Abstract
The nematode Caenorhabditis elegans is a widely adopted model organism for studying various neurobiological processes at the molecular and cellular level in vivo. With a small, flexible, and continuously moving body, the manipulation of C. elegans becomes a challenging task. In this review, we highlight recent advances in microfluidic technologies for the manipulation of C. elegans. These new family of microfluidic chips are capable of handling single or populations of worms in a high-throughput fashion and accurately controlling their microenvironment. So far, they have been successfully used to study neural circuits and behavior, to perform large-scale phetotyping and morphology-based screens as well as to understand axon regeneration after injury. We envision that microfluidic chips can further be used to study different aspects of the C. elegans nervous system, extending from fundamental understanding of behavioral dynamics to more complicated biological processes such as neural aging and learning and memory.
Introduction
For the past 30 years, Caenorhabditis elegans has been extensively used by scientists as a genetic model to address fundamental neurobiological questions because of its versatility in behavior, simple nervous system, short life cycle, and availability of powerful genetic tools [1,2]. With the appropriate transgenic manipulations, the nervous system of the worm is readily accessible for fluorescence imaging, thus permitting phenotyping and visual screening as well as precise targeting and ablation of axons. These ideal and unique properties of the worms, however, are difficult to be exploited using the conventional, time-consuming manual methods.
Microfluidics has recently emerged as a new tool for precise manipulations of C. elegans and their environment for neurobiological studies. This technology opened up a new range of experimental possibilities ranging from precise delivery of chemicals and environmental stimuli for behavioral studies to nerve regeneration, to calcium imaging of neuron activities, and to high-throughput genetic screenings for neural development studies. Together, C. elegans as a model organism, and microfluidic devices, provide a powerful combination to investigate today's many challenges in neurobiology.
Several unique properties of microfluidics make them ideal for their applications in C. elegans: first, the availability of simple and cheap microfabrication techniques since the revolution that soft lithography brought to microfabrication [3,4]; second, the use of transparent materials such as glass or poly-dimethyl-siloxane (PDMS), allowing transmission of light for optical imaging and manipulation; third, the ability to manipulate small amounts of liquid in small dimensions on the order of 10's to 100's of microns, providing precise and fast manipulation of small size C. elegans that can live in liquid media and reducing the use of chemicals and drugs to small amounts; fourth, the scalability to handle a large population of worms either in parallel or in series in high-throughput fashion; and fifth, the possibility to interface with available robotic handling technologies that use microtiter plates, thus enabling the screening of a large collection of RNAi libraries and chemical compounds in a rapid, automatic, and parallel fashion.
Thanks to these unique properties, several microfluidic devices have recently been developed for C. elegans, specifically for their precise immobilization (Figure 1) and for the control of their microenvironment. This review will illustrate the possibilities offered by microfluidic devices used on C. elegans in four classes of applications in neurobiology: behavior, phenotyping, functional imaging, and regeneration (following injury).
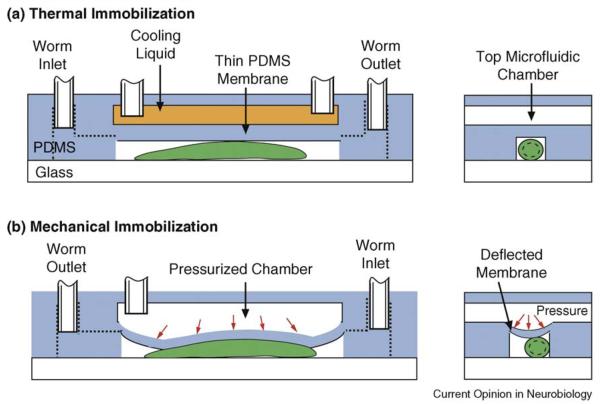
Figure 1.
Two microfluidic approaches to immobilize single worms using: (a) a cooling liquid to lower the worm's temperature to ~4°C [18••] and (b) a pressurized membrane to mechanically restrict the worm's movement [31••]. Both approaches incorporate a two-layer PDMS microfluidic architecture.
