Abstract
In many species, males can influence the amount of resources their mates invest in reproduction. Two favoured hypotheses for this observation are that females assess male quality during courtship or copulation and alter their investment in offspring accordingly, or that males manipulate females to invest heavily in offspring produced soon after mating. Here, we examined whether there is genetic variation for males to influence female short-term reproductive investment in Drosophila melanogaster, a species with strong sexual selection and substantial sexual conflict. We measured the fecundity and egg size of females mated to males from multiple isofemale lines collected from populations around the globe. Although these traits were not strongly influenced by the male's population of origin, we found that 22 per cent of the variation in female short-term reproductive investment was attributable to the genotype of her mate. This is the first direct evidence that male D. melanogaster vary genetically in their proximate influence on female fecundity, egg size and overall reproductive investment.
Keywords: maternal investment, fecundity, egg size, differential allocation hypothesis, sexual conflict, Drosophila melanogaster
1. Introduction
Resources necessary for survival and reproduction are often limiting in most systems. Consequently, the amount of resources females can invest in their offspring varies depending on a number of factors, such as female condition, life history or environmental variation. This altered investment can be manifest as a change in the number or size of offspring at birth or hatching and/or the amount of maternal care provided thereafter. Because variation in maternal investment directly impacts the number of surviving offspring, it can have strong implications for the inclusive fitness of both parents.
The idea that males can influence maternal investment is pervasive in many systems. One of the leading explanations for this influence is the ‘differential allocation hypothesis’ [1,2], which predicts that females will increase their overall fitness by investing more resources in the offspring of high-quality males (but see also the ‘reproductive compensation hypothesis’ [3]). In support of this hypothesis, female reproductive investment increases when they mate with males of larger size in seed beetles (Cercidium floridum) [4], dung beetles (Onthophagus taurus) [5], crayfish (Austropotamobius italicus) [6] and Australian rainbowfish (Melanotaenia australis) [7]. Also, male attractiveness influences female reproductive investment in several bird species, including zebra finches (Poephila guttata) [8], barn swallows (Hirundo rustica) [9], peafowl (Pavo cristatus) [10], Chinese quails (Coturnix chinensis) [11] and mallard ducks (Anas platyrhynchos) [12]. The majority of these examples provided females several opportunities to assess male quality, either by lengthy male–female interactions, nuptial gifts to the female or extensive parental care, enabling females to alter their reproductive investment accordingly.
An alternative explanation is that males could directly manipulate female reproductive investment, reflecting a potential sexual conflict associated with promiscuous systems. When females mate with multiple males and have a high likelihood of surviving to produce multiple broods, males will be selected to maximize female investment in the broods produced immediately following mating, when paternity confidence is generally the greatest. However, if the resources that females invest in a current brood reduce their ability to invest in future broods, they may be selected to invest less in individual broods to maximize their lifetime fitness. In animals, this conflict has been extensively studied in the context of the mammalian placenta, which provides nourishment from the mother's bloodstream to the developing foetus (e.g. [13,14]). The idea that paternally inherited genes expressed in the placenta are selected to increase maternal investment, while maternally inherited genes are selected to decrease placental investment, has received support both theoretically [15,16] and empirically (reviewed in [17]).
The fruitfly, Drosophila melanogaster, has a promiscuous mating system. Males and females only interact during courtship and copulation, and females lay eggs without providing any parental care. Courtship is very complex in this species [18] and copulation duration can vary substantially [19], providing several opportunities for females to assess male quality. In addition, males can influence the behaviour and physiology of their mates after mating by transferring a cocktail of seminal proteins in their ejaculates (reviewed in [20]). A single ejaculate contains at least 138 different seminal proteins [21], two of which have been studied extensively for their effects on female fecundity. Acp26Aa (ovulin) stimulates the release of oocytes from the ovaries, increasing female fecundity on the first day after mating [22,23]. In contrast, Acp70A (sex peptide) acts at oogenesis (reviewed in [24]) and is gradually released after binding to the tails of sperm in storage, increasing female fecundity for at least one week after mating [25]. Sex peptide is often harmful to females, decreasing their reproductive success and lifespan [26], but it can also be beneficial to females under certain nutritional conditions [27]. Thus, although males rarely interact with their mates after copulation, seminal proteins give them a potential avenue through which they could influence female investment during this time.
Previous studies have suggested that male D. melanogaster may vary genetically in their ability to influence female fecundity. For example, mating with smaller sized males has been reported to increase female fecundity on the first day following mating, relative to mating with larger males [28], although these results are not consistent [29]. Several studies focusing on variation in male sperm competitive ability seem to indicate that different male genotypes influence female fecundity [30–32], but these studies are potentially confounded by the number of times each female mated, viability differences between male genotypes and lengthy exposure of the females to male courtship and harassment, which is known to be costly to females [33].
