Abstract
We hypothesized that bronchodilator treatment not only improves hyperinflation and endurance capacity but also muscular efficiency in stable chronic obstructive pulmonary disease (COPD). We aimed to demonstrate that tiotropium and salmeterol improve muscular efficiency compared with placebo. Twenty-five COPD patients were studied, including 20 males of mean (standard deviation) age 62 years (7 years) with baseline forced expiratory volume in 1 second of 41% (10%) predicted, and maximal workload of 101 Watt (36 Watt). Subjects were randomized for 6-week treatment with tiotropium 18 μg once daily, salmeterol 50 μg twice daily, or placebo using a double-blind, crossover design. Muscular efficiency and endurance time were measured during cycling at 50% of maximal work load. Resting energy expenditure was measured using a ventilated hood. Muscular efficiency after tiotropium, salmeterol, and placebo treatment was 14.6%, 14.4%, and 14.4%, respectively (P > 0.05), and resting energy expenditure was 1485 kcal/24 hours, 1709 kcal/24 hours, and 1472 kcal/24 hours (P > 0.05), respectively. Endurance time after tiotropium treatment was significantly higher than that after placebo (27.0 minutes versus 19.3 minutes [P = 0.02]), whereas endurance time after salmeterol treatment was not higher than that after placebo (23.3 minutes [P = 0.22]). In this small study, we were not able to demonstrate that bronchodilator therapy improved muscular efficiency. Apparently, reduced costs of breathing relative to total energy expenditure were too small to be detected.
Keywords: bronchodilation, chronic obstructive pulmonary disease, energy expenditure, muscle energetics, muscular exercise
Introduction
Patients with chronic obstructive pulmonary disease (COPD) often have impaired exercise tolerance, leading to reduced physical activity and low quality of life. Impaired exercise tolerance is due to a complex interplay between limitations in ventilatory capacity, gas exchange, cardiovascular capacity, and peripheral muscle metabolism. Static and dynamic hyperinflation during exercise plays an important role and may initiate a vicious circle of decline that includes physical deconditioning and worsening of dyspnea.1 Lung deflation by means of bronchodilators reduces hyperinflation and improves submaximal exercise endurance, as was shown for both the long-acting-β-2 agonist salmeterol and the long-acting anticholinergic agent tiotropium.2,3 One study demonstrated that both agents were equally effective at improving functional residual capacity (FRC) and inspiratory capacity (IC) at rest and at increased breathing frequencies (15 breaths/minute, 30 breaths/minute, and 45 breaths/minute).4 No published direct comparison between the two agents’ effect on submaximal exercise tolerance exists.
Energy expenditure at a constant submaximal workload is another way to investigate hyperinflation and its consequences for exercise tolerance. “Muscular efficiency” of movement is frequently used to express the efficacy of skeletal muscles to transfer biochemical energy into external work or movement.5 Calculations of muscular efficiency during steady-state situations are based on open-circuit indirect calorimetry (oxygen uptake [VO2], carbon dioxide output [VCO2]) and the assumption that energy requirements in the muscles are met by respiration. Baarends et al6 showed that after correction for resting energy expenditure (REE), the net muscular efficiency in 33 patients with mild-to-moderately severe COPD ranged from 8.5% to 22.7% (median 15.5%). Interestingly, patients with a low net muscular efficiency (<17%) demonstrated enhanced ventilatory responses to exercise and higher airway resistance at rest. This suggests that decreased muscular efficiency is related to increased costs of breathing, probably due to hyperinflation.
We speculated that measurement of muscular efficiency in COPD can detect improvements in costs of breathing induced by tiotropium or salmeterol. We hypothesized that both treatments improve muscular efficiency, along with endurance capacity, in clinically stable patients with COPD who demonstrated ventilatory limitation during maximal exercise.
Methods
Patients
Outpatients with stable COPD were recruited from pulmonary departments of hospitals in the north of The Netherlands. Patients had to be ≥40 years old with a diagnosis of moderate-to-severe COPD (forced expiratory volume in 1 second [FEV1] ≤ 60% predicted; FEV1/forced vital capacity [FVC] ≤ 70%) and a smoking history of >10 pack-years. FEV1% predicted had to increase by >5% after inhalation with 480 μg salbutamol and 80 μg ipratropium (Combivent®, Boehringer Ingelheim, Ridgefield, CT, USA). The use of inhaled and oral steroids (maximally 10 mg a day) was allowed during the study. Patients who needed supplemental oxygen and who had three or more exacerbations per year were excluded. Ventilatory limitation of maximal exercise capacity was required (defined by an increase in oxygen tension in arterial blood [PaCO2] and/or an increase in minute ventilation [VE] > 80% [FEV1 × 37.5]). Additionally, patients required a maximal workload (Wmax) of ≥40 Watt during an incremental cycle test. The main exclusion criteria for the study included a 10 mmol/L increase in blood lactate during peak exercise or 2.5 mmol/L at ≥50% of Wmax and oxygen saturation <90% at 50% of Wmax.
