Abstract
Human babies and other young mammals prefer food odours and flavours of their mother's diet during pregnancy as well as their mother's individually distinctive odour. Newborn mice also prefer the individual odours of more closely related—even unfamiliar—lactating females. If exposure to in utero odorants—which include metabolites from the mother's diet and the foetus's genetically determined individual odour—helps shape the neuroanatomical development of the olfactory bulb, this could influence the perception of such biologically important odours that are preferred after birth. We exposed gene-targeted mice during gestation and nursing to odorants that activate GFP-tagged olfactory receptors (ORs) and then measured the effects on the size of tagged glomeruli in the olfactory bulb where axons from olfactory sensory neurons (OSNs) coalesce by OR type. We found significantly larger tagged glomeruli in mice exposed to these activating odorants in amniotic fluid, and later in mother's milk, as well as significant preferences for the activating odour. Larger glomeruli comprising OSNs that respond to consistently encountered odorants should enhance detection and discrimination of these subsequently preferred odours, which in nature would facilitate selection of palatable foods and kin recognition, through similarities in individual odours of relatives.
Keywords: odour exposure, olfactory bulb, odour preferences, neuroanatomical development, mice
1. Introduction
Babies' preferences for odours of foods their mothers ate during pregnancy and nursing [1–4] and for their own mother's odour [5–7] demonstrated, along with similar preferences of rabbits [8], lambs [9], dogs [10], rats [11,12] and mice [13], that infant mammals respond preferentially to familiar odours they encountered in utero and prior to weaning. At the same time, newborn mouse pups' preferences for the nipple odour of unfamiliar lactating females that are more genetically similar to themselves (whether paternal aunts to unrelated females or distant conspecific to heterospecific females) [14] demonstrated that newborns can assess degrees of genetic relatedness across a broad relatedness continuum [15,16]. Individual odours of more genetically similar conspecifics are perceptually similar compared with odours of less genetically similar conspecifics [15,17]. Rodents from different species and of all ages use this similarity to respond to unfamiliar individuals of differing degrees of genetic relatedness as though they were assessing the extent of the common qualities in their individual odours and then preferring individuals whose odours are more similar to their own [14–16]. Such preferences (for familiar odours and for genetically similar conspecifics' odours) depend on perceptual discriminations among alternative stimuli and then a response based on the relative attractiveness of the distinctive differences. We sought to investigate processes occurring during initial neuroanatomical development of the olfactory system that could prepare young mammals to make the perceptual discriminations underlying the described adaptive responses to odours of unfamiliar conspecifics and also to familiar food and maternal odours.
Odorants entering the nasal cavity through the nose during sniffing or through the mouth (as flavours) during eating are detected by olfactory receptor (OR) proteins in olfactory sensory neurons (OSNs) spread across the olfactory epithelium (OE) [18,19]. In the mouse genome, there are about 1000 different OR genes, each coding for a different type of OR protein [20]. Each OSN expresses only one type of OR protein, which determines the OR identity of the particular OSN [18,19] and which odorants will activate it. ORs are activated by multiple odorants, and odorants activate multiple ORs in different combinations [21]. The chemical composition and concentration of the odorants entering the nasal cavity determines which subsets of OSNs will be activated and how strongly the various types respond. During its development, each OSN extends a single axon that grows, over a period of days, from the OE to the olfactory bulb (OB) where it coalesces with other OSN axons of the same OR type in a neuropil mass, called a glomerulus, and synapses on dendrites of mitral/tufted cells and periglomerular cells [19,22,23]. Although the relative proportions of OSNs of the various OR types (among the millions of OSNs in the OE) are not known, these proportions must (because of differences in the accessibility of particular OR types and the extent to which the entering odorants activate them) influence both the sensation and the ultimate perception of the odour. If the proportions of particular OSN types that are expressed or survive in a mammal's developing olfactory system are affected by odorant exposure during foetal and early postnatal life, each individual—with its unique odour environment—would have its own unique proportion of each type of OSN. This would determine a unique pattern of activation across OSNs when an odorant enters the nose. Likewise, other odorant-exposure-dependent changes in the glomeruli would lead to an individualized, distinctive perception of the odour. Exposure to the individual's own genetically determined and diet-dependent odorous metabolites excreted into the amniotic fluid [5,6,12,24], which bathes the developing nasal cavity and OE, could modulate the proportions of mature OSNs and the neuroanatomical structure of the glomeruli, which would shape the odour perception during subsequent odour exposure. In short, odour exposure would configure the developing olfactory system to optimize perception based on individual experience. Moreover, although the processes may be somewhat different when OSNs are regenerated later in life [19,25], consistent exposure to odorants—such as dietary odours while eating and the individual's own odour while grooming or in the nest—could maintain or modify the proportion of OSN types and the structure of the glomeruli across the lifespan. It has long been suggested that prenatal and early postnatal experience could influence the development of the neural structures underlying observed behavioural effects (e.g. [26,27]), but the hypotheses about what and how this occurs have not previously been formulated or tested as they are here.
Glomeruli are visually distinct in histological sections, and most have acquired the characteristics of the mature olfactory system by the third week after birth [22,23,28]. With the advent of genetic fluorescence tagging [29,30], it became possible to identify a particular glomerulus by the type of OR expressed in the axons projecting to it by co-expression of τGFP with the OR protein, making the fluorescence evident in the glomeruli when they are formed.
