Abstract
Zoo animal husbandry aims at constantly improving husbandry, reproductive success and ultimately animal welfare. Nevertheless, analyses to determine factors influencing husbandry of different species are rare. The relative life expectancy (rLE; life expectancy (LE) as proportion of longevity) describes husbandry success of captive populations. Correlating rLE with biological characteristics of different species, reasons for variation in rLE can be detected. We analysed data of 166 901 animals representing 78 ruminant species kept in 850 facilities. The rLE of females correlated with the percentage of grass in a species' natural diet, suggesting that needs of species adapted to grass can be more easily accommodated than the needs of those adapted to browse. Males of monogamous species demonstrate higher rLE than polygamous males, which matches observed differences of sexual bias in LE in free-living populations and thus supports the ecological theory that the mating system influences LE. The third interesting finding was that rLE was higher in species managed by international studbooks when compared with species not managed in this way. Our method facilitates the identification of biological characteristics of species that are relevant for their husbandry success, and they also support ecological theory. Translating these findings into feeding recommendations, our approach can help to improve animal husbandry.
Keywords: animal husbandry, browser, artiodactyls, life expectancy, sexual bias, zoo
1. Introduction
In 2003, the international zoo community claimed ‘to exercise the highest standards of animal welfare’ [1]. The importance of this aim cannot be overvalued, as ethical considerations of zoo critics conclude that keeping animals in zoos is only acceptable if their welfare is guaranteed [2,3]. Important questions arise from the call for ‘highest standards of animal welfare’ in zoos: how can we measure welfare, and how can husbandry success be improved [4]? Even though an increasing number of articles have been published in this field, most articles are theoretical [5]. Behavioural patterns (occurrence of stereotypies), metabolic parameters (blood and faecal corticosteroid concentrations), health status (prevalence and incidence of diseases) and life-history data (breeding success, life expectancy (LE)) were discussed as feasible indicators of wellbeing in zoos [6,7].
In their collaborative effort to manage self-sustaining populations, the zoo community started pooling their population data in a common database, managed by the International Species Information System (ISIS). ISIS have collected individual animal data from approximately 850 member institutions in over 80 countries since 1973. Considering all single zoo populations of one species as parts of one metapopulation, ISIS data allow calculations of parameters characterizing the average zoo population. To estimate the development of a metapopulation, calculations of life-history parameters (e.g. annual mortality and LE) are required. For example, Clubb [8] calculated that adult female elephants (Elephas maximus and Loxodonta africana) had shorter life expectancies in zoos compared with wild and semi-wild reference populations.
Comparative analyses of different species' performance in captivity are particularly valuable to detect factors influencing husbandry success. Unfortunately, such analyses are still relatively rare. Clubb & Mason [9–11] demonstrated that frequencies of stereotypies and the extent of infant mortality in captive carnivores were higher in wide-ranging species when compared with species with smaller home range sizes. As LE of different species correlates generally with the body mass of the species (allometric principle [12]), such comparative analyses of LE require a correction for this factor. In one survey of life-history data from 20 deer species kept in captivity, the relative LE (rLE; average LE as proportion of maximum LE) of adult females correlated positively with the percentage of grass in a species' natural diet (%grass) [13]. These examples demonstrated that interspecies comparisons of behavioural measures or life-history data allow the detection of biological characteristics that are relevant for the adaptability of species to live under the conditions in captivity.
Here, we use such a comparative approach (rLE of metapopulations) to analyse biological factors correlated to husbandry success in 78 ruminant species. We expect that not only browsing deer, but also browsing ruminants, in general, perform less successfully in captivity compared with mixed feeders and grazers. Species from the tropics and subtropics should have more problems coping with climatic conditions in the temperate zone (where the majority of ISIS zoos are located), and thus should display a lower rLE compared with species originating from the temperate zones. Compared with wild populations, captive zoo animals are confronted with much higher population densities. Density-dependent influences on LE (social stress, contact with pathogens) should have a higher impact in solitary and pair-living species, which are less adapted to crowded conditions (as in zoos). Males that defend a harem have a higher investment in reproduction compared with monogamous species, so that males of polygamous species may have a lower LE. Additionally, we test the hypothesis that species intensively managed by an international studbook perform better than those unmanaged, assuming that husbandry of such focus species is performed with particular care.