Study design
This was a randomized, double-blind, double-dummy, placebo-controlled, crossover study, conducted over three 6-week periods. The effects of tiotropium, salmeterol, and placebo on muscular efficiency were compared. The primary endpoint was change in gross muscular efficiency following 6 weeks of randomized treatment. Secondary endpoints included REE, endurance capacity at 50% Wmax, net muscular efficiency, FEV1, FVC, peak expiratory flow (PEF) variability over 1 week, specific airway conductance (sGaw), FRC, and symptoms determined using the Clinical COPD Questionnaire (CCQ). The study was approved by the local independent ethics committee, and informed consent was obtained from all individuals.
Study protocol
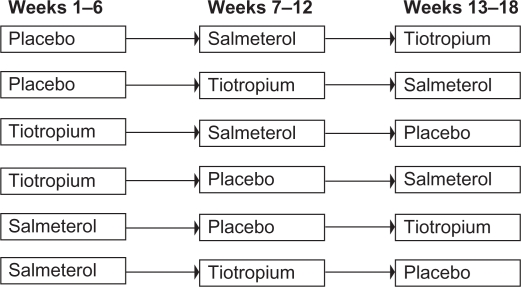
Following screening and a 4-week run-in period, 25 patients were randomized to one of six treatment sequences (Figure 1). During each 6-week treatment period, patients received either tiotropium 18 μg once daily, salmeterol 50 μg twice daily, or placebo twice daily. Salbutamol was taken as required, using a metered dose inhaler, but not during the 8 hours prior to testing. Testing occurred after each 6-week period between 8 a.m. and 10 a.m., 1 hour after drug administration. Visits were delayed for maximally 2 weeks in case of an exacerbation. PEF was measured twice daily during the 6-week periods.
Figure 1.
The six possible treatment sequences to which patients were randomly assigned. There was no washout period between successive treatment sequences.
Incremental bicycle ergometer test
Maximal exercise capacity was measured using an incremental symptom-limited cycle ergometer test. Patients inhaled four puffs of ipratropium/salbutamol 20/120 μg (Combivent) via an AeroChamber® (Boehringer Ingelheim, Ingelheim, Germany) 30 minutes before commencing the test. A brachial or radial artery catheter was inserted, and samples were drawn at rest and then every 2 minutes. VE, VO2, and VCO2 were measured from breath-by-breath analysis every 30 seconds (EOS Sprint; Jaeger, Würzburg, Germany). Dyspnea was assessed every minute using a modified Borg scale.7
The test required 2 minutes of seated rest on the ergometer when baseline measurements were collected. Patients were then instructed to pedal at 60–70 revs/minute. After 5 minutes, unloaded cycling power was increased every minute by work rate of 10% of the estimated Wmax (according to the European Respiratory Society criteria), until the patient was unable to maintain the pedaling frequency. Reasons for stopping were recorded. Peak values were the average over the last 20 seconds of the maximum completed work. Peak VO2 was predicted using equations for healthy subjects older than 55 years of age.8 Peak VE was predicted from the Carter et al’s9 equation: 37.5 × FEV1.
Muscular efficiency and REE
Gross muscular efficiency (%) was assessed by measuring energy expenditure (EE) during cycling at 50% of Wmax. Immediately after reaching steady state, EE was averaged over a period of at least 5 minutes. In addition, steady-state EE was measured at isotime, a time point that was determined by the shortest exercise time of each subject. After reaching the constant workload of 50% Wmax, patients were asked to continue cycling for as long as possible. Gross muscular efficiency (%) was calculated using the equation load (Watt) of exercise × 0.01433 kcal/min/EE kcal/min × 100%. REE was measured using an open-circuit indirect calorimetry system using a ventilated hood (Oxycon Beta®, Jaeger). Measurements were taken in the early morning, following an overnight fast, with patients in a supine position. Net muscular efficiency (%) was calculated using the values obtained for the gross muscular efficiency and REE using the equation10 load (W) of exercise × 0.01433 kcal/min/EE − REE kcal/min × 100%. In a pilot study, mean (standard deviation [SD]) differences of two measurements (interval of 1–2 weeks) of gross muscular efficiency, net muscular efficiency, and REE were 0.011% (0.82%), 0.035% (1.76%), and 7 kcal/24 hours (228 kcal/24 hours).