Here, we mated females a single time to males of different genotypes to test whether male D. melanogaster are genetically variable in their ability to influence female fecundity during the first 2 days following mating. Individuals from laboratory-adapted populations of D. melanogaster are generally not appropriate for this study, as they are often selected for only one episode of reproduction in their lifetime. To address this problem, and to maximize our likelihood of detecting variation for this trait, we used males from 50 different isofemale lines that were originally collected from five populations around the world (10 lines from each of five locations) and mated them to females with an experimentally controlled genotype (F1 hybrids between two inbred lines), such that any variation in female fecundity should be predominantly owing to their mates. However, since fecundity is negatively correlated with egg size in D. melanogaster [34], variation in fecundity induction may be offset by variation in egg size, causing it to be selectively neutral in terms of overall female reproductive investment. To control for this possibility, we also measured the volume of eggs produced by our experimental flies. We used egg measurements rather than offspring characteristics to determine female investment because the latter could be confounded by viability differences between male lines that are independent of the females. Thus, we incorporated both egg number and egg size to determine whether short-term reproductive investment varied among females mated to males from the 50 isofemale lines.
2. Material and methods
(a). Study populations and worldwide isofemale lines
To test for genetic variation in the ability of males to influence female reproductive investment, we used males from a series of D. melanogaster isofemale lines collected from five different populations. Ten lines each were selected from D. melanogaster populations in Ithaca (New York), The Netherlands, Zimbabwe, Beijing and Tasmania. These 50 lines, here referred to as the ‘worldwide lines’, were provided by A. G. Clark (Cornell University), and represent a subset of the isofemale lines described in Greenberg et al. [35]. Upon collection from the wild, each isofemale line underwent full-sibling inbreeding for 12 generations to create genetic uniformity. Since their receipt in November 2008, our 50 worldwide lines have been maintained on a two week culture cycle on standard cornmeal/molasses/killed-yeast medium. Because much of the genetic diversity in these lines was rapidly purged upon introduction to the laboratory, adaptation to the new environment and changing culture conditions was probably minimal. Therefore, we can use each line to test whether genetic variation for a specific trait was present in the original natural population.
To control the female genotype in our experiments, we created isogenic, heterozygous tester females by crossing two inbred lines that originated from a standard laboratory population. The two inbred lines (ibw2 and ibw6) were initially collected from a large, outbred replicate of our base population (LHM, for details, see [36]) into which a recessive, brown-eyed marker (bw) had been introgressed through repeated back-crossing (LHM − bw, for details, see [36]). This population is unrelated to any of the populations used to create the 50 worldwide lines that our males were collected from. To create each line, a single virgin female was collected from the LHM − bw population and was mated to a single male from the same population. Offspring were collected from this mating, and one male and one female (who were full siblings) were mated. Each line then underwent 10 further generations of full-sibling inbreeding. Since September 2008, these lines have been maintained on a two week culture cycle at a small population size (seven vials per line with 12 breeding individuals per vial).
To create the experimental females (ibw2/ibw6), we crossed virgin females from the ibw2 line to males from the ibw6 line, and collected their heterozygous, isogenic daughters as virgins. These females were always created in the same manner: by crossing females from the ibw2 inbred line with males from the ibw6 inbred line. Since we are only using these experimental females to create a static environment in which we can screen for an effect of male variation with high experimental power, the culture conditions of their source population is not relevant for our experimental design.
(b). Collecting experimental flies
We measured the ability of males from the 50 worldwide lines to influence female reproductive investment over five experimental replicates, with all 50 lines surveyed in each replicate. All replicates were conducted according to the experimental design described below.
To collect experimental males, two vials containing food medium and a small amount of live yeast added to the surface (to stimulate fecundity) were set up with 10–20 pairs of males and females from each of the 50 worldwide lines described above. After 3 days, the flies were transferred to fresh vials with food medium and live yeast for 2 additional days before being discarded. Larval density was regulated visually in both sets of vials 2–3 days after egg deposition by removing excess larvae from high-density vials. After 13–14 days, the flies from the four vials for each worldwide line were pooled, and 20–25 males per line were collected and held in fresh vials containing medium for 3–4 days until the experiments began.
To create isogenic ibw2/ibw6 females, virgin females were collected from the ibw2 inbred line as they eclosed and were held for 5–6 days to mature. At this time, they were combined in groups of eight with eight males from the ibw6 inbred line into 14 vials containing food medium supplemented with live yeast. After 24 h, the flies were transferred to fresh vials containing food medium and live yeast for another 24 h before being discarded. Excess eggs were culled from both sets of vials to obtain a density of 150–200 eggs per vial. Virgin ibw2/ibw6 females were collected from these vials 8–9 days later and were held in groups of 20 in vials containing food medium for 3–4 days until the experiments began. Experimental males and females were approximately the same age in all replicates.
