Abstract
Colours in feathers are produced by pigments or by nanostructurally organized tissues that interact with light. One of the simplest nanostructures is a single layer of keratin overlying a linearly organized layer of melanosomes that create iridescent colours of feather barbules through thin-film interference. Recently, it has been hypothesized that glossy (i.e. high specular reflectance) black feathers may be evolutionarily intermediate between matte black and iridescent feathers, and thus have a smooth keratin layer that produces gloss, but not the layered organization of melanosomes needed for iridescence. However, the morphological bases of glossiness remain unknown. Here, we use a theoretical approach to generate predictions about morphological differences between matte and glossy feathers that we then empirically test. Thin-film models predicted that glossy spectra would result from a keratin layer 110–180 nm thick and a melanin layer greater than 115 nm thick. Transmission electron microscopy data show that nanostructure of glossy barbules falls well within that range, but that of matte barbules does not. Further, glossy barbules had a thinner and more regular keratin cortex, as well as a more continuous underlying melanin layer, than matte barbules. Thus, their quasi-ordered nanostructures are morphologically intermediate between matte black and iridescent feathers, and perceived gloss may be a form of weakly chromatic iridescence.
Keywords: biophotonics, iridescence, plumage colour, structural colour
1. Introduction
Colours of animals can be produced by two broad categories of mechanisms. Pigment-based colours result from the wavelength-dependent absorbance of light by molecules, typically carotenoids and melanins [1,2]. By contrast, structural colours are produced by coherent scattering of incident light by tissues that periodically vary in refractive index [3], and can be iridescent (i.e. varying in hue with angle of observation) or non-iridescent. In feathers, iridescent coloration is usually produced by layered stacks of keratin and hollow or solid melanosomes in feather barbules [3,4], with colour properties deriving from the optical path travelled by light (determined by the layer's thickness and refractive index), as well as the difference in refractive index of the materials. In the simplest case, a single layer of keratin over a layer of ordered melanin granules creates the appropriate optical path to produce iridescent colours with discrete peaks [5–7]. These thin-film structures also result in a strong angle-dependence of colour properties, giving rise not only to the changes in hue that characterize iridescence, but also to strong specular (mirror-like) reflectance that accounts for their high brightness (overall reflectance) [8,9].
Most studies of animal coloration have focused on the function and mechanisms of hue, saturation and brightness [10], largely ignoring other colour and reflection attributes. Glossiness—loosely defined as the specular component of light reflecting from an object [11,12], and perhaps most familiar as a sought-after component of human hair—is one such attribute. Being a complex variable derived from both the material's physical attributes and the observer's interpretation of highlights and background illuminance [13], several definitions (as well as attempts to properly quantify it) have been posed, and the appropriate measure is likely to vary according to the system [12].
Feathers vary in gloss even more widely than hair, as can be seen most clearly in non-iridescent black feathers that range from the dull matte of house sparrow (Passer domesticus) bibs to the lustrous sheen of crows and ravens (Family Corvidae). Because iridescent feathers also have high gloss, Toomey et al. [14] recently hypothesized that glossy black colour might be an intermediate step in the evolution of iridescence from matte black [3,15], suggesting that gloss without iridescence may result from a smooth feather surface that precedes the organization of melanin granules underneath it. Before we can test this hypothesis, it is first necessary to understand the morphological and mechanistic basis of glossy black feathers.
Thus, here we investigate the micro- and nanostructural basis of glossy black feathers using both a theoretical and empirical approach. First, we use reflectance spectrometry to quantify and compare glossiness of feathers visually classified as either glossy or matte. Second, we develop a mathematical model of an idealized glossy black thick-film reflector. Finally, we empirically test this model by using electron microscopy to examine and compare the micro- and nanostructure of glossy and matte feathers.
2. Material and methods
(a). Sample collection
Study skins of a phylogenetically diverse group of birds from the Ornithological Collection at The University of Akron were visually classified as matte or glossy black by three independent observers. Between 5 and 10 contour feathers were obtained from each of eight species that had been unanimously classified as glossy or matte (electronic supplementary material, table S1). Feathers were numerically coded and stored in numbered envelopes to allow for data collection blind to both species and group. Three of the feathers were separated for reflectance measurements, and the remainder were used for scanning (SEM) and transmission electron microscopy (TEM).
(b). Glossiness quantification
For all reflectance measurements, we taped feathers to black construction paper, overlaying each other to simulate their natural organization on the bird's body. Reflectance was measured from these stacks using an Avantes AvaSpec-2048 spectrometer and AvaLight-XE pulsed xenon light source, relative to a WS-2 white reflectance standard (Avantes Inc., Boulder, CO, USA).
We used the ratio of specular to diffuse reflectance as our index of glossiness (Hunter's ‘contrast gloss’ [11,12]). To quantify specular reflectance, we took point-source reflectance measurements using two separate probes both placed at 75° from the plane normal using a block holder (AFH-15, Avantes Inc.). This geometry was chosen to maximize specular reflectance, minimizing scattering within the bulk material [16]. To quantify diffuse reflectance, we used an integrating sphere (AvaSphere-50-REFL, Avantes Inc.) equipped with a black gloss trap (AvaSphere-GT50, Avantes Inc.) to exclude specular reflectance.