Microfluidics for behavioral studies
Because of the many advantages C. elegans has over larger model organisms (transparent body, stereotypical nervous system, availability of mutants, and transgenic strains), many advancements have been made in behavioral neurogenetics over the years ([2,5-8]). The experimental platforms for performing behavior assays have not changed substantially since the conception of the field. One example is in standard chemotaxis experiments, where odors are delivered either passively by diffusion of the odor molecules from a droplet source on plates, or by a needle spritzer in solution. These techniques pose serious challenges in precisely controlling the shapes of the chemical concentration field or gradients and the timing of the delivery of chemicals without perturbing the animals in some other ways.
Microfluidics offer technical solutions to some of these challenges. Because microfluidic devices made with PDMS are transparent and practically nonfluorescent, different modes of microscopy (e.g. bright field and fluorescence) can be easily performed on animals inside the chips, and behaviors of animals can also be easily recorded. Additionally, microscale flow in microfluidic chips behaves interestingly; laminar flow is virtually guaranteed and mass transport is fast because the length scale materials have to diffuse through is small. These properties can be taken advantage of to create controlled environments for delivering odor or creating gradients (spatial or temporal). For example, Zhang et al. [9] uses directional diffusion to deliver (different) odors from multiple bacterial lawns to worms in a maze and studies learning behavior [9]. Gray et al. created an oxygen gradient in <1 min for populations of worms to study aerotaxis (hyperoxia and hypoxia avoidance) behavior [10]. In a different example, Lockery et al. [11] and Park et al. [12] used micropillars to create artificial soil to allow C. elegans crawl while immersed in liquid, which potentially enables a set of behavioral experiments that were originally difficult to perform because in liquid C. elegans tends to swim with inefficient motility rather than crawl. Park et al. [13] and Doll et al. [14] also took advantage of microfabrication to create small force sensors suitable for measuring mechanical properties and forces generated by worms when locomoting. In three other elegant methods for isolating worms into droplets/small wells and delivering chemicals, simple microscale manipulations also enable worms to be observed in a massively parallel fashion [15-17].
Microfluidics for phenotyping and morphology-based screens
In neural development and cell biology studies, morphologies of cells and subcellular structures such as synapses or organelles are often important phenotypes of interest. Fluorescence microscopy is the workhorse of modern cell biology and is the most prevalent method for phenotyping; additionally, a large number of genetic screens are based on fluorescent morphologies. However, most of the microscopy today is still performed with worms manually mounted on agar pads, involving several handling steps that are manual, serial, and nonquantitative in nature.
To address these issues, Chung et al. [18••] and Crane et al. [19] demonstrated a methodology stream-lining high-resolution imaging of thousands of worms; when worms of mutant phenotypes are detected, sorting can take place (Figure 2a). The microfluidic approach is compatible with any microscope setup, provides high-resolution images showing cellular and subcellular structures, for example axons, dendrites, and synapses (Figure 2b,c). These devices are fully automated, resulting in the ability to screen through thousands of worms reliably. Chung et al. [18••] uses on-chip local cooling to immobilize worms. This is enabled by the small thermal mass of the device; in other words, the channel containing the worm suspension can warm up and cool down extremely fast (<0.1 s) such that the time the worms experience 4°C is only ~2 s, the time required to complete the image acquisition. Crane et al. also demonstrated the first genetic screens with EMS-mutagenized worm population using microfluidic chips [19]. In these and other examples, the ability to integrate valves, flow regulators, and geometrical features for multistep manipulation of worms enables complex sorting schemes to take place with organisms like C. elegans that are non-spherical and can move. Moreover, worms of all sizes from larvae to adults can be manipulated on-chip for microscopy, as the various microfluidic features can be easily modified to accommodate the appropriate animal body size.