Lung function
Lung function tests were performed according to the guidelines of the European Respiratory Society11 and included FEV1 and FVC. Screening FEV1 was measured before and 40 minutes after 480 μg salbutamol and 80 μg ipratropium (Combivent). Total lung capacity, FRC, airway resistance, and sGaw were measured by body plethysmography (Masterlab®, Jaeger Würzburg, Germany). PEF values (MiniWright®, Clement Clarke International, Harlow, UK) were recorded twice daily during the treatment periods, immediately after waking up and just before going to sleep, and always before study medication. PEF day-to-day variability was defined by (highest morning PEF − highest morning PEF of the day before) × 100%, averaged over 7 successive days. PEF within-day variability was defined by (highest PEF − lowest PEF)/(mean value of 2) × 100%, averaged over 7 successive days.
Disease-specific symptoms were assessed using the CCQ. This is a self-administered questionnaire specifically developed to measure clinical control in patients with COPD.12 CCQ score <1 = no limitation, ≥1 and <2 = mild limitation, ≥2 and <3 = moderate limitation, and ≥3 = severe limitation. Adverse events occurring during the study period were monitored and recorded.
Statistical analysis
Statistics were computed using SAS® Version 8.02 (SAS Institute Inc., Cary, NC, USA). Characteristics of the study group are given as mean (SD). Statistical analysis was performed using a full analysis set and analysis of variance. Twenty-four patients were required in order to detect an absolute difference of 1% in mean gross muscular efficiency between the two treatments at the 5% significance level with 90% power. P < 0.05 was considered significant.
Results
Patients had moderate-to-severe COPD (Table 1). Four patients had an exacerbation during the study. One exacerbation led to discontinuation of the study. No hospitalizations occurred during the study period.
Table 1.
Screening characteristics (n = 25)a
| Screening characteristics | N (mean [SD]) |
|---|---|
| Clinical characteristics | |
| Age (years) | 62.0 (7.1) |
| Sex (% males) | 20 (80) |
| Smoking history (pack-years) | 45.3 (26.9) |
| Current smoker/exsmoker (%) | 40/60 |
| Number of corticosteroid users, inhaled/oral | 19/2 |
| Duration of COPD (years) | 7.4 (5.9) |
| Body mass index (kg/m2) | 24.6 (3.4) |
| Prebronchodilator FEV1 (% predicted) | 40.7 (10.4) |
| Postbronchodilator FEV1 (% predicted) | 48.1 (10.1) |
| FEV1/FVC × 100 (%) | 39.1 (8.8) |
| FVC (% predicted) | 3.9 (0.7) |
| Maximal incremental bicycle test | |
| Maximal workload (Watt) | 99.6 (37.2) |
| VO2 at maximum (L/minute) | 1128.9 (394.7) |
| PaO2 at rest (mmHg) | 50.6 (198.4) |
| PaO2 at maximum (mmHg) | 54.5 (181.5) |
| PaCO2 at rest (mmHg) | 25.0 (96.3) |
| PaCO2 at maximum (mmHg) | 34.5 (115.3) |
| Lactate rest (mmol/L) | 1.5 (1.2) |
| Lactate at maximum (mmol/L) | 6.5 (2.5) |
| Subjective reason for stopping exercise | |
| Discomfort with legs (%) | 2 (8) |
| Discomfort with breathing (%) | 19 (76) |
| Both discomfort with legs and breathing (%) | 3 (12) |
| Pain in the chest (%) | 0 (0) |
| Other (%) | 1 (4) |
| Dyspnea Borg before exercise | 0.2 (0.4) |
| Dyspnea Borg at end of exercise | 7.0 (1.9) |
Note:
Values are means (SD) or numbers (% proportion).
Abbreviations: BMI, body mass index; CO2, carbon dioxide; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; O2, oxygen; SD, standard deviation; VO2, oxygen uptake.
Of the 25 patients randomized, 23 received tiotropium, 24 salmeterol, and 24 placebo. Both tiotropium and salmeterol improved FEV1, FVC, FRC, and sGaw compared with placebo (Table 2). Differences between tiotropium and salmeterol were not statistically significant.
