Abstract
Understanding the many factors that underlie phenotypic variation is of profound importance to evolutionary biologists. The embryonic endocrine environment is one such factor that has received much attention. In placental amniotes, the dynamic interaction of maternal and embryonic steroid production and metabolism is critical to regulating the endocrine environment. Less is known about how embryos of oviparous amniotes regulate their endocrine environment because most studies have focused on relating initial steroid levels in the yolk at oviposition to offspring phenotype. We tested the hypothesis that embryos of oviparous amniotes regulate their endocrine environment by conjugating maternal steroids and subsequently using the metabolites as precursors for steroid production later in development. Using the red-eared slider turtle (Trachemys scripta), we first characterized the conjugation of exogenous oestradiol to either oestradiol glucuronide or oestradiol sulphate (E2-S) in ovo during the first 15 days of development. Results show that oestradiol is primarily conjugated to E2-S. We then examined whether E2-S influenced sex determination and report that E2-S increases the production of female offspring. These data demonstrate that oviparous amniotes can both sulphonate steroids and respond to sulphonated steroids during embryonic development in a manner similar to placental amniotes.
Keywords: yolk steroids, steroid metabolism, maternal effect, oestradiol sulphate
1. Introduction
One primary goal of evolutionary biology is to understand how phenotypic variation is created and lost in response to selective pressures. The phenotype of an individual will ultimately be the result of an interaction between its own genotype and the environment it encounters. Phenotypic variation resulting from differences in the endocrine environment experienced during embryonic development has received ample attention over the years [1,2]. This attention is derived from the fact that early endocrine signals, specifically steroid hormones, have the ability to produce effects that persist throughout an organism's lifetime and influence vital processes such as reproduction [3,4]. Most of our understanding of how steroids influence development comes from experimental manipulations or maternal conditions where steroid signalling is disrupted. Examples of this include exogenous steroid application [5], enzyme inhibitor application [6], enzyme deficiencies [7,8] and gene knockouts [9]. These studies illustrate how variation in steroid signals can lead to variation in offspring phenotypes.
In light of data demonstrating that early steroid signals can produce long-term phenotypic effects, research in placental mammals has examined how embryos undergo normal development in the endocrinologically active maternal environment present during pregnancy. These studies report that the mammalian placenta, which develops from one or more extra-embryonic membranes [10], possesses an abundance of steroidogenic enzymes [1]. In addition to enzymes, such as cytochrome P450s, that are responsible for the biotransformation of steroids [1], there are also enzymes responsible for conjugation of steroids present in the placenta [11]. Steroid conjugation is mostly carried out by two families of enzymes: UDP-glucuronosyl transferases or sulphotransferases, with sulphotransferases being the most prevalent during embryonic development [12]. The conjugated steroids are more hydrophilic than their parent steroid and are typically considered inactive owing to their inability to bind steroid receptors [13,14]. Importantly, these conjugated steroids, both sulphonated and glucuronidated, are potential precursors for steroid production as they can be enzymatically converted back to their original, active form. Thus, it appears that placental enzymes function to modulate maternal steroid signals prior to their passing into foetal circulation, and also to modulate foetal signals prior to their passing into maternal circulation [1,15].
While work in placental mammals has primarily focused on how offspring are protected from maternal steroid signals, most work done in oviparous amniotes has focused on maternal steroid signals as a means to influence offspring phenotypes in an adaptive manner [2,16]. In oviparous amniotes such as reptiles [17] and birds [18], maternal steroids accumulate in the yolk during folliculogenesis, and this results in a discrete contribution of maternal steroids being present at oviposition. In bird eggs, concentrations of androgens in the yolk can vary systematically [18] and have been shown to influence a suite of offspring traits [2,19,20]. Interestingly, not all steroid-sensitive traits are affected by yolk steroids and the effects can be context-dependent, but the reason for this phenomenon remains unresolved [21]. Recent research in birds has demonstrated that exogenous steroids are metabolized in vivo during the early part of embryonic development [22,23], concurrent with a decline in endogenous yolk steroid levels [23]. One commonality between both in vivo studies and several earlier in vitro studies [24,25] is that steroid conjugation is prevalent during the early part of embryonic development. And while steroid conjugation is known to provide precursors for steroid production in placental mammals [1], very little is known about the role of steroid conjugates in oviparous amniotes.
