Abstract
Host genetic factors responsible for the interindividual differences in naturally occurring antibody responses to the human epidermal growth factor receptor 2 (HER-2) in humans have not been identified. The aim of the present investigation was to determine whether GM and KM allotypes — genetic markers of IgG heavy chains and κ-type light chains, respectively — contribute to these differences. A total of 152 Estonian women with breast cancer were characterized for IgG antibodies to HER-2 and allotyped for several GM and KM markers. IgG3 determinant GM 13 was significantly associated with higher HER-2 IgG levels (median IgG titer 800 vs. 400, p = 0.007). Other GM allotypes, known to be in linkage disequilibrium with GM 13, were also associated with higher anti-HER-2 antibody levels, albeit not as strongly. These results show that GM allotypes are associated with humoral immunity to HER-2, a finding with potentially significant implications for immunotherapy of breast cancer.
Keywords: breast cancer, HER-2, humoral immune response, genetic regulation, GM allotypes, KM allotypes, IgG antibodies
1. Introduction
Human epidermal growth factor receptor (HER-2) protooncogene is amplified in 25 to 30% of breast cancer patients and is associated with poor prognosis. High titer antibodies to the HER-2 protein are an effective means of tumor control, as evidenced by the success of trastuzumab — a recombinant humanized monoclonal antibody directed against HER-2—in HER-2 overexpressing breast cancer. Such therapeutic antibodies could potentially be generated by active immunization, which would circumvent many of the problems associated with passive antibody therapy, including the build-up of resistance. Designing an effective vaccine would, however, require a better understanding of the genetic and immunological factors that influence natural immunity to tumor-associated antigens. There are significant interindividual differences in naturally occurring antibody responses to HER-2 [reviewed in ref. #1]. Studies using human HER-2 transgenic mice suggest that the generation of anti-HER-2 antibody responses is genetically controlled [2], but as yet no host genetic factors that might contribute to the differences in immune responsiveness in humans have been identified. The aim of the present investigation was to determine whether particular alleles of a major gene complex of the immune system, GM allotypes—which, individually and/or epistatically with KM allotypes, have been shown to influence antibody responses to some tumor-associated antigens [3–5]—are also associated with the level of naturally occurring antibodies to HER-2 in patients with breast cancer.
2. Patients and methods
Serum samples were obtained before treatment from 152 patients (median age, 57 years; range, 33–85) with histologically verified breast cancer diagnosed at the Cancer Center of the North Estonian Regional Hospital. The number of subjects in various stages (pathologic tumor-node-metastasis classification of malignant tumors), were as follows: I (25), IIa (45), IIb (42), IIIa (29), IIIb (10), IV (1). All subjects were Estonians. The study protocol was approved by the Medical Research Ethics Committee/IRB of the respective institutions. All subjects provided informed consent.
A recombinant 43.4 kDa protein of 397 amino acids from human herstatin, produced in E. coli, was used as a source of HER-2 antigen (ProSpec-Tany TechnoGene Ltd., Rehovot, Israel). The aminoacid residues in this recombinant protein are identical to those in the extracellular domains (I and II) of HER-2. Ninety-six-well flat bottom microtiter plate wells were coated with 100ul (100ng/ml) of human HER-2 in bicarbonate buffer, pH 9.6, and incubated at 37°C for 1 hr. Wells were washed 5 times with phosphate buffered saline containing 0.05% tween 20 (PBS-T) and unbound sites were blocked with 1% BSA in PBS-T (BSA-PBST). Wells were again washed and incubated with twofold dilutions of serum from breast cancer patients (1:100 to 1:6400) in BSA-PBST and incubated for 1 hr at 37°C. Wells incubated with BSA-PBST alone (without patients’ serum) were used as blank. Wells were washed further and incubated with anti-human IgG HRP conjugate for 30 min at 37°C. After final wash, 100ul HRP substrate, hydrogen peroxide, along with TMB as chromagenic substrate were added to each well and incubated in the dark at room temperature for 20 minutes. The reaction was stopped by the addition of 100ul of 2N HCl and the absorbance values were measured at 495 nm in an ELISA reader. Anti-HER-2 antibody titer was expressed as the highest dilution of the patient serum that gave an absorbance value above the background value (BSA-PBST).