We took three measurements each of diffuse and specular reflectance from the feathers using AvaSoft v. 7.2, with the probe holder completely removed and placed at a different location on the feather surface before each measurement. For point-source specular reflectance measurements, we also rotated the holding block in relation to the proximo-distal axis to ensure that readings were taken at the azimuth angle of maximum reflectance [9]. We then interpolated measurements to 1 nm bins within the avian visible spectrum (300–700 nm), averaged reflectance across all wavelengths for each species and divided average specular by diffuse reflectance to obtain glossiness [11,12].
(c). Thin-film optical modelling
Iridescent colours in feather barbules are characterized by discrete, saturated reflectance peaks and relatively high brightness [3]. By contrast, all black colours are characterized by low and uniform reflectance with no saturated peaks (e.g. [15,17]), and glossy blacks should have higher specular reflectance than matte blacks. We hypothesized that this enhanced specular reflectance, without saturated peaks, could be produced by simple thin-film nanostructures with certain optical path lengths. To identify these path lengths, we conducted thin-film simulations using the transfer matrix method (as outlined in [18]), implemented in a script developed by Maia et al. [5] for the programming language R [19]. Since these models assume ideal reflectors (i.e. perfectly specular reflectance, smooth and regular interfaces) and only consider the specular component of reflection, they define an upper-bound hypothetical model to be compared with empirical measurements. Light was modelled at normal incidence, thus minimizing any surface effects that would not be accounted for by the model. Complex refractive indices (incorporating the extinction coefficient, which reflects the wavelength-dependent absorption of materials) for keratin (ñ = 1.56 − 0.03i) and melanin (ñ = 2.00 − 0.6i) were used [6,7,15], and both the outermost keratin layer and the melanin layer were allowed to vary in thickness from 50 to 1000 nm in 10 nm bins.
All possible combinations of keratin and melanin layer thicknesses mentioned above were considered in an automated simulation to calculate brightness and contrast (a measure of saturation, calculated as the maximum minus the minimum reflectance values from the spectrum [10]) of the predicted spectra. We then divided average reflectance in contrast to obtain a brightness-to-saturation ratio. Because they lack discrete reflectance peaks but have relatively high specular reflectance, the specular component of glossy black colours should have high brightness and low contrast, and thus high brightness-to-saturation ratios. We used this model to predict the thickness values of keratin and melanin layers that would produce such high values, then compared these with the empirical values obtained from transmission electron micrographs (see below).
(d). Electron microscopy measurements
TEM samples were prepared following Shawkey et al. [20]. Reflectance stereomicroscopy suggested that differences in gloss resulted mostly from barbule (not barb rami) reflection, so we focused our morphological analyses on these structures. Three barbule images of each species were used to measure the barbule morphology. From each of these images, the following measurements were taken using ImageJ software [21]: (i) barbule width (the distance between the outermost edges of the barbule); (ii) barbule thickness (the distance between the top and bottom surfaces of the barbule cross-section, taken at three points per barbule); (iii) keratin cortex thickness (the distance from the edge of the barbule to the outermost melanin granule, taken at six different points per barbule); (iv) keratin cortex coefficient of variation (CV, obtained from the six measurements per barbule); (v) melanin layer thickness (the distance from the melanin granule defining the edge of the keratin cortex to the adjacent innermost granule); (vi) melanin density (the area occupied by melanin granules, obtained by a threshold procedure, divided by the cross-sectional barbule area). Finally, we drew a transect connecting all outermost granules in the perimeter of the barbule cross-section. The portion of this transect not intersecting melanin granules was used to quantify (vii) the number of gaps on the melanin layer and (viii) the discontinuity of the melanin layer (percentage of the transect not covered by melanin granules).
Variables (i) and (ii) were used to quantify size and shape of barbules, since such factors have been shown to affect glossiness in human hair [22,23]. Variables (iii–v) were used to compare barbule nanostructural morphology to the simulated thin-film models, and variable (vi) was obtained to quantify the importance of pigmentation on glossiness [24]. Variables (vii) and (viii) were used to quantify the level of barbule nanostructural organization, since an organized melanin layer ultimately defines the keratin cortex thickness and is essential for iridescent structural colour to be produced in dark glossy species [5,6,25].
(e). Evaluation of surface roughness
Irregularities in reflecting surfaces can lead to out-of-phase reflectance and thus low gloss [16]. We therefore used SEM to assess surface roughness of barbules of glossy and matte feathers. Based on the Rayleigh criterion, at the specular reflectance angle of 75°, irregularities in the surface must be over approximately 145 nm to interfere with scattering of light within the avian visible spectrum [16]. At 4000×, features of this size and over are easily visible, so we used this magnification in our analyses. We developed a five-step graphic scale of textures (electronic supplementary material, figure S1) that ranged from smooth (=1) to rough (=5) based on surface irregularities. Five participants, blind to the identity of the species, were asked to rate one SEM micrograph for each species according to the roughness scale. Within-species repeatability of these rankings was evaluated [26], and average values were used in the analysis.