In this work, we studied the effects of odour exposure during gestation and prior to weaning on the neuroanatomy of developing glomeruli—the site of the first sensory synapse—in the olfactory bulbs of mice. The findings reported here indicate that glomeruli formed from OSNs that respond to particular activating odorants are larger in mice exposed to these odorants during the initial development of their olfactory system. Our findings using these same odorants also confirm previous findings [1–13] that odour exposure in utero and/or during nursing elicits a preference for that odour at the time of weaning.
2. Methods
(a). Animals
Breeding pairs of gene-targeted mice with either the M71 (M71-IRES-τGFP) or the M72 (M72-IRES-τGFP) olfactory receptor gene tagged with GFP were purchased from Jackson Laboratories and housed in the animal facility at the University of Colorado Denver in a reversed light cycle (11.00 h off; 23.00 h on). The offspring from the initial pairs were used as parents for the pups (M71: n = 46; M72: n = 26) in the experimental litters. For the M71s, there were three litters for each group; for the M72s, owing to breeding difficulties, there was one litter for each group.
(b). Procedure
(i). Odour exposure and experimental design
Genetically determined individual odour constituents in the uterine environment cannot be altered in a natural way, but the composition of dietary metabolites changes with the mother's diet. Thus, for this experiment, we used mice in which OSNs expressing a specific OR express GFP and selected OSN types with receptors that respond strongly to an odorant that can safely be added as a supplemental flavour to the mother's diet: M71 receptors [29,31] are among the receptors activated by acetophenone (Sigma), described as ‘cherry’; M72 receptors [32] are among the receptors activated by isopropyl tiglate (Sigma), described as ‘mint’. Standard flavour laboratory mouse chow (Harlan) was scented (1 ml per 100 g crushed chow) with acetophenone or isopropyl tiglate suspended in filtered water. The flavour-supplemented pellets (reformed in ice cube trays) were dried for 3 days under a fume hood to reduce the odour to a suitable level—as judged by a human nose—before presenting them to the mothers.
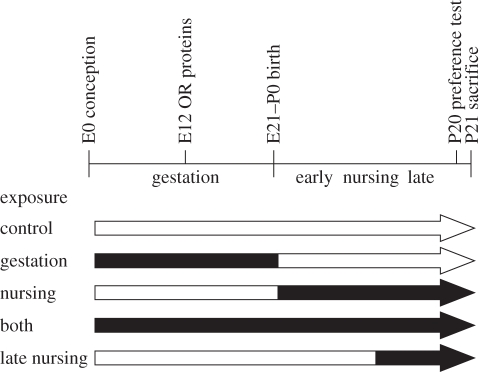
For this initial study, we chose a parsimonious design (figure 1): giving mothers of pups in the four experimental groups a flavour-supplemented diet instead of the standard flavour diet throughout ‘gestation’ (M71: n = 13; M72: n = 7), throughout ‘nursing’ (M71: n = 11; M72: n = 7), throughout ‘both’ pregnancy and nursing (M71: n = 11) or during the last 10 days of ‘late nursing’ (M72: n = 6) and then comparing the sizes of the tagged glomeruli of their pups with those of control group pups (M71: n = 11; M72: n = 6) from mothers that ate the standard diet. (We chose to feed the mothers of the control pups the standard flavour chow rather than chow supplemented with a different flavour that did not activate the tagged glomerulus. This avoids confounding effects from additional odour exposure that cannot be quantified in the untagged glomeruli. This design also enables comparisons of each experimental group with a single control group.)
Figure 1.
Filled portions of the arrows under the developmental timeline indicate when the mother of the tested pups ate the flavour-supplemented diet. OR proteins become evident from E12.
(ii). Preference tests
Pups at postnatal day (P) 20 were tested in clean cages under red light in the first 2 h of their dark phase. One 6 g flavour-supplemented pellet of each type was placed at each end (varied randomly). The time spent sniffing within 1 cm of each pellet during the 3 min test was recorded with stopwatches by experimenters who were blind to the pellet flavours. Pups from the control litters were tested to confirm that there was no intrinsic preference for either flavour-supplemented diet when both flavours were novel. Pups from the experimental litters were tested to determine whether they preferred the flavour their mothers ate to the novel flavour.
(iii). Perfusion
To enable comparison of the effects of odour exposure during gestation and nursing, all pups were sacrificed at three weeks old. Mice (P21) were anaesthetized and perfused transcardially with 4 per cent paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Olfactory bulbs were harvested and post-fixed for 2 h and then transferred to 25 per cent sucrose in phosphate-buffered saline (PBS) for cryoprotection overnight. Brains were embedded in Neg50 (Richard-Allan Scientific) cutting medium on dry ice and sliced at 20 µm on a Leica cryostat at −13°C. Slides were washed (2 × 5 min) with 0.1 M PBS before coverslipping with Flouromount-G (SouthernBiotech).
