Abstract
Anthropogenic noise is prevalent across the globe and can exclude birds from otherwise suitable habitat and negatively influence fitness; however, the mechanisms responsible for species' responses to noise are not always clear. One effect of noise is a reduction in effective acoustic communication through acoustic masking, yet some urban songbirds may compensate for masking by noise through altering their songs. Whether this vocal flexibility accounts for species persistence in noisy areas is unknown. Here, we investigated the influence of noise on habitat use and vocal frequency in two suboscine flycatchers using a natural experiment that isolated effects of noise from confounding stimuli common to urban habitats. With increased noise exposure, grey flycatcher (Empidonax wrightii) occupancy declined, but vocal frequency did not change. By contrast, ash-throated flycatcher (Myiarchus cinerascens) occupancy was uninfluenced by noise, but individuals in areas with greater noise amplitudes vocalized at a higher frequency, although the increase (≈200 kHz) may only marginally improve communication and may represent a secondary effect from increased vocal amplitude. Even so, the different flycatcher behavioural responses suggest that signal change may help some species persist in noisy areas and prompt important questions regarding which species will cope with an increasingly noisy world.
Keywords: acoustic communication, acoustic masking, anthropogenic noise, habitat use, signal change, suboscine
1. Introduction
Ecologists and evolutionary biologists have long recognized the role of the physical environment as a selective force in the evolution of vocal communication [1,2]. All environments are also characterized by background sounds, or noise, which can interfere with important acoustic signals. As background noise amplitude increases, it reduces a receiver's ability to detect and discriminate relevant signals from other sounds (acoustic masking). Many animals have evolved signal characteristics that minimize acoustic masking from sounds within their natural habitats [3], yet given the rapid and continued spread of human-altered landscapes, animals are now faced with new environmental acoustics that influence acoustic communication.
Anthropogenic ambient noise in cities, along roadways and adjacent to industrialized wildlands presents particular challenges for animals that rely on acoustic communication, especially birds. Because anthropogenic noise is louder and often more continuous than sounds in most natural habitats, it presents an evolutionarily novel condition for many species and a potentially important force influencing the ecology and evolution of wild populations [4]. Noisy areas also provide a unique opportunity to understand how animals adjust or fail to adjust their acoustic signals to reduce masking effects [5].
Several recent studies provide correlative evidence that some birds change spectral, amplitude and temporal features of their acoustic signals in noisy habitats [6–8]. Urban habitats differ from natural habitats in many respects other than noise. Although these other features may also influence the structure of acoustic signals (e.g. fewer frequency absorbing and reverberating features) [5,9], investigators have argued that signal changes may occur in response to masking by noise to improve communication via an increase in the signal-to-noise ratio (SNR). For example, in the presence of urban noise, several species, including the great tit (Parus major) [6] and reed bunting (Emberiza schoeniclus) [10], sing with a higher minimum frequency, presumably to ‘sing-above’ low-frequency noise and decrease masking effects. Individual nightingales (Luscinia megarhynchos) adjust the amplitude of their songs in response to different levels of background noise (Lombard effect) [7]. In terms of temporal adjustments, European robins (Erithacus rubecula) inhabiting areas with high levels of traffic noise sing at night, when background noise amplitudes are an order of magnitude lower [8]. Another mechanism by which species may maintain a suitable SNR for signal transmission is through avoidance of noisy areas. Many species may have limited vocal flexibility to reduce the acoustic masking of important signals, potentially explaining patterns of reduced avian densities [11] and reductions in species richness and community diversity in areas exposed to noise [12]. These patterns suggest that increased exposure of habitat to noise may represent habitat loss for many species owing to unfavourable environmental acoustics for signal transmission, although knowledge of which species are sensitive to noise and which may be more tolerant owing to vocal signal change is currently limited.
Amplitude adjustments that increase SNR in noisy environments appear quite common among birds and mammals [3]; however, correlative evidence for changes to spectral and temporal signal features in response to noise among avian species are restricted to oscine birds (order Passeriformes, suborder Oscines). There is currently a need to understand whether other avian taxa are capable of those same strategies observed in Oscines that reduce the masking potential of noise, especially among suboscine birds (order Passeriformes, suborder Tyranni), which constitute approximately 20 per cent of Passeriformes [13]. Unlike songs of oscine birds, suboscine song appears to develop in the absence of learning [14,15]. Given this distinction, suboscines have typically been thought to have little intraspecific song variation and individuals are expected to have little vocal plasticity, yet recent data suggest that previous dogma regarding limited vocal variation and flexibility in suboscines may be invalid. For example, intraspecific song variation in suboscines has been recognized as important for individual recognition and discrimination [15,16]. Additionally, ocellated antbirds (Phaenostictus mcleannani) change frequency features depending on social interactions [17]. These examples of song variation among and within individuals may reflect a degree of vocal flexibility that permits individual birds to adjust signals to a variable acoustic environment. However, knowledge of whether suboscines are capable of noise-dependent signal change is lacking.