Steroid conjugation has been proposed as a crucial process in the regulation of maternal steroid signals in oviparous amniotes [26], with most of the empirical support for this hypothesis coming from work done in the red-eared slider turtle (Trachemys scripta). Although not necessarily interpreted within the context of maternal steroid effects, the effects of early steroid manipulations, specifically 17β-oestradiol (E2), are well characterized in this species. Trachemys scripta possesses temperature-dependent sex determination where embryos incubated at cooler temperatures (less than 28°C) develop as males. The percentage of female embryos starts to increase as temperatures exceed 28°C, with temperatures above 30°C resulting in all embryos developing as females [5]. In this system, exogenous E2 has consistently and repeatedly been used to override the temperature cue to experimentally produce females at temperatures that would normally produce males [5], providing the initial evidence that early oestradiol levels play a role in sex determination. Embryos are most sensitive to exogenous manipulation during the middle third of incubation when gonadal differentiation is occurring [27]. The application of 1.0 µg of E2 to T. scripta eggs incubating at a male-producing temperature (26°C) did not produce any females when applied at embryonic stage 12, but produced over 80 per cent females when applied at embryonic stage 18 [27]. However, application of higher doses of E2 (10 µg) at both of these stages produced 100 per cent females [27]. Natural variation in endogenous yolk E2 levels is also associated with an increased production of female offspring in the closely related painted turtle (Chrysemys picta) [17]. Concentrations of E2 in the yolk decline significantly by day 10 of development in T. scripta [28]. During this same period, sulphotransferase enzyme activity increases significantly in the yolk and extra-embryonic membranes, and exogenous E2 is conjugated in ovo [26]. Overall, the data from turtles suggest that yolk E2 is conjugated to oestradiol sulphate (E2-S) during the early part of development. Combining the turtle data with those from placental mammals demonstrating that embryos use sulphonated steroids as precursors for steroid production, we hypothesized that yolk E2 effects in turtles result from the sulphonation of E2 to E2-S early in development and the subsequent reactivation to E2 later in development. To test this hypothesis, we first characterized how exogenous E2 is conjugated to confirm that sulphonation is the primary conjugation pathway in T. scripta eggs. We also examined the potential for E2 to be glucuronidated as an alternative route of conjugation. We then examined whether T. scripta embryos were capable of responding to exogenous E2-S. These studies provide data that allow important comparisons to be made between placental and oviparous amniotes with regard to how they regulate maternal steroid signals during embryonic development.
2. Material and methods
(a). Experiment 1: characterization of oestradiol conjugation
To characterize the mode of oestradiol conjugation, we used 15 frozen yolk samples from five clutches that were previously used to quantify E2 levels in egg following exogenous E2 application [28]. Briefly, eggs were treated within 24 h of oviposition with 10 µg of E2 dissolved in 10 µl of ethanol, except for one egg from each clutch that was immediately frozen (day 0) to allow characterization of initial endogenous levels of conjugated E2. Eggs were then incubated at 31°C, and one egg from each clutch was sampled on day 5 and day 15 of development. A single female-producing temperature was chosen for this experiment because the rate of yolk oestradiol metabolism is not influenced by incubation temperature [5]. At the time of sampling, yolks were isolated, homogenized and then frozen at −20°C.
Oestradiol glucuronide (E2-G) and E2-S were quantified using a modified procedure of Geisler et al. [29]. From each yolk, two separate 100 mg aliquots were dissolved in 200 µl of phosphate-buffered saline (pH = 7.4) in a 1.5 ml micro-centrifuge tube. Aliquots (n = 6) containing 250 pg of E2-3-G (Steraloids, Newport, RI, USA) or E2-3-S (Sigma-Aldrich, St Louis, MO, USA) were included in the assay as standards to quantify percentage recovery and calculate intra-assay variation. Then, 800 µl of 80 per cent methanol was added to each aliquot, vortexed and transferred to a 12 × 75 mm glass test tube. Each micro-centrifuge tube was then rinsed with 200 µl of methanol, vortexed and added to the test tube. Tubes were centrifuged at 3000 r.p.m. for 15 min at 0°C, followed by a decanting of the supernatant into a fresh test tube. The tube containing the yolk sample was then rinsed with an additional 1 ml of methanol, vortexed and spun again. The supernatants were combined and stored at 0°C to allow sedimentation of the neutral lipids. The following day, samples were spun at 2000 r.p.m. for 10 min at 0°C to pellet neutral lipids. The supernatants, containing conjugated and unconjugated steroids, were decanted into a fresh test tube and dried under nitrogen gas.
