Abstract
Cancer pain impairs the quality of life of cancer patients, but opioid intervention can cause significant side effects that further decrease quality of life. Although electroacupuncture (EA) has been used to treat cancer pain, its mechanisms are largely unknown. To examine its effects and underlying mechanisms on cancer pain, we injected AT-3.1 prostate cancer cells into the tibia to induce bone cancer in the male Copenhagen rat. The resulting pain was treated with 10 Hz/ 2 mA/ 0.4 ms pulse EA for 30 min daily at the point equivalent to the human acupoint GB30 (Huantiao) between days 14 and 18 after the injection. For sham control, EA needles were inserted into GB30 without stimulation. Thermal hyperalgesia, a decrease in paw withdrawal latency (PWL) to a noxious thermal stimulus, and mechanical hyperalgesia, a decrease in paw withdrawal pressure threshold (PWPT), was measured at baseline and 20 min after the EA treatment. Preprodynorphin mRNA and dynorphin were respectively determined by RT-PCR and immunohistochemistry. Thermal and mechanical hyperalgesia developed ipsilaterally between days 12 and 18 after cancer cell inoculation. EA significantly (P<0.05) attenuated this hyperalgesia, increasing PWL and PWPT, and inhibited up-regulation of preprodynorphin mRNA and dynorphin compared to sham control. Intrathecal injection of antiserum against dynorphin A (1-17) also significantly inhibited the cancer-induced hyperalgesia.
These results suggest that EA alleviates bone cancer pain at least in part by suppressing dynorphin expression, and they support the clinical use of EA in the treatment of cancer pain.
Keywords: Cancer pain, Acupuncture, Hyperalgesia, Spinal cord, Dynorphin
1. Introduction
The aim of the present study was to evaluate the efficacy and mechanisms of EA on bone cancer pain in an established rat model (Zhang et al. 2005c).
Cancer pain resulting from metastasis to skeletal bone is the most common physical symptom of cancer patients and is extremely disruptive to patients’ quality of life. Bone metastases have been identified at autopsy in up to 90% of patients dying from prostate cancer (Rana et al. 1993; Bubendorf et al. 2000) and 85% of those dying from breast or lung cancer (Nielsen et al. 1991). Opioid treatment can cause such significant side effects as sedation, nausea, vomiting, constipation, hallucinations, nightmares, urinary retention, multifocal myoclonus and dizziness (Pasternak 1988; McNicol et al. 2003). Reportedly, 36-50% of cancer patients suffer from pain severe enough to compromise their daily lives (Strang 1998; Cleary 2000). Consequently, there is a clinical need for new therapies, with few side effects, that can be used to treat and alleviate tumor-induced pain.
Acupuncture, in which filiform needles are inserted into acupuncture points and manually twirled, has been used in China and other Asian countries for thousands of years to treat a variety of diseases and symptoms, including pain (Cheng 1999). Recent studies demonstrate that electroacupuncture (EA), in which an electrode is attached to the acupuncture needle to provide pulsating electrical stimulation, has significant therapeutic effects on rat models of chronic inflammatory (Lao et al. 2004), neuropathic (Dai et al. 2001), and ankle sprain pain (Koo et al. 2002) as well as on a mouse model of cutaneous cancer pain (Mao-Ying et al. 2006). Our study with an animal model of inflammatory pain showed that 10 Hz EA at acupoint Huantiao (GB30) significantly attenuates hyperalgesia (Lao et al. 2004). However, the effects of EA on bone cancer pain have not previously been scientifically investigated.
Acupuncture has been used clinically to treat cancer-related pain, but the data on the efficacy of acupuncture/EA in cancer pain are ambiguous and the mechanisms of the effects of acupuncture on such pain are not clear (Lee et al. 2005; Bardia et al. 2006); It has, however, been well demonstrated that EA provides significantly greater improvement than sham control in function and pain scores in patients with osteoarthritis of the knee (Berman et al. 2004).
Prodynorphin, whose 2400 base mRNA is encoded by four exons, is the precursor of a group of opioid peptides, including alpha-neo-endorphin, dynorphin A, and dynorphin B. These peptides may modulate a variety of responses such as pain perception, intestinal peristalsis, feeding, sleep and general activities (Civelli et al. 1985). Dynorphin acts on kappa opioid receptors to impede nociceptive signals (Laughlin et al. 2001). However, intrathecal dynorphin induces pain behavior (Laughlin et al. 1997; Lai et al. 2006). It has been demonstrated that spinal preprodynorphin (PPD) mRNA and dynorphin peptides are significantly up-regulated in inflammatory and neuropathic pain models (Ruda et al. 1988; Kajander et al. 1990; Zhang et al. 2005a). A study with prodynorphin knock-out mice showed that up-regulated spinal dynorphin is pronociceptive and is required for the maintenance of persistent neuropathic pain (Wang et al. 2001). It has been reported that a competitive N-methyl-D-aspartate (NMDA) receptor antagonist, D(-)-2-amino-5-phosphonovaleric acid (D-APV), and an NMDA ion-channel blocker, MK-801, inhibited dynorphin-induced pain behavior (Tan-No et al. 2002). Dynorphin acts at a polyamine site of the NMDA receptor to produce its excitatory effects (Caudle and Dubner 1998). The data suggest that dynorphin interacts with the NMDA receptor leading to nociceptive effect (Laughlin et al. 2001; Wollemann and Benyhe 2004). Recent studies also demonstrate that spinal dynorphin promotes pain through its action in sensory neurons by activating bradykinin receptors (Lai et al. 2006). All these data imply that spinal cord dynorphin plays an important role in persistent pain.