(c). Testing for a male influence on female fecundity
Each experiment began by setting up a series of ‘mating observation vials’. Each vial contained a small amount of food medium with a cardstock paper divider placed vertically down its centre, dividing it into two separated halves. This divider was designed to be slightly shorter than the vial, such that when the bottom end was pushed into the food medium, there was approximately 30 mm between the top of the divider and the top of the vial. Mating observation vials were set up by placing a single female on one side of the divider and two males on the other using light CO2 anaesthesia. To keep the flies separated, a foam plug was pushed down into the vial until it reached the top of the divider.
Within each replicate, we set up seven mating observation vials for each of the 50 worldwide lines (350 vials in total). Each vial had a single ibw2/ibw6 female on one side of the divider, and two males from the same worldwide line on the other side. These observation vials allowed us to set up mating vials (while keeping the sexes separated) 24 h before observations began, giving the flies ample time to recover from CO2 anaesthetization. We also created 20 vials containing only a single ibw2/ibw6 female (no males) to measure virgin fecundity. These virgin females allowed us to confirm that any variation in fecundity associated with the male genotype was due to variation in fecundity induction, rather than the inability of some genotypes to sufficiently stimulate females.
Mating observations began by lifting the foam plugs above the divider, such that the males and female could interact. We observed the vials for copulations over a 2 h period, during which time none of the females mated more than once. When five females from each line (out of seven total) had mated, these females were transferred individually into oviposition test tubes (with a scored surface to promote oviposition). The 20 virgin females were also transferred to oviposition tubes during this time. All females were held in the test tubes for 22 h, at which time we transferred them into fresh, individual oviposition tubes for another 22 h. The number of eggs laid in the first 22 h post-mating was counted and recorded as ‘day-1 fecundity’, and the number of eggs laid in the second 22 h post-mating (hours 23–44) was counted and recorded as ‘day-2 fecundity’. For simplicity, we refer to the first 22 h post-mating as ‘day-1’ and the second 22 h as ‘day-2’, but these two periods only account for 44 h in total.
(d). Testing for a male influence on egg volume
At the end of the day-2 fecundity survey (the second 22 h), we combined the five females that had mated to males from the same worldwide line into an egg-laying chamber containing a Petri dish filled with food medium. The females were given 4 h to oviposit on the dish, at which time they were discarded. For each dish (i.e. each worldwide line), we arranged 10 eggs on their dorsal side and photographed them using an Olympus MicroFire digital camera and PictureFrame 2.0 software. We measured egg size (V) using ImageJ software (v. 1.43u), and calculated egg volume using the formula for a prolate spheroid: V = 1/6πW2L, where W is the length of the equatorial diameter and L is the length of the polar axis. We measured the eggs produced at the end of the second day following mating (hours 44–48) because previous work indicated that male effects on egg volume are not detectable immediately following mating, but are detectable on the second day (A. D. Stewart, T. A. F. Long & W. R. Rice 2009, unpublished data).
(e). Testing for a male influence on female reproductive investment
To measure overall female reproductive investment, we multiplied mean day-2 fecundity by the mean egg volume for each worldwide line. Day-2 fecundity was used for this index because the egg volume measurements were taken immediately following this time period (and on the same day).
(f). Data analysis
To measure whether males differed in their ability to influence female fecundity, egg volume and overall reproductive investment based on their population of origin or genotype, we performed a nested random effects analysis of variance (ANOVA) with ‘population’ as the main random effect and ‘line nested within population’ (‘line[population]’) as an additional random factor. Because there was variation attributable to experimental replicate for each trait measured (all p < 0.05), we first controlled for replicate effects by fitting a one-way ANOVA (with replicate as the main effect) and then performing all subsequent analyses on the residuals. To further determine how much variation in these traits could be attributed to the female's mate, we obtained bounded variance components from our random-effects ANOVAs. For all analyses of fecundity, the unit of replication was the average fecundity of the five females mated to males from the same worldwide line within each replicate. For egg volume analyses, the unit of replication was the average egg volume of the 10 eggs sired by males from the same worldwide line (taken at random from a pool of eggs produced by the five females mated to the same male genotype). For female reproductive investment, the unit of replication was the product of mean day-2 fecundity and mean egg volume for each worldwide line. All analyses were performed using JMP v. 8 software.
3. Results
(a). Genetic variation for male fecundity induction
Day-1 fecundity (number of eggs laid by females in the first 22 h after mating) and day-2 fecundity (number of eggs laid by females in the second 22 h post-mating, i.e. hours 23–44) were positively correlated over the 50 worldwide lines (r = 0.50, n = 50, p = 0.0002). Day-1 fecundity increased significantly for females mated to males from each of the 50 worldwide lines, compared with the fecundity of virgin females (electronic supplementary material, figure S1; Dunnet's test of day-1 fecundity for each line versus virgin females; all p ≤ 0.001), indicating that all male genotypes were able to sufficiently stimulate fecundity in their mates.