(c). Data analysis
Fluorescence of axons and their coalescence into glomeruli are readily distinguishable in coronal slices when viewed under 4× magnification. Images of single glomeruli (four per mouse) were captured at 40× on a Nikon Eclipse 600 microscope/camera using Nikon Imaging Software. In ImageJ software, the fluorescent glomerulus in each image was delineated using ‘adjust > threshold’ from the image menu (then delineating the boundary between the glomerulus and the incoming axons using ‘freehand selection’ from the draw menu when necessary), and areas were calculated using ‘analyse particles’ from the analyse menu. The volume (µm3) of each glomerulus was determined by summing the areas of serial coronal sections and multiplying by the depth of the slice (20 µm).
Main effects were assessed with ANOVA, and post hoc comparisons were assessed with Tukey HSD tests using Statistica software. The glomerulus types were analysed separately because the M72s were larger. The initial analysis showed a main effect of litter on weight and glomerular volume. This was because the average weight of pups in the control groups was significantly higher than that of pups in the gestation groups. We did not consider this an issue because if higher weight had been associated with larger glomeruli, this would have worked against our finding significantly larger glomeruli in the gestation group. Although the findings are comparable either way at the reported level of precision, the weight range was broad enough (6–12.6 g) that we normalized volumes by multiplying the individual glomerulus volume by the average animal weight (8.9 g) and dividing by the individual animal's weight. There were no differences in the volumes of medial versus lateral glomeruli or those in the left or right OB. There were no differences in the findings when using the average volume per group or average volume per mouse in the group as the statistical unit. (Because the M71 glomerulus matures later than the M72 [28], there was a possibility of finding doublets in M71 mice sacrificed at weaning. We excluded the 7.2 per cent (n = 13) doublets found in the M71 pups from the analysis because we could not be sure that the combined volume of the doublets was comparable to that of the single glomeruli. There was one doublet in the control group and the other 12 doublets were distributed across the three treatment groups, but the sample was too small to find statistically significant differences in occurrences of doublets across groups (F3,172 = 0.835, p = 0.4765).) We used non-parametric Wilcoxon matched pairs tests to analyse the preference data because the data were not normally distributed.
3. Results and discussion
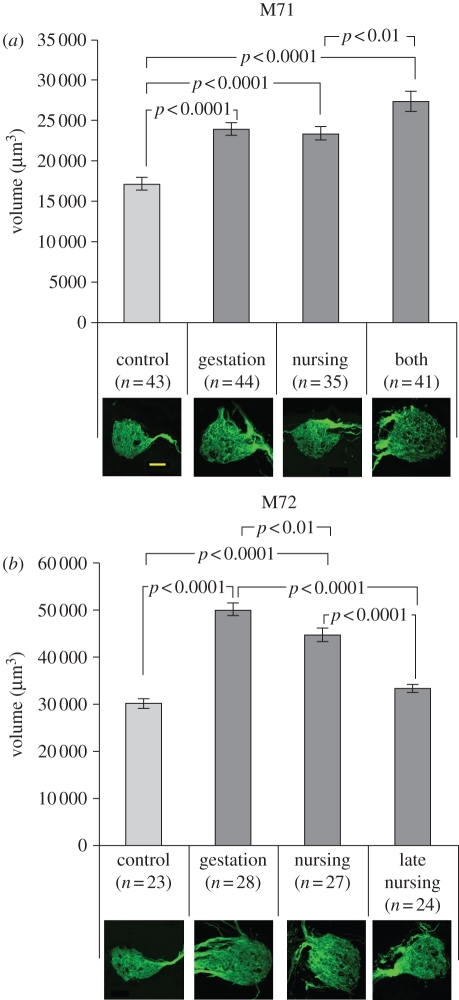
By exposing foetal and pre-weaning mouse pups with GFP-tagged OSNs to odorants that activate the tagged receptors and measuring the effect of this exposure on the sizes of developing glomeruli where these OSN axons coalesce, we have shown that odour exposure in utero and/or during the first few weeks after birth shapes neuroanatomical development in the olfactory system. The fluorescent glomeruli of three-week-old mice whose mothers had eaten lightly flavour-supplemented chow during gestation or nursing or both (figure 1) were significantly larger than those whose mothers had eaten the standard flavour chow (figure 2a,b; ANOVA: M71: F3,159 = 22.57, p = 0.000001; M72: F3,98 = 55.20, p = 0.000001), demonstrating the efficacy of odour exposure in modulating the neuroanatomical development of both types of tagged glomeruli. For M72s, the effects were significantly larger in pups exposed to mint during gestation than during nursing (figure 2b), suggesting that, although OR proteins are expressed during the last 10 embryonic days [33], exposure to odorants in the womb could be decisive in establishing the dimensions of the developing glomeruli. Fluorescent glomeruli of M71 pups exposed to cherry during gestation plus nursing were not significantly larger than those exposed only during gestation (figure 2a), further emphasizing the importance of in utero exposure. Odour exposure for just the last 10 days of nursing did not significantly affect the size of fluorescent glomeruli compared with controls (although the average weight of pups in the late nursing group was significantly higher than that of the control pups), and these glomeruli were significantly smaller than those of pups exposed throughout nursing (figure 2b), either pointing to a perinatal critical period in OSN/glomerular development or indicating that 10 days of odour exposure just prior to performing histology is not sufficient to establish increases in the volume of glomeruli.
Figure 2.
