Abstract
Tarsius is an extant genus of primates endemic to the islands of Southeast Asia that is characterized by enormously enlarged orbits reflecting its nocturnal activity pattern. Tarsiers play a pivotal role in reconstructing primate phylogeny, because they appear to comprise, along with Anthropoidea, one of only two extant haplorhine clades. Their fossils are extremely rare. Here, we describe a new species of Tarsius from the Middle Miocene of Thailand. We reconstructed aspects of its orbital morphology using a geometric–morphometric method. The result shows that the new species of Tarsius had a very large orbit (falling within the range of variation of modern Tarsius) with a high degree of frontation and a low degree of convergence. Its relatively divergent lower premolar roots suggest a longer mesial tooth row and therefore a longer muzzle than in extant species. The new species documents a previous unknown Miocene group of Tarsius, indicating greater taxonomic diversity and morphological complexity during tarsier evolution. The current restriction of tarsiers to offshore islands in Southeast Asia appears to be a relatively recent phenomenon.
Keywords: Tarsius, Tarsiidae, morphometrics, Miocene, orbit, Thailand
1. Introduction
The fossil record of Tarsiidae is very scant, consisting of only two genera, Xanthorhysis and Tarsius, that were apparently restricted to Asia. The oldest tarsiid ever discovered, Middle Eocene Xanthorhysis tabrumi from the Heti Formation, Shanxi Province, China, is documented by a dentary with P3–M3 and alveoli for C1 and P2 [1]. Tarsius eocaenus, from the Shanghuang fissure-fillings, Jiangsu Province, China, was initially represented by five isolated teeth [2]. More recently, a facial fragment with the crown of P3 and complete or partial alveoli for I2, C1, P2 and the mesial roots of P4 was described [3]. It demonstrates that the large orbit characteristic of living Tarsius was already present in the Middle Eocene and that T. eocaenus also possessed a haplorhine oronasal configuration. Tarsius has often been considered a living fossil, displaying no evolutionary changes in the orbit and dentition since the Eocene [4]. A Middle Miocene tarsiid, Tarsius thailandicus [1,5,6], is documented by six isolated teeth from the Li Basin, northern Thailand. It corresponds to a small-sized species showing modern dental characters. Afrotarsius chatrathi from the Oligocene of Fayum, Egypt, which consists of a lower jaw with part of P3–P4 and M1–M3 [7], has been described as an African tarsiiform primate. Its tarsiid affinities have been widely challenged, and Afrotarsius is more plausibly interpreted as a basal anthropoid primate [1,5,8]. A tibiofibula specimen attributed to A. chatrathi by Rasmussen et al. [9] was considered as probably not belonging to a primate [10].
Here we describe a new species of Middle Miocene Tarsius from northern Thailand. The new species is represented by a maxillary fragment preserving the partial orbital floor with P3, P4 and M1, 17 mandibular fragments and two isolated molars. As a result, this Miocene species of Tarsius is now the best-documented fossil tarsier currently known. Its orbit is poorly preserved, but the morphology of this region can be reconstructed using a geometric–morphometric method. Our main objective is to compare the orbital morphology of the new Miocene tarsier with that of extant Tarsius in order to assess the antiquity of the peculiarly enlarged orbits of living species of Tarsius. If our results confirm the antiquity of modern tarsier orbital morphology (and their secondarily nocturnal adaptations), this will support the proposal that haplorhines were primitively diurnal, a condition retained in early anthropoids. Alternatively, these distinctive adaptations to nocturnal life may have originated relatively recently during the course of tarsier evolutionary history. The peculiar dental characters displayed by the new species will also permit an assessment of its phylogenetic relationships with extant species of Tarsius, which are considered to cluster into two distinct groups. This also raises questions about the diversity of Tarsius during the Miocene.
2. Geological background
The new Tarsius specimens were recovered from the Q and K coal zones of the Na Khaem Formation, Mae Moh coal mine, Mae Moh District, Lampang Province, northern Thailand. The Na Khaem Formation is composed of the main lignite-bearing units, interbedded with lacustrine mudstones and minor sandstones, indicating sedimentation under the alternation of freshwater lake and swamp/marsh in a calcium-rich lacustrine environment [11]. The Q and K coal zones, the main coal-producing layers, range in thickness from 10 to 30 m and 15 to 30 m, respectively. The new Tarsius species was discovered through surface survey, and it is associated with a diversified vertebrate fauna including bony fishes, snakes, turtles, crocodilians and a variety of other mammals such as Stegolophodon [12,13], Gomphotherium cf. browni [14], Gaindatherium [15], Siamogale thailandica [16], Maemohcyon potisati [17], Neocometes cf. orientalis, Prokanisamys benjavuni [18] and Siamoadapis maemohensis [19].
The age of the Q and K coal zones of the Na Khaem Formation is conventionally considered as Middle Miocene and magnetostratigraphic data correlate these strata with chron C5Aar, between 13.3 and 13.1 Ma [20,21].
3. Systematic palaeontology
Primates Linnaeus, 1758: Haplorhini Pocock, 1918, Tarsiiformes Gregory, 1915, Tarsiidae Gray, 1825, Tarsius Storr, 1870.
Tarsius sirindhornae sp. nov.
Holotype. TF 6260 (Department of Mineral Resources, Bangkok), left maxilla preserving partial orbit floor with P3–M1 (figures 1a, 2i–l).