Conjugated steroids were separated from unconjugated steroids using solid-phase extraction. Sep Pak C18 cartridges (Waters Inc., Milford, MA, USA) were conditioned by washing with 5 ml of methanol, then 5 ml of Nanopure water. Samples were reconstituted in 1 ml of water and added to the cartridges. Once the samples were loaded, cartridges were washed with 5 ml of water, and unconjugated steroids were eluted with 5 ml of diethyl ether. Finally, conjugated steroids were eluted with 5 ml of methanol. Conjugated steroids were converted back to their free form by enzymatic hydrolysis. One aliquot was hydrolysed with β-glucuronidase purified from bovine liver to convert E2-G back to E2 (Sigma-Aldrich) [30] while the other was hydrolysed with steroid sulphatase (STS) purified from abalone entrails (Sigma-Aldrich) to convert E2-S back to E2 [29]. Purified enzymes were dissolved in sodium acetate buffer (pH = 5.0) at a concentration of 5 mg ml−1 for β-glucuronidase and 1 mg ml−1 for sulphatase. Hydrolysis was carried out by adding 2 ml of the enzyme solution to the conjugated steroids and incubating for 24 h at 37°C. The newly freed steroids were extracted with 3 ml diethyl ether (2×), dried under nitrogen gas and reconstituted in 1 ml of 10 per cent ethyl acetate in isooctane.
E2 was then quantified in each sample by radioimmunoassay (RIA) [26,31]. Tritiated oestradiol (2000 cpm) was added to each sample to calculate percentage recovery during the RIA process, and steroids were fractionated using column chromatography to minimize any effects of antibody cross-reactivity. E2 was quantified in its respective fraction with a competitive binding assay using an antibody specific to E2 (reported cross-reactivities are 14% with oestrone, 5% with oestriol and less than 0.01% with all other reported steroids; Antibody 7010, Biogenesis, Kingston, NH, USA). Samples were run in duplicate and compared with a standard curve that ranged from 5 to 500 pg g−1. E2-G levels are quantified as the amount of E2 within the fraction hydrolysed with β-glucuronidase, while E2-S levels are quantified as the amount of E2 within the fraction hydrolysed with sulphatase.
(b). Experiment 2: biological activity of oestradiol sulphate
The second experiment characterized the biological activity of E2-S by examining its ability to influence sex determination in T. scripta. To do this, 24 clutches of eggs were obtained from females collected at Banner Marsh Fish and Wildlife Area in central Illinois. Clutches were either treated within 24 h of oviposition (n = 12) or after 20 days of incubation (n = 12) as this is the end of the first third of embryonic development when gonadal differentiation begins in this species [5]. At the time of treatment, eggs from each clutch were randomized and received one of four doses of E2-S (0, 0.1, 1.0 or 10 µg) dissolved in 70 per cent ethanol and applied topically in a 5 µl bolus. Eggs were partially buried in moist vermiculite and incubated at 28°C for the entirety of development. Incubating eggs at this male-biased temperature allowed us to detect any increase in the number of female hatchlings following treatment. The sex of each hatchling was then characterized by macroscopic inspection of the gonads and Müllerian ducts a minimum of 60 days post-hatch [32]. Procedures were approved by the Illinois State University Institutional Animal Care and Use Committee, and eggs collected under permission of the Illinois Department of Natural Resources.
(c). Statistical analyses
To test for differences in conjugated steroid levels, we performed a repeated-measures MANOVA with steroid levels (corrected for percentage recovery) as dependent variables, day of development as a fixed factor and clutch of origin as a random factor. Steroid levels were log-transformed prior to analysis. To compare sex ratios between the different doses of E2-S, Fisher's exact tests were used. All statistical tests were conducted in SAS v. 9.1 (SAS Institute, Cary, NC, USA).
3. Results
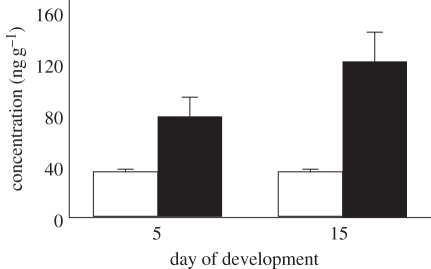
Endogenous levels of both E2-G and E2-S were low to undetectable within the yolk at the time of oviposition. Following E2 treatment, E2-G and E2-S were detectable within the yolk, with E2-S concentrations being significantly higher than those of E2-G (F1,3 = 188, p = 0.001; figure 1). Concentrations of E2-G and E2-S did not change from day 5 to day 15 (F1,3 = 1.41, p = 0.32; figure 1). Mean corrected values (ng g−1) for E2-G were 34.6 on day 5 and 32.9 on day 15, while values for E2-S were 81.2 and 134.8, respectively. Percentage recovery for E2-G and E2-S was 37 and 51 per cent, respectively. Intra-assay variation was 15 per cent for E2-G and 13 per cent for E2-S.