Table 2.
Pulmonary function values after 6 weeks of treatmenta
| Function | Tiotropium | Salmeterol | Placebo |
|---|---|---|---|
| FEV1 (L) | 1.32 (0.03)* | 1.27 (0.03)** | 1.14 (0.03) |
| FVC (L) | 3.27 (0.06)* | 3.12 (0.06)* | 2.81 (0.06) |
| FEV1/FVC (%) | 40.70 (0.7) | 40.81 (0.7) | 41.20 (0.73) |
| TLC (L) | 7.72 (0.09)*** | 7.85 (0.09)** | 8.13 (0.09) |
| RV (L)1 | 3.97 (0.10)* | 4.37 (0.10)*** | 4.90 (0.11) |
| FRC (L) | 5.13 (0.12)** | 5.13 (0.12)** | 5.61 (0.13) |
| Raw (kPa sec/L) | 0.49 (0.03)* | 0.54 (0.03)*** | 0.68 (0.03) |
| sGaw 1/(kPa sec) | 0.51 (0.03)* | 0.45 (0.03)* | 0.31 (0.03) |
| Diary data | |||
| Morning PEFb (L/min) | 248.91 (3.63)* | 250.24 (3.52)* | 229.98 (3.63) |
| Evening PEF (L/min) | 260.24 (3.26)* | 253.63 (3.14)* | 231.91 (3.26) |
| Day-to-day PEF variability | 5.55 (0.53) | 5.45 (0.53) | 6.72 (0.56) |
| Within-day PEF variability | 11.25 (1.40) | 7.91 (1.34) | 10.92 (1.44) |
Notes:
Values are means (standard deviation [SD]) adjusted for patient and cycle using analysis of covariance;
PEF values were assessed in the 6-week treatment periods, whereas the other lung functions were assessed 1 hour after drug administration and 1 hour in advance of the submaximal bicycle tests;
P < 0.001 versus placebo;
P < 0.05 versus placebo;
P < 0.01 versus placebo.
Abbreviations: FEV1, forced expiratory volume in 1 second; FRC, functional residual capacity; FVC, forced vital capacity; PEF, peak expiratory flow; Raw, airway resistance; RV, residual volume; sGaw, specific airway conductance; TLC, total lung capacity.
Compared with placebo, neither tiotropium nor salmeterol significantly improved the primary endpoint of mean gross muscular efficiency after 6 weeks of treatment (Table 3). Active treatment did not significantly changed mean net muscular efficiency, VO2, VCO2, VE, and tidal volume. Mean (SD) REE values after tiotropium, salmeterol, and placebo treatment were 1485 kcal/24 hours (112 kcal/24 hours), 1709 kcal/24 hours (105 kcal/24 hours), and 1472 kcal/24 hours (120 kcal/24 hours), respectively.
Table 3.
Constant-load exercise after 6 weeks of treatmenta
| Tiotropium | Salmeterol | Placebo | |
|---|---|---|---|
| Immediately after reaching steady state | |||
| Gross muscular efficiency (%) | 13.01 (0.32)* | 13.17 (0.30)* | 14.14 (0.34) |
| Net muscular efficiency (%) | 16.03 (0.57) | 17.15 (0.51) | 17.12 (0.65) |
| EE (Kcal/min) | 5.43 (0.24) | 5.32 (0.22) | 5.40 (0.25) |
| VO2 (mL/min) | 1018.05 (18.22) | 1019.30 (17.38) | 1015.51 (19.59) |
| VCO2 (mL/min) | 897.65 (13.80) | 898.20 (13.16) | 888.32 (14.84) |
| At isotime (shortest exercise time) | |||
| Gross muscular efficiency (%) | 14.55 (0.31) | 14.35 (0.30) | 14.42 (0.33) |
| Net muscular efficiency (%) | 19.34 (0.54) | 18.51 (0.51) | 19.07 (0.62) |
| EE (Kcal/min) | 5.02 (0.10) | 5.07 (0.09) | 5.01 (0.10) |
| VO2 (mL/min) | 1026.30 (20.72) | 1037.91 (19.75) | 1026.18 (21.96) |
| VCO2 (mL/min) | 898.40 (15.83) | 904.32 (15.08) | 893.35 (16.77) |
Notes:
Values are means (standard error);
P < 0.05 versus placebo;
P < 0.01 versus placebo;
P < 0.001 versus placebo.