(f). Statistical analyses
We tested the distribution of all variables for departures from the normal distribution by visually inspecting their normal probability plots and comparing them to those obtained from random samples of the same size from normal distributions with the same parameters (mean and standard deviation). This approach is considered preferable to formal normality tests, especially in the case of small samples [27]. After log-transforming glossiness and keratin cortex thickness values, all variables were normally distributed. Unless otherwise noted, means are presented with their standard errors.
We used two-sample t-tests to compare variables between glossy and matte groups, and Pearson correlation tests to assess the relationship between the morphological variables and glossiness. Given our sample sizes and the number of variables tested, we had to account for and minimize chances of incurring both type I and type II errors. The inherent loss of power associated with Bonferroni corrections and similar procedures [28,29] led us to present all our results as standardized effect sizes with their associated confidence intervals [30]. Through this approach, the magnitude of the differences and associations, as well as the associated uncertainties, can be evaluated directly, providing a more objective interpretation of both significant and non-significant results [31–33].
We calculated all effect sizes and confidence intervals according to Nakagawa & Cuthill [30]. For t-tests, we calculated Hedge's d, and the associated confidence interval was calculated using non-central t distributions. For correlations, Pearson's correlation coefficient (r) was used, but since this statistic has a known non-normal distribution, Fisher's Z transformation was used to obtain standardized values (Zr) for the calculation of confidence intervals. For ease of interpretation, Zr values for the effect size and confidence limits were back-calculated to r values for the figures.
3. Results
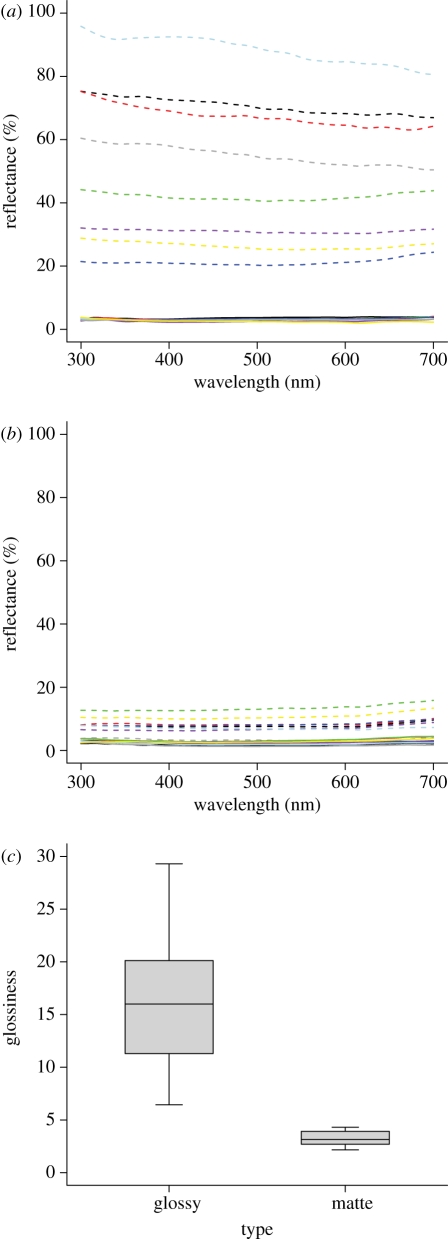
As expected, diffuse reflectance values for black feathers were low—all under 5 per cent average reflectance (solid lines in figure 1a,b). However, specular reflectance curves from matte and glossy species (as assessed visually before spectral data were obtained) were distinctive: while the average reflectance of matte species was under 15 per cent, that of glossy species was highly variable and above 20 per cent (dashed lines in figure 1a,b). Glossiness, as measured by the specular-to-diffuse reflectance ratio, was significantly higher in glossy than in matte species (t14 = 8.25, p < 0.001; figure 1c).
Figure 1.
(a) Diffuse (solid lines) and (b) specular (dashed lines) smoothed reflectance spectra of species with (a) glossy and (b) matte black plumage. (a) Black, double-crested cormorant; red, common raven; green, fish crow; blue, magpie-lark; light blue, acorn woodpecker; purple, Caspian tern; yellow, turkey vulture; grey, yellow-bellied sapsucker. (b) Black, yellow-billed cacique; red, horned grebe; green, Baltimore oriole; blue, northern flicker; light blue, black-mandibled toucan; purple, California quail; yellow, house sparrow; grey, American goldfinch. (c) Boxplot of glossiness values for glossy and matte species.
(a). Thin-film optical modelling
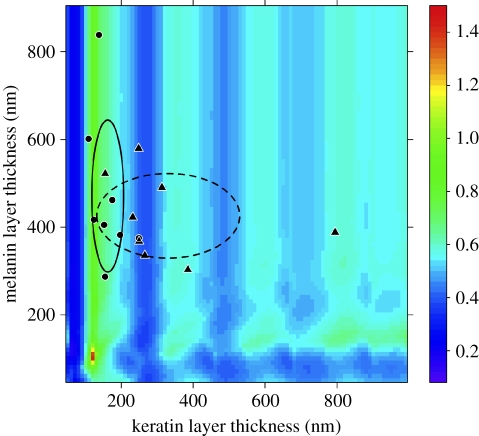
Our simulations predicted that the largest values for the brightness-to-saturation ratio should occur when the keratin layer is between 110 and 180 nm thick, peaking when it is approximately 120 nm thick and the melanin layer 115 nm thick (figure 2). A thinner cortex would result in very little interference within the visual range, and a thicker cortex produces higher-order harmonic peaks that are narrow and saturated (electronic supplementary material, figure S2). Simulations also predicted that variation in the keratin cortex thickness should play the most significant role in producing gloss, with the melanin layer thickness serving a greatly reduced role. Indeed, melanin has no discernible effect when its thickness is greater than approximately 150 nm (figure 2), smaller than the minimum diameter of most avian melanosomes thus far studied [34,35]. Keratin thickness, on the other hand, strongly affected ratios in an oscillatory manner when its thickness was less than approximately 500 nm, with a more level effect at values greater than 500 nm.