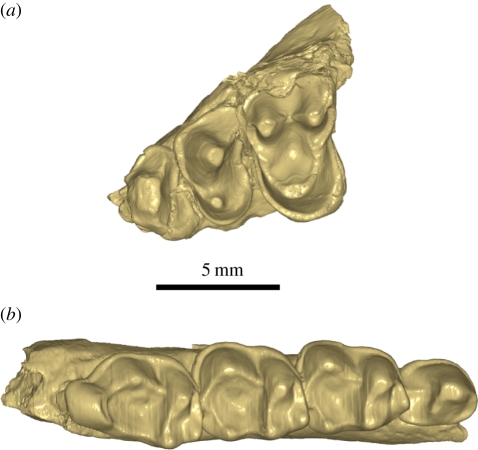
Figure 1.
Occlusal view of Tarsius sirindhornae n. sp. (a) Maxillar fragment with P3–M1 holotype (TF 6260); (b) lower jaw with P4–M3 (TF 6247).
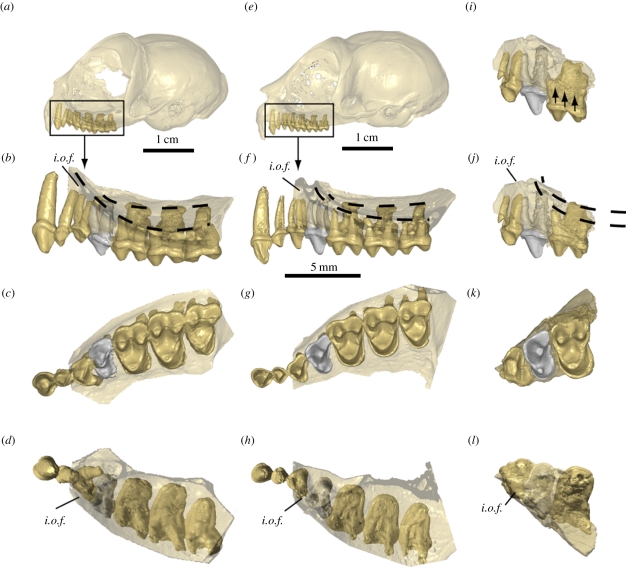
Figure 2.
Micro-CT scan views of the orbital floors and premolar and molar root patterns in extant Tarsius (a–h) compared with those of T. sirindhornae n. sp. (i–l). The two dashed lines join the tips of the lingual roots and of the labial roots in the orbital floor. P4 is represented in a grey colour. i.o.f., infraorbital foramen. (a–d) Tarsius bancanus skull (PAL-44), with orbital floor expanding until above P3 root. (e–h) Tarsius spectrum skull (AS 1821), with P2 and P3 roots excluded from orbital floor. (i–l) Maxillary fragment image of T. sirindhornae n. sp. holotype (TF 6260) in its original configuration (i), then with the M1 lifted upwards to bring its labial cingulum in line with that of P4 as in extant Tarsius (j), suggesting that according to the long roots of P4, the orbital floor must have raised abruptly in front of M1, indicating a less anteriorly expanded orbit compared with those of extant Tarsius. (b,f,j) Labial view. (c,g,k) Occlusal view. (d,h,l) Apical view of the orbital floor.
Etymology. In honour of Her Royal Highness Princess Maha Chakri Sirindhorn, who has repeatedly demonstrated her great interest in the palaeontological richness of her country.
Type series. TF 6244, right mandible with P3–M3 (figure 3e); TF 6245, left mandible with P4–M2; TF 6246, right mandible with P4 (figure 3d); TF 6247, left mandible with P4–M3 (figures 1b and 3c); TF 6248 right mandible with M1–M3; TF 6249, right mandible with P4–M1; TF 6250, left mandible with fragment of P3–P4 and M1–M3; TF 6251, right mandible with M1–M3; TF 6252, right mandible with M1-M3; TF 6253, left mandible with fragment of M1–M2 and M3; TF 6254, left mandible with M2 and fragment of M3; TF 6255, right mandible with fragment of M2 and M3; TF 6256, right mandible with M1–M2 and fragment of M3; TF 6257, left mandible with P4–M1; TF 6258, left mandible with fragment of M2 and M3; TF 6259, left mandible with P4–M3; TF 6261, left mandible with fragment of M2 and M3; TF 6262, right M2; TF 6263, right M3. All specimens are from the same locality as the holotype. Tooth dimensions are given in table 1.
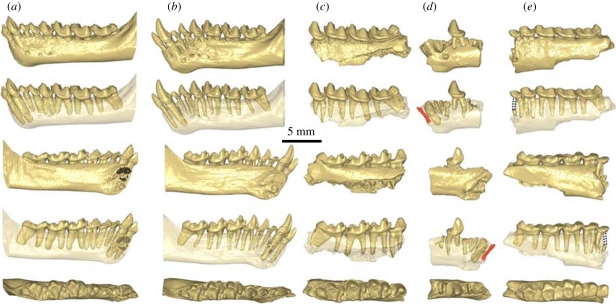
Figure 3.
Micro-CT scan views of the lower jaws of extant Tarsius (a,b) compared with those of fossil T. sirindhornae n. sp. (c–e). Lower jaws of fossil fragments are represented from top to bottom in respective buccal, buccal transparent, lingual, lingual transparent and occlusal views. (a) Tarsius bancanus (PAL-44) left lower jaw, (b) T. spectrum (AS 1821) left lower jaw, (c) T. sirindhornae n. sp. left lower jaw fragment with P4–M1 (TF 6247), (d) mirror image of the right lower jaw fragment of T. sirindhornae n. sp. with crown of P4 and roots of I (in red), C, P2 and P3 (TF 6246), (e) mirror image of the right lower jaw fragment of T. sirindhornae n. sp. with P3–M3 (TF 6244) displaying unreduced premolars and vertical, parallel and not appressed roots of P3 (mesial root completed with dotted lines) and P4.