2. Material and methods
For this investigation, data from 166 901 animals, representing 78 species held in captivity (suborder Ruminantia) were analysed. The data were collected by the ISIS. Data preparation followed the same procedure as described by Müller et al. [13]. LE of a species' birth cohort was expressed separately for both sexes as rLE (LE of a cohort as a proportion of the record longevity of the species) to exclude allometric influences. Only animals that lived 2 years from date of birth were included, to exclude a bias owing to the culling of surplus young animals. Ranging from 0 to 1, an rLE of 0 would denote the death of all individuals at birth, whereas an rLE of 1 implies that all individuals reached the maximum lifespan.
To analyse the influence of biological and husbandry factors on the rLE, literature data on body mass, geographical origin, social behaviour (in case of females), mating system (in case of males), percentage of grass in the natural diet of a species, as well as the existence of an international studbook, were included in a step down linear regression approach as independent variables (separately for both sexes; see the electronic supplementary material for details). To achieve normality, some of the variables were log-transformed in advance. In order to avoid the false interpretation of ancestry-based correlations in these models as adaptation (i.e., finding a significant result simply because related species behave in a similar manner) [14,15], the analyses were controlled for phylogenetic influences using the ‘phylogenetic generalized least-squares’ method (PGLS; [16,17]; see the electronic supplementary material for details and phylogenetic tree). This procedure estimates a covariance matrix of the species owing to their ancestral roots and includes these inter-relationships in a generalized least-squares algorithm to determine the model parameters. For comparison purposes, respective generalized linear models (GLMs) without phylogenetic control were set-up. The statistical calculations were performed with SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and COMPARE 4.6 programme [17]. The significance level was set to α < 0.05.
3. Results
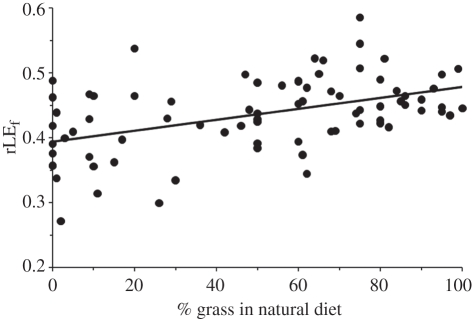
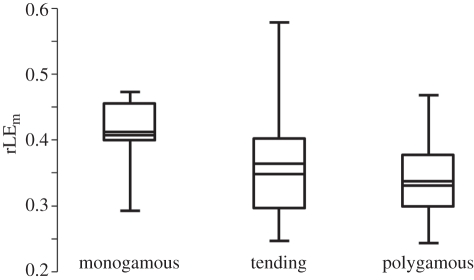
Within species, an rLE of females was significantly higher than an rLE of males (paired t-test, p < 0.001, n = 78 species; see the electronic supplementary material for details). In females, the step-down procedure identified %grass (figure 1) and the presence of an international studbook as the only significant factors influencing rLE. In males, we identified mating type (figure 2) and also the presence of an international studbook as the only significant factors influencing rLE (table 1). The resulting models were identical for analyses with and without PGLS.
Figure 1.
Positive correlation of the relative life expectancy (rLE) of females that lived 2 years from date of birth (rLEf) with the percentage of grass in a species' natural diet (%grass). Note that species with low %grass (browsers) demonstrated lower rLEf compared with species with medium and high %grass (intermediate feeding type and grazers). The relationship was significant (see the electronic supplementary material, table S1).
Figure 2.
Range, arithmetic mean and quartiles of the rLE of males that lived 2 years from date of birth (rLEm) according to mating type. Note that males of monogamous species had a higher rLE compared with polygamous species. The relationship was significant (see the electronic supplementary material, table S1).
Table 1.