Abbreviations: EE, energy expenditure; VCO2, carbon dioxide production; VO2, oxygen consumption.
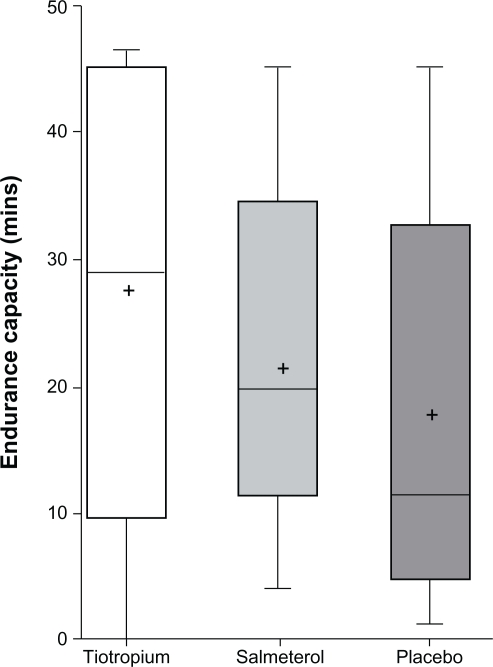
Irrespective of the absence of change in the primary outcome, a significant improvement above placebo in exercise endurance was observed after 6 weeks with tiotropium but not with salmeterol (Figure 2). After 6 weeks, mean (SD) endurance time was 26.96 minutes (2.26 minutes) with tiotropium compared with 19.30 minutes (2.34 minutes) with placebo (P = 0.02). Mean (SD) endurance time after salmeterol was 23.33 minutes (2.26 minutes) (P = 0.22 versus placebo).
Figure 2.
Endurance time at 50% maximal workload after 6 weeks of treatment.
No correlations were present between changes in endurance time and FEV1, FRC, or gross muscular efficiency.
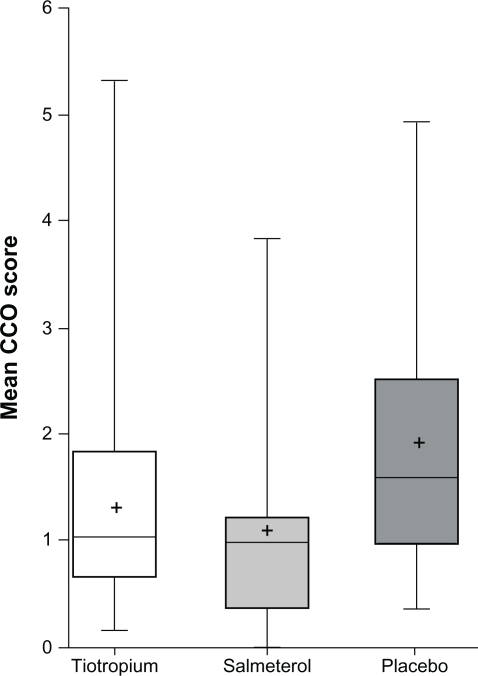
Assessment of patients’ symptoms and functional state by total CCQ scores suggested a significant reduction with both tiotropium (P = 0.011) and salmeterol (P = 0.0003) (Figure 3, Table 4). This was mainly due to significant changes in the symptoms and functional state domains. Differences between tiotropium and salmeterol were not statistically significant (P = 0.20).
Figure 3.
Clinical COPD Questionnaire after 6 weeks of treatment.
Table 4.
Clinical COPD Questionnaire items after 6 weeks of treatmenta
| Tiotropium | Salmeterol | Placebo | |
|---|---|---|---|
| Symptom domain | |||
| Short of breath at rest | 0.69 (0.18)* | 0.47 (0.18)* | 1.69 (0.18) |
| Short of breath during physical exercise | 2.63 (0.21)** | 2.18 (0.21)* | 3.67 (0.22) |
| Did you cough? | 1.77 (0.19) | 1.69 (0.19) | 2.02 (0.20) |
| Did you produce phlegm? | 1.59 (0.21) | 1.32 (0.21) | 1.76 (0.22) |
| Mental state domain | |||
| Concerned about getting a cold/exacerbation | 0.50 (0.14) | 0.28 (0.14) | 0.65 (0.15) |
| Depressed due to breathing problems | 0.68 (0.19) | 0.65 (0.19) | 0.92 (0.19) |
| Functional state domain | |||
| Daily activities at home | 1.10 (0.21) | 0.73 (0.21)** | 1.53 (0.21) |
| Social activities | 0.77 (0.20) | 0.64 (0.20) | 1.21 (0.21) |
| Moderate physical activities | 2.54 (0.24)*** | 2.09 (0.24)** | 3.27 (0.25) |
| Strenuous physical exercises | 1.59 (0.22) | 1.24 (0.22)** | 2.22 (0.23) |
Notes:
Values are means (standard error) adjusted for patient and cycle using analysis of covariance;
P < 0.001 versus placebo;
P < 0.01 versus placebo;
P < 0.05 versus placebo.