Table 1.
Dental measurements (mm) in MD (mesiodistal) × BL (buccolingual) of T. sirindhornae n. sp. compared with other Tarsiidae. (Numbers in parentheses are BL of talonid.)
| taxa | P3 | P4 | M1 | M2 | M3 | estimated body weight (g) |
|---|---|---|---|---|---|---|
| T. sirindhornae | ||||||
| TF 6244 | 1.62 × 1.66 | 2.06 × 1.92 | 2.67 × 2.46 | 2.82 × 2.66 | 3.44 × 2.15 | 119 |
| TF 6245 | 2.42 × 1.88 | 2.98 × 2.60 | 3.19 × 2.92 | 153 | ||
| TF 6246 | 2.30 × 1.77 | |||||
| TF 6247 | 2.09 × 1.66 | 2.94 × 2.40 | 2.99 × 2.60 | 3.81 × 2.52 | 133 | |
| TF 6248 | 2.77 × 2.42 | 3.00 × 2.55 | 3.73 × 2.50 | 123 | ||
| TF 6249 | 2.48 × 1.99 | 3.21 × 2.74 | 185 | |||
| TF 6250 | — × 2.64 | 3.25 × 2.62 | 3.83 × 2.42 | |||
| TF 6251 | 3.20 × 2.33 | 3.06 × 2.42 | 3.53 × 2.10 | 144 | ||
| TF 6252 | 2.92 × 2.48 | 2.89 × 2.59 | 3.54 × 2.30 | 138 | ||
| TF 6253 | — × 2.73 | — × 2.92 | 3.70 × 2.37 | |||
| TF 6254 | 3.27 × 2.50 | 3.63 × 2.32 | ||||
| TF 6255 | — × 2.47 | 3.50 × 2.27 | ||||
| TF 6256 | 2.62 × 2.11 | 2.67 × 2.16 | 92 | |||
| TF 6257 | 2.17 × 1.93 | 3.14 × 2.48 | 154 | |||
| TF 6258 | — × 2.51 | 3.48 × 2.25 | ||||
| TF 6259 | 2.18 × 1.90 | 2.75 × 2.47 | 2.98 × 2.59 | 3.52 × 2.39 | 130 | |
| TF 6261 | — × 2.77 | 3.57 × 2.39 | ||||
| TF 6262 | 3.35 × 2.71 | |||||
| TF 6263 | 3.83 × 2.56 | |||||
| T. thailandicusa | ||||||
| T Li43 | 1.25 × 1.27 | |||||
| T Li44 | 1.27 × 1.35 | |||||
| T Li45 | 2.43 × 1.83 (0.89) | 66 | ||||
| T Li46 | 2.52 × 2.10 (1.02) | |||||
| T Li47 | 2.58 × 1.83 (0.89) | |||||
| T. eocaenusb | 29 | |||||
| IVPP V11030 | 1.65 × 1.55 | |||||
| X. tabrumic | ||||||
| IVPP V12063 | 1.55 × 1.20 | 2.10 × 1.55 | 2.25 × 1.85 | 2.20 × 1.80 | 2.35 × 1.60 | 60 |
| T. bancanusd | 2.97 × 2.67 | 2.72 × 2.90 | 158 | |||
| T. syrichtad | 2.59 × 2.47 | 2.44 × 2.62 | 114 | |||
| T. spectrumd | 2.18 × 2.20 | 2.17 × 2.33 | 74 | |||
| T. pumilusd | 2.00 × 1.85 | 50 | ||||
| taxa | P2 | P3 | P4 | M1 | ||
| T. sirindhornae | ||||||
| TF 6260 (holotype) | 1.77 × 2.58 | 2.11 × 3.17 | 3.19 × 4.15 | |||
| T. thailandicusa | ||||||
| T Li42 | 1.06 × 0.93 | |||||
| T. eocaenuse | ||||||
| IVPP V14563 | 1.39 × 1.63 | |||||
| T. bancanusd | 2.73 × 4.42 | |||||
| T. syrichtad | 2.52 × 3.83 | |||||
| T. spectrumd | 2.26 × 3.44 | |||||
| T. pumilusd | 1.85 × 2.90 | |||||
Type locality and age. Q and K coal layers of the Na Khaem Formation, Mae Moh coal mine, Mae Moh District, Lampang Province, northern Thailand; Middle Miocene (13.3–13.1 Myr ago).
Diagnosis. Differs from extant species of Tarsius by its anteriorly less expanded orbit, less elevated molar cusps, P4 with variably developed metaconid, more robust protoconid and hypoconid on M1 and M2, and larger hypoconulid on M3; P3 and P4 roots well separated, as in Tarsius spectrum, not fused or appressed as in Tarsius bancanus. Mesial root of P3 vertical, not procumbent as in extant species. Less reduced P3/3 and P4/4 · P3 nearly the size of P4 and P4 roots significantly longer than those of M1. Differs from T. thailandicus by its larger size, less elevated cusps and divergent and non-appressed P3 and P4 roots. Differs from other fossil Tarsius and Xanthorhysis by it larger size, less elevated cusps, better defined hypoconulid, less developed protocristid, shallower trigonid basin and more mesial position of entoconid relative to hypoconid.
Measurements. All measurements of T. sirindhornae and extant Tarsius are given in table 1 and electronic supplementary material, table S1.