Recently, it was reported that dynorphin was up-regulated in a mouse bone cancer model (Schwei et al. 1999). But the involvement of dynorphin in bone cancer-induced pain has not been previously investigated. Therefore in this study we investigated the effect of EA on bone cancer pain, dynorphin involvement in such pain and the effects of EA on bone cancer-induced PPD mRNA expression and dynorphin production. We hypothesized that EA would significantly inhibit bone cancer-induced hyperalgesia and suppress PPD mRNA and dynorphin up-regulation in the spinal cord.
2. Methods
Male Copenhagen rats weighing 200-220g (Harlan) were kept under controlled conditions (22°C ± 0.5°C, relative humidity 40-60%, 7:00am to 7:00pm alternate light-dark cycles, food and water ad libitum). The commonly used Sprague–Dawley rats (Harlan) do not respond well to AT-3.1 cells (unpublished observations). The animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine.
2.1 Experimental design
The study consisted of the following three experiments. Experiment 1: Effects of EA on hyperalgesia. Bone cancer rats were divided into the EA treatment (n=7) and sham EA (n=7) groups. Thirty-minute EA treatment was given on days 14-18, by which time cancer rats showed significant hyperalgesia in our previous study (Zhang et al. 2005c). Hind paw withdrawal latency (PWL) was determined at baseline and on day 12 and twenty minutes after EA treatment on days 15 and 18. Hind paw withdrawal pressure threshold (PWPT) was determined at baseline and on day 12 and twenty minutes after EA treatment on days 14 and 17. The investigator performing the behavioral tests was blind to the treatment assignments. After behavioral testing, rats were deeply anesthetized with pentobarbital sodium (60 mg/kg, i.p.) and the lumbar4-5 spinal cord was removed.
Experiment 2: Effect of EA on PPD expression in the spinal cord. The spinal cord from experiment 1 was used to measure relative dynorphin and its mRNA levels using immunohistochemistry and reverse transcription-polymerase chain reaction (RT-PCR), respectively.
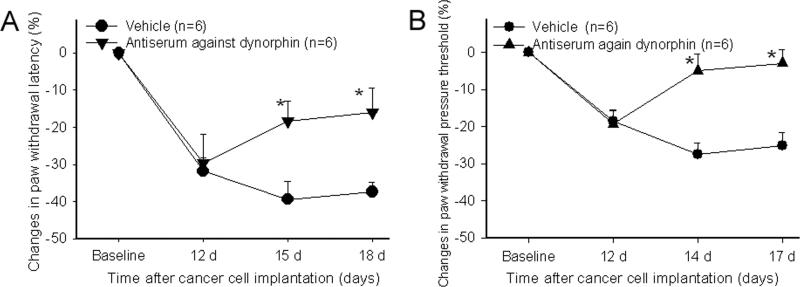
Experiment 3: Effects of antiserum against dynorphin A1-17 on hyperalgesia. Rats were prepared for intrathecal injection under pentobarbital sodium anesthesia (45 mg/kg, i.p.) before cancer cell implantation (Zhang et al. 2004a). Cancer rats fitted with intrathecal catheters were divided into dynorphin antiserum treatment (n=6) and saline control (n=6) groups. Intrathecal antiserum (Peninsula laboratories Inc., 50 μg/5 μl per rat) or saline administration was started 14 days post-cancer cell inoculation and continued daily until day 18. PWL and PWPT tests were conducted at baseline and 30 minutes post-drug on days 15 and 18 for PWL and days 14 and 17 for PWPT.