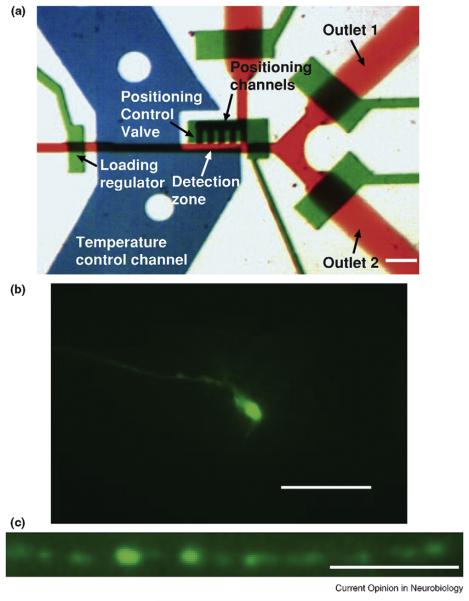
Figure 2.
(a) The functional part of a microfluidic device for imaging, phenotyping, and sorting of C. elegans. The red channels are for delivering worm sample suspensions and sorting exits; the green layer is for control valves; the blue is for the temperature-regulating fluids. Scale bar 100 m. (b) One image from a stack of (z-scan) images showing an AWC neuron expressing GFP (Pstr-2::GFP). The neuronal processes are clearly visible, similar to fluorescence microscopy on a slide using conventional method. Scale bar 20 m. (c) Punctal structures along the nerve cord (Punc-25::GFP::RAB-5) in a mutant animal. The structures are subcellular, and the intensities can be easily quantified for a variety of studies, including synapse development. Scale bar 10 m (adapted from [18••]).
Microfluidics for neural functional imaging
Microfluidic chips that can be used to manipulate individual worms and accurately control locally their microenvironment have greatly benefited the study of the worm's nervous system. Various ‘imaging’ chips, combined with the recently developed calcium-sensitive fluorescent indicators [20] have enabled optical monitoring of neuronal activity from intact worms, in an effort to understand how neuronal circuits generate behavior at the single neuron level.
The typical calcium imaging experiment is based on the so-called ‘glued worm preparation’ procedure [21]. That procedure requires the immobilization of single worms by ice-freezing and gluing them on an agar pad. A chemical [22], thermal [23], or mechanical [21] stimulus is subsequently delivered to the worm body to trigger a neuronal response while optical recording takes place. Concerns arise on the toxicity of the glue and the effect of the temperature shock to the functionality of the nervous system. Moreover, the delivery of the stimulus cannot be accurately spatiotemporally controlled, while the stimulus-evoked behavior cannot be analyzed. Combined imaging/behavior setups for tracking free-moving worms have also been developed [24], but their use for imaging single neurons is limited by the low magnification-low numerical aperture microscope objective used, resulting in weak fluorescent signal.
To overcome these problems, Chronis et al. [25••] recently demonstrated that it is possible to replace the glue approach by microfludic technology. ‘Imaging-behavior’ chips were used to generate behavior patterns that can be further analyzed and correlated with calcium imaging data, while ‘trap and stimulate’ microfluidic chips [26] were developed to immobilize single worms and accurately deliver a chemical stimulus. These devices are made out of a single or double PDMS layers that are bonded to a glass coverslip to obtain a tight seal. The PDMS layer is microfabricated using standard single-layer or multi-layer soft lithographic processes [27].
Imaging-behavior chips consist of a worm microtrap that permits lateral body movement in a predefined 2D microfluidic geometry. The worm is slightly compressed in the vertical direction and thus not allowed to escape the field of view. That is achieved by fabricating a microtrap with a thickness slightly smaller than the diameter of the worm (typically a 35–40 μmm in diameter young adult fits into a ~28-μm-thick microtrap). Imaging/behavior chips are powerful tools for correlating behavior output with neuronal activity through calcium imaging. It has been recently shown that the AVA interneuron is active only during backward locomotion, an indication that AVA is key player in initiating reversals [25••].