Here, we add to the limited understanding of which species can cope with signal interference from anthropogenic noise and which may disappear from the increasing number of areas exposed to noise. We investigate vocal frequency change and habitat use in response to continuous anthropogenic noise in two suboscine tyrant flycatchers (family Tyranidae): the ash-throated flycatcher (Myiarchus cinerascens) and grey flycatcher (Empidonax wrightii). In a previous nesting study, we isolated anthropogenic noise from other confounding stimuli often associated with noisy areas and controlled for habitat differences by using study sites located in woodland adjacent to natural gas wells with and without noisy compressors [12]. There we found the grey flycatcher avoiding noisy areas in its nest-site selection, yet the ash-throated flycatcher appeared uninfluenced by noise in its nest placement (n = 15; C. D. Francis 2007, unpublished data). Here, we use the same natural experiment to further investigate these species' responses to noise in their habitat use. We also test for changes in song and call spectral features in response to noise as potential mechanisms responsible for any differences in habitat use and the observed differences in each species' nest placement with respect to noise. We hypothesize that ash-throated flycatchers are noise tolerant and show no change in occupancy in response to noise amplitude because they modify frequency characteristics of their vocal signals with increases in compressor noise. By contrast, we hypothesize that grey flycatcher occupancy decreases with increased noise amplitude because they do not adjust frequency characteristics of their vocalizations as noise amplitude increases.
2. Material and methods
(a). Study species
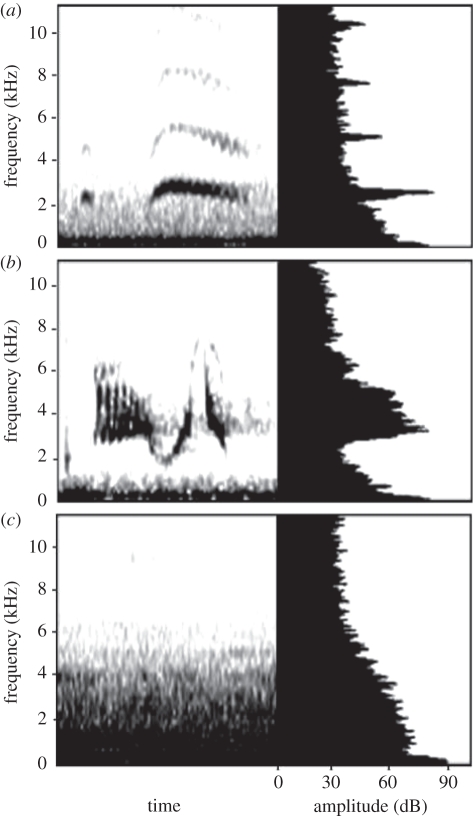
We focused on two flycatchers that breed in open woodlands of western North America. The ash-throated flycatcher is the larger of the two species (≈28 g) and is a secondary cavity nester that persists in human-altered habitats [18]. The grey flycatcher is ≈12.5 g and is an open cup-nesting species common to piñon (Pinus edulis)–juniper (Juniperus osteosperma) woodlands [19]. Vocalizations of the two species are characterized by different, but overlapping frequencies: grey flycatcher songs and calls are higher pitched (range ≈1.5–7.0 kHz) than those of ash-throated flycatchers (range ≈1.0–4.0 kHz; figure 1 and the electronic supplementary material, figure S1). Given this difference in vocal frequency range, lower frequency ash-throated flycatcher vocalizations are expected to suffer more from acoustic masking and although the strength of acoustic masking by noise may be less severe at higher frequencies, the higher pitched grey flycatcher vocalizations may still be masked by background noise because noise amplitudes at our study sites contain considerable energy as high as 5 kHz (see below; figure 1 and electronic supplementary material, figure S2).
Figure 1.
Spectrograms (left) and power spectra (right) of (a) ash-throated and (b) grey flycatcher songs and (c) of background noise on a treatment site at 200 m from the compressor. Darker shades in spectrograms indicate more acoustic energy located at those frequencies, which is reflected by higher amplitude values in the power spectra. See figure S2 in the electronic supplementary material for an example of background noise at 50 m from the compressor.
(b). Study area
We conducted our study within Rattlesnake Canyon Habitat Management Area (RCHMA), which is located within the San Juan Basin in northwestern New Mexico, USA, and managed by the Bureau of Land Management. RCHMA is dominated by piñon–juniper woodlands and is within one of the nation's most developed energy-producing regions. Gas wells are often coupled with compressors, which aid in the transportation of gas through pipelines and run 24 h a day, 365 days a year aside from periodic maintenance and our bird surveys and nest searches [12]. These compressors generate noise at amplitude levels that are hazardous to humans (figure 1 and electronic supplementary material, figure S2) [20]. Because noisy compressors are present on some well pads (treatment sites) and absent on others (control sites), RCHMA provides a unique opportunity to determine the influence of noise on natural populations and communities. Additionally, human activity and vegetation do not differ on and around the well pads with and without noisy compressors that are used in this study [12]; thus, effects of noise are separated from other confounding variables that complicate some other studies.