Figure 2.
Contour plot of the average reflectance and contrast of thin-film model simulations varying in both keratin cortex and melanin layer thicknesses. Measured values for glossy (circles) and matte (triangles) species are displayed, and ovals represent the standard deviation in both keratin cortex and melanin layer thicknesses (solid line, glossy; dashed line, matte).
Because melanosome diameter constrains the achievable melanin layer thickness, and the thickness for the highest brightness-to-saturation ratio (115 nm) is much smaller than the observed melanosome diameter in our sample (260.83 ± 13.53 nm), we determined that a keratin thickness between 110 and 180 nm should produce the greatest amount of gloss. We thus predicted that the thickness of the cortex in glossy feathers should be constrained within this range, while the range of the melanin layer thickness would be unconstrained because it serves no optical function. Further, we predicted that both cortex and melanin layer thickness of matte feathers should not be restricted within this range.
(b). Structural measurements
As predicted, glossy species showed a high variation in melanin layer thickness, but variation of keratin cortex thickness was restricted and largely within our predicted values (table 1 and figure 2). Matte species, on the other hand, did not show this pattern, with highly variable keratin cortex thicknesses largely outside those predicted for glossy species, as well as highly variable melanin layer thicknesses (figure 2).
Table 1.
Means (and s.e.) and t-test results for barbule morphological variables of matte and glossy black feathers. Significant (p < 0.05) results are bolded.
| variable | glossy | matte | t (d.f. = 14) | p |
|---|---|---|---|---|
| barbule width (µm) | 12.92 (1.47) | 14.01 (1.16) | 0.39 | 0.69 |
| barbule thickness (µm) | 3.05 (0.19) | 3.26 (0.19) | 0.55 | 0.59 |
| barbule aspect ratio | 4.41 (0.51) | 4.36 (0.35) | 0.95 | 0.36 |
| keratin cortex thickness (nm)a | 162.56 (10.82) | 330.65 (48.22) | 3.25 | 0.005 |
| keratin cortex CV (%) | 23.91 (2.45) | 44.02 (5.66) | 2.24 | 0.04 |
| melanin layer thickness (nm) | 471.15 (42.02) | 426.04 (23.37) | −0.64 | 0.53 |
| melanin layer discontinuity (%) | 16.44 (1.49) | 32.92 (2.27) | 4.16 | <0.001 |
| number of gaps in the melanin layer | 8.36 (0.54) | 12.73 (0.83) | 3.04 | 0.008 |
| proportion of melanin (%) | 51.68 (2.35) | 45.11 (2.75) | −1.25 | 0.23 |
aLog-transformed for statistical analyses.
Descriptive statistics for the variables measured are summarized in table 1. Glossy and matte species differed in the nanostructural characteristics of their barbules (figure 3): glossy species had thinner and less variable keratin cortex, and a more continuous melanin layer with fewer gaps (figure 4a). However, glossy and matte species did not differ in microstructural characteristics of their barbules, with no statistical differences in barbule width, thickness or aspect ratio (t-tests; all p > 0.05). These variables had a point estimate effect size close to zero, further supporting a lack of difference in those variables between the groups (table 1 and figure 4a). There were no differences between the groups in the proportion of melanin within the barbule or the thickness of the melanin layer (figure 4a).
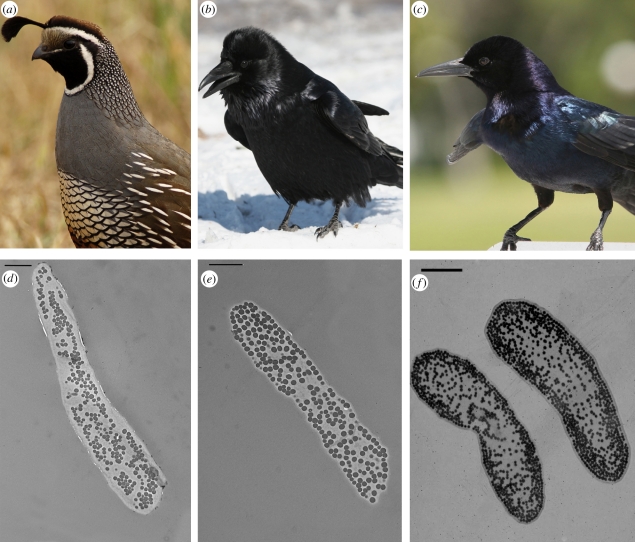
Figure 3.