As mentioned above, sinusoidal-shape worm microtraps of larger thickness, termed ‘artificial dirt’, have also been proposed to generate an crawl-like behavior [11]. These devices can potentially be used to monitor interneuronal and motorneuronal activity of free-moving nematodes, but maintaining the worm in the field of view can be a challenging task.
‘Trap and stimulate’ microfluidic chips incorporate a modified version of the worm microtrap described above and a microfluidic stimulus delivery system. The shape of the microtrap is designed to match the size of the head or tail of the worm (where most sensory neurons and inter-neurons are located) to minimize their lateral movement. The worm microtrap is further integrated with a four-flow microfluidic network [25••] or a thin gas-permeable membrane with a two-layer architecture [28] to deliver a liquid or a gas stimulus, respectively. These ‘trap and stimulate’ devices have greatly facilitated the study of neuronal circuits. The four-flow design was used to record stimulus-evoked responses from chemosensory sensory neurons and interneurons, revealing the role of each neuron in the worm olfactory circuit [26]. In a similar set of experiments, a novel property of the ASH poly-modal neuron was observed: ASH responded to the removal of a hyperosmolarity shock, a property not previously described with the glue approach indicating the combined benefit of microfluidically immobilizing and stimulating the worm. Finally, the thin-membrane chip design was used to identify the molecular basis of rapid O2-dependent behavior through calcium imaging of two distinct classes of O2-sensitive neurons.
Microfluidics for nerve regeneration studies
Ultrafast laser nanosurgery has emerged as a new neurosurgical tool that is capable of precisely ablating neuronal tissue in living organisms in a minimally invasive fashion [29,30]. Combining laser nanosurgery in model organisms, such as C. elegans, with microfluidic technology permits the user to control both the environment of the whole organism, either chemical-free or containing specific drugs, and its immobilization — the latter being particularly imperative because of the accuracy requirement of nanosurgery. Moreover, microfluidic devices can be easily automated to bring high-throughput capabilities to drug or gene screenings.
Guo et al. [31••] recently proposed and demonstrated a novel approach that adopts a two-layer microfluidic configuration for rapid immobilization of C. elegans for precise laser nanosurgery of their axons. Figure 3 shows an illustration of this method and the microfluidic device developed specifically for nerve regeneration studies. The bottom layer against the glass slide houses the worms in liquid and the upper layer contains the pressurized air that controls the mechanical trap. In between these two layers, a thin PDMS membrane exits in the trapping area that is deflected downward, pressing the animal against the glass when pressure is applied in the upper micro-channel. That approach made it possible to completely immobilize worms and perform the first in vivo nanoaxotomy and subsequent time-lapse imaging of regrowing axons, in the absence of anesthetics. The precision and accuracy of this mechanical trap was similar to those achieved on agar pads with anesthetics. An additional study utilized a similar two-layer design and showed that their original immobilization technique based on suction [32] was improved greatly, finally providing the precision necessary to perform nanosurgery [33]. On-chip axonal regeneration studies revealed faster regrowth than previously obtained for both touch and motor neurons showing how microfluidic devices can be effective in avoiding the unwanted side effects of anesthetics [31••]. On the other hand, microfluidic trapping did not have statistically significant effect on the axonal regeneration yield.
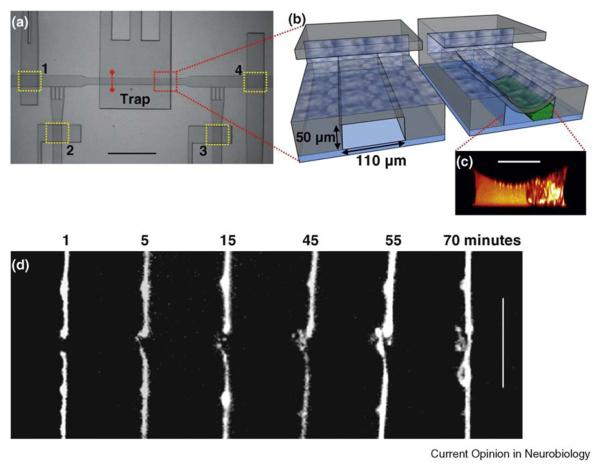
Figure 3.