Results of final GLMs. (Independent variables remained after a step-down procedure starting with %grass, studbook, mating system (or social system, alternatively), habitat and ln(body mass). Results given for GLMs without and with phylogenetic generalized least squares (PGLS; likelihood ratio test). Independent variables assessed: body mass, percentage of grass in the natural diet, social system or mating system (alternatively), natural habitat (temperate versus subtropical/tropical) and presence of an international studbook.)
| GLM | GLM (PGLS) | |
|---|---|---|
| F, p | χ2, p | |
| dependent variable: rLE2f | ||
| %grass | F1,75 = 19.84, p < 0.001 | χ2 = 8.28, d.f. = 1, p = 0.004 |
| studbook | F1,75 = 7.69, p = 0.007 | χ2 = 8.80, d.f. = 1, p = 0.003 |
| dependent variable: rLE2m | ||
| mating system | F2,74 = 6.719, p = 0.002 | χ2 = 9.92, d.f. = 2, p = 0.007 |
| studbook | F1,74 = 6.745, p = 0.011 | χ2 = 5.52, d.f. = 1, p = 0.019 |
| dependent variable: rLE2m | ||
| social system | F2,74 = 5.420, p = 0.006 | χ2 = 9.76, d.f. = 2, p = 0.008 |
| studbook | F1,74 = 5.177, p = 0.026 | χ2 = 4.34, d.f. = 1, p = 0.037 |
4. Discussion
Our study identified biological characteristics of species that had an influence on husbandry success in the past, allowing suggestions for improvements in husbandry. The data on rLE generated in this study (see the electronic supplementary material for details) can serve as global averages, against which a zoo can compare its populations, in the form of an in-house quality control and warning system.
So far, the effect of particular husbandry measures is rather assessed by approaches investigating single species than by comparative analyses between species. Different studies demonstrate that environmental enrichment, feeding management or exposure to the public influence the excretion of corticoids, indicating different stress responses [19]. Our analysis does not test for such husbandry-related factors, but identifies biological characteristics that describe the adaptability of a species to live under captive conditions. The results allow two different conclusions whether species with a low rLE should be kept: either try to optimize husbandry, or focus on species in which a higher husbandry success can more easily be achieved.
Contrary to our prediction that social behaviour of a species (measure to live under crowded conditions) influences rLE of female ruminants, such a correlation could not be detected. It is conceivable that the common practice of keeping solitary species in pairs in large enclosures prevents a negative impact on rLE. Additionally, no relationship between the geographical origin of a species and an rLE was observed, indicating that climatic stress in (sub-)tropic species that are kept in the temperate zone does either not play an important role, or that winter housing in heated stables eliminates the influence on rLE.
In adult female ruminants, the percentage of grass in a species' natural diet was positively correlated to the rLE in captivity. This parameter characterizes the diet a species is physiologically adapted to (not the one fed in zoos), and indicates whether a species is a browser (very low percentage of grass in the natural diet), mixed feeder or grazer [20]. Our results corroborate the subjective experience that browsers demonstrate a higher nutrition-related mortality in captivity and are more challenging to keep when compared with grazing species, owing to the complex logistics of providing browse [21]. In captivity, browsers are often offered grass hay and/or lucerne hay as surrogate roughage sources. The reluctance of browsers to ingest such roughage sources in larger amounts, as either their teeth or their stomachs are not adapted to the physical properties of these materials [22,23], leads to an increased proportion of concentrate feeds in the ingested diet. This will cause chronic forestomach acidosis, which in turn leads to a higher incidence of a variety of diseases [24] and ultimately to a shorter average LE.