Comparison of (a,d) matte (California quail), (b,e) glossy (common raven) and (c,f) iridescent (boat-tailed grackle Quiscalus major, not included in this study) for (a–c) plumage properties and (d–f) barbule morphology, as observed through TEM (scale bars, 2 µm). Note the increasing order and continuity of the outermost melanin granules from right to left. Photo credits: (a) Matthew Knoth; (b) Jean-Guy Dallaire; and (c) Tom Friedel.
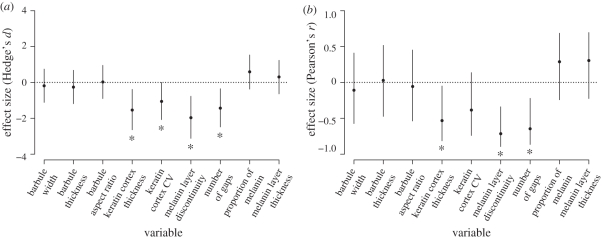
Figure 4.
Standardized effect sizes for (a) type of colour (glossy or matte) and (b) association with glossiness on the tested variables. In (a), effect sizes are relative to glossy species (i.e. positive values indicate higher values for glossy than matte species). Asterisks indicate (a) significant t-tests or (b) Pearson correlation tests (and 95% confidence intervals for effect size that do not overlap zero).
Glossiness decreased with increasing keratin cortex thickness, melanin layer discontinuity and the number of gaps in the melanin layer, and, consistent with the comparative results above, was not correlated to any barbule microstructural morphology variables (figure 4b). Further, confidence intervals of the effect size of the correlation between glossiness and both measures of organization were narrower than in any other measure, indicating the smaller uncertainty upon the estimates of these significant effects (figure 4b).
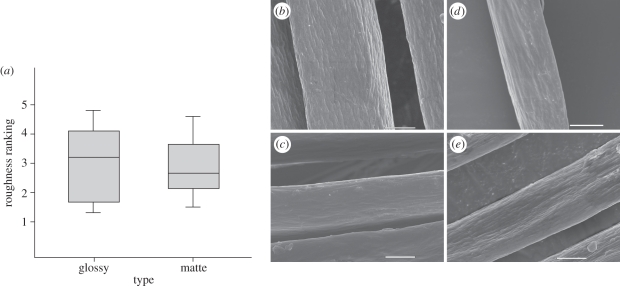
Surface roughness rankings were highly repeatable (r = 0.67, F15,60 = 11.57, p < 0.001) and, though they were also highly variable between species, glossy and matte species were not statistically different (t14 = 0.21, p = 0.84; figure 5). Indeed, one of the glossiest species (yellow-bellied sapsucker) had an extremely rough surface (ranking = 4.8), while a species with one of the darkest matte feathers (California quail) had the second smoothest surface (ranking = 1.5, figure 5). Further, surface roughness rankings were not correlated to glossiness (r = 0.03, d.f. = 14, p = 0.90). Although more precise methods for evaluating roughness, like atomic force microscopy (AFM), should be used for verification, these data suggest that feather gloss is unrelated to surface roughness.
Figure 5.
(a) Boxplot for the roughness measurements obtained from visual evaluation of SEM images of glossy and matte feather barbules. (b–e) Examples of SEM images from glossy ((b) yellow-bellied sapsucker; (c) common raven) and matte ((d) horned grebe; (e) American goldfinch) barbules. Scale bars, 5 µm.
4. Discussion
As far as we are aware, this is the first attempt to identify the anatomical basis of glossiness in feathers. Our theoretical and empirical data suggest that it is produced by a simple arrangement of an extremely thin keratin cortex over a quasi-ordered layer of melanin granules in barbules. This structure thus appears to be morphologically intermediate between matte black and iridescent colours. This intermediacy is also seen in the faint peaks in spectral curves from many glossy black samples, which suggest that they have weak or washed-out iridescent colour. These results have important implications for the mechanisms and evolution of iridescent structural colours.
Physical models of thin-film structures predicted that glossy black colours should result from a keratin cortex between 110 and 180 nm thick. Although an organized layer of melanosomes is necessary to delineate this layer, its thickness should not affect the reflected spectra owing to melanin's high absorbance. Consistent with these predictions, the keratin cortex of glossy species averaged approximately 160 nm thick and was considerably less variable in thickness than the melanin layer. Matte species, on the other hand, showed high variation in both keratin and melanin layer thicknesses, suggesting that matte colour can be produced by a broad range of morphologies.
Interestingly, the keratin cortex dimensions found in glossy species should produce single-peak spectral curves within the avian visual limits, from a UV-violet peak (388 nm hue for a 100-nm-thick keratin cortex) to a copper-yellow (632 nm hue for a 180-nm-thick cortex), which would account for the weak saturation of their spectral curves (electronic supplementary material, figure S3). Such morphology, producing saturated iridescent colours, has been described in several species, where the melanin granules form a continuous and uninterrupted layer beneath the keratin cortex (figure 4) [3–5,15,36]. However, the organization of melanosomes in glossy black feathers is more irregular than in iridescent species with similar structures, with more gaps and very few rows of granules delineating the layer, making it a suboptimal thin-film reflector. Gaps and irregularities in the melanin layer deviate them from these ideal conditions, affecting the saturation of the reflected colour by reducing the layer's average refractive index and/or by interrupting the thin-film reflector [37,38], and has been shown to affect intraspecific and interspecific patterns of iridescence in this predicted direction [6,15,25]. Thus, the nanostructures in glossy black feathers would require only an increased level of organization to become fully iridescent.