Weekly mean (SD) number of puffs of rescue medication over the last 3 weeks on treatment were 11.9 (18.5), 12.0 (17.2), and 15.5 (18.5) for tiotropium, salmeterol, and placebo, respectively.
Discussion
This study investigated the effects of two long-acting bronchodilators on working energy expenditure and REE in COPD. The mean gross muscular efficiency and REE did not differ significantly from placebo with either tiotropium or salmeterol treatment. Only tiotropium significantly improved exercise endurance. Consistent with the results of other studies, both drugs significantly improved symptoms, functional state, and lung function, including parameters of hyperinflation.
Cost of breathing during maximal exercise may rise to 35%–40% of the maximal oxygen consumption and contribute significantly to the limited physical performance of patients with COPD.13 Assuming that effective bronchodilator therapy reduces the work of breathing during submaximal cycling by 25%, total energy expenditure during exercise would be anticipated to be reduced by 8%–10%. This study was statistically powered to detect a change in gross muscular efficiency of 1%, which reflects a relative change of approximately 7%. With a sample size of 24 subjects, we feel 95% confident that both salmeterol and tiotropium improved total energy expenditure by no more than 7%. Our results are in line with a study investigating the effects of pressure support ventilation in severe COPD patients who cycled at a mean constant work load of 33 Watts.14 Despite significantly improved VE and inspiratory effort, no changes were found in oxygen consumption. The authors concluded that the anticipated reduction in oxygen cost of breathing relative to total oxygen consumption was too small to be detected.
Recently, the underlying mechanisms of decreased muscular efficiency in COPD have been examined and may explain, at least in part, our findings. Arterial and venous blood flow in the legs during a two-leg cycle exercise and single knee-extensor exercise was compared between COPD patients and age-matched healthy controls.15 At a given workload, local oxygen consumption in leg muscles was significantly higher in COPD patients than in healthy controls. In addition, a higher proportion of type II muscle fibers in the vastus lateralis muscle from COPD patients suggested that the reduced muscular efficiency of the leg muscles was due to the contribution of less effective muscle fibers. In contrast, respiratory muscles in COPD are suggested to have an elevated proportion of very efficient type I fibers.16 This suggests that, in exercising COPD patients, the contribution of peripheral muscle dysfunction to pathologically increased total energy expenditure might be more important than the increased work of breathing.
Tiotropium significantly improved airway conductance (±60%) and FRC (±9%) as well as endurance time (±40%), yet without significant correlations between these parameters. Other studies demonstrated modest correlations between changes in endurance capacity and changes in FEV1 or IC induced by ipratropium17 and tiotropium,18 whereas 20% of patients failed to improve endurance time despite positive effects on lung function.17 Consequently, bronchodilators may alter rate-limiting factors other than lung function. In this respect, a rate-limiting blood flow has been demonstrated in the lower limbs of some COPD patients during cycling exercise.19 In healthy subjects, a local reflex vasoconstriction due to the increased work of breathing has been suggested to reduce leg blood flow and to promote quadricep fatigue during cycling at relatively high work loads.20,21 Unloading of the respiratory muscles improved time to exhaustion and exercise-induced quadricep muscle fatigue.20–23 Similar mechanisms may have improved the endurance time in our tiotropium-treated COPD patients. Redistribution of blood to the legs after tiotropium inhalation may also be the result of an improved cardiac performance. Travers et al24 demonstrated that heart rate was lower with tiotropium at rest and during exercise, with a corresponding increase in oxygen pulse (surrogate measure of cardiac stroke volume) and decrease in the rate pressure product (surrogate measure of myocardial work). Together, these findings give rise to a new research question: is tiotropium-improved endurance capacity in COPD the consequence of reduced hyperinflation as well as increased leg blood flow?