Description and comparisons. TF 6260 is a left maxillary fragment with P3–M1 and a preserved partial orbital floor. P3 and P4 generally resemble those of extant species but display larger paracone on P3 and stronger protocone on P4. The antero-buccal corner of P3 is damaged, but the crown is less reduced than that of extant species. The paracone of P4 is inclined more lingually and located more mesially than is the case in extant species of Tarsius. The preparacrista of P4 is better developed among extant species, in which it is strongly connected to the parastyle. This crest is absent on the fossil. M1 is significantly larger than P4. It differs from M1 in other species of Tarsius in having a distal margin with a more waisted outline and a stronger metastyle. The postprotocrista splits into two crests, a premetaconule crista joining the mesiolingual slope of the metacone and a postmetaconule crista connecting the metastyle, but among extant Tarsius conules are generally less well expressed and the metaconule cristae are reduced, especially the distal ones. Horizontal wear facets can be observed on the paracone, metacone, protocone, paraconule and metaconule. Cingula surround the entire perimeter of the crown's base, but the lingual cingulum is remarkably developed.
The teeth described above are part of a maxillary fragment that includes the floor of the orbital cavity (figure 2i–l). In the lateral view, a small area in its uppermost mesial part corresponds to the lower edge of the infra-orbital foramen. The three root apices of M1 pierce the floor of the orbital cavity as in modern Tarsius. Such is not the case for the three roots of P4, which are much longer than those of M1, indicating that the floor of the orbital cavity rises at the level of the distal roots of P4. Therefore, the great length of the P4 roots indicates that the orbit extended less anteriorly in TF 6260 than in any extant species. Among modern species of Tarsius, the roots of the posterior upper cheek teeth, including P4, are always in contact with the floor of the orbital cavity (figure 2b,f). As the size and morphology of the orbital cavity constrain the imprints of the root apices on the orbital floor, we hypothesize that the dimension and orientation of the orbital contour can be partially inferred from the length and position of the roots of P3, P4 and M1.
The dentary of the new species resembles those of extant species of Tarsius (figure 3a,b), differing chiefly in being more robust. A single foramen mentale can be observed on one specimen (TF 6246) under the mesial root of P3, but there are two such foramina in Xanthorhysis, beneath the mesial part of the alveolus of P2 and the mesial root of P3, and also in extant Tarsius (beneath P3 and P4). Images produced by micro computed tomography (micro-CT) scans image show that T. sirindhornae has only one lower incisor (figure 3d), documented by a partial alveolus located mesial to the canine alveolus. It is tiny and rather procumbent, being inclined about 55° relative to the tooth row. The diameter of C (figure 3d) can be estimated on the basis of a partial alveolus in TF 6246. It shows an estimated mesiodistal dimension of 1.47 mm. The canine root is massive and procumbent, oriented about 60° relative to the tooth row. P2 is represented only by a partial alveolus (TF 6246) and a micro-CT scan image confirms that it is single-rooted and inclined like the incisor and canine (figure 3d). P3 is double-rooted with vertical and well-separated roots. Its crown displays a large protoconid (figure 3e). Mandible ramus depth beneath P3 measures 4.38 mm on TF 6244 and 3.53 mm on TF 6246, similar to extant Tarsius (3.66–4.23 mm). P4 shows a variably developed metaconid and is double-rooted as in T. spectrum but in contrast to T. bancanus, the P4 of which has a single root or two closely appressed roots (figure 3c–e). The lower molars of T. sirindhornae are similar to those of extant Tarsius, with the exception of their less elevated cusps and the notably enlarged M3, which bears a larger hypoconulid than in extant Tarsius. The talonid of M1 is broad and bears an enlarged hypoconid and a smaller entoconid as in all extant Tarsius, differing also from the Eocene Tarsius that shows equal size of these two cusps. The paraconid of M2 is more lingually situated than on M1, yielding a longer paracristid and a wider trigonid than on M1. Its entoconid is more reduced than on M1. M3 is mesiodistally longer than those of any extant species of Tarsius. The entoconid, located at the same level as the hypoconid, is tiny to absent. This cusp is usually more strongly expressed in extant Tarsius, as it is in Xanthorhysis. The symphysis was unfused and its surface is notable for the depth of the pit for the genioglossus muscle (figure 3d). The coronoid process rises from the occlusal surface of the molars at an angle of 55°. Its lateral side bears a strong anterior masseteric ridge. A retromolar space is present. The angular region shows a slight downward inclination, starting at the level posterior to the beginning of the vertical branch.
4. Method and results
(a). Methods
We used geometric–morphometric methods to estimate the root and orbital morphology of T. sirindhornae. The comparative sample consists of 12 crania of extant Tarsius (T. bancanus: n = 4; T. syrichta: n = 4; T. spectrum: n = 1; T. sp. indet.: n = 3), nine of which are adults, the three remaining ones being subadults exhibiting relatively smaller orbits, with P4 and M1 erupted and a P3 almost fully erupted (see the electronic supplementary material, table S2, for a list of the specimens). Though limited in size, the comparative sample was chosen to be representative of modern Tarsius variability, with the aim to build a satisfactorily model of Tarsius orbital morphology. The analysis protocol involved 10 landmarks corresponding to the centre of the root apices of P3, P4 and M1 and to the floor of the infraorbital foramen, which were digitized on the reconstruction of T. sirindhornae TF 6260 and on the left maxillae of extant specimens (see the electronic supplementary material, figure S1a). Patterns of radicular shape variation were analysed using principal components (PC) analysis of shape in linearized Procrustes space [22].