The diet a species is naturally adapted to was not a predictor of LE in male ruminants. Instead, mating type had a significant influence on LE, with males of monogamous species demonstrating a higher LE than territorial males, or males defending a harem. Lower annual survival rates in males when compared with females are a common characteristic in population dynamics of free-living wild ungulates [25]. The here-described lower rLE of male ruminants in captivity proves that this pattern can also be observed in captive populations. This is particularly interesting, as the pressure of the rut is expected to be much lower in captivity, where usually only one adult male is kept in a harem, when compared with the situation in the wild, where several males compete for the females. In one experimental study on wild-living soay sheep (Ovis aries), castrated males demonstrated a prolonged LE compared with intact males and even females [26]. Both findings together support speculations that not only an intensive intraspecific competition for females during the rut, but also reproductive physiology per se has a negative influence on male LE. In free-living mammal populations, the degree of male-biased adult mortality correlates positively to the degree of sexual size dimorphism [27]. Sex differences in adult longevity are more pronounced in polygynous (degree of sexual dimorphism correlates with degree of polygyny [28]) when compared with monogamous species [29]. Two results of our analysis support the theory that sexual dimorphism and mating system explain the pattern of sexual bias in adult LE of ungulates: (i) rLE in captive males of ruminant species with lower male reproductive investment (monogamous species) was higher compared to species with higher investment (polygynous species), and (ii) the difference between the rLE of females and males of monogamous species was significantly smaller than the difference between female and male rLE in polygynous species. Nevertheless, an influence of culling measures on the observed sexual bias of adult LE with respect to mating systems cannot be completely excluded, although recommendations for population management of the World Association of Zoos and Aquariums [30] and the results of Müller et al. [13]. suggest that culling is performed before animals are sexually mature.
One major past achievement of zoos was the conservation of species that went extinct in the wild, including Przewalski's horse (Equus caballus przewalskii) and Père David's deer (Elaphurus davidianus). A major key to this success was the breeding coordination of many zoos with international studbooks. Nowadays, endangered species' conservation by ex situ breeding programmes is one of the most important aims of zoological institutions [1], and over 150 international studbooks have been established. The principle aim of such studbooks is to maintain a broad genetic diversity by reducing inbreeding to a minimum. Additionally, detailed husbandry recommendations including spatial requirements, housing facilities, group composition and feeding regimes are often an integral part of these studbooks. In both, male and female ruminants, rLE was higher in species managed with the help of an international studbook. Newborn mortality of several species in captivity was higher in inbreed compared with non-inbreed individuals [31–33], suggesting that inbreeding may also have an influence on adult LE. It is possible that both, the effort to reduce inbreeding in studbook-managed populations (as compared with non-managed species), or the implementation of the detailed husbandry guidelines, resulted in the higher rLE values of the respective species. The success of such an intensive population management should encourage more widespread use of studbook coordination in additional species.
Further analyses will demonstrate whether factors like inbreeding or geographical distribution of zoo populations also influence LE in captivity, and whether analyses of other taxa identify more parameters that are relevant for the husbandry success of wild species in captivity.
Acknowledgements
We thank the Georg and Bertha Schwyzer-Winiker-Stiftung and the Vontobel-Stiftung for financial support, the World Association of Zoos and Aquariums for making the data transfer from ISIS possible, and all participating zoos for their consistent data collection. The text and the explanatory power of the results were improved owing to the helpful comments of two anonymous reviewers.