Figure 1.
Mean concentrations (corrected values) of E2-G (white) and E2-S (black) in the yolk. E2-S concentrations were significantly higher than E2-G (p = 0.001), with concentrations remaining stable during development (p = 0.32).
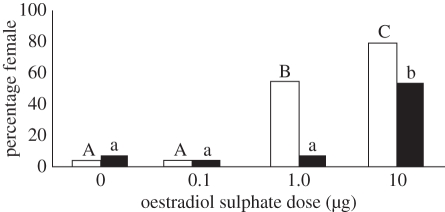
Exogenous E2-S had a significant effect on hatchling sex ratios. For eggs treated at oviposition (day 0), both the 1 and 10 µg dose led to an increased production of female hatchlings compared with the 0 and 0.1 µg dose (figure 2; p < 0.001). For eggs treated on day 20, only the 10.0 µg dose led to an increased production of female hatchlings (figure 2; p < 0.001). All treatment groups contained 26–30 hatchlings.
Figure 2.
Sex ratios of hatchlings treated with E2-S at day 0 (white) or at day 20 (black). Significant differences between groups are indicated for eggs treated at day 0 (capital letters) and those treated on day 20 (lower-case letters). Groups not sharing a similar letter are significantly different (p < 0.05).
4. Discussion
We hypothesized that embryos of oviparous amniotes primarily sulphonate maternal steroids and use the resulting sulphonated steroids as precursors for steroid production later in development, similar to embryos of placental mammals. Data from the first experiment demonstrate that (i) levels of conjugated steroids are very low at oviposition, (ii) E2 is conjugated during the first 5 days of development and (iii) sulphonation is the primary conjugation pathway (figure 1). Results from the second experiment suggest that T. scripta embryos are capable of responding to E2-S, as evidenced by the increased production of female hatchlings (figure 2). These results also provide support for the idea that the sulphotransferase/sulphatase pathway plays an important role in the modulation of maternal steroid signals in oviparous amniotes just as it does in placental amniotes. Demonstrating that embryos can sulphonate steroids, and that sulphonated steroids can influence offspring development, has important implications for how we interpret the effects of maternally derived yolk steroids by advancing our knowledge of how embryos regulate their endocrine environment.
Maternally derived yolk steroids have been extensively studied since they were first proposed as a means by which females could influence offspring phenotype [2,18,19]. Numerous studies have shown that concentrations of various steroids in the yolk decline during development [23,28,33–36]. In T. scripta, concentrations of yolk E2 are significantly higher in the female's second clutch of the season, but, despite this initial difference, concentrations by day 10 of development are low/undetectable in both groups, and this decline is not influenced by incubation temperature [5]. In fact, E2 concentrations in the same yolks used in this current study were shown to significantly decline from day 5 to day 15 [26]. When calculating the total amount of E2 measured within these eggs (yolk + albumen + embryo), amounts declined from 0.217 µg on day 5 to 0.024 µg on day 15, which only represents 2 and 0.02 per cent, respectively, of the initial 10 µg that was initially applied on day 0 [26]. The current study demonstrated that some of this E2 was conjugated to E2-G and E2-S. The total amount of E2-G in just the yolk was 0.178 µg on day 5 and 0.161 µg on day 15, while the total amount of E2-S was 0.419 µg on day 5 and 0.661 µg on day 15. These levels still only account for less than 10 per cent of the initial E2 that was applied. Potential explanations for the unaccounted-for E2 include, but are not limited to, incomplete transfer of steroid through the eggshell, presence of E2-G and E2-S in the albumen or embryo (only yolk was examined in the present study) and metabolism of E2 to different oestrogens. While it is possible that some of the initial E2 remained on the eggshell, a previous study using tritiated E2 demonstrated that this topical route of administration resulted in over 70 per cent transfer of tritiated E2 into the egg [26]. This study also demonstrated that water-soluble metabolites of E2 were present in the yolk and albumen in approximately equal amounts, suggesting that E2-G and E2-S would be present in the albumen in addition to the yolk [26]. At this point, we have no measure of the conversion of E2 to other oestrogens such as oestrone, but work in the European starling (Sturnus vulgaris) has shown that exogenous testosterone is converted to other androgen metabolites, with less than 5 per cent of the initial testosterone detectable after 6 days of incubation [23]. More work is needed to characterize E2 metabolites throughout the egg during embryonic development in T. scripta. Regardless of the ultimate fate of the steroid, the data from this study demonstrate that even a supraphysiological dose of E2 (10 µg) can be almost completely metabolized during the first third of development and result in levels of E2-S in the yolk that are 85 times higher than E2 levels in the entire egg.