We anticipated that patients with modest reversibility after Combivent would improve notably, as one study demonstrated that work of breathing improved by >25% after inhaling a single dose of formoterol in COPD patients with a mean reversibility in FEV1 of 5% predicted.25 Retrospectively, it may have been better to have selected patients on the basis of volume (hyperinflation) instead of flow (FEV1) response, although both frequently occur together.26 Additionally, we selected COPD patients with a proven ventilatory limitation at maximal work load, assuming that reduced muscular efficiency due to dynamic hyperinflation is more common in patients with a cardiovascular limitation or oxygen uptake disorder. However, muscular efficiency was tested at 50% of Wmax, at which breathing frequency may have been too low to significantly increase hyperinflation and the work of breathing. Finally, we excluded subjects with oxygen desaturation and/or lactate production at 50% of Wmax, as energy release due to anaerobic combustion is not measured by indirect calorimetry. However, arterial blood samples may not reflect local anaerobic combustion in the leg and respiratory muscles.
Net muscular efficiency in our study was somewhat higher than the 15.5% (8.5%–22.7%) values of Baarends et al.6 Their study took place in a pulmonary rehabilitation center and included COPD patients with similar age (61 years) and FEV1 (37% predicted), yet with a lower Wmax (58 Watt). Muscular efficiency in their study was measured during submaximal cycling at a work load of (on average) 30 Watt versus (on average) 50 Watt in our study, suggesting that the lower values of net muscular efficiency reported by Baarends et al6 reflect methodological differences and more severe disease. Interestingly, Perrault et al27 demonstrated similar delta muscular efficiency in COPD patients (FEV1 36% predicted) and age-matched controls; however, COPD patients and controls did not cycle at the same workloads. To our knowledge, no studies compared net muscular efficiency of COPD patients and age-matched healthy controls during cycling at similar workloads.
We hypothesized that salmeterol would increase REE and therefore decrease gross muscular efficiency. This was based on the finding of a 5% increase in REE after inhaling 5 mg salbutamol in patients with COPD.28 Another study showed a positive correlation between 24-hour total energy expenditure and the daily dose of inhaled β-2-agonists.29 Such findings have not been demonstrated for the anticholinergic drug ipratropium bromide.30 Our nonsignificant finding (P = 0.15 for salmeterol versus placebo) may be due to insufficient statistical power or may reflect adaptation in resting metabolism after 6-week treatment with salmeterol.
Conclusion
We conclude that muscular efficiency and REE do not improve after 6-week treatment with tiotropium or salmeterol in clinically stable COPD. This lack of effect occurs despite significant improvements in lung function, including parameters of hyperinflation, and, in cases of tiotropium treatment only, significant improvements in exercise endurance. We assume that the reduced costs of breathing relative to total energy expenditure were too small to be detected. Future studies should focus on the effects of bronchodilators on cardiovascular and local leg performance during submaximal exercise.
Acknowledgments
The authors thank Therese van Hoogdalem and Monica Leever for assisting during the cycle tests, as well as Judith Cohen, Margot Gosman, Eric Bathoorn, and Frank Volbeda for guiding the patients through the study. We thank the participating patients and the hospitals that helped recruit them: Scheper Ziekenhuis in Emmen (Dr Steven Rutgers), Refaja Ziekenhuis in Stadskanaal (Dr Gerard Versijp and Dr Tineke Renkema), Wilhelmina Ziekenhuis in Assen (Dr Peter Hengel), Nij Smellinghe Ziekenhuis in Drachten (Dr Henk Los), and Bethesda Ziekenhuis in Hoogeveen (Dr Jos Horsch).
Footnotes
Disclosure
This investigator-initiated study was supported by Boehringer Ingelheim bv, Alkmaar, The Netherlands.