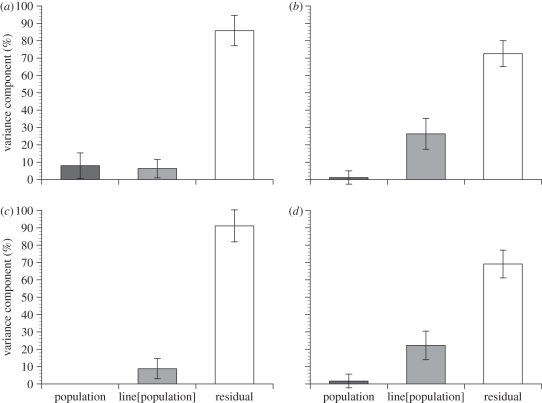
We found a significant influence of the male's population of origin for day-1 fecundity (F4,193 = 4.17, p = 0.0058), such that 7.95 + 7.37% of the variation in this trait was attributable to the male's population (figure 1a; variance components are reported throughout as mean percentage of total variation + s.e.). We did not, however, find an effect of male population on female day-2 fecundity (figure 1b; variance component estimate of 1.12 + 3.83%; F4,193 = 1.33, p = 0.27).
Figure 1.
The percentage of variation in female (a) day-1 fecundity, (b) day-2 fecundity, (c) egg volume and (d) total reproductive investment that can be attributed to her mate's population of origin (‘population’) or genotype within each population (‘line[population]’). Error bars indicate standard errors.
We also examined whether there was genetic variation within locations for male ability to induce female fecundity among the 10 isofemale lines collected from each population (electronic supplementary material, figure S1). Although we were unable to detect an influence of male genotype on day-1 fecundity (F45,193 = 1.34, p = 0.09), there was a highly significant effect of male genotype on day-2 fecundity (F45,193 = 2.77, p < 0.0001). The percentage of total variation in female day-1 fecundity attributable to sire genotype was estimated to be only 6.26 + 5.28% (figure 1a), while this metric was considerably larger (26.31 + 8.93%) for day-2 fecundity (figure 1b).
(b). Genetic variation for male influence on egg size
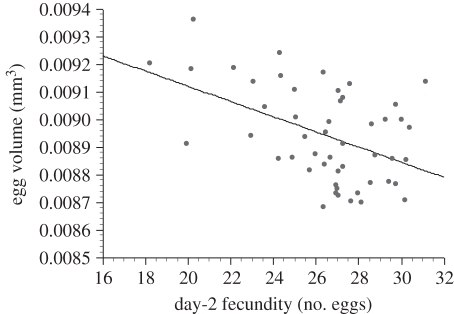
We did not detect any influence of the male's population of origin on egg volume (F4,193 = 0.51, p = 0.73), indicating that males from different populations did not produce differently sized eggs on average (figure 1c). However, within each population, male genotype accounted for 8.85 + 5.85% of the total variation in egg volume (figure 1c and electronic supplementary material, figure S2; F45,193 = 1.53, p = 0.027). As expected, egg volume and day-2 fecundity (measured immediately before egg volume) were negatively correlated across the 50 worldwide lines (figure 2; r = −0.4696, p = 0.0006).
Figure 2.
Negative correlation between day-2 fecundity and egg volume for females mated to males from the 50 worldwide lines. Each point represents the mean value for 25 females mated to males from a single line.
(c). Genetic variation for male influence on short-term female reproductive investment
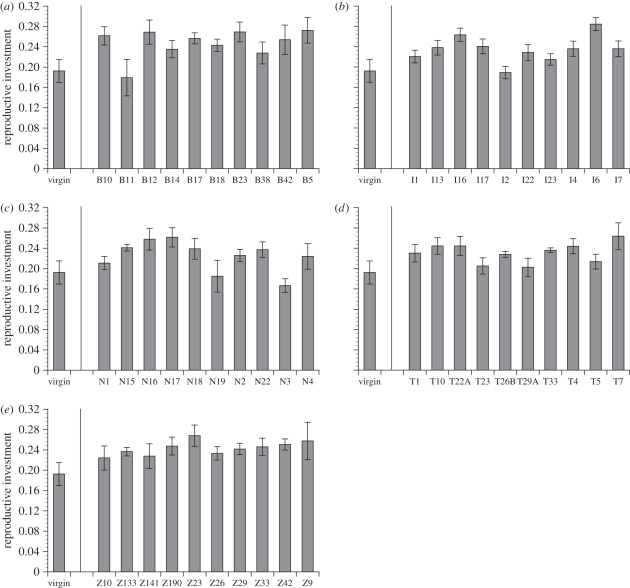
We measured female reproductive investment as mean day-2 fecundity multiplied by mean egg volume. In spite of the negative correlation between fecundity and egg size, we found a large influence of male genotype within populations on female reproductive investment (figure 3; F45,193 = 2.43, p < 0.0001): sire genotype accounted for 22.2 + 8.22% of the total variation in this trait (figure 1d). This pattern of genetic variation was consistent within each population (all p < 0.04) except for Zimbabwe (F9,40 = 0.63, p = 0.76), and there was no influence of the male's population on female investment (figure 1d; variance component of 1.74 + 3.99%; F4,193 = 1.54, p = 0.21). Removing the Zimbabwe population from the model did not strongly affect the variation in female reproductive investment owing to male population (F3,153 = 1.42, p = 0.25; variance component = 1.54 + 4.90%) or genotype (F36,153 = 3.02, p < 0.0001; variance component = 29.05 + 10.49%).