Owing to the small number of specimens, we could not predict accurately orbital parameters such as diameter, convergence and frontation directly from Procrustes coordinates. As such, in order to estimate the orbital morphology of T. sirindhornae, we first reduced the dimensionality of the system describing shape variation. We only considered the components of shape variation that relate the best to orbital diameter, convergence and frontation. To do so, we performed multivariate regressions of root apex shape against orbital diameter, the degree of frontation and the degree of convergence on the extant specimen sample, following the protocol described by Frost et al. [23]. In extant Tarsius, the centroid size of the landmark configuration was found to be only weakly and insignificantly correlated with orbital diameter (r2 = 0.25; p = 0.11). This is the reason why the estimation of the orbital diameter of T. sirindhornae was derived from a measure of shape, and not size. This procedure yielded regression shape vectors of orbital diameter (RSVo), frontation (RSVf) and convergence (RSVc). Variation in orbital diameter and in the degrees of frontation and convergence accounted for substantial parts of radicular apex shape variation (23.44, 19.57 and 21.09%, respectively), confirming the hypothesis that jugal teeth root morphology and the position of the infraorbital foramen of extant Tarsius are correlated with the orbital geometry. RSVo, RSVf and RSVc projecting scores of extant specimens were well correlated with their measured orbital diameters (r2 = 0.89; p < 0.0001), degrees of frontation (r2 = 0.69, p = 0.0008) and convergence (r2 = 0.72, p = 0.0005). This allowed for estimations of orbital diameter, frontation and convergence for T. sirindhornae derived from its RSVo, RSVf and RSVc projecting scores, as well as 95% confidence and prediction intervals (see the electronic supplementary material, figure S2). We used Morphotools [24,25] to perform the geometric–morphometric analysis and the multivariate regressions, and R [26] to predict orbital diameter, frontation and convergence.
(b). Results
Visualizing patterns of root apices shape variation in morphospace permits characterization of taxon-specific morphologies (see the electronic supplementary material, figure S3a). PC1 describes the curvature of the dental root arch, the lingual root apex line being more curved lingually for high PC1 values (see the electronic supplementary material, figure S3b). Specimens with low PC1 scores, such as T. spectrum, exhibit a less compressed mesial tooth row and a more laterally and posteriorly placed infraorbital foramen. PC2 reflects mainly differences in P3 root apex shape (see the electronic supplementary material, figure S3b). Specimens with high PC2 scores (the three subadults) exhibit a lingual root apex of P3 placed more lingually, and an infraorbital foramen placed in a relatively lower and more lateral position. Tarsius sirindhornae exhibits a curved dental root arch and lies inside the morphological variation of extant adult Tarsius and resembles more T. bancanus and T. syrichta than T. spectrum. The position of T. sirindhornae within PC1–PC2 subspace gives further support to the hypothesis that extant Tarsius is an adequate model to estimate the orbital morphology of this fossil.
Tarsius sirindhornae orbit lies within the extant sample range variation and exhibits a high degree of frontation (64.5°; 95% confidence intervals (CI): 63.5–65.4°; 95% pred. int.: 60.97–67.98°) and a low degree of convergence (50.6°; 95% CI: 49.2–52.0°; 95% pred. int.: 45.65–55.59°) and a large diameter (18.3 mm; 95% CI: 17.9–18.6 mm; 95% pred. int.: 17.06–19.46 mm) (see the electronic supplementary material, figure S2 and table S2). But the orbit of T. sirindhornae was relatively less expanded anteriorly than what can be observed in extant Tarsius.
5. Discussion and conclusion
Tarsiers are among the smallest haplorhine primates (80–150 g), and their diet is based on insects and other small animal prey [27]. Tarsius sirindhornae was a large species of Tarsius, as large as or possibly larger than T. bancanus, the largest extant Tarsius. We used the regression equation of the M1 area against body weight proposed by Gingerich [28] for Tarsius and obtained an estimated body weight of T. sirindhornae of 90–180 g. Its body mass places it below ‘Kay's threshold’ of 500 g, below which primates cannot be obligate folivores owing to fundamental metabolic constraints [29]. Some specimens of T. sirindhornae display a high degree of premolar and molar wear with horizontal wear facets, suggesting a dietary difference from most other tarsiers. Its large premolars and molars, with enlarged M3 and low and less acute molar cusps, also point towards a less insectivorous and carnivorous diet than the extant and fossil Tarsius and even other fossil Tarsiidae. The meaning of such an odd wear pattern has previously been discussed concerning Tarsius pumilus without conclusive evidence [30], gum feeding observed for that species being incapable of producing such a peculiar wear pattern and the small body weight of both species making it unlikely to have included significant amounts of plant food [31]. The diet of T. sirindhornae (like that of T. pumilus) must nevertheless have included some kind of food with an abrasive component. Microwear analysis may deliver additional information concerning the diet of this peculiar Tarsius species.
Fragmentary cranial remains of T. eocaenus demonstrate that tarsiers already possessed greatly enlarged orbits during the Middle Eocene [3]. The new species from Thailand confirms that the very large orbits which are associated with the nocturnal activity pattern of modern Tarsius were already developed 13 Ma. This extreme orbital enlargement is considered to be the consequence of a secondary shift to nocturnality from a diurnal ancestor [32]. The most primitive known primates, including basal haplorrhines, had relatively small orbits, suggesting a diurnal activity pattern [33]. Indeed, most primitive anthropoids for which orbit anatomy is partially known, including Bahinia [34] and Phenacopithecus [35], display small orbits, corresponding to a diurnal activity pattern. However, Biretia from the oldest Fayum locality BQ2, Egypt, displays greatly enlarged orbits, which are interpreted as a derived feature [36]. This indicates that orbital enlargement occurred several times during the evolution of haplorhines. Tarsius apparently displays exceptionally stable craniodental anatomy over tens of millions of years. Such stasis is exceptional among eutherian mammals over such a long time interval.