Mean (±s.e.m.) glomerulus volume (µm3), normalized to average weight pup. (a,b) Mothers of ‘control’ pups ate standard flavour chow (light grey bars); mothers of treatment pups ate flavour-supplemented chow (dark grey bars) during gestation, nursing or both. Late nursing mothers ate flavour-supplemented chow for the last 10 days of nursing. Cross-sections of exemplary GFP-tagged glomeruli from each group appear below each bar. Note that there are more OSN axons entering the glomeruli in the treated than the control groups. The scale bar (20 µm) applies to all images.
It is reasonable to infer, although it could not be measured, that the odour-exposure-induced increases in glomerular size occurred in all the glomeruli that were activated by the supplemental odorant and not just the tagged glomeruli. It is a logical inference, noting again that all odorants activate multiple glomeruli and that all glomeruli are activated by multiple odorants, and that this odour-exposure-dependent neuroanatomical tuning occurs broadly across the OE and OB irrespective of the odour source. Because glomeruli formed by OSNs that are activated by consistently encountered odorants become larger than glomeruli that do not receive such activation and because each individual foetus or infant encounters a unique combination of odorants—including metabolites from the mother's diet and its own genetically determined metabolites—the relative sizes of the 1000 types of glomeruli in each individual's developing OB depend to some extent on the relative presence of odorants that activate their constituent OSNs. Individually distinctive combinations of odorants during development help configure the olfactory system by establishing individually distinctive patterns of differentially sized glomeruli.
There are a number of possible explanations for the odour-exposure-induced increase in glomerular volume. A glomerulus could become larger because there are more OSNs in the OE with axons projecting to it. During foetal OSN development, growing dendritic knobs reach the OE surface in the nasal cavity before OR proteins are evident in the cell, but they are detectable in the dendritic knobs before the cilia start growing the following day [33]. This developmental sequence, which also must occur later in life during OSN regeneration and replacement [25], could allow exposure to environmental odorants to influence which OR gene is expressed and the functional identity of the maturing OSN, as has been suggested in adult mice [34]. It is also possible that OR gene expression is determined by intrinsic genomic factors [35,36] and that differential odour exposure during their continued development affects their survival as the OSN axons grow from the epithelium to the glomerulus [37]. Another possibility is that activation through odour exposure modulates branching of the axon terminals once they reach the glomerulus [38]. Activation could also enhance dendritic arborizations of mitral cells in the glomerulus [39]. Future studies will be necessary to distinguish among these and other possibilities and thus to shed additional light on the exact processes by which odour exposure determines the proportions of OSN types in the individual's OE and/or the sizes of developing glomeruli. Nonetheless, it is clear from the results of the gestation groups in comparison with the controls (figure 2a,b) that exposure to activating odorants makes a substantial contribution to the increase in the size of the glomerulus even though it reaches maturity two or more weeks after birth [22,23,28]. Ongoing studies in our laboratory are also addressing the longevity of the effects of odour exposure during gestation in older mice that either have or have not experienced the activating odour after birth and the effects of constant versus intermittent odour exposure during other stages of the life cycle.
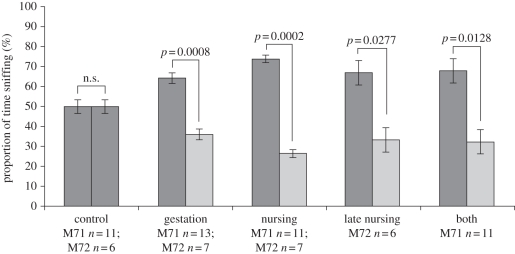
Pups at P20 whose mothers had eaten the standard flavour chow showed no preference between the two flavour-supplemented chows (figure 3). In contrast, P20 pups from both mouse lines whose mothers had eaten flavour-supplemented chow during gestation, nursing or both preferred the odour of their mother's diet to a differently flavoured diet (figure 3). Note that this preference was robust even if their only exposure to the odour had been during gestation and thus they had not had any opportunity to smell the odour after birth (figure 3). Interestingly, the pups in the late nursing group showed a clear preference for the familiar odour even though their tagged glomeruli were not significantly bigger than those of the control group, indicating that preferences for familiar food odours over readily discriminable novel food odours can be established in the absence of enhanced glomerular volume. The preferences described here are consistent with those reported in previous studies of prenatal and early postnatal learning [1–3,8–11]. The association between odour or flavour exposure and preferences for those odours or flavours appears robust, but the neurocircuitry connecting the stimulus to the response has never been clear. In the gestation, nursing and both groups, the preferences parallel increased volume of tagged glomeruli that are activated by the exposed flavour, but the late nursing group showed a significant preference without a significant increase in the volume of the tagged glomeruli (figures 2 and 3). This suggests that odour or flavour preferences can be established before the odour exposure has enhanced the volume of the glomeruli, uncoupling the preferential response and the perception of the odour stimulus and raising new questions about the role of a process that configures the neuroanatomy through odour exposure during initial development.
Figure 3.
Odour exposure elicits odour preferences. Mean (± s.e.m.) proportion of time during a 3 min preference test that pups spent investigating the cherry- and mint-flavoured pellets. Pups that had not been exposed to either flavour showed no preference (grey bars). Pups whose mothers had eaten a flavoured diet (cherry for M71s; mint for M72s) during gestation, nursing or both significantly preferred the flavour their mother ate (dark grey bars) to the novel flavour—mint for M71s; cherry for M72s (light grey bars).