(c). Point counts
In 2007, we conducted surveys for ash-throated and grey flycatchers in woodlands surrounding gas wells at eight control sites and five treatment sites. Within two concentric circles around each well (50 m and 150 m from the well), we surveyed 13–16 randomly generated point count locations. Each point count location was visited twice during the study. At each point count location, we conducted a 7 min bird survey, and all surveys were completed between 7.00 and 12.00 h. Because of increases in identification error with large distances, we truncated all observations at 50 m from the point count location, using the closest distance from which each individual was detected. Additionally, and perhaps most importantly, treatment site compressors were turned off approximately 20 min prior to surveys and remained off for the duration of surveys to eliminate the negative effect of noise on bird detections [21].
Background noise amplitude was measured on the second of two surveys at all control site point count locations. Because compressors were turned off during surveys on treatment sites, we returned to each treatment point count location on a third visit to measure background noise amplitude with the compressors on. Noise amplitude measurements were taken with NIST-certified sound pressure metres (Casella model CEL 320 and CEL 1002 converter) only when there were no birds vocalizing within ≈30 m that could bias measurements of the compressor noise and when wind conditions were below category three (≈13–18 km h−1) on the Beaufort Wind Scale. At each location, we measured mean amplitude (equivalent continuous noise level (Leq), fast response time) with A- and C-weighting. Here, we used A-weighted decibels (dB(A)) values in all analyses because A-weighting filters much of the low frequency compressor noise (less than 0.5 kHz) that most birds hear poorly [22] and provides a better representation of acoustic energy at the frequencies at which the two species in this study vocalize (≈1.0–7.0 kHz; electronic supplementary material, figure S1).
Because there were no systematic differences in habitat characteristics, such as canopy cover, tree species and ratios, shrubs and ground cover on treatment and control sites [12], we assumed a constant detection probability on all surveys. Additionally, compressor noise was turned off during surveys on treatment sites so as to not bias our ability to locate birds [12]. To estimate the influence of background noise amplitude on habitat use, we used generalized linear mixed models with the lme4 package in program R [23]. For each flycatcher species, we modelled habitat occupancy using binomial logistic regression with mean background noise amplitude treated as a fixed effect and gas well site as a random effect. We used likelihood-ratio tests to compare models with the fixed effect of mean background noise amplitude to null models containing only the random effect of gas well site. Occurrence at a point count location was defined as whether a species was detected during any of the survey visits.
(d). Vocalization measurements
Ash-throated and grey flycatcher vocalizations were recorded at 37 sites spanning our study area between 11 May and 2 July 2009. Because noise is a permanent feature in our study system that does not vary throughout the day, such as traffic noise, we were able to control for temporal adjustments to vocalizations to overcome masking effects, such as vocalizing during relatively quiet time periods during the day [8], and focus on frequency characteristics. To ensure for independence of samples, we only sampled one individual per species at each site or, when we did sample more than one individual per species on a site, we only sampled individuals that maintained non-adjacent territories.
We recorded vocalizations using a Marantz PMD 660 Digital recorder using a directional shotgun microphone (Audio-technica AT-815) pointed directly at the vocalizing individual, typically from a distance of 5–15 m. We recorded vocalizations for entire song or call bouts (i.e. duration that an individual vocalized from a single perch). After each recording, we also recorded background noise and measured the amplitude for 2 min from as close to the perch as possible, recording mean amplitude values with a sound pressure metre as specified above for point counts. For each individual sampled, we also noted the number of singing males on adjacent territories, distance to the individual, and cardinal direction of the projected vocalization. All measurements and recordings were made when wind speed was less than category three on the Beaufort Wind Scale.
For each individual sampled, we randomly selected five strophes or calls from each recording and measured the following: minimum and maximum frequency, peak frequency (the frequency vocalized at the highest amplitude), peak frequency of the lowest note (highest amplitude of the call or song's lowest note) and vocalization bandwidth (calculated as the minimum frequency subtracted from the maximum frequency). All measurements were performed in RavenPro v. 1.3 [24]. We used a sampling rate of 48 kHz and a Hamming window with a fast Fourier transformation length set to 1024, providing a spectral resolution of 47 Hz. Vocalization peak frequency and peak frequency of the lowest note were calculated automatically. Measurements of minimum and maximum frequencies were performed manually using cursor measurements at the margin of notes on spectrograms [10,25] with the aid of waveform and power spectrum views to guide precise cursor placement. Despite the overlap with compressor noise, vocalization minimum frequencies were easily distinguished on spectrograms, even from recordings with considerable background noise (electronic supplementary material, figure S3). All spectral variables were averaged for each vocalizing individual so that there was a mean value representing each variable for calls and songs of each male.