Figure 3.
Mean reproductive investment, measured as total egg volume, for females mated to males of different isofemale lines collected from populations in (a) Beijing, (b) Ithaca, New York, (c) The Netherlands, (d) Tasmania, and (e) Zimbabwe. The mean reproductive investment for 100 virgin females is shown in each panel for comparison. Error bars indicate standard errors.
4. Discussion
In this study, we set out to investigate whether there is genetic variation in D. melanogaster that enables males to influence the short-term reproductive investment of their mates. Although several studies in other species indicate that a male's phenotype can influence maternal investment (e.g. [5,7,10,12]), none of these studies documented or quantified the genetic variation among sires for this trait. In addition, the majority of these studies only examined one aspect of female reproductive investment, such as the size or numbers of eggs or offspring or the amount of maternal care. Here, we measured both egg size and egg number in a promiscuous species with no parental care to show that male genotype strongly influences female reproductive investment.
Our finding that males from different populations differ in their ability to stimulate female fecundity on the first day following mating (although to a small degree; figure 1a) demonstrates broad, regional differences in rapid fecundity induction by males, a pattern that may be explained by different life histories and/or selective pressures in the populations. Within populations, the effects of sire genotype on female fecundity differed depending on the time since copulation. Although there was no variation among male genotypes for fecundity induction on the first day following mating, we found a substantial effect of the male genotype on the second day (figure 1b and electronic supplementary material, figure S1), demonstrating abundant genetic variation among males within populations for female fecundity induction. This delayed effect of male genotype may occur because virgin females in our study (and commonly in nature) produce and retain mature oocytes for several days before mating, at which time ovulation is initiated before sperm storage is completed (reviewed in [37]). Consequently, any male- or female-mediated change in fecundity that occurs by increasing the rate of oogenesis would not be manifest until the supply of previously matured oocytes was exhausted.
To provide a more complete picture of maternal investment in D. melanogaster, we also measured the volume of eggs produced by females when they were mated to males of different genotypes. Eggs produced by female Drosophila vary in size depending on species [38], populations within species [39] and temperature [40,41]. In D. melanogaster, large eggs are advantageous, as they increase embryonic viability and larval development rate [42]. Since past studies of egg size in Drosophila focused almost exclusively on potential maternal influences, a male-mediated effect on egg size has not previously been reported (although Pitnick et al. [39] reports a male by female interaction for egg size resulting from interpopulation crosses in D. mojavensis). In our study, males from different populations did not vary in the size of eggs their mates produced on average, but nearly 9 per cent of the variation in egg size was associated with the genotype of the sire within each population (figure 1c and electronic supplementary material, figure S2). This finding indicates that a male's genotype influences not only the fecundity of his mate, but the size of eggs she produces as well. Consistent with previous studies [34], egg size was strongly negatively correlated with female fecundity (figure 2), demonstrating a trade-off between these traits.
As a result of this trade-off, both egg size and egg number need to be considered when measuring reproductive investment by females. If the variation in one trait is largely offset by variation in the other, there may be no net variation in overall reproductive investment. As expected from our measures of fecundity and egg size, the overall reproductive investment of females did not vary depending on the population that her mate originated from; it is possible that gene flow between these populations, owing to factors like international travel and fruit trade, may be sufficient to prevent differentiation for this trait. Conversely, in spite of the negative correlation between egg size and egg number, we found strong genetic variation among sires within populations for their effect on female investment; sire genotype accounted for 22 per cent of the total variation in female reproductive investment (figures 1d and 3). Interestingly, while fecundity was much lower in virgin females, their large egg size (electronic supplementary material, figure S2) produced an investment level surprisingly similar to mated females.