References
- 1.Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med. 2006;119:21–31. doi: 10.1016/j.amjmed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell DE, Voduc N, Fitzpatrick M, Webb KA. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J. 2004;24:86–94. doi: 10.1183/09031936.04.00072703. [DOI] [PubMed] [Google Scholar]
- 3.Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128:1168–1178. doi: 10.1378/chest.128.3.1168. [DOI] [PubMed] [Google Scholar]
- 4.Van Noord JA, Smeets JJ, Otte A, et al. The effect of tiotropium, salmeterol and its combination on dynamic hyperinflation in COPD. Proc Am Thorac Soc. 2005;2:A542. [Google Scholar]
- 5.Perrault H. Efficiency of movement in health and chronic disease. Clin Invest Med. 2006;29:117–121. [PubMed] [Google Scholar]
- 6.Baarends EM, Schols AM, Akkermans MA, Wouters EF. Decreased mechanical efficiency in clinically stable patients with COPD. Thorax. 1997;52:981–986. doi: 10.1136/thx.52.11.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 8.Blackie SP, Fairbarn MS, McElvaney GN, et al. Prediction of maximal oxygen uptake and power during cycle ergometry in subjects older than 55 years of age. Am Rev Respir Dis. 1989;139:1424–1429. doi: 10.1164/ajrccm/139.6.1424. [DOI] [PubMed] [Google Scholar]
- 9.Carter R, Peavler M, Zinkgraf S, et al. Predicting maximal exercise ventilation in patients with chronic obstructive pulmonary disease. Chest. 1987;92:253–259. doi: 10.1378/chest.92.2.253. [DOI] [PubMed] [Google Scholar]
- 10.Gaesser GA, Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. J Appl Physiol. 1975;38:1132–1139. doi: 10.1152/jappl.1975.38.6.1132. [DOI] [PubMed] [Google Scholar]
- 11.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society [see comments] Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 12.Van Der Molen T, Willemse BW, Schokker S, et al. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes. 2003;1:13. doi: 10.1186/1477-7525-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scano G, Grazzini M, Stendardi L, Gigliotti F. Respiratory muscle energetics during exercise in healthy subjects and patients with COPD. Respir Med. 2006;100:1896–1906. doi: 10.1016/j.rmed.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Maltais F, Reissmann H, Gottfried SB. Pressure support reduces inspiratory effort and dyspnea during exercise in chronic airflow obstruction. Am J Respir Crit Care Med. 1995;151:1027–1033. doi: 10.1164/ajrccm/151.4.1027. [DOI] [PubMed] [Google Scholar]
- 15.Richardson RS, Leek BT, Gavin TP, et al. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med. 2004;169:89–96. doi: 10.1164/rccm.200305-627OC. [DOI] [PubMed] [Google Scholar]
- 16.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168:10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:542–549. doi: 10.1164/ajrccm.160.2.9901038. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell DE, Fluge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23:832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 19.Simon M, LeBlanc P, Jobin J, et al. Limitation of lower limb VO(2) during cycling exercise in COPD patients. J Appl Physiol. 2001;90:1013–1019. doi: 10.1152/jappl.2001.90.3.1013. [DOI] [PubMed] [Google Scholar]
- 20.Harms CA, Babcock MA, McClaran SR, et al. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 21.Harms CA, Wetter TJ, St Croix CM, et al. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- 22.Harms CA, Wetter TJ, McClaran SR, et al. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 23.Romer LM, Lovering AT, Haverkamp HC, et al. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol. 2006;571:425–439. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Travers J, Laveneziana P, Webb KA, et al. Effect of tiotropium bromide on the cardiovascular response to exercise in COPD. Respir Med. 2007;101:2017–2024. doi: 10.1016/j.rmed.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Maesen BL, Westermann CJ, Duurkens VA, van den Bosch JM. Effects of formoterol in apparently poorly reversible chronic obstructive pulmonary disease. Eur Respir J. 1999;13:1103–1108. doi: 10.1034/j.1399-3003.1999.13e27.x. [DOI] [PubMed] [Google Scholar]
- 26.Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121:1042–1050. doi: 10.1378/chest.121.4.1042. [DOI] [PubMed] [Google Scholar]
- 27.Perrault H, Gravel G, Ofir D, et al. Cycling efficiency is not compromised for moderate exercise in moderately severe COPD. Med Sci Sports Exerc. 2007;39:918–925. doi: 10.1249/mss.0b013e3180383d50. [DOI] [PubMed] [Google Scholar]
- 28.Creutzberg EC, Schols AM, Bothmer-Quaedvlieg FC, et al. Acute effects of nebulized salbutamol on resting energy expenditure in patients with chronic obstructive pulmonary disease and in healthy subjects. Respiration. 1998;65:375–380. doi: 10.1159/000029298. [DOI] [PubMed] [Google Scholar]
- 29.Hugli O, Schutz Y, Fitting JW. The daily energy expenditure in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:294–300. doi: 10.1164/ajrccm.153.1.8542132. [DOI] [PubMed] [Google Scholar]
- 30.Burdet L, de Muralt B, Schutz Y, Fitting JW. Thermogenic effect of bronchodilators in patients with chronic obstructive pulmonary disease. Thorax. 1997;52:130–135. doi: 10.1136/thx.52.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]