We used linear regression to examine the influence of background noise amplitude on each of the spectral variables for each species' vocalizations. All frequency data were log transformed prior to analyses to stabilize variance and normalize distributions. For each vocalization type (song and call) per species, the significance threshold was adjusted to 0.01 following a Bonferroni correction for multiple comparisons. All analyses were performed in program R [23].
3. Results
(a). Noise measurements and species occupancies
Mean point count location amplitudes ranged from 32.1–45.8 dB(A) on control sites and 46.0–68.2 dB(A) on treatment sites. Mean noise amplitude was significantly higher at treatment point count locations (56.1 ± 0.6 s.e. dB(A)) than on control sites (37.4 ± 0.3 s.e. dB(A); two-sample t-test: two-tailed-t195 = 33.309, p < 0.001).
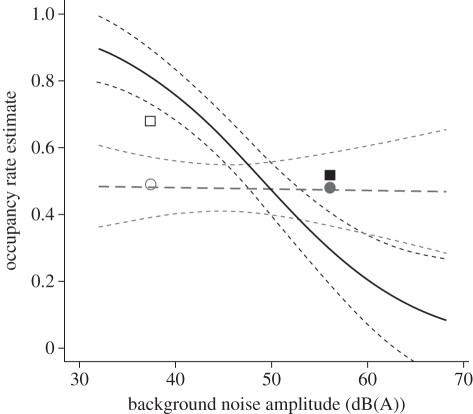
Ash-throated flycatchers were detected at 49 per cent of the control (no compressor noise) point count locations (n = 125) and 48 per cent of the treatment (compressor noise present, except during surveys) point count locations (n = 72). Grey flycatchers were detected at 68 per cent of the control point count locations and 52 per cent of the treatment point count locations. For ash-throated flycatchers, noise amplitude did not influence habitat occupancy estimates (likelihood-ratio test: χ2 = 0.005, p = 0.942). By contrast, noise amplitude had a significant negative effect on grey flycatcher habitat occupancy (likelihood-ratio test: χ2 = 15.958, p < 0.001). Specifically, grey flycatcher occupancy decreased with respect to increased noise amplitude (βamplitude = −0.125 ± 0.030 s.e.; figure 2).
Figure 2.
The occupancy rate estimate for grey flycatchers declined significantly with increased noise amplitude at the point count locations (solid black line). Ash-throated flycatcher occupancy was not significantly affected by noise amplitude (bold grey long-dashed line). Small-dashed lines denote 95% confidence intervals for occupancy estimates with respect to noise amplitude. Points are located at the mean noise amplitude on each site-type and represent the proportion of point count locations where grey flycatchers (black square) and ash-throated flycatchers (grey circle) were detected on treatment sites (filled symbols) and control sites (open symbols).
(b). Noise amplitudes and vocal frequencies
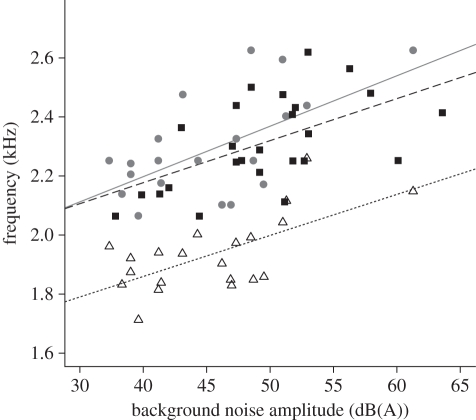
Vocalizing individuals of the two species experienced similar background noise amplitudes. Ash-throated flycatcher vocalizations were recorded within a range of background noise between 37.3–63.6 dB(A). We recorded grey flycatcher vocalizations in background noise as low as 35.6 dB(A) and as high as 62.6 dB(A). For the ash-throated flycatcher, peak frequency of the lowest note for songs and calls, plus call minimum frequency, were all positively related to noise amplitude (table 1 and figure 3). This increase in the call minimum frequency resulted in a significant reduction in call bandwidth with increased noise amplitude, despite no change in call maximum frequency with respect to noise (table 1). No other significant relationships between noise and spectral characteristics were identified for ash-throated flycatchers (table 1). For grey flycatchers, no song or call spectral characteristics were related to noise amplitude (all p > 0.110; electronic supplementary material, table S1).
Table 1.
Results from regression analyses using background noise amplitude to predict spectral characteristics for ash-throated flycatcher song (n = 26) and call (n = 21). (Significant values, after Bonferroni correction was applied for multiple comparisons, are indicated with asterisks. See table S1 in the electronic supplementary material for complete results for the grey flycatcher.)
| song |
call |
|||
|---|---|---|---|---|
| spectral feature | r2 | p | r2 | p |
| minimum frequency | 0.123 | 0.045 | 0.382 | 0.002* |
| maximum frequency | 0.024 | 0.216 | 0.042 | 0.670 |
| peak frequency | 0.091 | 0.073 | 0.052 | 0.932 |
| lowest note peak frequency | 0.304 | 0.002* | 0.296 | 0.006* |
| bandwidth | 0.033 | 0.657 | 0.261 | 0.010* |
Figure 3.