While our study demonstrates that males vary in their influence on maternal investment, it does not address the underlying mechanism(s) causing this variation. There are two favoured hypotheses, which are not mutually exclusive. First, this variation may be driven by cryptic female choice [43] and/or adaptive maternal effects in response to male quality, as predicted by the differential allocation hypothesis [1,8]. For example, females may screen aspects of male courtship behaviour and/or copulation duration and alter their reproductive investment according to the perceived quality of their mate. Alternatively, variation in female reproductive investment may be predominantly male-mediated, such that males vary in their ability to manipulate female short-term investment. Under this scenario, females will invest more in the offspring of a specific mate, potentially at the expense of her future reproduction. Our observed male influence on female reproductive investment could thus represent an additional facet of sexual conflict in D. melanogaster.
If variation in female investment is directly caused by males, it could potentially occur via variation in male seminal proteins [20]. In promiscuous species where males transfer seminal proteins that stimulate female fecundity, we would expect selection to favour males who can turn this ‘on/off switch’ into a variable ‘dimmer switch’ to maximize the proximate fecundity of their mate. Although our current study cannot address this hypothesis, the next step in this investigation will be to survey the seminal proteins transferred by each of these male genotypes (and the genes coding for them) to determine whether they vary in the types and/or quantities of proteins they transfer [21]. If we can find an association between male seminal fluid composition and the reproductive investment of their mates, this would suggest that a male-mediated effect (via seminal proteins) is at least partially responsible for our observed variation in maternal investment.
It is also possible that female reproductive investment is controlled by both sexes. In D. melanogaster, females are known to influence the outcome of sperm competition, and a strong female genotype by male genotype interaction has been reported for this trait [44]. This interaction suggests that sperm competition is a complex post-copulatory trait that is probably influenced by intersexual coevolution [45]. Although our use of a single female genotype in this study greatly increased the power of our experimental design, it does not indicate how changes in female genotypes might affect the degree to which males influence maternal investment. In future work, it would be interesting to determine whether the performance of a specific male genotype is independent of his mate, or whether maternal investment changes in a complex manner depending on the combination of parental genotypes, as is the case for sperm competition.
In summary, our study demonstrates that there is substantial genetic variation among sires for their influence on female short-term reproductive investment. Most of this variation was found within populations and not among distant geographical locations. Although future studies are necessary to determine the degree to which males and/or females control this effect, this is the first direct evidence that males vary genetically in their proximate influence on female fecundity, egg size and overall female reproductive investment.
Acknowledgements
We thank A. G. Clark for providing the worldwide isofemale lines used in this study. We also thank the members of the EEMB Evolution Group for helpful discussions and T. Long for technical assistance with egg volume measurements. This article was greatly improved by the comments and suggestions of Associate Editor Locke Rowe and two anonymous reviewers. This work was supported by a National Institutes of Health grant to W.R.R. (1R01HD057974-01). A.P. was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Postgraduate Scholarship, a Broida-Hirschfelder Fellowship awarded by the UCSB Faculty Women's Club and a Rosati Dissertation Fellowship in Science and Engineering awarded by the UCSB Graduate Division.
References
- 1.Sheldon B. C. 2000. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 15, 397–402 10.1016/S0169-5347(00)01953-4 (doi:10.1016/S0169-5347(00)01953-4) [DOI] [PubMed] [Google Scholar]
- 2.Harris W. E., Uller T. 2009. Reproductive investment when mate quality varies: differential allocation versus reproductive compensation. Phil. Trans. R. Soc. B 364, 1039–1048 10.1098/rstb.2008.0299 (doi:10.1098/rstb.2008.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gowaty P. A. 2008. Reproductive compensation. J. Evol. Biol. 21, 1189–1200 10.1111/j.1420-9101.2008.01559.x (doi:10.1111/j.1420-9101.2008.01559.x) [DOI] [PubMed] [Google Scholar]