Tarsius sirindhornae is the second recorded species of fossil Tarsius found in the Miocene strata of Thailand. Tarsius thailandicus, a Middle Miocene fossil from the Li Basin of northern Thailand, is significantly smaller but similar in age [18] to T. sirindhornae. It displays appressed roots on its P4 and probably on P3, which further distinguishes it from T. sirindhornae and brings it closer to the extant species of Tarsius. This shows the occurrence of at least two distinct Miocene groups of Tarsius in Thailand. We compared the possible relationships of T. sirindhornae with the two morphological groups of extant tarsiers: a Philippine-Western group (from the Philippines and the Greater Sunda Islands) and an Eastern group (from Sulawesi) [37]. The Philippine-Western group shows extremely enlarged orbits, enlarged molars (especially the third molar), narrow molar talonids and prominent cingula around maxillary incisors and canines. P3 is one rooted and roots of P4 are strongly appressed. No metaconid seems present on the P4. The Eastern group shows relatively smaller orbits, a shorter tooth row, reduced third molars, broad molar talonids and poor development of cingula on maxillary incisors and canines. P3 and P4 have two well-separated roots as in T. sirindhornae. By its unreduced lower premolar roots, interpreted here as a plesiomorphic character, T. sirindhornae appears to be closer to the Eastern group. But other derived characters, such as the enlarged M3 and the large orbit, are shared with the other group. Recent discoveries of new extant species of Tarsius have increased the number of taxa, thereby complicating their phylogenetic relationships [37–39]. The preliminary genetic analysis published by Shekelle [40] suggested an unresolved trichotomy among the Philippine, Western and Eastern tarsiers, with a divergence possibly dating to the Middle Miocene. Shekelle [41] also proposed that the small size of T. pumilus represents a primitive character and that the large size of some island species is the consequence of an island effect. The large size of our Miocene tarsier does not support this interpretation.
Tarsius sirindhornae displays several autapomorphic characters, such as less elevated molar cusps, enlarged M3 hypoconulid, robust lower jaw, in addition to its peculiar orbit. It also displays some primitive characters, such as retention of a strong postmetacrista and postmetaconule crista on upper molars and the divergent roots of premolars and molars that suggest a less mesio-distally compressed lower mesial tooth row. This unusual combination of primitive and derived characters indicates that it belongs to a distinct group of Miocene Tarsius that is probably not specially related to any of the extant ones. This suggests a higher diversification of Tarsius clades during the Miocene and its reduction through time. The same is true for their geographical range. During the Middle Eocene, their distribution reached as far north as central China. In the Miocene, they are only recorded in Thailand and remain undocumented in China, even in nearby Yunnan Province. They disappeared from Thailand prior to the Plio-Pleistocene, because no trace of fossil Tarsius was found in the rich Plio-Pleistocene micromammal assemblages collected in caves and fissure-fillings across Thailand [42]. At that time, they probably had reached their present refuge distribution.
To conclude, T. sirindhornae represents a new species and also the largest known fossil assemblage of Tarsius. It significantly expands the taxonomic and anatomical diversity of this intriguing primate. Its unique combination of primitive and derived dental characters excludes T. sirindhornae from the two main clusters of extant Tarsius, supporting that it belongs to an extinct Miocene group. This fossil evidence therefore suggests a reduction in taxonomic diversity that accompanied the contraction of the geographical range of Tarsius during its Neogene evolutionary history in Southeast Asia. A geometric–morphometric analysis demonstrates that the new fossil tarsier had an enlarged orbit, a high degree of frontation and a low degree of convergence, similar to that shown by modern species of Tarsius. However, according to the great length of its P4 roots, the orbit of the new species was less expanded anteriorly than those of extant Tarsius, and its muzzle was longer, documenting morphological differences from the extant forms. Nevertheless, the modern orbit characters associated with a nocturnal activity pattern are still present, confirming the great antiquity and stability of these characters during Tarsius evolution within the last 45 Ma. This, in turn, supports the inference that haplorhines are primitively diurnal and that this condition was retained in early anthropoids.
Acknowledgements
This work has been supported by the Department of Mineral Resources, Bangkok, the C.N.R.S.-T.R.F. ‘Caenozoic Biodiversity' Programme, the C.N.R.S. Eclipse II Programme, the ANR-09-BLAN-0238-02 Programme and the University of Poitiers. The authors would like to thank EGAT (Electricity Generating Authority of Thailand) for providing scientific assistance, information and access to the mine. We are grateful to Chris Beard (Carnegie Museum) and Franck Guy (IPHEP, University of Poitiers) for their comments and their help in providing comparative materials, Marian Dagosto for providing data on extant Tarsius, Mana Rugbumrung for assistance in fieldwork, Peter Wyss (E.M.P.A.) and Christoph Zollikofer (A.I.M.) for the access to their µCT facilities and Marcia Ponce de Lèon (A.I.M.) (University of Zürich) for access to the extant tarsier specimens.