Serum samples were typed for G1M (1/a, 2/x, 3/f, 17/z), G2M (23/n), G3M (5/b1, 6/c3, 13/b3, 21/g), and KM 1 and 3 allotypes by a standard hemagglutination-inhibition method [6]. In brief, a mixture containing human blood group ORh+ erythrocytes coated with anti-Rh antibodies of known GM/KM allotypes, the test sera, and monospecific anti-allotype antibodies were incubated in a microtiter plate. Test sera containing IgG of particular allotype inhibited hemagglutination by the anti-allotype antibody, whereas negative sera did not. Allotyping reagents were purchased from the Central Laboratory of the Netherlands in Amsterdam and from the Université Paul Sabatier, Toulouse, France. Some GM markers (e.g. GM 10, 11, 26, 27) could not be typed due to the scarcity/unavailability of typing reagents. However, because of almost absolute linkage disequilibrium between particular GM allotypes within a race, not all markers need to be typed to determine the haplotypes responsible for a phenotype. The GM markers determined in this investigation are sufficient to detect the major haplotypes segregating in various human populations.
Nonparametric Wilcoxon rank sum tests were used to determine whether there were statistically significant differences in antibody levels corresponding to different allotypes. Statistical significance was defined as p < 0.05 (two-sided).
3. Results and discussion
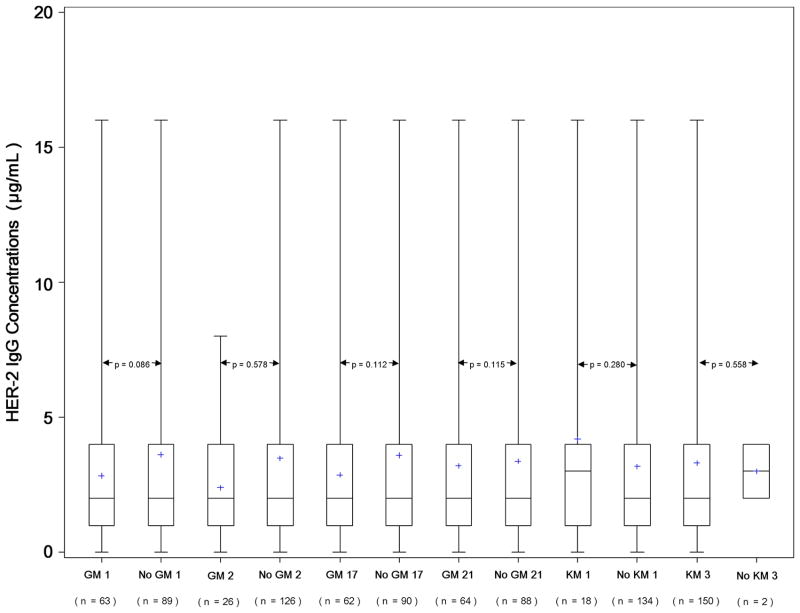
The distribution of GM phenotypes among the 152 study subjects in relation to the median levels (titer) of IgG antibodies to HER-2 is given in Table 1. Figure 1 presents boxplots showing the distribution of HER-2 IgG antibody levels corresponding to the presence and absence of IgG1 (GM 1, GM 2, GM 17) and IgG3 (GM 21) allotypes that are known to be in significant linkage disequilibrium in Caucasians [7–9]. It also presents the antibody levels corresponding to KM (1,3) allotypes. None of the GM or KM allotypes were associated with HER-2 IgG levels.
Table 1.
Distribution (number of subjects) of GM phenotypes in relation to levels (titer) of IgG antibodies to HER-2 in patients with breast cancer
| GM Phenotypes | Number of Subjects | Antibody titer Median (IQR) |
|---|---|---|
| 1,17 13,21 | 1 | 6400 (6400–6400) |
| 1,17 21 | 4 | 300 (150–400) |
| 1,2,17 21 | 5 | 400 (400–800) |
| 1,2,3,17 23 5,13,21 | 11 | 1600 (400–1600) |
| 1,2,3,17 5,13,21 | 8 | 1200 (400–1600) |
| 1,2,3,17 21 | 1 | 400 (400–400) |
| 1,2,3 23 5,13,21 | 1 | 400 (400–400) |
| 1,3,17 23 5,13,21 | 15 | 800 (200–1600) |
| 1,3,17 5,13,21 | 17 | 800 (400–1600) |
| 3 23 5,13 | 77 | 800 (400–1600) |
| 3 23 5,13,21 | 1 | 1600 (1600–1600) |
| 3 5,13 | 11 | 800 (200–1600) |
| Total | 152 | 800 (400–1600) |
IQR: Interquartile range
Fig. 1.