- 4.Fox C. W., McLennan L. A., Mousseau T. A. 1995. Male body size affects female lifetime reproductive success in a seed beetle. Anim. Behav. 50, 281–284 10.1006/anbe.1995.0242 (doi:10.1006/anbe.1995.0242) [DOI] [Google Scholar]
- 5.Kotiaho J. S., Simmons L. W., Hunt J., Tomkins J. L. 2003. Males influence maternal effects that promote sexual selection: a quantitative genetic experiment with dung beetles Onthophagus taurus. Am. Nat. 161, 852–859 10.1086/375173 (doi:10.1086/375173) [DOI] [PubMed] [Google Scholar]
- 6.Galeotti P., Rubolini D., Fea G., Ghia D., Nardi P. A., Gherardi F., Fasola M. 2006. Female freshwater crayfish adjust egg and clutch size in relation to multiple male traits. Proc. R. Soc. B 273, 1105–1110 10.1098/rspb.2005.3345 (doi:10.1098/rspb.2005.3345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans J. P., Box T. M., Brooshooft P., Tatler J. R., Fitzpatrick J. L. 2010. Females increase egg deposition in favor of large males in the rainbowfish, Melanotaenia australis. Behav. Ecol. 21, 465–469 10.1093/beheco/arq006 (doi:10.1093/beheco/arq006) [DOI] [Google Scholar]
- 8.Burley N. 1988. The differential-allocation hypothesis: an experimental test. Am. Nat. 132, 611–628 10.1086/284877 (doi:10.1086/284877) [DOI] [Google Scholar]
- 9.De Lope F., Møller A. P. 1993. Female reproductive effort depends on the degree of ornamentation of their mates. Evolution 47, 1152–1160 10.2307/2409981 (doi:10.2307/2409981) [DOI] [PubMed] [Google Scholar]
- 10.Petrie M., Williams A. 1993. Peahens lay more eggs for peacocks with larger trains. Proc. R. Soc. Lond. B 251, 127–131 10.1098/rspb.1993.0018 (doi:10.1098/rspb.1993.0018) [DOI] [Google Scholar]
- 11.Uller T., Eklöf J., Andersson S. 2005. Female egg investment in relation to male sexual traits and the potential for transgenerational effects in sexual selection. Behav. Ecol. Sociobiol. 57, 584–590 10.1007/s00265-004-0886-2 (doi:10.1007/s00265-004-0886-2) [DOI] [Google Scholar]
- 12.Cunningham E. J. A., Russell A. F. 2000. Egg investment is influenced by male attractiveness in the mallard. Nature 404, 74–77 10.1038/35003565 (doi:10.1038/35003565) [DOI] [PubMed] [Google Scholar]
- 13.Haig D., Westoby M. 1989. Parent-specific gene expression and the triploid endosperm. Am. Nat. 134, 147–155 10.1086/284971 (doi:10.1086/284971) [DOI] [Google Scholar]
- 14.Moore T., Haig D. 1991. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 7, 45–49 [DOI] [PubMed] [Google Scholar]
- 15.Haig D. 1996. Placental hormones, genomic imprinting, and maternal-fetal communication. J. Evol. Biol. 9, 357–380 10.1046/j.1420-9101.1996.9030357.x (doi:10.1046/j.1420-9101.1996.9030357.x) [DOI] [Google Scholar]
- 16.Mochizuki A., Takeda Y., Iwasa Y. 1996. The evolution of genomic imprinting. Genetics 144, 1283–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkins J. F., Haig D. 2003. What good is genomic imprinting: the function of parent-specific gene expression. Nat. Rev. Genet. 4, 359–368 10.1038/nrg1062 (doi:10.1038/nrg1062) [DOI] [PubMed] [Google Scholar]
- 18.Bastock M., Manning A. 1955. The courtship of Drosophila melanogaster. Behaviour 8, 85–111 10.1163/156853955X00184 (doi:10.1163/156853955X00184) [DOI] [Google Scholar]
- 19.Friberg U. 2006. Male perception of female mating status: its effect on copulation duration, sperm defence and female fitness. Anim. Behav. 72, 1259–1268 10.1016/j.anbehav.2006.03.021 (doi:10.1016/j.anbehav.2006.03.021) [DOI] [Google Scholar]
- 20.Chapman T. 2001. Seminal fluid-mediated fitness traits in Drosophila. Heredity 87, 511–521 10.1046/j.1365-2540.2001.00961.x (doi:10.1046/j.1365-2540.2001.00961.x) [DOI] [PubMed] [Google Scholar]
- 21.Findlay G. D., Yi X., MacCoss M. J., Swanson W. J. 2008. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 6, e178. 10.1371/journal.pbio.0060178 (doi:10.1371/journal.pbio.0060178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herndon L. A., Wolfner M. F. 1995. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc. Natl Acad. Sci. USA 92, 10 114–10 118 10.1073/pnas.92.22.10114 (doi:10.1073/pnas.92.22.10114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heifetz Y., Lung O., Frongillo E. A., Jr, Wolfner M. F. 2000. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 10, 99–102 10.1016/S0960-9822(00)00288-8 (doi:10.1016/S0960-9822(00)00288-8) [DOI] [PubMed] [Google Scholar]
- 24.Kubli E. 2003. Sex-peptides: seminal peptides of the Drosophila male. Cell. Mol. Life Sci. 60, 1689–1704 10.1007/s00018-003-3052 (doi:10.1007/s00018-003-3052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng J., Chen S., Büsser S., Liu H., Honegger T., Kubli E. 2005. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 15, 207–213 10.1016/j.cub.2005.01.034 (doi:10.1016/j.cub.2005.01.034) [DOI] [PubMed] [Google Scholar]