References
- 1.Beard K. C. 1998. A new genus of Tarsiidae (Mammalia: Primates) from the middle Eocene of Shanxi Province, China, with notes on the historical biogeography of tarsiers. Bull. Carnegie Mus. Nat. Hist. 34, 260–277 [Google Scholar]
- 2.Beard K. C., Qi T., Dawson M. R., Wang B., Li C. K. 1994. A diverse new primate fauna from middle Eocene fissure-fillings in southeastern China. Nature 368, 604–609 10.1038/368604a0 (doi:10.1038/368604a0) [DOI] [PubMed] [Google Scholar]
- 3.Rossie J. B., Ni X. J., Beard K. C. 2006. Cranial remains of an Eocene tarsier. Proc. Natl Acad. Sci. USA 103, 4381–4385 10.1073/pnas.0509424103 (doi:10.1073/pnas.0509424103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jablonski N. G. 2003. The evolution of the Tarsii niche. In Tarsiers past, present, and future (eds Wright P. C., Simons E. L., Gursky S.), pp. 35–49 London, UK: Rutgers University Press [Google Scholar]
- 5.Ginsburg L., Mein P. 1987. Tarsius thailandica nov. sp., first fossil Tarsiidae (Primates, Mammalia) of Asia. C. R. Acad. Sci. 304, 1213–1215 [Google Scholar]
- 6.Mein P., Ginsburg L. 1997. Early Miocene mammals from Li Mae Long, Thailand: systematic, biostratigraphic and paleoenvironment (translated from French). Geodiversitas 19, 783–844 [Google Scholar]
- 7.Simons L. S., Bown T. M. 1985. Afrotarsius chatrathi, first tarsiiform primate (? Tarsiidae) from Africa. Nature 313, 475–477 10.1038/313475a0 (doi:10.1038/313475a0) [DOI] [Google Scholar]
- 8.Ross C., Williams B., Kay R. F. 1998. Phylogenetic analysis of anthropoid relationships. J. Hum. Evol. 35, 221–306 10.1006/jhev.1998.0254 (doi:10.1006/jhev.1998.0254) [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen D. T., Conroy G. C., Simons E. L. 1998. Tarsier-like locomotor specializations in the Oligocene primate Afrotarsius. Proc. Natl Acad. Sci. USA 95, 14 848–14 850 10.1073/pnas.95.25.14848 (doi:10.1073/pnas.95.25.14848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White J. L., Gebo D. L. 2004. Unique proximal tibial morphology in strepsirrhine primates. Am. J. Primatol. 64, 293–308 10.1002/ajp.20079 (doi:10.1002/ajp.20079) [DOI] [PubMed] [Google Scholar]
- 11.Corsiri R., Crouch A. 1985. Mae Moh coal deposit: geologic report. Thailand/Australia Lignite Mines Development Project, Electricity Generating Authority of Thailand, vol. 1, pp. 1–448
- 12.von Koenigswald G. H. R. 1959. A mastodon and other fossil mammals from Thailand. Report of Investigation of Royal Department of Mines, pp. 25–31 [Google Scholar]
- 13.Tassy P., et al. 1992. A new Stegolophodon (Proboscidea, Mammalia) from the early Miocene of Northern Thailand. Geobios 25, 511–523 10.1016/S0016-6995(92)80079-S (doi:10.1016/S0016-6995(92)80079-S) [DOI] [Google Scholar]
- 14.Chavasseau O., Chaimanee Y., Yamee C., Tian P., Rugbumrung M., Marandat B., Jaeger J.-J. 2009. New Proboscideans (Mammalia) from the middle Miocene of Thailand. Zool. J. Linn. Soc. 155, 703–721 10.1111/j.1096-3642.2008.00456.X (doi:10.1111/j.1096-3642.2008.00456.X) [DOI] [Google Scholar]
- 15.Ginsburg L., Tassy P. 1985. The fossil mammals and the age of the lignite beds in the intramontane basins of Northern Thailand. J. Geol. Soc. Thailand 8, 13–27 [Google Scholar]
- 16.Ginsburg L., Ingavat R., Tassy P. 1983. Siamogale thailandica, a new mustelid (Carnivora, Mammalia) from the Neogene of Southeast Asia (translated from French). Bull. Soc. Geol. France 25, 953–956 [Google Scholar]
- 17.Peigné S., Chaimanee Y., Yamee C., Tian P., Jaeger J.-J. 2006. A new Amphicyonid (Mammalia, Carnivora, Amphicyonidae) from the late middle Miocene of northern Thailand and a review of the amphicyonine record in Asia. J. Asian Earth Sci. 26, 519–532 10.1016/j.jseaes.2004.11.003 (doi:10.1016/j.jseaes.2004.11.003) [DOI] [Google Scholar]
- 18.Chaimanee Y., Yamee C., Marandat B., Jaeger J.-J. 2007. First middle Miocene rodents from the Mae Moh Basin (Thailand): biochonological and paleoenvironmental implications. Bull. Carnegie Mus. Nat. Hist. 39, 157–163 10.2992/0145-9058(2007)39 (doi:10.2992/0145-9058(2007)39) [DOI] [Google Scholar]
- 19.Chaimanee Y., Yamee C., Tian P., Chavasseau O., Jaeger J.-J. 2008. First middle Miocene sivaladapid primate from Thailand. J. Hum. Evol. 54, 434–443 10.1016/j.jhevol.2007.10.001 (doi:10.1016/j.jhevol.2007.10.001) [DOI] [PubMed] [Google Scholar]
- 20.Benammi M., et al. 2002. Magnetostratigraphy of the Middle Miocene continental sedimentary sequences of the Mae Moh Basin in northern Thailand: evidence for counterclockwise block rotation. Earth Planet Sci. Lett. 204, 373–383 10.1016/S0012-821X(02)01002-6 (doi:10.1016/S0012-821X(02)01002-6) [DOI] [Google Scholar]