Relationship between ash-throated flycatcher vocal frequency (kHz) and background noise amplitude (dB(A)) measured at the location of the individual. Peak frequency of the lowest note for songs (black squares and long-dashed line) and calls (grey circles and solid grey line), plus call minimum frequency (open triangles and short-dashed line), all increased with background noise amplitudes.
4. Discussion
This study is the first, to our knowledge, to show a link between noise-dependent habitat occupancy and signal variation in birds and the first, also to our knowledge, to examine changes in signal structure in suboscine birds exposed to anthropogenic noise. Vocal frequency characteristics of the noise-sensitive grey flycatcher appear uninfluenced by ambient noise amplitudes. By contrast, the noise-tolerant ash-throated flycatcher vocalizes at a higher frequency with increased background noise amplitude. The differences observed for these two species suggest that signal frequency change may be a mechanism that permits some species to inhabit noisy environments, yet because the ash-throated flycatcher's increase in song frequency is relatively small (≈200 kHz), it raises important questions regarding the cause of this change and whether the change improves communication in noise. We elaborate on these possibilities below.
The two species examined here vocalize at different frequencies; therefore, noise may not represent an equivalent source of acoustic interference for each. The ash-throated flycatcher's lower frequency vocalizations should suffer from a greater degree of acoustic masking by low frequency noise than the grey flycatcher, and thus it might be expected to have a stronger negative response to noise in terms of habitat selection, or it may alter vocal attributes in response to acoustic masking. For example, a recent study examining the influence of traffic noise on Australian songbirds found the low-frequency singing grey shrike-thrush (Colluricincla harmonica) to sing at a higher frequency in the presence of traffic noise, yet the higher pitched singing grey fantail (Rhipidura fuliginosa) did not shift song frequency in noise [26]. Detections of both species declined with increased traffic noise, but the authors were unable to determine whether this pattern was the result of declines in abundance as a result of traffic noise or a reduced probability of detection by the observer, which can be severely affected by increased noise [21].
Here, we controlled for the negative influence of noise on detections by turning compressors off during surveys and though we did not notice a change in bird behaviour when compressors were turned off, both during this study and in a related 3 year study involving nest searching and monitoring [12], we cannot rule out that the lack of compressor noise during our surveys did not increase or decrease avian acoustic behaviour. Thus, with the caveat that turning compressors off may have influenced bird behaviour, we found that ash-throated flycatchers did not avoid noisy areas, but vocalized at a higher pitch with increased background noise. By contrast, grey flycatcher occupancy declined with increased noise exposure, as expected from the pattern of noise avoidance in their nest placement [12], yet even those grey flycatchers vocalizing in noisy areas (as high as 62.6 dB(A)) did not have different vocal spectral features than those vocalizing in quiet areas. Though the grey flycatcher's higher pitched vocalizations may suffer less acoustic masking from low-frequency compressor noise, their vocalizations may still be functionally masked when vocalizing at low amplitudes or when near gas wells compressors where background noise has considerable energy above 5 kHz (see the electronic supplementary material, figure S2).
The frequency shifts observed for low-pitched features of ash-throated flycatcher vocalizations may be expected because low frequencies suffer most from acoustic masking from low-pitched anthropogenic noise. Several recent studies have also found frequency shifts among low-frequency song features in oscine birds and the magnitude of the frequency change observed in this study (≈200 Hz) is similar to other reported shifts. For example, the minimum frequency of urban great tit songs was approximately 200 Hz higher than that of great tit songs in forested habitats [25], and two separate studies report that urban European blackbird's (Turdus merula) sing the low-pitched motif section of their song at roughly 120–200 Hz higher than blackbirds from forested areas [9,27]. A shift of 200 Hz has also been observed for chiffchaffs (Phylloscopus collybita) near highways relative to those near rivers [28] and reed buntings appear to shift the minimum frequency of their songs up 500 Hz in noisy areas [10]. Whether other species may be capable of larger noise-dependent frequency shifts is unknown, but the repeated documentation of relatively small frequency shifts may reflect common constraints to frequency change in passerine birds in response to masking by anthropogenic noise, or may represent a physiological side effect of changes in vocal amplitude (see below [29]). Additionally, these small frequency shifts may only slightly improve communication in noisy environments [29], and because compressor noise in our study has substantial acoustic energy as high as 5 kHz, it is probable that an increase of 200 Hz may only marginally improve ash-throated flycatcher communication. More research documenting whether other oscine and suboscine birds are capable of noise-dependent signal shifts, plus the magnitude of such shifts, will greatly improve our understanding of what range of shifts may be expected in songbirds and whether such shifts effectively mitigate masking effects of noise.