We had hypothesized that exposure to odorants in the womb (and later prior to weaning) may regulate the maturation of the sensory apparatus in each individual's developing olfactory system so that it becomes especially sensitive to consistently encountered in utero odorants. These odorants would be a unique combination of the growing foetus's excreted genetically determined ‘individual odour’ metabolites and the mother's dietary metabolites [5,6,12,24]. Such enhanced sensitivity could help young mammals make more finely tuned discriminations [40–43] among odours of conspecifics and familiar foods, enabling them to demonstrate more subtle preferential responses. Although the results of the current study do not address direct connections between the reported odour-exposure-modulated neuroanatomical changes during olfactory system development and enhanced sensitivity to the activating odours, it is possible to draw some inferences by putting our results in the context of findings from other studies. A previous study in rabbit pups linked postnatal preferences for the odour of juniper berries with increased electro-olfactogram (EOG) activity in the OE to juniper berry odour after in utero exposure to the odour through the mother's juniper berry-supplemented diet [8]. This suggests not only that in utero odour exposure enhances sensitivity but also that the effects are evident in the OE as well as in the OB. There is more substantial evidence of such connections in adult mammals. Adenovirus-driven OR gene expression in adult rats led to greater EOG responsiveness in the OE to the exposed odorants [44]. In humans, the enhanced EOG response following repetitive odour exposure was also significantly correlated with lower detection thresholds [45]. Previous studies with adult M71 GFP-tagged mice showed an increase in the number of M71 OSNs in the OE and larger M71 glomeruli after exposure to the odour of acetophenone [31]. In addition, in mice and humans, consistent odour exposure has enhanced both the sensitivity to those odorants [34,40,45–48] and the discriminability of similar odours [41–42]. This leads us to speculate (recognizing the complexity of the underlying neurocircuitry) that having larger glomeruli following consistent odorant exposure (figure 2) facilitates both detection and discrimination of odorants that activate them.
The enhanced sensitivity and discriminability afforded by larger glomeruli would not be necessary (or obvious) in distinctions between readily discriminable odours, particularly if one of the odours is familiar and the other novel, as was the case in previous studies [1–3,8–11] and in the preference tests reported here, for example in the late nursing group, between the familiar mint-scented chow and the unfamiliar cherry-scented chow. The enhanced sensitivity and discriminability would be advantageous, however, in subtle distinctions between similar, unfamiliar odours such as those made by newborn mouse pups that were differentially attracted to the nipple odour of their lactating paternal aunt over that of an unrelated lactating female [14]. Additional studies will be necessary to reveal whether these speculations are correct.
Whatever the precise circuitry from odour detection through discrimination to preferential response may be, it would usually be adaptive to shape the neuroanatomy of the developing olfactory system to enhance the perceptual sensitivity to in utero odorants if this sensitivity contributes in any way to facilitating subtle odour preferences. The mother's dietary metabolites typically indicate the safety as well as the availability of foods in the environment. Enhanced sensitivity to their odours would be advantageous in locating these foods and distinguishing them from similar but less suitable items, and inborn preferences should promote acceptance of these available palatable foods after weaning. In humans, enhanced sensitivity and inborn preferences may not always be advantageous, however, because even when ‘available’ and ‘palatable’ do not equate with ‘healthy’ foods, the mother's food choices may influence her unborn child's preferences nonetheless. This could explain how the process can go awry, leading to maladaptive preferences, such as the attraction to ethanol odour and flavour in humans and other mammals [49] whose mothers consumed alcohol during pregnancy. It is sobering to recognize from the findings presented here that odour exposure during gestation and nursing affects not only early flavour learning [50] but the neuroanatomical development of the olfactory system as well.
The described tuning of the developing olfactory system is certainly advantageous for the genetic relatedness assessment mechanism underlying newborn mouse pups' attraction to the odour of more genetically similar females [14]: the constant presence of the foetus's genetically determined individual odour metabolites in the amniotic fluid bathing the nasal cavity should enhance the size of glomeruli of OSNs that are activated by these metabolites. These larger glomeruli should enhance the newborn or infant's ability to detect and discriminate between subtle differences in conspecifics' individual odours, which are composed of genetically determined odorous metabolites that are similar to their own but in differing proportions (see discussion in [15]). The greater the overlap between the newborn's odour and the odour of the encountered individual, the stronger the sensory activation would be when the newborn smells the other's odour. Thus, stronger sensation from receptors in the network of glomeruli that respond to individual odours would indicate closer genetic relatedness of the encountered individual, and subsequent perceptual distinctions could facilitate the process of using individual odour similarities to assess degrees of genetic relatedness demonstrated in newborn [14] and adult mice [16] and other rodents [15]. If this enhanced sensitivity could be linked—in some yet to be determined way—to differential attraction, it could also facilitate an adaptive preferential choice. Perhaps human babies [5,6] and rodent pups [12] prefer their mother's odour not simply because it is familiar but because her proportion of genetically determined odorous compounds, being more similar to their own, provides a better activating match in their olfactory system. In any case, the reported findings help clarify genetic relatedness assessment (kin recognition) mechanisms [15] by suggesting a likely neuroanatomical basis of differential individual odour perception that occurs at the sensory periphery.