Surface smoothness is typically considered the primary mechanism for achieving glossiness [39,40]. Interestingly, we found no effect of surface texture on gloss, suggesting that the nanostructure of these feathers may produce gloss independent of surface roughness. However, the thickness of the keratin cortex within barbules of glossy species was less variable than that of matte species, suggesting that uniform thickness of the surface may be critical to gloss production even if smoothness is not. Feathers may have uniformly smooth surfaces, and thus any variation in gloss may only originate from variation in internal structure and pigment deposition pattern. Alternatively, the surface of iridescent barbules may be even smoother than that of glossy ones, and this hypothesis should be tested.
Though not directly causing or producing gloss, scattering or absorption of light within the material may also influence perceived gloss by affecting the proportion of diffusely reflected light [23,24,41]. We found no evidence for the influence of pigmentation in glossiness, nor for a difference in patterns of melanization between glossy and matte species. These results are consistent with the fact that there was little interspecific variation in diffuse reflectance, as well as similar patterns of diffuse reflectance for matte and glossy species.
Neither barbule size nor shape had an effect on feather gloss. In human hair, both fibre size and shape have been suggested to influence gloss, by increasing the specular reflectance area and facilitating fibre alignment [22]. In feathers, barbules compose most of the feather vane surface, with hooklets that bind them together in position [42]. Therefore, it is probable that, in the case of feathers, barbule shape may not be as important in producing gloss, since barbules are already interwoven and held in place to provide the smooth vane that is critical to feather function.
Coloration of bird feathers is considered to be extremely labile, leading to fast divergence of patch colour and shape [43–45]. Here, we show that glossy black feathers share properties with both melanin-based matte and structurally coloured feathers. Since all these colours are produced by the same material components, differing only in their relative concentration and organization, it is possible that glossy black feathers represent a potential intermediate on the evolution of iridescence. Toomey et al. [14] suggested that a smooth keratin surface would produce glossy feathers and that the rearrangement of granules within barbules would then lead to iridescence. Our results show, however, no relationship between surface smoothness and glossiness, and in fact suggest that some degree of nanostructural organization is already present in glossy black feathers.
The formation of a more organized outer layer of melanosomes during the barbule development may provide a scaffold for drying of keratin and thereby promote the growth of a keratin layer with uniform thickness [46]. Thus, the organization of granules may actually precede the production of a uniform cortex. Therefore, we suggest that one mechanism for the evolution of iridescence may be an interaction between melanosome organization and keratin cortex formation during feather development. Further, under this scenario, ‘thick-film structures’ (with a fundamental peak reflectance in the infrared spectrum and one or several harmonic peaks in the visible spectrum) should either be derived from this thin-film condition or represent an entirely different evolutionary and developmental pathway to iridescent coloration in feathers. These hypotheses can be tested through examination of developing matte, glossy and iridescent feathers from closely related species. Furthermore, the hypothesis that gloss is evolutionarily intermediate between matte and iridescent colour can be tested through rigorous phylogenetic comparisons. This work thus lays the groundwork for examination of the mechanisms and evolution of structural plumage colour at new levels of detail.
Acknowledgements
We would like to thank Matthew Toomey and Melissa Meadows for important discussions on the application and interpretation of some methods employed herein, including sharing of programming codes. We would also like to thank Chad Eliason, Ian Horn and two anonymous reviewers for insightful comments during the elaboration and on previous versions of the manuscript. Pictures in figure 4a–c obtained through Creative Commons Licenses (a,b: CC-BY-NC-ND; c: CC-BY). This work was supported by University of Akron startup funds and AFOSR grant FA9550-09-1-0159 (both to M.D.S.).