A growing body of literature comparing songs of urban and rural birds suggests that birds modify the pitch of their song in response to noise, yet differences in song pitch between urban and rural populations may instead be the result of a change in the physical structure of the environment or motivational state of the signaller [9]. Cities have less frequency absorbing and reverberating features, such as those in forests where lower frequencies are optimal for sound transmission. Additionally, motivational state could be higher during aggressive social interactions, such as in territory defence in high-density urban bird populations. Here, we show that ash-throated flycatcher vocalizations are higher pitched with increased background noise independent of changes to the physical structure of the habitat because vegetation features, such as tree density or canopy cover, do not differ on treatment and control sites [12]. We also found no evidence for changes in ash-throated flycatcher occupancy with respect to noise amplitude, suggesting no change in density that may influence aggressive social interactions and motivational state of the signaller.
There are several other potential mechanisms that may explain the higher minimum frequencies observed for ash-throated flycatchers vocalizing in increased background noise and include evolutionary, ontogenetic or behavioural modifications [30,31]. Because song is largely thought to develop in the absence of learning in tyrant flycatchers [14,15], ontogenetic changes during song acquisition may be unlikely. A long-term adaptive explanation via natural selection is also possible, but this mechanism may also be improbable owing to the scattered spatial arrangement of compressors throughout our study area. Unlike urban areas, where vast regions may have elevated background noise amplitudes relative to the surrounding landscape, our study area is characterized by point sources of elevated background noise in a relatively quiet landscape; thus this patchy distribution of noise exposure is unlikely to support a divergent population. Another possible explanation for the observed patterns could be intraspecific differences in vocal frequencies at the population level, where individuals with particular vocal frequencies settle in areas where their signals may be successfully dispatched. For example, larger bodied birds with lower pitched vocalizations may tend to settle in relatively quiet areas and smaller individuals that vocalize at a higher pitch occupy noisier areas. Unfortunately, data on individual body sizes are not available and we could not evaluate this possibility.
Short-term behavioural modifications may be a more likely explanation for the observed frequency changes, and noise-dependent modifications at the level of the individual have been documented in several oscine birds, including great tits, chiffchaffs and reed buntings [10,28,31]. Though tyrant flycatcher song may develop normally in the absence of learning, this does not necessarily mean that individuals may be incapable of small adjustments to innate signals in response to environmental conditions and other stimuli. For example, ocellated antbirds (P. mcleannani) increase the pitch of their vocalizations during aggressive encounters [17]. In the case of noise-dependent signal adjustments, signal modifications would require a signaller to detect masking of a signal and alter the vocalization in such a way that it increases detection by receivers [30]. However, critical tests to determine whether ash-throated flycatcher and other suboscine individuals adjust vocal frequency in response to acoustic masking are needed.
Another plausible mechanistic explanation for the frequency shift in background noise is that frequency shifts are by-products of shifts to a different vocal attribute: amplitude. Increases in frequency coupled with increased vocal amplitude have been observed in humans, frogs and non-passerine birds [32–35], and increases in vocal amplitude with increased noise (Lombard effect) appear common in mammals and many birds [3]. It is possible that the small frequency shifts observed in this and other studies may be consequences of increases in vocal amplitude, rather than short- or long-term adaptations to overcome the masking effects of noise [29]. Unfortunately, however, accurate measurement of vocal amplitude in the field is challenging and requires measurement from directly below the individual to control for the directional radiation of vocal sound waves [7]. Studies using captive birds that can simultaneously measure amplitude, spectral and temporal changes to vocalizations in response to noise may prove to be especially fruitful in identifying which vocal features may covary with signal adjustments.
Our data show a clear difference in species' responses to noise in terms of habitat use, plus differences in patterns of vocal frequency with respect to background noise amplitude and masking potential. These results suggest that generalizations across species regarding sensitivities to noise and vocal changes in response to acoustic masking may be limited. Growing correlative evidence from single-species studies suggest that noise-dependent signal change may be quite common in oscine birds [6,10,28]. In this study, we see very different responses to noise from representatives of two different subfamilies within Tyrannidae (Tyranninae and Fluvicolinae), suggesting that not all tyrant flycatchers respond to anthropogenic noise in the same manner, both in terms of habitat use and vocal frequency patterns. Whether more closely related species tend to have similar responses is still unknown.
A fundamental next step is to begin to evaluate the phylogenetic distribution of responses to anthropogenic noise through multi-species studies, both in terms of habitat selection and vocal change. We expect that closely related species will have a shared suite of similar vocal traits, and that members of individual lineages might show comparable responses to noise. Understanding if and to what degree responses are phylogenetically conserved will greatly improve our ability to determine which lineages and species can cope with acoustic interference from anthropogenic noise and which are muted by industrial clamour and disappear from the increasing number of areas afflicted by human noise.
Acknowledgements
This study was completed in compliance with the University of Colorado Animal Care and Use Guidelines.