References
- 1.WAZA 2003. WAZA code of ethics and animal welfare. In 58th Annual Meeting, of the World Association of Zoos and Aquariums San José, CA: WAZA; See www.waza.org/en/site/conservation/code-of-ethics-animal-welfare; accessed 26 November 2010 [Google Scholar]
- 2.Wickins-Dražilová D. 2006. Zoo animal welfare. J. Agric. Environ. Ethics 19, 27–36 10.1007/s10806-005-4380-2 (doi:10.1007/s10806-005-4380-2) [DOI] [Google Scholar]
- 3.Hutchins M. 2003. Zoo and aquarium animal management and conservation: current trends and future challenges. Int. Zoo Yearbook 38, 14–28 10.1111/j.1748-1090.2003.tb02060.x (doi:10.1111/j.1748-1090.2003.tb02060.x) [DOI] [Google Scholar]
- 4.Dawkins A. S. 2006. A user's guide to animal welfare science. Trends Ecol. Evol. 21, 77–82 10.1016/j.tree.2005.10.017 (doi:10.1016/j.tree.2005.10.017) [DOI] [PubMed] [Google Scholar]
- 5.Goulart V. D., Azevedo P. G., Van de Schepop J. A., Teixeira C. P., Barçante L., Azevedo C. S., Young R. J. 2009. GAPs in the study of zoo and wild animal welfare. Zoo Biol. 28, 561–573 [DOI] [PubMed] [Google Scholar]
- 6.Hosey G., Melfi V., Pankhurst S. 2009. Animal welfare. In Zoo animals behaviour, management, and welfare (eds Hosey G., Melfi V., Pankhurst S.), pp. 219–258 New York, NY: Oxford University Press Inc [Google Scholar]
- 7.Hill S. P., Broom D. M. 2009. Measuring zoo animal welfare: theory and practice. Zoo Biol. 28, 531–544 [DOI] [PubMed] [Google Scholar]
- 8.Clubb R., Rowcliffe M., Lee P., Mar K. U., Moss C., Mason G. J. 2008. Compromised survivorship in zoo elephants. Science 322, 1649. 10.1126/science.1164298 (doi:10.1126/science.1164298) [DOI] [PubMed] [Google Scholar]
- 9.Clubb R., Mason G. J. 2007. Natural behavioural biology as a risk factor in carnivore welfare: how analysing species differences could help zoos improve enclosures. Appl. Anim. Behav. Sci. 102, 303–328 10.1016/j.applanim.2006.05.033 (doi:10.1016/j.applanim.2006.05.033) [DOI] [Google Scholar]
- 10.Clubb R., Mason G. J. 2003. Captivity effects on wide-ranging carnivores. Nature 425, 473–474 10.1038/425473a (doi:10.1038/425473a) [DOI] [PubMed] [Google Scholar]
- 11.Mason G. J. 2010. Species differences in responses to captivity: stress, welfare and the comparative method. Trends Ecol. Evol. 25, 713–721 10.1016/j.tree.2010.08.011 (doi:10.1016/j.tree.2010.08.011) [DOI] [PubMed] [Google Scholar]
- 12.Western D. 1979. Size, life history and ecology in mammals. Afr. J. Ecol. 17, 185–204 10.1111/j.1365-2028.1979.tb00256.x (doi:10.1111/j.1365-2028.1979.tb00256.x) [DOI] [Google Scholar]
- 13.Müller D. W. H., Bingaman Lackey L., Streich W. J., Hatt J.-M., Clauss M. 2010. Relevance of management and feeding regimens on life expectancy in captive deer. Am. J. Vet. Res. 71, 275–280 10.2460/ajvr.71.3.275 (doi:10.2460/ajvr.71.3.275) [DOI] [PubMed] [Google Scholar]
- 14.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15 10.1086/284325 (doi:10.1086/284325) [DOI] [Google Scholar]
- 15.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884 10.1038/44766 (doi:10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 16.Martins E. P., Hansen T. F. 1997. Phylogenesis and the comperative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667 10.1086/286013 (doi:10.1086/286013) [DOI] [Google Scholar]
- 17.Rohlf F. 2001. Comparative methods for the analysis of continuous variables: geometric interpretations. Evolution 55, 2143–2160 [DOI] [PubMed] [Google Scholar]
- 18.Martins E. 2004. COMPARE, version 4.6. Computer programs for the statistical analysis of comparative data. Department of Biology, Indiana University, Bloomington IN, Distributed by the author at http://compare.bio.indiana.edu/