Two of the most important decisions that animals make are what to eat and with whom to affiliate. Previous research has confirmed the importance of odour memory in differential responses to familiar as opposed to novel foods and individuals. It is now clear that the neuroanatomical development of the olfactory system enables distinctions, even early in life, at the sensory level—apart from odour learning—that could affect both these types of choices. The mechanisms that fine-tune the neural circuitry in the olfactory bulb and the higher cortical processes through which these sensory discriminations are manifest in adaptive behaviours await further elucidation. Nonetheless, the new conceptualization of the interaction between environmental odours and neuroanatomical development and the behavioural implications of the individualized tuning tested and reported here represent a substantial advance in understanding the importance of experience in the concurrent shaping of neuroanatomy and behaviour.
Acknowledgements
Animals were maintained in accordance with NIH and institutional guidelines.
We thank M. Block, N. Busquet, T. Finger and S. Wagner for helpful discussions and comments on an earlier version of the manuscript and K. Magee for assistance in animal breeding and care. This study was supported by National Institute of Health Award numbers F33DC009137 (J.T.), DC04657 (D.R.) and DC006070 (D.R.).
References
- 1.Schaal B., Marlier L., Soussignan R. 2000. Human foetuses learn odours from their pregnant mother's diet. Chem. Senses 25, 729–737 10.1093/chemse/25.6.729 (doi:10.1093/chemse/25.6.729) [DOI] [PubMed] [Google Scholar]
- 2.Mennella J. A., Jagnow C. P., Beauchamp G. K. 2001. Prenatal and postnatal flavor learning by human infants. Pediatrics 107, e88. 10.1542/peds.107.6.e88 (doi:10.1542/peds.107.6.e88) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hepper P. G. 1995. Human fetal olfactory learning. Int. J. Prenatal Perinatal Psychol. Med. 7, 147–151 [Google Scholar]
- 4.Mennella J. A., Beauchamp G. K. 1991. Maternal diet alters the sensory qualities of human milk and the nursling's behavior. Pediatrics 88, 737–744 [PubMed] [Google Scholar]
- 5.Schaal B., Marlier L., Soussignan R. 1998. Olfactory function in the human fetus: evidence from selective neonatal responsiveness to the odor of amniotic fluid. Behav. Neurosci. 112, 1438–1449 10.1037/0735-7044.112.6.1438 (doi:10.1037/0735-7044.112.6.1438) [DOI] [PubMed] [Google Scholar]
- 6.Marlier L., Schaal B., Soussignan R. 1998. Neonatal responsiveness to the odor of amniotic and lacteal fluids: a test of perinatal chemosensory continuity. Child Dev. 69, 611–623 [PubMed] [Google Scholar]
- 7.Porter R. H., Winberg J. 1999. Unique salience of maternal breast odors for newborn infants. Neurosci. Biobehav. Rev. 23, 439–449 10.1016/S0149-7634(98)00044-X (doi:10.1016/S0149-7634(98)00044-X) [DOI] [PubMed] [Google Scholar]
- 8.Semke E., Distel H., Hudson R. 1995. Specific enhancement of olfactory receptor sensitivity associated with foetal learning of food odours in the rabbit. Naturwissenschaften 82, 148–149 10.1007/BF01177279 (doi:10.1007/BF01177279) [DOI] [PubMed] [Google Scholar]
- 9.Schaal B., Orgeur P., Arnould C. 1995. Olfactory preferences in newborn lambs: possible influence of prenatal experience. Behaviour 132, 351–365 10.1163/156853995X00603 (doi:10.1163/156853995X00603) [DOI] [Google Scholar]
- 10.Wells D. L., Hepper P. G. 2006. Prenatal olfactory learning in the domestic dog. Anim. Behav. 72, 681–686 10.1016/j.anbehav.2005.12.008 (doi:10.1016/j.anbehav.2005.12.008) [DOI] [PubMed] [Google Scholar]
- 11.Hepper P. G. 1988. Adaptive fetal learning: prenatal exposure to garlic affects postnatal preferences. Anim. Behav. 36, 935–936 10.1016/S0003-3472(88)80177-5 (doi:10.1016/S0003-3472(88)80177-5) [DOI] [Google Scholar]
- 12.Hepper P. G. 1987. The amniotic fluid: an important priming role in kin recognition. Anim. Behav. 35, 1343–1346 10.1016/S0003-3472(87)80006-4 (doi:10.1016/S0003-3472(87)80006-4) [DOI] [Google Scholar]
- 13.Nolte D. L., Mason J. R. 1995. Maternal ingestion of ortho-aminoacetophenone during gestation affects intake by offspring. Physiol. Behav. 58, 925–928 10.1016/0031-9384(95)00152-9 (doi:10.1016/0031-9384(95)00152-9) [DOI] [PubMed] [Google Scholar]