References
- 1.McGraw K. 2006. Mechanics of carotenoid-based coloration. In Bird coloration (eds Hill G., McGraw K.), pp. 177–242 Cambridge, MA: Harvard University Press [Google Scholar]
- 2.McGraw K. 2006. Mechanics of melanin-based coloration. In Bird coloration (eds Hill G., McGraw K.), pp. 243–294 Cambridge, MA: Harvard University Press [Google Scholar]
- 3.Prum R. 2006. Anatomy, physics and evolution of structural colors. In Bird coloration (eds Hill G., McGraw K.), pp. 295–353 Cambridge, MA: Harvard University Press [Google Scholar]
- 4.Durrer H. 1986. The skin of birds: coloration. In Biology of the integument 2: vertebrates (eds Matoltsy A., Richards K.), pp. 239–247 Berlin, Germany: Springer [Google Scholar]
- 5.Maia R., Caetano J. V. O., Bao S. N., Macedo R. H. 2009. Iridescent structural colour production in male blue-black grassquit feather barbules: the role of keratin and melanin. J. R. Soc. Interface 6, S203–S211 10.1098/rsif.2008.0460.focus (doi:10.1098/rsif.2008.0460.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doucet S., Shawkey M., Hill G., Montgomerie R. 2006. Iridescent plumage in satin bowerbirds: structure, mechanisms and nanostructural predictors of individual variation in colour. J. Exp. Biol. 209, 380–390 10.1242/jeb.01988 (doi:10.1242/jeb.01988) [DOI] [PubMed] [Google Scholar]
- 7.Brink D., van der Berg N. 2004. Structural colours from the feathers of the bird Bostrychia hagedash. J. Phys. D Appl. Phys. 37, 813–818 10.1088/0022-3727/37/5/025 (doi:10.1088/0022-3727/37/5/025) [DOI] [Google Scholar]
- 8.Kinoshita S., Yoshioka S., Miyazaki J. 2008. Physics of structural colors. Rep. Prog. Phys. 71, 076 401. 10.1088/0034-4885/71/7/076401 (doi:10.1088/0034-4885/71/7/076401) [DOI] [Google Scholar]
- 9.Osorio D., Ham A. 2002. Spectral reflectance and directional properties of structural coloration in bird plumage. J. Exp. Biol. 205, 2017–2027 [DOI] [PubMed] [Google Scholar]
- 10.Montgomerie R. 2006. Analyzing colors. In Bird coloration (eds Hill G., McGraw K.), pp. 90–147 Cambridge, MA: Harvard University Press [Google Scholar]
- 11.Rasmussen P., Dyck J. 2000. Silkiness in brown mink pelts characterized with optical methods. J. Anim. Sci. 78, 1697–1709 [DOI] [PubMed] [Google Scholar]
- 12.Nickerson D. 1957. A new cotton lustermeter for yarns and fibers. Text. Res. J. 27, 111–123 10.1177/004051755702700204 (doi:10.1177/004051755702700204) [DOI] [Google Scholar]
- 13.Anderson B. L., Kim J. 2009. Image statistics do not explain the perception of gloss and lightness. J. Vis. 9, 10–17 10.1167/9.11.10 (doi:10.1167/9.11.10) [DOI] [PubMed] [Google Scholar]
- 14.Toomey M. B., Butler M. W., Meadows M. G., Taylor L. A., Fokidis H. B., McGraw K. J. 2010. A novel method for quantifying the glossiness of animals. Behav. Ecol. Sociobiol. 64, 1047–1055 10.1007/s00265-010-0926-z (doi:10.1007/s00265-010-0926-z) [DOI] [Google Scholar]
- 15.Shawkey M. D., Hauber M. E., Estep L. K., Hill G. E. 2006. Evolutionary transitions and mechanisms of matte and iridescent plumage coloration in grackles and allies (Icteridae). J. R. Soc. Interface 3, 777–786 10.1098/rsif.2006.0131 (doi:10.1098/rsif.2006.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willmouth F. M. 1986. Transparency, translucency and gloss. In Optical properties of polymers (ed. Meeten G. H.), pp. 265–334 London, UK: Elsevier Applied Science Publishers [Google Scholar]
- 17.Doucet S., Shawkey M., Rathburn M., Mays H., Montgomerie R. 2004. Concordant evolution of plumage colour, feather microstructure and a melanocortin receptor gene between mainland and island populations of a fairy-wren. Proc. R. Soc. Lond. B 271, 1663–1670 10.1098/rspb.2004.2779 (doi:10.1098/rspb.2004.2779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jellison G. 1998. Spectroscopic ellipsometry data analysis: measured versus calculated quantities. Thin Solid Films 313, 33–39 [Google Scholar]
- 19.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 20.Shawkey M., Estes A., Siefferman L., Hill G. 2003. Nanostructure predicts intraspecific variation in ultraviolet-blue plumage colours. Proc. R. Soc. Lond. B 270, 1455–1460 10.1098/rspb.2003.2390 (doi:10.1098/rspb.2003.2390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasband W. 1997–2004. ImageJ. Bethesda, MD: National Institutes of Health [Google Scholar]
- 22.Keis K., Ramaprasad K., Kamath Y. 2004. Studies of light scattering from ethnic hair fibers. J. Cosmet. Sci. 55, 49–63 [PubMed] [Google Scholar]
- 23.Stamm R. F., Garcia M. L., Fuchs J. J. 1977. The optical properties of human hair I. Fundamental considerations and goniophotometer curves. J. Soc. Cosmet. Chem. 28, 571–599 [Google Scholar]