We are grateful to our many research assistants for field support, especially R. Kennedy, N. Kleist and P. Nylander. We also thank two anonymous reviewers, R. Guralnick, N. Kleist, R. Safran and S. Wagner for useful suggestions and comments on earlier versions of this article. This work was supported by NSF DDIG (no. IOS 0910092), the United States Bureau of Land Management, ConocoPhillips, Williams Energy, and the University of Colorado Department of Ecology and Evolutionary Biology and Graduate School.
References
- 1.Richards D. G., Wiley R. H. 1980. Reverberations and amplitude fluctuations in the propagation of sound in a forest: implications for animal communication. Am. Nat. 115, 381–399 10.1086/283568 (doi:10.1086/283568) [DOI] [Google Scholar]
- 2.Slabbekoorn H., Smith T. B. 2002. Habitat-dependent song divergence in the little greenbul: analysis of environmental selection pressures on acoustic signals. Evolution 56, 1849–1858 10.1111/j.0014-3820.2002.tb00199.x (doi:10.1111/j.0014-3820.2002.tb00199.x) [DOI] [PubMed] [Google Scholar]
- 3.Brumm H., Slabbekoorn H. 2005. Acoustic communication noise. Adv. Study Behav. 35, 151–209 10.1016/S0065-3454(05)35004-2 (doi:10.1016/S0065-3454(05)35004-2) [DOI] [Google Scholar]
- 4.Slabbekoorn H., Ripmeester E. A. 2008. Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83 10.1111/j.1365-294X.2007.03487.x (doi:10.1111/j.1365-294X.2007.03487.x) [DOI] [PubMed] [Google Scholar]
- 5.Warren P. S., Katti M., Ermann M., Brazel A. 2006. Urban bioacoustics: it's not just noise. Anim. Behav. 71, 491–502 10.1016/j.anbehav.2005.07.014 (doi:10.1016/j.anbehav.2005.07.014) [DOI] [Google Scholar]
- 6.Slabbekoorn H., Peet M. 2003. Birds sing at a higher pitch in urban noise. Nature 424, 267. 10.1038/424267a (doi:10.1038/424267a) [DOI] [PubMed] [Google Scholar]
- 7.Brumm H. 2004. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 73, 434–440 10.1111/j.0021-8790.2004.00814.x (doi:10.1111/j.0021-8790.2004.00814.x) [DOI] [Google Scholar]
- 8.Fuller R. A., Warren P. H., Gaston K. J. 2007. Daytime noise predicts nocturnal singing in urban robins. Biol. Lett. 3, 368–370 10.1098/rsbl.2007.0134 (doi:10.1098/rsbl.2007.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemeth E., Brumm H. 2009. Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization? Anim. Behav. 78, 637–641 10.1016/j.anbehav.2009.06.016 (doi:10.1016/j.anbehav.2009.06.016) [DOI] [Google Scholar]
- 10.Gross K., Pasinelli G., Kunc H. P. 2010. Behavioral plasticity allows short-term adjustment to a novel environment. Am. Nat. 176, 456–464 10.1086/655428 (doi:10.1086/655428) [DOI] [PubMed] [Google Scholar]
- 11.Bayne E. M., Habib L., Boutin S. 2008. Impacts of chronic anthropogenic noise from energy-sector activity on abundance of songbirds in the boreal forest. Conserv. Biol. 22, 1186–1193 10.1111/j.1523-1739.2008.00973.x (doi:10.1111/j.1523-1739.2008.00973.x) [DOI] [PubMed] [Google Scholar]
- 12.Francis C. D., Ortega C. P., Cruz A. 2009. Noise pollution changes avian communities and species interactions. Curr. Biol. 19, 1415–1419 10.1016/j.cub.2009.06.052 (doi:10.1016/j.cub.2009.06.052) [DOI] [PubMed] [Google Scholar]
- 13.Sibley C. G., Monroe B. L., Jr 1990. Distribution and taxonomy of birds of the world. New Haven, CT: Yale University Press [Google Scholar]
- 14.Kroodsma D. E. 1984. Songs of the alder flycatcher (Empidonax alnorum) and willow flycatcher (Empidonax traillii) are innate. Auk 101, 13–24 [Google Scholar]
- 15.Kroodsma D. E. 2004. The diversity and plasticity of birdsong. In Nature's music: the science of birdsong (eds Marler P., Slabbekoorn H.), pp. 108–131 San Diego, CA: Elsevier [Google Scholar]
- 16.Ríos-Chelén A. A., Garcia C. M., Riebel K. 2005. Variation in the song of a sub-oscine, the vermilion flycatcher. Behaviour 142, 1115–1132 10.1163/156853905774405326 (doi:10.1163/156853905774405326) [DOI] [Google Scholar]