Sulphonation has been established as the primary steroid conjugation pathway during early embryonic development in mammals for some time [12]. With regard to the sulphonation of E2, the enzyme primarily responsible for this conversion is oestrogen sulphotransferase (SULT1E1) (EC 2.8.2.4). The resulting metabolite, E2-S, has increased water solubility and is considered inactive [13,14]. However, E2-S (along with other sulphonated steroids) can be reactivated by the enzyme STS (EC 3.1.6.2), highlighting the importance of STS as a producer of active steroids. The ability of E2-S to influence sex determination in T. scripta suggests the presence of STS activity during embryonic development, and STS expression has been reported in embryonic [37] and foetal [38] tissues. With evidence mounting that maternal steroids are sulphonated during the early part of embryonic development in oviparous amniotes [23,26,28], embryonic STS activity becomes a critical part of understanding when and where maternal steroid effects can arise. The sulphonation of maternal steroids by developing embryos results in the conversion of lipophilic, active steroids to water-soluble, inactive forms. The temporary inactivation of steroids via sulphonation may help reconcile the apparent contradiction that maternal steroids can influence individual differentiation without influencing other processes (such as sexual differentiation [21]) by allowing for tissue-specific reactivation of sulphonated steroids by STS.
In T. scripta, this specific contradiction is not present, as gonadal differentiation is sensitive to E2 [5]. But this system does provide a steroid-sensitive trait that is dichotomous (male versus female), allowing us to clearly demonstrate that sulphonated steroids can influence development in an oviparous amniote. And despite the difference between turtles and birds in the relative influence of steroids on gonadal differentiation, other aspects of yolk steroid signalling are similar. For example, both taxa exhibit a decline in yolk steroid concentrations across development as well as in ovo steroid metabolism early in development [39]. Because of their sensitivity to E2, it may be even more important for turtles to convert maternal steroids to inactive metabolites to prevent a potential skewing of sex ratios. Our finding that the lowest dose of E2-S did not influence sex ratios suggests that embryos can buffer themselves from a maternal effect on gonadal differentiation when steroid levels are low. Consistent with this finding, endogenous steroids primarily influence offspring sex ratios late in the nesting season [17], when yolks from second clutches contain almost 10 times the amount of E2 as first clutches [28,40]. Embryos appear more sensitive to E2-S when applied immediately at oviposition versus day 20 of development, but the reasons for this remain unknown. Numerous factors that influence E2-S movement and reactivation are likely to differ between these two points of development. Interestingly, we did not detect any E2-G or E2-S in the yolks at the time of oviposition, suggesting that these substances are not transferred to the yolk during folliculogenesis. At this point, data suggest that both turtles and birds, and probably oviparous vertebrates more generally, are able to modulate maternal steroid signals, thus demonstrating an important embryonic contribution to the manifestation of maternal steroid effects.
By demonstrating that embryos of T. scripta can both sulphonate steroids and respond to sulphonated steroids, this study illustrates the complex and dynamic nature of yolk steroid effects. The interaction between maternal signal and embryonic response that we report is very similar to what is found in placental amniotes and suggests that a common pathway is used in the modulation of maternal steroids. Since embryonic steroid milieus during sexual differentiation can produce long-term phenotypic effects and disruption of these milieus often leads to abnormal reproductive behaviour in adults [21], sulphonation may allow embryos to use maternal steroids as precursors for steroid production without the risk associated with transporting active steroid signals. The presence of steroid sulphonation during embryonic development in an oviparous amniote may also help explain how viviparity evolved in vertebrates [41]. One potential issue with the transition from oviparity to viviparity is that the embryo would be subjected to the maternal environment, including steroid signalling, throughout development. Sulphonation is the mechanism by which placental amniotes are thought to protect themselves from the potentially detrimental effects of maternal steroids [11], and thus our finding of a similar mechanism in an oviparous system suggests that one major barrier to viviparity is gone. Overall, emerging data indicate that oviparous and placental amniotes modulate maternal steroid signals in a similar manner. More studies are needed to characterize the role of sulphonated steroids during embryonic development if we are to fully understand how maternal steroid signals create phenotypic variation in offspring, but it now appears that oviparous amniotes provide a powerful system in which to study the regulation of maternal steroid signals. The discrete nature of the egg facilitates investigation into the mechanisms of how embryos regulate their endocrine environment because there is a separation from the maternal endocrine environment. By advancing our knowledge of how embryos regulate their endocrine environment, we will develop a more complete understanding as to how phenotypic variation is created.