Laser axotomy chip for nerve regeneration studies. (a) Top view of the trapping area on the chip. The worms are loaded into the chip through an inlet on the left-hand side (not shown in the picture) and then immobilized in the microtrap. After axotomy, worms are either transferred to the recovery chambers or unloaded for follow-up studies. Microfluidic valves are highlighted by yellow rectangles and numbered from 1 to 4. Valve 1 regulates the injections of worms into the trapping area. Valves 2 and 3 control the side channels allowing a fine positioning of the worm. Valve 4 is the gate to the recovery chambers. The dashed red line indicates the cross-section that was imaged by two-photon microscopy. (b) A conceptual 3D sectional rendering of the two-layer microtrap showing a trapped worm. (c) A two-photon image of cross-sectional profile of a trapped worm. Scale bar, 50 μm. (d) Time-lapse imaging of nerve regeneration of an ALM neuron after axotomy. Distal ends are on the lower part of the pictures, proximal ones on the upper. After 70 min, the proximal end regrew and reconnected to the distal end. Scale bar, 10 μm (adapted from [31••]).
Another trapping approach utilizes single-layer geometry where worms are squeezed in tapering down channels in a microfluidic maze structure [34]. This approach allows for precise immobilization of a large number of worms in parallel. It has been shown that the worms can be recovered after being trapped for a short period of time without any effect on viability. Such approach was later used to keep the worms immobilized for the ablation of single synapses and for the duration of the follow-up observations up to five hours [35].
Microfluidic trapping provides many advantages over the conventional immobilization techniques that were formerly used in nerve regeneration studies of C. elegans, such as anesthesia on agar pads or glue, including: first, no chemicals other than the liquid growth medium will interfere with the physiological processes of the worms; second, the worms do not need a recovery period after surgery, permitting immediate behavioral study of the postaxotomy functionality; and third, the sample population is well contained and experiment conditions are easily reproducible since the trap for surgery and the environment for recovery could be on the same chip.
The deflected-membrane trapping approach described above (termed ‘compressive immobilization’ [36] and ‘serial trapping’ [37]) can easily be adapted to immobilize other model organisms and to incorporate other manipulation techniques, such as optical trapping [38] and light stimulation [39,40]. This method can also provide an additional benefit of serial immobilization where model organisms can be trapped and manipulated sequentially, widening the possibilities of high-throughput biological investigations. For example, the incorporation of full automation and the interfacing of the microfluidic device to multiwell plates [41,32] will greatly facilitate genome-wide reverse screening using RNA interference.
Conclusions
Designing appropriate microfluidic systems for studying morphology, development, physiology, behavior, and nerve regeneration of C. elegans has thus far shown clear advantages in some applications as we have summarized here. Perhaps three important lessons can be learned from these examples: understanding the needs and bottlenecks of the biological research is critical for identifying the engineering solution; playing the strengths of microfluidics appropriately based on the biological system of interest is critical for successful applications; lastly, considering each applications separately as the needs are different and hence the tools should be different. As the existing microfluidic systems become popularized and new applications are identified, we believe the utility of these systems will eventually yield invaluable biological data.