- 17.Araya-Ajoy Y., Chaves-Campos J., Kalko E. K. V., DeWoody J. A. 2009. High-pitched notes during vocal contests signal genetic diversity in ocellated antbirds. PLoS ONE 4, e8137. 10.1371/journal.pone.0008137 (doi:10.1371/journal.pone.0008137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardiff S. W., Dittmann D. L. 2002. Ash-throated flycatcher (Myiarchus cinerascens), no. 664. In The birds of North America (ed. Poole A.), pp. 1–32 Ithaca, NY: Cornell Laboratory of Ornithology; 10.2173/bna.664 (doi:10.2173/bna.664) [DOI] [Google Scholar]
- 19.Sterling J. C. 1999. Gray flycatcher (Empidonax wrightii). In The birds of North America, vol. 458 (eds Poole A., Gill F.), pp. 1–16 Philadelphia, PA: The Birds of North America, Inc; 10.2173/bna.458 (doi:10.2173/bna.458) [DOI] [Google Scholar]
- 20.Habib L., Bayne E. M., Boutin S. 2007. Chronic industrial noise affects pairing success and age structure of overbirds Seiurus aurocapilla. J. Appl. Ecol. 44, 176–184 10.1111/j.1365-2664.2006.01234.x (doi:10.1111/j.1365-2664.2006.01234.x) [DOI] [Google Scholar]
- 21.Pacifici K., Simons T. R., Pollock K. H. 2008. Effects of vegetation and background noise on the detection process in auditory avian point count surveys. Auk 125, 600–607 10.1525/auk.2008.07078 (doi:10.1525/auk.2008.07078) [DOI] [Google Scholar]
- 22.Dooling R. J., Popper A. N. 2007. The effects of highway noise on birds. See http://www.dot.ca.gov/hq/env/bio/files/caltrans_birds_10-7-2007b.pdf
- 23.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 24.Charif R. A., Waack A. M., Strickman L. M. 2008. Raven Pro 1.3 user's manual. Ithaca, NY: Cornell Laboratory of Ornithology [Google Scholar]
- 25.Slabbekoorn H., den Boer-Visser A. 2006. Cities change the songs of birds. Curr. Biol. 16, 2326–2331 10.1016/j.cub.2006.10.008 (doi:10.1016/j.cub.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 26.Parris K. M., Schneider A. 2009. Impacts of traffic noise and traffic volume on birds of roadside habitats. Ecol. Soc. 14, 29 See http://www.ecologyandsociety.org/vol14/iss1/art29/ [Google Scholar]
- 27.Ripmeester E. A. P., Kok J. S., Van Rijssel J. C., Slabbekoorn H. 2010. Habitat-related birdsong divergence: a multi-level study on the influence of territory density and ambient noise in European blackbirds. Behav. Ecol. Sociobiol. 64, 409–418 10.1007/s00265-009-0857-8 (doi:10.1007/s00265-009-0857-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verzijden M. N., Ripmeester E. A., Ohms V. R., Snelderwaard P., Slabbekoorn H. 2010. Immediate spectral flexibility in singing chiffchaffs during experimental exposure to highway noise. J. Exp. Biol. 213, 2575–2581 10.1242/jeb.038299 (doi:10.1242/jeb.038299) [DOI] [PubMed] [Google Scholar]
- 29.Nemeth E., Brumm H. 2010. Birds and anthropogenic noise: are urban songs adaptive? Am. Nat. 176, 465–475 10.1086/656275 (doi:10.1086/656275) [DOI] [PubMed] [Google Scholar]
- 30.Patricelli G. L., Blickley J. L. 2006. Avian communication in urban noise: causes and consequences of vocal adjustment. Auk 123, 639–649 10.1642/0004-8038(2006)123[639:ACIUNC]2.0.CO;2 (doi:10.1642/0004-8038(2006)123[639:ACIUNC]2.0.CO;2) [DOI] [Google Scholar]
- 31.Halfwerk W., Slabbekoorn H. 2009. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim. Behav. 78, 1301–1307 10.1016/j.anbehav.2009.09.015 (doi:10.1016/j.anbehav.2009.09.015) [DOI] [Google Scholar]
- 32.Junqua C. 1993. The Lombard reflex and its role on human listeners and automatic speech recognizers. J. Acoust. Soc. Am. 93, 510–524 10.1121/1.405631 (doi:10.1121/1.405631) [DOI] [PubMed] [Google Scholar]
- 33.Traunmüller H., Eriksson A. 2000. Acoustic effects of variation in vocal effort by men, women, and children. J. Acoust. Soc. Am. 107, 3438–3451 10.1121/1.429414 (doi:10.1121/1.429414) [DOI] [PubMed] [Google Scholar]
- 34.Lopez P. T., Narins P. M., Lewis E. R., Moore S. W. 1988. Acoustically induced call modification in the white-lipped frog, Leptodactylus albilabris. Anim. Behav. 36, 1295–1308 [Google Scholar]
- 35.Beckers G. J. L., Suthers R. A., ten Cate C. 2003. Mechanisms of frequency and amplitude modulation in ring dove song. J. Exp. Biol. 206, 1833–1843 10.1242/jeb.00364 (doi:10.1242/jeb.00364) [DOI] [PubMed] [Google Scholar]