- 14.Todrank J., Busquet N., Baudoin C., Heth G. 2005. Preferences of newborn mice for odours indicating closer genetic relatedness: is experience necessary? Proc. R. Soc. B 272, 2083–2088 10.1098/rspb.2005.3187 (doi:10.1098/rspb.2005.3187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todrank J., Heth G. 2003. Odor-genes covariance and genetic relatedness assessments: rethinking odor-based ‘recognition’ mechanisms in rodents. Adv. Study Behav. 32, 77–130 10.1016/S0065-3454(03)01002-7 (doi:10.1016/S0065-3454(03)01002-7) [DOI] [Google Scholar]
- 16.Heth G., Todrank J., Busquet N., Baudoin C. 2003. Genetic relatedness assessment through individual odour similarities (G-ratios) in mice. Biol. J. Linn. Soc. 78, 595–603 10.1046/j.0024-4066.2002.00194.x (doi:10.1046/j.0024-4066.2002.00194.x) [DOI] [Google Scholar]
- 17.Tzur S., Todrank J., Juergens A., Nevo E., Heth G. 2009. Odour-genes covariance within a natural population of subterranean Spalax galili blind mole rats. Biol. J. Linn. Soc. 96, 483–490 10.1111/j.1095-8312.2008.01155.x (doi:10.1111/j.1095-8312.2008.01155.x) [DOI] [Google Scholar]
- 18.Firestein S. 2001. How the olfactory system makes sense of scents. Nature 413, 211–218 10.1038/35093026 (doi:10.1038/35093026) [DOI] [PubMed] [Google Scholar]
- 19.Mombaerts P. 2006. Axonal wiring in the mouse olfactory system. Annu. Rev. Cell Dev. Biol. 22, 713–737 10.1146/annurev.cellbio.21.012804.093915 (doi:10.1146/annurev.cellbio.21.012804.093915) [DOI] [PubMed] [Google Scholar]
- 20.Godfrey P. A., Malnic B., Buck L. B. 2004. The mouse olfactory receptor gene family. Proc. Natl Acad. Sci. USA 101, 2156–2161 10.1073/pnas.0308051100 (doi:10.1073/pnas.0308051100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malnic B., Hirono J., Sato T., Buck L. B. 1999. Combinatorial receptor codes for odors. Cell 96, 713–723 10.1016/S0092-8674(00)80581-4 (doi:10.1016/S0092-8674(00)80581-4) [DOI] [PubMed] [Google Scholar]
- 22.Treloar H. B., Purcell A. L., Greer C. A. 1999. Glomerular formation in the developing rat olfactory bulb. J. Comp. Neurol. 413, 289–304 (doi:10.1002/(SICI)1096-9861(19991018)413:2<289::AID-CNE9>3.0.CO;2-U) [DOI] [PubMed] [Google Scholar]
- 23.Kim H., Greer C. A. 2000. The emergence of compartmental organization in olfactory bulb glomeruli during postnatal development. J. Comp. Neurol. 422, 297–311 (doi:10.1002/(SICI)1096-9861(20000626)422:2<297::AID-CNE10>3.0.CO;2-M) [DOI] [PubMed] [Google Scholar]
- 24.Mennella J. A., Johnson A., Beauchamp G. K. 1995. Garlic ingestion by pregnant women alters the odor of amniotic fluid. Chem. Senses 20, 207–209 10.1093/chemse/20.2.207 (doi:10.1093/chemse/20.2.207) [DOI] [PubMed] [Google Scholar]
- 25.Farbman A. I. 1990. Olfactory neurogenesis: genetic or environmental controls? Trends Neurosci. 13, 362–365 10.1016/0166-2236(90)90017-5 (doi:10.1016/0166-2236(90)90017-5) [DOI] [PubMed] [Google Scholar]
- 26.Nijhuis J. G. (ed.) 1992. Fetal behaviour, developmental and perinatal aspects. Oxford, UK: Oxford University Press [Google Scholar]
- 27.Lecanuet J.-P., Fifer W. P., Krasnegor N. A., Smotherman W. P. (eds) 1995. Fetal development, a psychobiological perspective. Hillsdale, NJ: Lawrence Erlbaum Associates [Google Scholar]
- 28.Zou D.-J., Feinstein P., Rivers A. L., Mathews G. A., Kim A., Greer C. A., Mombaerts P., Firestein S. 2004. Postnatal refinement of peripheral olfactory projections. Science 304, 1976–1979 10.1126/science.1093468 (doi:10.1126/science.1093468) [DOI] [PubMed] [Google Scholar]
- 29.Bozza T., Feinstein P., Zheng C., Mombaerts P. 2002. Odorant receptor expression defines functional units in the mouse olfactory system. J. Neurosci. 22, 3033–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter S. M., Zheng C., Koos D. S., Feinstein P., Fraser S. E., Mombaerts P. 2001. Structure and emergence of specific olfactory glomeruli. J. Neurosci. 21, 9713–9723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones S. V., Choi D. C., Davis M., Ressler K. J. 2008. Learning-dependent structural plasticity in the adult olfactory pathway. J. Neurosci. 28, 13 106–13 111 10.1523/JNEUROSCI.4465-08.2008 (doi:10.1523/JNEUROSCI.4465-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soucy E. R., Albeanu D. F., Fantana A. L., Murthy V. N., Meister M. 2009. Precision and diversity in an odor map on the olfactory bulb. Nat. Neurosci. 12, 210–220 10.1038/nn.2262 (doi:10.1038/nn.2262) [DOI] [PubMed] [Google Scholar]