Acknowledgements
The authors would like to acknowledge Dr Frederic Bourgeois for his valuable help in the preparation of the manuscript. This work was supported by grants R21-NS058646 (AB and NC), R01-NS060129 (AB), R21-AG033259 (NC), and R21-NS058465 (HL) from the National Institute of Health, and DBI-0149833 (HL) from the National Science Foundation. HL is a Sloan Research Fellow in Neuroscience.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
** of outstanding interest
- 1.Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 3.Xia Y, Whitesides GM. Soft lithography. Angew Chem Int Ed. 1998;37:550–575. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Quake SR, Scherer A. From micro- to nanofabrication with soft materials. Science. 2000;290:1536–1540. doi: 10.1126/science.290.5496.1536. [DOI] [PubMed] [Google Scholar]
- 5.Bargmann CI. Chemosensation in C., 10.1895/wormbook.1.123.1. The C. elegans Research Community, WormBook; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobert O. Specification of the Nervous System. The C. elegans Research Community, WormBook; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Y. Synaptogenesis 10.1895/wormbook.1.44.1. The C. elegans Research Community, WormBook; 2005. [Google Scholar]
- 8.de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 10.Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 11.Lockery SR, Lawton KJ, Doll JC, Faumont S, Coulthard SM, Thiele TR, Chronis N, McCormick KE, Goodman MB, Pruitt BL. Artificial dirt: microfluidic substrates for nematode neurobiology and behavior. J Neurophysiol. 2008;99:3136. doi: 10.1152/jn.91327.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park S, Hwang H, Nam SW, Martinez F, Austin RH, Ryu WS. Enhanced Caenorhabditis elegans locomotion in a structured microfluidic environment. PLoS ONE. 2008;3:e2550. doi: 10.1371/journal.pone.0002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SJ, Goodman MB, Pruitt BL. Analysis of nematode mechanics by piezoresistive displacement clamp. Proc Natl Acad Sci U S A. 2007;104:17376. doi: 10.1073/pnas.0702138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doll JC, Harjee N, Klejwa N, Kwon R, Coulthard SM, Petzold B, Goodman MB, Pruitt BL. SU-8 force sensing pillar arrays for biological measurements. Lab Chip. 2009;9:1449–1454. doi: 10.1039/b818622g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo L, Gabel CV, Ha HI, Zhang Y, Samuel ADT. Olfactory behavior of swimming C. elegans analyzed by measuring motile responses to temporal variations of odorants. J Neurophysiol. 2008;99:2617. doi: 10.1152/jn.00053.2008. [DOI] [PubMed] [Google Scholar]
- 16.Shi W, Qin J, Ye N, Lin B. Droplet-based microfluidic system for individual Caenorhabditis elegans assay. Lab Chip. 2008;8:1432–1435. doi: 10.1039/b808753a. [DOI] [PubMed] [Google Scholar]
- 17.Clausell-Tormos J, Lieber D, Baret JC, El-Harrak A, Miller OJ, Frenz L, Blouwolff J, Humphry KJ, Köster S, Duan H, et al. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem Biol. 2008;15:427–437. doi: 10.1016/j.chembiol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 18••.Chung K, Crane MM, Lu H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat Methods. 2008;5:637–643. doi: 10.1038/nmeth.1227. By integrating the manipulation of nematodes in a microfluidic device and automated multiparametric analysis of morphological features, the authors managed to identify and sort worms depending on their phenotypes. For the first time, high throughput and precise manipulation of C. elegans has been achieved on a microfluidic device. [DOI] [PubMed] [Google Scholar]
- 19.Crane MM, Chung K, Lu H. Computer-enhanced high-throughput genetic screens of C. elegans in a microfluidic system. Lab Chip. 2009;9:38–40. doi: 10.1039/b813730g. [DOI] [PubMed] [Google Scholar]
- 20.Mank M, Griesbeck O. Genetically encoded calcium indicators. Chem Rev. 2008;108:1550. doi: 10.1021/cr078213v. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H, Kerr R, Bianchi L, Frøkjær-Jensen C, Slone D, Xue J, Gerstbrein B, Driscoll M, Schafer WR. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 2003;39:1005–1017. doi: 10.1016/j.neuron.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhara A, Okumura M, Kimata T, Tanizawa Y, Takano R, Kimura KD, Inada H, Matsumoto K, Mori I. Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science. 2008;320:803–807. doi: 10.1126/science.1148922. [DOI] [PubMed] [Google Scholar]