Boxplots of HER-2 IgG levels and GM 1, GM 2, GM 17, GM 21, KM 1, and KM 3 allotypes. Bold horizontal lines indicate the median IgG levels corresponding to the presence or absence of particular allotype, while boxes are indicative of the interquartile ranges. Dotted lines extend from the 5th to the 95th percentiles, and plus (+) signs indicate minima and maxima.
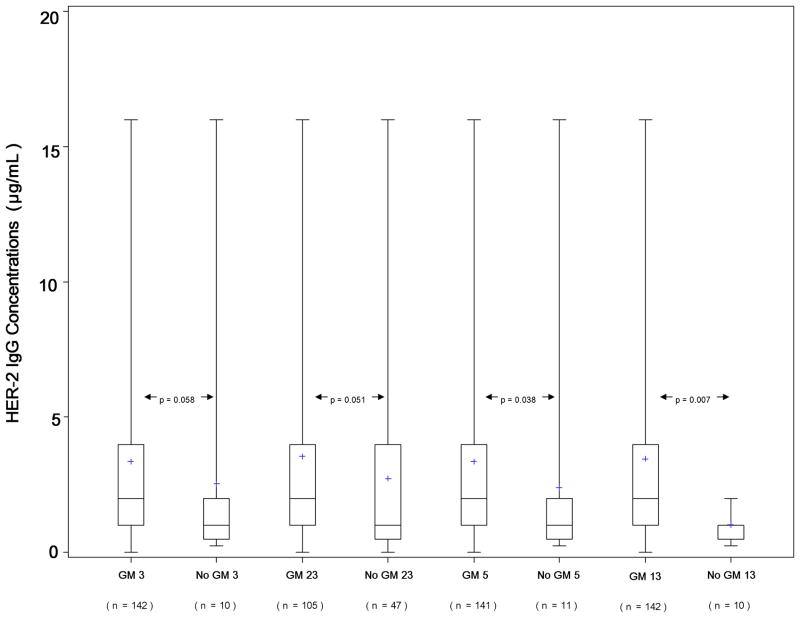
Figure 2 presents boxplots showing the distribution of HER-2 IgG antibody levels corresponding to the presence and absence of IgG1 (GM 3), IgG2 (GM 23) and IgG3 (GM 5, GM 13) allotypes known to be in significant linkage disequilibrium in Caucasians [7–9]. GM 13 allotype was highly significantly associated with HER-2 IgG levels: subjects with this allotype had higher antibody levels compared to those lacking this marker (median IgG titer 800 vs. 400, p = 0.007). The same twofold difference in the antibody levels was associated with the presence or absence of GM determinants in linkage disequilibrium with GM 13, and the p values were either significant (GM 5 vs. non-GM 5, p = 0.038) or at border-line significance, 0.058 and 0.051 (GM 3 and GM 23), reflecting slight differences in the marker frequencies.
Fig. 2.
Boxplots of HER-2 IgG levels and GM 3, GM 23, GM 5, and GM 13 allotypes. Bold horizontal lines indicate the median IgG levels corresponding to the presence or absence of particular allotype, while boxes are indicative of the interquartile ranges. Dotted lines extend from the 5th to the 95th percentiles, and plus (+) signs indicate minima and maxima.
The results presented here show that breast cancer patients with GM 3 and13 allotypes had a twofold higher median anti-HER-2 IgG antibody concentration than those lacking these allotypes. These markers could influence the antibody responsiveness to HER-2 by being part of the recognition structure for the HER-2 epitopes on the B-cell membrane-bound IgG. Perhaps membrane-bound IgG molecules with GM 3 and 13 are more compatible receptors for HER-2—and provoke stronger humoral immunity—than IgG with other GM determinants. These constant (C) region determinants could also influence the conformation of the Ig variable (V) regions involved in antigen binding and thus cause changes in antibody specificity. Some elegant studies in mice investigating the contribution of C-region determinants to the expression of certain idiotypes and their participation in other conformational changes in the V region support this interpretation. Involvement of both C and V regions in the formation of idiotypic determinants was documented for the T15 system many years ago [10]. A more recent study has clearly established that amino acid sequence polymorphisms in the C region of the Ig molecule affect the secondary structure of the antigen-binding site in the V region [11]. Amino acid substitutions associated with GM allotypes cause structural changes in the C region, which could impose structural constraints (conformation) on the V region, resulting in variation in antibody specificity to HER-2. It is also possible that the associations we have observed are due to linkage disequilibrium between particular GM alleles determined in this investigation and those not determined here (e.g. GM 10, 11) or alleles of another locus, as-yet-unidentified, for humoral immune responsiveness to HER-2.