- 33.Schwarzenbacher K., Fleischer J., Breer H. 2005. Formation and maturation of olfactory cilia monitored by odorant receptor-specific antibodies. Histochem. Cell Biol. 123, 419–428 10.1007/s00418-005-0790-5 (doi:10.1007/s00418-005-0790-5) [DOI] [PubMed] [Google Scholar]
- 34.Wang H. W., Wysocki C. J., Gold G. H. 1993. Induction of olfactory receptor sensitivity in mice. Science 260, 998–1000 10.1126/science.8493539 (doi:10.1126/science.8493539) [DOI] [PubMed] [Google Scholar]
- 35.Serizawa S., Miyamichi K., Sakano H. 2004. One neuron-one receptor rule in the mouse olfactory system. Trends Genet. 20, 648–653 10.1016/j.tig.2004.09.006 (doi:10.1016/j.tig.2004.09.006) [DOI] [PubMed] [Google Scholar]
- 36.Imai T., Sakano H. 2008. Odorant receptor-mediated signaling in the mouse. Curr. Opin. Neurobiol. 18, 251–260 10.1016/j.conb.2008.07.009 (doi:10.1016/j.conb.2008.07.009) [DOI] [PubMed] [Google Scholar]
- 37.Watt W. C., Sakano H., Lee Z.-Y., Reusch J. E., Trinh K., Storm D. R. 2004. Odorant stimulation enhances survival of olfactory sensory neurons via MAPK and CREB. Neuron 41, 955–967 10.1016/S0896-6273(04)00075-3 (doi:10.1016/S0896-6273(04)00075-3) [DOI] [PubMed] [Google Scholar]
- 38.Gong Q., Chen H., Farbman A. I. 2009. Olfactory sensory axon growth and branching is influenced by sonic hedgehog. Dev. Dyn. 238, 1768–1776 10.1002/dvdy.22005 (doi:10.1002/dvdy.22005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imamura F., Greer C. A. 2009. Dendritic branching of olfactory bulb mitral and tufted cells: regulation by TrkB. PLoS ONE 4, e6729. 10.1371/journal.pone.0006729 (doi:10.1371/journal.pone.0006729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandairon N., Stack C., Kiselycznyk C., Linster C. 2006. Broad activation of the olfactory bulb produces long-lasting changes in odor perception. Proc. Natl Acad. Sci. USA 103, 13 543–13 548 10.1073/pnas.0602750103 (doi:10.1073/pnas.0602750103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandairon N., Stack C., Kiselycznyk C., Linster C. 2006. Enrichment to odors improves olfactory discrimination in adult rats. Behav. Neurosci. 120, 173–179 10.1037/0735-7044.120.1.173 (doi:10.1037/0735-7044.120.1.173) [DOI] [PubMed] [Google Scholar]
- 42.Mandairon N., Stack C., Linster C. 2006. Olfactory enrichment improves recognition of individual components in mixtures. Physiol. Behav. 89, 379–384 10.1016/j.physbeh.2006.07.013 (doi:10.1016/j.physbeh.2006.07.013) [DOI] [PubMed] [Google Scholar]
- 43.Moreno M. M., Linster C., Escanilla O., Sacquet J., Didier A., Mandairon N. 2009. Olfactory perceptual learning requires adult neurogenesis. Proc. Natl Acad. Sci. USA 106, 17 980–17 985 10.1073/pnas.0907063106 (doi:10.1073/pnas.0907063106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao H., Ivic L., Otaki J. M., Hashimoto M., Mikoshiba K., Firestein S. 1998. Functional expression of a mammalian odorant receptor. Science 279, 237–242 10.1126/science.279.5348.237 (doi:10.1126/science.279.5348.237) [DOI] [PubMed] [Google Scholar]
- 45.Wang L., Chen L., Jacob T. 2003. Evidence for peripheral plasticity in human odour response. J. Physiol. 554, 236–244 10.1113/jphysiol.2003.054726 (doi:10.1113/jphysiol.2003.054726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wysocki C. J., Dorries K. M., Beauchamp G. K. 1989. Ability to perceive androstenone can be acquired by ostensibly anosmic people. Proc. Natl Acad. Sci. USA 86, 7976–7978 10.1073/pnas.86.20.7976 (doi:10.1073/pnas.86.20.7976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalton P., Doolittle N., Breslin P. A. S. 2002. Gender-specific induction of enhanced sensitivity to odors. Nat. Neurosci. 5, 199–200 10.1038/nn803 (doi:10.1038/nn803) [DOI] [PubMed] [Google Scholar]
- 48.Yee K. K., Wysocki C. J. 2001. Odorant exposure increases olfactory sensitivity: olfactory epithelium is implicated. Physiol. Behav. 72, 705–711 10.1016/S0031-9384(01)00428-0 (doi:10.1016/S0031-9384(01)00428-0) [DOI] [PubMed] [Google Scholar]
- 49.Molina J. C., Spear N. E., Spear L. P., Mennella J. A., Lewis M. J. 2007. The International Society for Developmental Psychobiology 39th Annual Meeting symposium: alcohol and development: beyond fetal alcohol syndrome. Dev. Psychobiol. 49, 227–242 10.1002/dev.20224 (doi:10.1002/dev.20224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beauchamp G. K., Mennella J. 2009. Early flavor learning and its impact on later feeding behavior. J. Pediatr. Gastroenterol. Nutr. 48, S25–S30 10.1097/MPG.0b013e31819774a5 (doi:10.1097/MPG.0b013e31819774a5) [DOI] [PubMed] [Google Scholar]