958. Retrieved from http://www.smj.org.sa/ [PubMed] [Google Scholar]
- Shafagoj YA, Mohammed FI, Hadidi KA. Hubble-bubble (water pipe) smoking: Levels of nicotine and cotinine in plasma, saliva and urine. International Journal of Clinical Pharmacology & Therapeutics. 2002;40:249–255. doi: 10.5414/cpp40249. Retrieved from http://www.dustri.com/nc/journals-in-english/mag/int-journal-of-clinical-pharmacology-and-therapeutics.html. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Antonios C, Azar S. A portable, low-resistance puff topography instrument for pulsating, high-flow smoking devices. Behavior Research Methods. 2005;37:186–191. doi: 10.3758/bf03206414. Retrieved from http://brm.psychonomic-journals.org/ [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food and Chemical Toxicology. 2005;43:655–661. doi: 10.1016/j.fct.2004.12.013. doi:10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Smith-Simone S, Maziak W, Ward KD, Eissenberg T. Waterpipe tobacco smoking: knowledge, attitudes, beliefs, and behaviors in two U.S. samples. Nicotine & Tobacco Research. 2008;10:393–398. doi: 10.1080/14622200701825023. doi:10.1080/14622200701825023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward KD, Eissenberg T, Gray JN, Srinivas V, Wilson N, Maziak W. Characteristics of U.S. waterpipe users: A preliminary report. Nicotine & Tobacco Research. 2007;9:1339–1346. doi: 10.1080/14622200701705019. doi:10.1080/14622200701705019. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Waterpipe tobacco smoking: health effects, research needs and recommended actions by regulators. Geneva, Switzerland: Author; 2005. Retrieved from http://www.who.int/tobacco/global_interaction/tobreg/Waterpipe%20recommendation_Final.pdf. [Google Scholar]