As mentioned earlier, HER-2 transgenic mice, which differ in their genetic backgrounds, show differential response to HER-2 vaccination [2]. Based on these results, the authors have argued that a personalized vaccination regimen may be more appropriate for the genetically heterogeneous patient population. The results presented here support this view. If confirmed by an independent study, they would suggest that the subjects carrying the responder GM 3 23 5,13 haplotype are more likely to make therapeutic responses to HER-2-based vaccines. For the nonresponders, the vaccine could be fused with appropriate adjuvants (e.g. heat shock proteins) to overcome the allotypic restriction in immune responsiveness.
One limitation of our study is that we do not have data on HER-2 expression, as all patients in the study, except one, were treated about 12 years ago, when these measurements were not performed. Despite this limitation, it is hoped that the results presented here would inspire further studies on the genetic control of naturally occurring immune responses to tumor antigens.
Acknowledgments
This work was supported in part by grants from the US Department of Defense (W81XWH-08-1-0373), the National Center for Research Resources (UL1RR029882), and the Estonia Science Foundation (# 6726). The authors thank Shannon Stockham and Anna Marshall for expert technical assistance.
Abbreviations
- KM
kappa light chain marker
- GM
Immunoglobulin gamma heavy chain marker
References
- 1.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52:715–38. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radkevich-Brown O, Jacob J, Kershaw M, Wei WZ. Genetic regulation of the response to Her-2 DNA vaccination in human Her-2 transgenic mice. Cancer Res. 2009;69:212–18. doi: 10.1158/0008-5472.CAN-08-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandey JP, Shannon BT, Tsang KY, Fudenberg HH, Camblin JG. Heterozygosity at Gm loci associated with humoral immunity to osteosarcoma. J Exp Med. 1982;155:1228–32. doi: 10.1084/jem.155.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandey JP, Nietert PJ, Mensdorff-Pouilly S, Klaamas K, Kurtenkov O. Immunoglobulin allotypes influence antibody responses to mucin 1 in patients with gastric cancer. Cancer Res. 2008;68:4442–46. doi: 10.1158/0008-5472.CAN-07-5607. [DOI] [PubMed] [Google Scholar]
- 5.Pandey JP, Nietert PJ, Klaamas K, Kurtenkov O. A genetic variant of immunoglobulin γ2 is strongly associated with natural immunity to mucin 1 in patients with breast cancer. Cancer Immunol Immunother. 2009;58:2025–29. doi: 10.1007/s00262-009-0709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schanfield MS, van Loghem E. Human immunoglobulin allotypes. In: Weir DM, editor. Genetics and Molecular Immunology, [Handbook of Experimental Immunology. 4. Vol. 3. Boston: Blackwell; 1986. pp. 94.1–18. [Google Scholar]
- 7.Steinberg AG, Cook CE. The Distribution of Human Immunoglobulin Allotypes. New York: Oxford University Pres; 1981. [Google Scholar]
- 8.Grubb R. Advances in human immunoglobulin allotypes. Exp Clin Immunogenet. 1995;12:191–97. doi: 10.1159/000424871. [DOI] [PubMed] [Google Scholar]
- 9.Pandey JP. Genetics of immunoglobulins. In: Virella G, editor. Medical Immunology. New York: Marcel Dekker; 2007. pp. 73–81. [Google Scholar]
- 10.Morahan G, Berek C, Miller JFAP. An idiotypic determinant formed by both immunoglobulin constant and variable regions. Nature. 1983;301:720–22. doi: 10.1038/301720a0. [DOI] [PubMed] [Google Scholar]
- 11.Torres M, Fernandez-Fuentes N, Fiser A, Casadevall A. The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J Biol Chem. 2007;282:13917–27. doi: 10.1074/jbc.M700661200. [DOI] [PubMed] [Google Scholar]