Acknowledgements
We are grateful to Sandrine Clairardin and Steve Juliano for their assistance with the project. We would also like to thank the Illinois Department of Natural Resources for granting access to Banner Marsh. Financial support for this research was provided by the Beta Lambda Chapter of Phi Sigma to R.T.P. and from the Illinois State University School of Biological Sciences to R.M.B. R.T.P. was partially supported by NSF grant IOS-0952840.
References
- 1.Pepe G. J., Albrecht E. D. 1995. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr. Rev. 16, 608–648 [DOI] [PubMed] [Google Scholar]
- 2.Groothuis T. G. G., Müller W., von Engelhardt N., Carere C., Eising C. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352 10.1016/j.neubiorev.2004.12.002 (doi:10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 3.Gerall A., Moltz H., Ward I. 1992. Sexual differentiation. Handbook of Behavioral Neurobiology. New York, NY: Plenum [Google Scholar]
- 4.Balthazart J., Adkins-Regan E. 2002. Sexual differentiation of brain and behavior in birds. In Hormones, brain and behavior (ed. Pfaff D.), pp. 223–301 New York, NY: Academic Press [Google Scholar]
- 5.Wibbels T., Bull J. J., Crews D. 1994. Temperature-dependent sex determination: a mechanistic approach. J. Exp. Zool. 270, 71–78 10.1002/jez.1402700108 (doi:10.1002/jez.1402700108) [DOI] [PubMed] [Google Scholar]
- 6.Rhen T., Lang W. L. 1994. Temperature-dependent sex determination in the snapping turtle: manipulation of the embryonic sex steroid environment. Gen. Comp. Endocrinol. 96, 243–254 10.1006/gcen.1994.1179 (doi:10.1006/gcen.1994.1179) [DOI] [PubMed] [Google Scholar]
- 7.Shozu M., Akasofu K., Harada T., Kubota Y. 1991. A new cause of female pseudohermaphroditism—placental aromatase deficiency. J. Clin. Endocrinol. Metab. 72, 560–566 10.1210/jcem-72-3-560 (doi:10.1210/jcem-72-3-560) [DOI] [PubMed] [Google Scholar]
- 8.Harada N., Ogawa H., Shozu M., Yamada K., Suhara K., Nishida E., Takagi Y. 1992. Biochemical and molecular genetic analyses on placental aromatase (P-450arom) deficiency. J. Biol. Chem. 267, 4781–4785 [PubMed] [Google Scholar]
- 9.Tong M. H., Jiang H., Liu P., Lawson J. A., Brass L. F., Song W. 2005. Spontaneous fetal loss caused by placental thrombosis in estrogen sulfotransferase-deficient mice. Nat. Med. 11, 153–159 [DOI] [PubMed] [Google Scholar]
- 10.Rothchild I. 2003. The yolkless egg and the evolution of eutherian viviparity. Biol. Reprod. 68, 337–357 10.1095/biolreprod.102.004531 (doi:10.1095/biolreprod.102.004531) [DOI] [PubMed] [Google Scholar]
- 11.Levitz M. 1966. Conjugation and transfer of fetal–placental steroid hormones. J. Clin. Endocrinol. Metab. 26, 773–777 10.1210/jcem-26-7-773 (doi:10.1210/jcem-26-7-773) [DOI] [PubMed] [Google Scholar]
- 12.Pasqualini J. R. 1970. Metabolic conjugation and hydrolysis of steroid hormones in the fetoplacental unit. In Metabolic conjugation and metabolic hydrolysis (ed. Fishman W. H.), pp. 153–259 New York, NY: Academic Press [Google Scholar]
- 13.Pasqualini J. R., Gelly C., Lecerf F. 1986. Estrogen sulfates—biological and ultrastructural responses and metabolism in Mcf-7 human breast cancer cells. Breast Cancer Res. Treat. 8, 233–240 10.1007/BF01807336 (doi:10.1007/BF01807336) [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K., Kubushiro K., Iwamori Y., Okairi Y., Kiguchi K., Ishiwata I., Tsukazaki K., Nozawa S., Iwamori M. 2003. Estrogen sulfotransferase and sulfatase: roles in the regulation of estrogen activity in human uterine endometrial carcinomas. Cancer Sci. 94, 871–876 10.1111/j.1349-7006.2003.tb01369.x (doi:10.1111/j.1349-7006.2003.tb01369.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller W. L. 1998. Steroid hormone biosynthesis and actions in the materno-feto-placental unit. Clin. Perinatol. 25, 799–817 [PubMed] [Google Scholar]