- 24.Keis K., Ramaprasad K., Kamath Y. 2004. Effect of hair color on luster. J. Cosmet. Sci. 55, 423–436 [PubMed] [Google Scholar]
- 25.Lee E., Aoyama M., Sugita S. 2009. Microstructure of the feather in Japanese Jungle Crows (Corvus macrorhynchos) with distinguishing gender differences. Anat. Sci. Int. 84, 141–147 10.1007/s12565-009-0022-5 (doi:10.1007/s12565-009-0022-5) [DOI] [PubMed] [Google Scholar]
- 26.Lessells C., Boag P. T. 1987. Unrepeatable repeatabilities: a common mistake. The Auk. 104, 116–121 [Google Scholar]
- 27.Maindonald J., Braun J. 2007. Data analysis and graphics using R: an example-based approach, 2nd edn. Cambridge, UK: Cambridge University Press [Google Scholar]
- 28.Garcia L. 2004. Escaping the Bonferroni iron claw in ecological studies. Oikos 105, 657–663 10.1111/j.0030-1299.2004.13046.x (doi:10.1111/j.0030-1299.2004.13046.x) [DOI] [Google Scholar]
- 29.Nakagawa S. 2004. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 15, 1044–1045 10.1093/beheco/arh107 (doi:10.1093/beheco/arh107) [DOI] [Google Scholar]
- 30.Nakagawa S., Cuthill I. C. 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605 10.1111/j.1469-185X.2007.00027.x (doi:10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- 31.Colegrave N., Ruxton G. 2003. Confidence intervals are a more useful complement to nonsignificant tests than are power calculations. Behav. Ecol. 14, 446–447 10.1093/beheco/14.3.446 (doi:10.1093/beheco/14.3.446) [DOI] [Google Scholar]
- 32.Garamszegi L. 2006. Comparing effect sizes across variables: generalization without the need for Bonferroni correction. Behav. Ecol. 17, 682–687 10.1093/beheco/ark005 (doi:10.1093/beheco/ark005) [DOI] [Google Scholar]
- 33.Garamszegi L. Z., et al. 2009. Changing philosophies and tools for statistical inferences in behavioral ecology. Behav. Ecol. 20, 1363–1375 10.1093/beheco/arp137 (doi:10.1093/beheco/arp137) [DOI] [Google Scholar]
- 34.Li Q., Gao K.-Q., Vinther J., Shawkey M. D., Clarke J. A., D'Alba L., Meng Q., Briggs D. E. G., Prum R. O. 2010. Plumage color patterns of an extinct dinosaur. Science 327, 1369–1372 10.1126/science.1186290 (doi:10.1126/science.1186290) [DOI] [PubMed] [Google Scholar]
- 35.Clarke J. A., Ksepka D. T., Salas-Gismondi R., Altamirano A. J., Shawkey M. D., D'Alba L., Vinther J., DeVries T. J., Baby P. 2010. Fossil evidence for evolution of the shape and color of penguin feathers. Science 330, 954–957 10.1126/science.1193604 (doi:10.1126/science.1193604) [DOI] [PubMed] [Google Scholar]
- 36.Doucet S. M., Shawkey M. D., Hill G. E., Montgomerie R. 2006. Iridescent plumage in satin bowerbirds: structure, mechanisms and nanostructural predictors of individual variation in colour. J. Exp. Biol. 209, 380–390 10.1242/jeb.01988 (doi:10.1242/jeb.01988) [DOI] [PubMed] [Google Scholar]
- 37.Nakamura E., Yoshioka S., Kinoshita S. 2008. Structural color of rock dove's neck feather. J. Phys. Soc. Jpn 77, 124801. 10.1143/JPSJ.77.124801 (doi:10.1143/JPSJ.77.124801) [DOI] [Google Scholar]
- 38.Kinoshita S., Yoshioka S. 2005. Structural colors in nature: the role of regularity and irregularity in the structure. Chem. Phys. Chem. 6, 1442–1459 10.1002/cphc.200500007 (doi:10.1002/cphc.200500007) [DOI] [PubMed] [Google Scholar]
- 39.Yonehara M., Matsui T., Kihara K., Isono H., Kijima A., Sugibayashi T. 2004. Experimental relationships between surface roughness, glossiness and color of chromatic colored metals. Mater. Trans. 45, 1027–1032 10.2320/matertrans.45.1027 (doi:10.2320/matertrans.45.1027) [DOI] [Google Scholar]
- 40.Okada A., Uno Y., Rahario P., Furukawa T. 2003. Surface modification of EDMed surface by wide-area electron beam irradiation. Proc. ASPE Annual Conf. 18, 172–175 [Google Scholar]
- 41.Nagase S., Shibuichi S., Ando K., Kariya E., Satoh N. 2002. Influence of internal structures of hair fiber on hair appearance. I. Light scattering from the porous structure of the medulla of human hair. J. Cosmet. Sci. 53, 89–100 [PubMed] [Google Scholar]
- 42.Gill F. 2007. Ornithology, 3rd edn. New York, NY: W. H. Freeman & Co [Google Scholar]
- 43.Badyaev A., Hill G. 2003. Avian sexual dichromatism in relation to phylogeny and ecology. Annu. Rev. Ecol. Evol. Syst. 34, 27–49 10.1146/annurev.ecolsys.34.011802.132441 (doi:10.1146/annurev.ecolsys.34.011802.132441) [DOI] [Google Scholar]
- 44.Owens I., Hartley I. 1998. Sexual dimorphism in birds: why are there so many different forms of dimorphism? Proc. R. Soc. Lond. B 265, 397–407 10.1098/rspb.1998.0308 (doi:10.1098/rspb.1998.0308) [DOI] [Google Scholar]
- 45.Bennett P., Owens I. 2002. Evolutionary ecology of birds. Oxford, UK: Oxford University Press [Google Scholar]
- 46.Dyck J. 1973. Feather structure: the surface of barbs and barbules. Zool Jahrb Abt Anat Ontogenie Tiere 90, 550–566 [Google Scholar]