- 21.Coster P., Benammi M., Chaimanee Y., Yamee C., Chavasseau O., Emonet E.-G., Jaeger J.-J. 2010. A complete magnetic-polarity stratigraphy of the Miocene continental deposits of Mae Moh Basin, Northern Thailand and a reassessment of the age of hominoid bearing localities in Northern Thailand. Geol. Soc. Am. Bull. 122, 1180–1191 10.1130/B26568.1 (doi:10.1130/B26568.1) [DOI] [Google Scholar]
- 22.Dryden I. L., Mardia K. V. 1998. Statistical shape analysis. Chichester, UK: John Wiley & Sons [Google Scholar]
- 23.Frost S. R., Marcus L. F., Bookstein F. L., Reddy D. P., Delson E. 2003. Cranial allometry, phylogeography, and systematics of large-bodied papionins (Primates: Cercopithecinae) inferred from geometric morphometric analysis of landmark data. Anat. Rec. A 275, 1048–1072 [DOI] [PubMed] [Google Scholar]
- 24.Lebrun R. Evolution and Development of the Strepsirrhine Primate Skull. PhD thesis, Université Montpellier II & University Zürich-Irchel; 2008. [Google Scholar]
- 25.Specht M., Lebrun R., Zollikofer C. P. E. 2007. Visualizing shape transformation between chimpanzee and human braincases. Vis. Comput. 23, 743–751 [Google Scholar]
- 26.Ihaka R., Gentelman R. 1996. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5, 299–314 [Google Scholar]
- 27.Wright P. C. 2003. Are tarsiers silently leaping into extinction? In Tarsiers past, present, and future (eds Wright P. C., Simons E. L., Gursky S.), pp. 296–308 London, UK: Rutgers University Press [Google Scholar]
- 28.Gingerich P. D. 1984. Paleobiology of tarsiiform primates. In Biology of tarsiers (ed. Niemitz C.), pp. 33–44 Stuttgart, Germany: Gustav Fischer Verlag [Google Scholar]
- 29.Kay R. F., Covert H. H. 1984. Anatomy and behavior of extinct primates. In Food acquistion and processing in primates (eds Chivers D. J., Wood B. A., Bilsborough A.), pp. 467–508 Cambridge, UK: Cambridge University Press [Google Scholar]
- 30.Musser G. G., Dagosto M. 1987. The identity of Tarsius pumilus, a pygmy species endemic to the montane mossy forests of central Sulawesi. Am. Mus. Nov. 2867, 1–53 [Google Scholar]
- 31.Kay R. F. 1975. The functional adaptations of primate molar teeth. Am. J. Phys. Anthropol. 43, 195–216 10.1002/ajpa.1330430207 (doi:10.1002/ajpa.1330430207) [DOI] [PubMed] [Google Scholar]
- 32.Cartmill M. 1980. Morphology, function, and evolution of the anthropoid postorbital septum. In Evolutionary biology of the New World monkeys and continental drift (eds Ciochon R. L., Chiarelli A. B.), pp. 243–274 New York, NY: Plenum Press [Google Scholar]
- 33.Ni X., Wang Y., Hu Y., Li C. 2004. An euprimate skull from the early Eocene of China. Nature 427, 65–68 10.1038/nature02126 (doi:10.1038/nature02126) [DOI] [PubMed] [Google Scholar]
- 34.Jaeger J.-J., Thein T., Benammi M., Chaimanee Y., Soe A. N., Lwin T., Tun T., Wai S., Ducrocq S. 1999. A new primate from the middle Eocene of Myanmar and the Asian early origin of the anthropoids. Science 286, 528–530 10.1126/science.286.5439.528 (doi:10.1126/science.286.5439.528) [DOI] [PubMed] [Google Scholar]
- 35.Beard K. C., Wang J. 2004. The eosimid primates (Anthropoidea) of the Heti Formation, Yanqu Basin, Shanxi and Henan Provinces, People's Republic of China. J. Hum. Evol. 46, 401–432 10.1016/j.jhevol.2004.01.002 (doi:10.1016/j.jhevol.2004.01.002) [DOI] [PubMed] [Google Scholar]
- 36.Seiffert E. R., Simons E. L., Clyde W. C., Rossie J. B., Attia Y., Bown T. M., Chatrath P., Mathison M. E. 2005. Basal anthropoids from Egypt and the antiquity of Africa's higher primate radiation. Science 310, 300–304 10.1126/science.1116569 (doi:10.1126/science.1116569) [DOI] [PubMed] [Google Scholar]
- 37.Brandon-Jones D., et al. 2004. Asian primate classification. Int. J. Primatol. 25, 97–164 10.1023/B:IJOP.0000014647.18720.32 (doi:10.1023/B:IJOP.0000014647.18720.32) [DOI] [Google Scholar]
- 38.Groves C. P. 1998. Systematics of tarsiers and lorises. Primates 39, 13–27 [Google Scholar]
- 39.Shekelle M., Groves C., Merker S., Supriatna J. 2008. Tarsius tumpara: a new tarsier species from Siau Island, North Sulawesi. Primate Conserv. 23, 55–64 10.1896/052.023.0106 (doi:10.1896/052.023.0106) [DOI] [Google Scholar]
- 40.Shekelle M. Saint Louis, Missouri, USA: 2003. Taxonomy and biogeography of Eastern tarsiers. PhD thesis, Washington University, [Google Scholar]
- 41.Shekelle M. 2008. The history and mystery of the mountain tarsier Tarsius pumilus. Primate Conserv. 23, 121–124 10.1896/052.023.0113 (doi:10.1896/052.023.0113) [DOI] [Google Scholar]
- 42.Chaimanee Y. 1998. Plio-Pleistocene rodents of Thailand. Thai. Stud. Biodivers. 3, 1–303 [Google Scholar]