- 16.Groothuis T. G. G., Schwabl H. 2008. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Phil. Trans. R. Soc. B 363, 1647–1661 10.1098/rstb.2007.0007 (doi:10.1098/rstb.2007.0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden R. M., Ewert M. A., Nelson C. E. 2000. Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc. R. Soc. Lond. B 267, 1745–1749 10.1098/rspb.2000.1205 (doi:10.1098/rspb.2000.1205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwabl H. 1993. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA 90, 11 446–11 450 10.1073/pnas.90.24.11446 (doi:10.1073/pnas.90.24.11446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil D. 2008. Hormones in avian eggs: physiology, ecology, and behavior. In Advances in the study of behavior (eds Brockmann H. J., Roper T. J., Naguib M.), pp. 337–395 San Diego, CA: Academic Press [Google Scholar]
- 20.Navara K. J., Mendonca M. T. 2008. Yolk androgens as pleiotropic mediators of physiological processes: a mechanistic review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 150, 378–386 10.1016/j.cbpa.2008.05.002 (doi:10.1016/j.cbpa.2008.05.002) [DOI] [PubMed] [Google Scholar]
- 21.Carere C., Balthazart J. 2007. Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrinol. Metab. 18, 73–80 [DOI] [PubMed] [Google Scholar]
- 22.von Engelhardt N., Henriksen R., Groothuis T. G. G. 2009. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. Gen. Comp. Endocrinol. 163, 175–183 10.1016/j.ygcen.2009.04.004 (doi:10.1016/j.ygcen.2009.04.004) [DOI] [PubMed] [Google Scholar]
- 23.Paitz R. T., Bowden R. M., Casto J. M. 2011. Embryonic modulation of maternal steroids in European starlings (Sturnus vulgaris). Proc. R. Soc. B 278, 99–106 10.1098/rspb.2010.0813 (doi:10.1098/rspb.2010.0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons I. C. 1970. Metabolism of testosterone by early chick embryonic blastoderm. Steroids 16, 59–65 10.1016/S0039-128X(70)80095-2 (doi:10.1016/S0039-128X(70)80095-2) [DOI] [PubMed] [Google Scholar]
- 25.Antila E., Leikola A., Tahka S. 1984. Early steroid metabolism by chick blastoderm in vitro. Steroids 43, 315–323 10.1016/0039-128X(84)90049-7 (doi:10.1016/0039-128X(84)90049-7) [DOI] [PubMed] [Google Scholar]
- 26.Paitz R. T., Bowden R. M. 2008. A proposed role of the sulfotransferase/sulfatase pathway in modulating yolk steroid effects. Integr. Comp. Biol. 48, 419–427 10.1093/icb/icn034 (doi:10.1093/icb/icn034) [DOI] [PubMed] [Google Scholar]
- 27.Wibbels T., Bull J. J., Crews D. 1991. Synergism between temperature and estradiol: a common pathway in turtle sex determination? J. Exp. Zool. 260, 130–134 10.1002/jez.1402600117 (doi:10.1002/jez.1402600117) [DOI] [PubMed] [Google Scholar]
- 28.Paitz R. T., Bowden R. M. 2009. Rapid decline in the concentrations of three yolk steroids during development: is it embryonic regulation? Gen. Comp. Endocrinol. 161, 246–251 10.1016/j.ygcen.2009.01.018 (doi:10.1016/j.ygcen.2009.01.018) [DOI] [PubMed] [Google Scholar]