- 24.Nehrke K, Denton J, Mowrey W. Intestinal Ca2+ wave dynamics in freely moving C. elegans coordinate execution of a rhythmic motor program. AJP: Cell Physiol. 2008;294:C333–344. doi: 10.1152/ajpcell.00303.2007. [DOI] [PubMed] [Google Scholar]
- 25••.Chronis N, Zimmer M, Bargmann CI. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods. 2007;4:727–731. doi: 10.1038/nmeth1075. This is the first paper that came out describing the use of microfluidic devices to perform in vivo calcium imaging from sensory neurons and interneurons. It was showed that by using novel microfluidic chips it is possible not only to reveal unknown properties of sensory neurons, but also to correlate behavior patterns with neuronal activation events. [DOI] [PubMed] [Google Scholar]
- 26.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63. doi: 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- 27.Unger MA, Chou H-P, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- 28.Zimmer M, Gray JM, Pokala N, Chang AJ, Karow DS, Marletta MA, Hudson ML, Morton DB, Chronis N, Bargmann CI. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432:822–1822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- 30.Bourgeois F, Ben-Yakar A. Femtosecond laser nanoaxotomy properties and their effect on axonal recovery in C. elegans: erratum. Opt Expr. 2008;16:5963. doi: 10.1364/oe.16.005963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Guo SX, Bourgeois F, Chokshi T, Durr NJ, Hilliard MA, Chronis N, Ben-Yakar A. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nat Methods. 2008;5:531–533. doi: 10.1038/nmeth.1203. For the first time, femtosecond laser surgery was successfully performed on nematodes trapped in a microfluidic device. It was also shown that the absence of anesthetics for immobilization improved the speed of nerve regeneration in C. elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohde CB, Zeng F, Gonzalez-Rubio R, Angel M, Yanik MF. Microfluidic system for on-chip high-throughput whole-animal sorting and screening at subcellular resolution. Proc Natl Acad Sci U S A. 2007;104:13891–13895. doi: 10.1073/pnas.0706513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng F, Rohde CB, Yanik MF. Sub-cellular precision on-chip small-animal immobilization, multi-photon imaging and femtosecond-laser manipulation. Lab Chip. 2008;8:653–656. doi: 10.1039/b804808h. [DOI] [PubMed] [Google Scholar]
- 34.Hulme SE, Shevkoplyas SS, Apfeld J, Fontana W, Whitesides GM. A microfabricated array of clamps for immobilizing and imaging C. elegans. Lab Chip. 2007;7:1515–1523. doi: 10.1039/b707861g. [DOI] [PubMed] [Google Scholar]
- 35.Allen P, Sgro A, Chao D, Doepker B, Edgar JS, Shen K, Chiu D. Single-synapse ablation and long-term imaging in live C. elegans. J Neurosci Methods. 2008;173:20. doi: 10.1016/j.jneumeth.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chokshi TV, Ben-Yakar A, Chronis N. CO2 and compressive immobilization of C. elegans on-chip. Lab Chip. 2009;9:151–157. doi: 10.1039/b807345g. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Yakar A, Bourgeois F. Ultrafast laser nanosurgery in microfluidics for genome-wide screenings. Curr Opin Biotechnol. 2009;20:100–105. doi: 10.1016/j.copbio.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeffries GDM, Edgar JS, Zhao Y, Shelby JP, Fong C, Chiu DT. Using polarization-shaped optical vortex traps for single-cell nanosurgery. Nano Lett. 2006;7:415–420. doi: 10.1021/nl0626784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang F, Wang L-P, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Szobota S, Wang Y, Volgraf M, Liu Z, Sun C, Trauner D, Isacoff EY, Zhang X. All optical interface for parallel, remote, and spatiotemporal control of neuronal activity. Nano Lett. 2007;7:3859. doi: 10.1021/nl072783t. [DOI] [PubMed] [Google Scholar]
- 41.Fredrickson C, Fan ZH. Macro-to-micro interfaces for microfluidic devices. Lab Chip. 2004;4:526–533. doi: 10.1039/b410720a. [DOI] [PubMed] [Google Scholar]