- 19.Wielebnowski N. 2003. Stress and distress: evaluating their impact for the well-being of zoo animals. J. Am. Vet. Med. Assoc. 223, 973–976 10.2460/javma.2003.223.973 (doi:10.2460/javma.2003.223.973) [DOI] [PubMed] [Google Scholar]
- 20.Clauss M., Kaiser T., Hummel J. 2008. The morphophysiological adaptations of browsing and grazing mammals. In The ecology of browsing and grazing (eds Gordon I. J., Prins H. H. T.), pp. 47–88 Heidelberg, Germany: Springer [Google Scholar]
- 21.Clauss M., Dierenfeld E. S. 2008. The nutrition of browsers. In Zoo and wild animal medicine. Current therapy 6 (eds Fowler M. E., Miller R. E.), pp. 444–454 St Louis, MO: Saunders Elsevier [Google Scholar]
- 22.Clauss M., Lechner-Doll M., Streich W. J. 2003. Ruminant diversification as an adaptation to the physicomechanical characteristics of forage. A reevaluation of an old debate and a new hypothesis. Oikos 102, 253–262 10.1034/j.1600-0706.2003.12406.x (doi:10.1034/j.1600-0706.2003.12406.x) [DOI] [Google Scholar]
- 23.Hummel J., Fritz J., Kienzle E., Medici E. P., Lang S., Zimmermann W., Streich W. J., Clauss M. 2008. Differences in fecal particle size between free-ranging and captive individuals of two browser species. Zoo Biol. 27, 70–77 10.1002/zoo.20161 (doi:10.1002/zoo.20161) [DOI] [PubMed] [Google Scholar]
- 24.Kleen J. L., Hooijer G. A., Rehage J., Noordhuizen J. P. T. M. 2003. Subacute ruminal acidosis (SARA): a review. J. Vet. Med. Ser. A 50, 406–414 10.1046/j.1439-0442.2003.00569.x (doi:10.1046/j.1439-0442.2003.00569.x) [DOI] [PubMed] [Google Scholar]
- 25.Toïgo C., Gaillard M. 2003. Cause of sex-biased adult survival in ungulates: sexual size dimorphism, mating tactic or environment harshness? Oikos 101, 376–384 10.1034/j.1600-0706.2003.12073.x (doi:10.1034/j.1600-0706.2003.12073.x) [DOI] [Google Scholar]
- 26.Jewell P. A. 1997. Survival and behaviour of castrated Soay sheep (Ovis aries) in a feral island population on Hirta, St Kilda, Scotland. J. Zool. 243, 623–636 10.1111/j.1469-7998.1997.tb02806.x (doi:10.1111/j.1469-7998.1997.tb02806.x) [DOI] [Google Scholar]
- 27.Promislow D. E. L. 1992. Costs of sexual selection in natural populations of mammals. Proc. R. Soc. Lond. B 247, 203–210 10.1098/rspb.1992.0030 (doi:10.1098/rspb.1992.0030) [DOI] [Google Scholar]
- 28.Weckerly F. W. 1998. Sexual-size dimorphism: influence of mass and mating systems in the most dimorphic mammals. J. Mammal. 79, 33–52 10.2307/1382840 (doi:10.2307/1382840) [DOI] [Google Scholar]
- 29.Clutton-Brock T. H., Isvaran K. 2007. Sex differences in ageing in natural populations of vertebrates. Proc. R. Soc. B 274, 3097–3104 10.1098/rspb.2007.1138 (doi:10.1098/rspb.2007.1138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WAZA 2003. Responsible reproductive management: guiding principles. In Rigi Symposium, Ramifications of the reproductive management of animals in zoos. Goldau-Rigi, Switzerland: WAZA [Google Scholar]
- 31.Ralls K., Ballou J. D. 1982. Effects of inbreeding on infant mortality in captive primates. Int. J. Primatol. 3, 491–505 10.1007/BF0269347 (doi:10.1007/BF0269347) [DOI] [Google Scholar]
- 32.Ralls K., Ballou J. D. 1982. Effect of inbreeding on juvenile mortality in some small mammal species. Lab. Anim. 16, 159–166 10.1258/002367782781110151 (doi:10.1258/002367782781110151) [DOI] [PubMed] [Google Scholar]
- 33.Ralls K., Brugger K., Ballou J. 1979. Inbreeding and juvenile mortality in small populations of ungulates. Science 206, 1101–1103 10.1126/science.493997 (doi:10.1126/science.493997) [DOI] [PubMed] [Google Scholar]