- 29.Geisler J., Ekse D., Helle H., Duong N. K., Lonning P. E. 2008. An optimised, highly sensitive radioimmunoassay for the simultaneous measurement of estrone, estradiol and estrone sulfate in the ultra-low range in human plasma samples. J. Steroid Biochem. Mol. Biol. 109, 90–95 10.1016/j.jsbmb.2007.12.011 (doi:10.1016/j.jsbmb.2007.12.011) [DOI] [PubMed] [Google Scholar]
- 30.Ferchaud V., Courcoux P., Le Bizec B., Monteau F., Andre F. 2000. Enzymatic hydrolysis of conjugated steroid metabolites: search for optimum conditions using response surface methodology. Analyst 125, 2255–2259 10.1039/b003421p (doi:10.1039/b003421p) [DOI] [PubMed] [Google Scholar]
- 31.Wingfield J. C., Farner D. S. 1975. Determination of 5 steroids in avian plasma by radioimmunoassay and competitive-protein-binding. Steroids 26, 311–327 10.1016/0039-128X(75)90077-X (doi:10.1016/0039-128X(75)90077-X) [DOI] [PubMed] [Google Scholar]
- 32.Paitz R. T., Gould A. C., Holgersson M. C. N., Bowden R. M. 2010. Temperature, phenotype, and the evolution of temperature-dependent sex determination: how do natural incubations compare to laboratory incubations? J. Exp. Zool. B Mol. Dev. Evol. 314B, 86–93 10.1002/jez.b.21312 (doi:10.1002/jez.b.21312) [DOI] [PubMed] [Google Scholar]
- 33.Elf P. K., Fivizzani A. J. 2002. Changes in sex steroid levels in yolks of the leghorn chicken, Gallus domesticus, during embryonic development. J. Exp. Zool. 293, 594–600 10.1002/jez.10169 (doi:10.1002/jez.10169) [DOI] [PubMed] [Google Scholar]
- 34.Bowden R. M., Ewert M. A., Nelson C. E. 2002. Hormone levels in yolk decline throughout development in the red-eared slider turtle (Trachemys scripta elegans). Gen. Comp. Endocrinol. 129, 171–177 10.1016/S0016-6480(02)00530-0 (doi:10.1016/S0016-6480(02)00530-0) [DOI] [PubMed] [Google Scholar]
- 35.Eising C. M., Muller W., Dijkstra C., Groothuis T. G. G. 2003. Maternal androgens in egg yolks: relation with sex, incubation time and embryonic growth. Gen. Comp. Endocrinol. 132, 241–247 10.1016/S0016-6480(03)00090-X (doi:10.1016/S0016-6480(03)00090-X) [DOI] [PubMed] [Google Scholar]
- 36.Gilbert L., Bulmer E., Arnold K. E., Graves J. A. 2007. Yolk androgens and embryo sex: maternal effects or confounding factors? Horm. Behav. 51, 231–238 10.1016/j.yhbeh.2006.10.005 (doi:10.1016/j.yhbeh.2006.10.005) [DOI] [PubMed] [Google Scholar]
- 37.Compagnone N. A., Salido E., Shapiro L. J., Mellon S. H. 1997. Expression of steroid sulfatase during embryogenesis. Endocrinology 138, 4768–4773 10.1210/en.138.11.4768 (doi:10.1210/en.138.11.4768) [DOI] [PubMed] [Google Scholar]
- 38.Miki Y., et al. 2002. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J. Clin. Endocr. Metab. 87, 5760–5768 10.1210/jc.2002-020670 (doi:10.1210/jc.2002-020670) [DOI] [PubMed] [Google Scholar]
- 39.Paitz R. T., Bowden R. M. 2010. Progesterone metabolites, ‘xenobiotic-sensing’ nuclear receptors, and the metabolism of maternal steroids. Gen. Comp. Endocrinol. 166, 217–221 10.1016/j.ygcen.2009.11.011 (doi:10.1016/j.ygcen.2009.11.011) [DOI] [PubMed] [Google Scholar]
- 40.Bowden R. M., Paitz R. T., Janzen F. J. In press The ontogeny of post-maturation resource allocation in a turtle. Physiol. Biochem. Zool. [DOI] [PubMed] [Google Scholar]
- 41.Blackburn D. G. 2000. Reptilian viviparity: past research, future directions, and appropriate models. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 127, 391–409 10.1016/S1095-6433(00)00272-5 (doi:10.1016/S1095-6433(00)00272-5) [DOI] [PubMed] [Google Scholar]