- 26.Chapman T., Liddle L. F., Kalb J. M., Wolfner M. F., Partridge L. 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244 10.1038/373241a0 (doi:10.1038/373241a0) [DOI] [PubMed] [Google Scholar]
- 27.Fricke C., Bretman A., Chapman T. 2010. Female nutritional status determines the magnitude and sign of responses to a male ejaculate signal in Drosophila melanogaster. J. Evol. Biol. 23, 157–165 10.1111/j.1420-9101.2009.01882.x (doi:10.1111/j.1420-9101.2009.01882.x) [DOI] [PubMed] [Google Scholar]
- 28.Pitnick S. 1991. Male size influences mate fecundity and remating interval in Drosophila melanogaster. Anim. Behav. 41, 735–745 10.1016/S0003-3472(05)80340-9 (doi:10.1016/S0003-3472(05)80340-9) [DOI] [Google Scholar]
- 29.Pitnick S., García-González F. 2002. Harm to females increases with male body size in Drosophila melanogaster. Proc. R. Soc. Lond. B 269, 1821–1828 10.1098/rspb.2002.2090 (doi:10.1098/rspb.2002.2090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark A. G., Aguade M., Prout T., Harshman L. G., Langley C. H. 1995. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics 139, 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiumera A. C., Dumont B. L., Clark A. G. 2005. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics 169, 243–257 10.1534/genetics.104.032870 (doi:10.1534/genetics.104.032870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friberg U., Lew T. A., Byrne P. G., Rice W. R. 2005. Assessing the potential for an ongoing arms race within and between the sexes: selection and heritable variation. Evolution 59, 1540–1551 [PubMed] [Google Scholar]
- 33.Partridge L., Fowler K. 1990. Non-mating costs of exposure to males in female Drosophila melanogaster. J. Insect Physiol. 36, 419–425 10.1016/0022-1910(90)90059-O (doi:10.1016/0022-1910(90)90059-O) [DOI] [Google Scholar]
- 34.Schwarzkopf L., Blows M. W., Caley M. J. 1999. Life history consequences of divergent selection on egg size in Drosophila melanogaster. Am. Nat. 154, 333–340 10.1086/303242 (doi:10.1086/303242) [DOI] [PubMed] [Google Scholar]
- 35.Greenberg A. J., Hackett S. R., Harshman L. G., Clark A. G. 2010. A hierarchical Bayesian model for a novel sparse partial Diallel crossing design. Genetics 185, 361–373 10.1534/genetics.110.115055 (doi:10.1534/genetics.110.115055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice W. R., Linder J. E., Friberg U., Lew T. A., Morrow E. H., Stewart A. D. 2005. Inter-locus antagonistic coevolution as an engine of speciation: assessment with hemiclonal analysis. Proc. Natl Acad. Sci. USA 102, 6527–6534 10.1073/pnas.0501889102 (doi:10.1073/pnas.0501889102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bloch Qazi M. C., Heifetz Y., Wolfner M. F. 2003. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev. Biol. 256, 195–211 10.1016/S0012-1606(02)00125-2 (doi:10.1016/S0012-1606(02)00125-2) [DOI] [PubMed] [Google Scholar]
- 38.Markow T. A., Beall S., Matzkin L. M. 2009. Egg size, embryonic development time and ovoviviparity in Drosophila species. J. Evol. Biol. 22, 430–434 10.1111/j.1420-9101.2008.01649.x (doi:10.1111/j.1420-9101.2008.01649.x) [DOI] [PubMed] [Google Scholar]
- 39.Pitnick S., Miller G. T., Schneider K., Markow T. A. 2003. Ejaculate–female coevolution in Drosophila mojavensis. Proc. R. Soc. Lond. B 270, 1507–1512 10.1098/rspb.2003.2382 (doi:10.1098/rspb.2003.2382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avelar T. 1993. Egg size in Drosophila: standard unit of investment of variable response to environment? The effect of temperature. J. Insect Physiol. 39, 283–289 10.1016/0022-1910(93)90058-Y (doi:10.1016/0022-1910(93)90058-Y) [DOI] [Google Scholar]
- 41.Azevedo R. B. R., French V., Partridge L. 1996. Thermal evolution of egg size in Drosophila melanogaster. Evolution 50, 2338–2345 10.2307/2410702 (doi:10.2307/2410702) [DOI] [PubMed] [Google Scholar]
- 42.Azevedo R. B. R., Partridge L., French V. 1997. Life-history consequences of egg size in Drosophila melanogaster. Am. Nat. 150, 250–282 10.1086/286065 (doi:10.1086/286065) [DOI] [PubMed] [Google Scholar]
- 43.Eberhard W. G. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press [Google Scholar]
- 44.Clark A. G., Begun D. J., Prout T. 1999. Female × male interactions in Drosophila sperm competition. Science 283, 217–220 10.1126/science.283.5399.217 (doi:10.1126/science.283.5399.217) [DOI] [PubMed] [Google Scholar]
- 45.Manier M. K., Belote J. M., Berben K. S., Novikov D., Stuart W. T., Pitnick S. 2010. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science 328, 354–357 10.1126/science.1187096 (doi:10.1126/science.1187096) [DOI] [PubMed] [Google Scholar]