2.2. Cell culture and implantation
Detailed procedures for cell culture and cancer cell implantation into the tibia have been described previously (Zhang et al. 2005c). For cell culture, the AT-3.1 prostate cancer cell line, obtained from American Type Culture Collection (ATCC, Rockville, MD), was maintained in T-75 plastic flasks (Corning Glass), grown in RPMI 1640 medium (Sigma) supplemented with 250 nM dexamethasone and 10% fetal bovine serum (Sigma), and cultured in a water-saturated incubator in an atmosphere of 5% CO2:95% air. Cells were detached by a trypsin solution containing 0.05% trypsin and 0.02% EDTA and first collected by centrifuging 10 ml of medium for 3 min at 1200 rpm. The resulting pellet was washed twice with 10 ml of calcium- and magnesium-free Hank's solution and re-centrifuged for 3 minutes at 1200 rpm. The final pellet was diluted at final concentration of 3×105 cells/10 μl Hank's solution for injection and kept on ice until injection. For surgery, following complete induction of anesthesia with sodium pentobarbital (45 mg/kg, i.p.), one leg of the rat was shaved and the skin was disinfected with 7% tincture of iodine and 70% ethanol. A 1-cm long rostro-caudal incision was made in the skin over the upper medial half of the tibia. The tibia was carefully exposed and pierced with a 23-gauge needle 5 mm below the knee joint medial to the tibial tuberosity. A 10 μl volume of prostate cancer cells (3×105 cells) or vehicle (Hank's solution only) was injected into the bone cavity with a 50 μl Hamilton syringe. After a 2-minute delay while the cells filled the space in the bone cavity, the syringe was removed and the injection site was closed using bone wax (Ethicon). The muscle was stitched and the skin wound was closed using 3–0 silk threads. Each rat was monitored for general condition and changes in body weight during the 20-day experiment.
2.3 Thermal and mechanical hyperalgesia
The rats were tested for PWL by a previously described method (Hargreaves et al. 1988; Zhang et al. 2004b). Briefly, they were placed under an inverted clear plastic chamber on the glass surface of the Paw Thermal Stimulator System (UCSD, San Diego, CA) and allowed to acclimatize for 30 minutes before the test. A radiant heat stimulus was applied to the plantar surface of each hind paw from underneath the glass floor with a projector lamp bulb (CXL/CXR, 8 V, 50 W). PWL to the nearest 0.1 s was automatically recorded when the rat withdrew its paw from the stimulus. Stimulus intensity was adjusted to derive an average baseline PWL of approximately 10.0 s in naive animals. Paws were alternated randomly to preclude “order” effects. A 20-second cut-off was used to prevent tissue damage. Mean PWL was established by averaging the latency of four tests with a 5-minute interval between each test.
The rats used in the PWL test were also tested for mechanical hyperalgesia by determining the nociceptive PWPT with a Paw Pressure Analgesia Instrument (Ugo Basile, Italy) (Zhang et al. 2005c). The minimum paw pressure (in grams) that elicited paw withdrawal was defined as PWPT. A cut-off of 250 g was employed. Mean PWPT was established by averaging the values of four consecutive tests separated by intervals of 30 seconds.
2.4 EA treatment procedures
The EA treatment procedure was the same as that reported in our previous study (Lao et al. 2004). Ten Hz EA, which in prior studies showed significant anti-hyperalgesic effects in the rat inflammation model (Lao et al. 2004; Zhang et al. 2004a), was used in the present study. The equivalent of human acupoint GB30 on the rat's hind limbs was treated bilaterally. In humans, GB30 is located at the junction of the lateral 1/3 and medial 2/3 of the distance between the greater trochanter and the hiatus of the sacrum (Cheng 1999). The comparable landmarks were used to locate GB30 on the rat. GB30 was chosen based on traditional Chinese medicine (TCM) meridian theory (O'Connor and Bensky 1981), its successful use in our previous studies, and its use in studies by others (Xu et al. 1993; Lao et al. 2001; Lao et al. 2004).
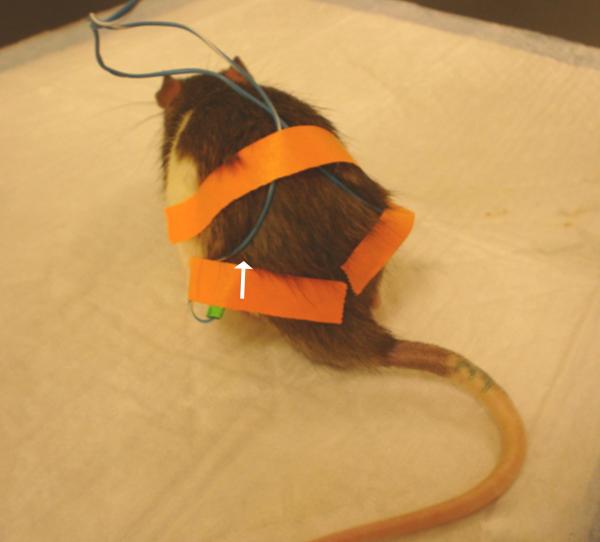
The animals were gently handled for 30 minutes each day and habituated to the plastic chamber for two to three days. After cleaning the skin with alcohol swabs, two acupuncture needles (gauge # 32, 0.5 inch in length) were swiftly inserted approximately one-half inch deep into each hind limb of the rat at GB30 by one investigator while another gently held the animal. The needles were stabilized with adhesive tape (Fig. 1) (Lao et al. 2004). EA was delivered by a stimulator (Electrostimulator 8-C, Pantheon Research Inc., Huntington Beach, CA) via two electrodes at 10Hz, 2 mA, 0.4 ms pulse width for 30 minutes on days 14-18 post-surgery. One end of each electrode was soldered to the needle handle in advance, and its other end was connected to the output channel of the Electrostimulator. A symmetrical biphasic wave was delivered to the electrodes so that the stimulation to the acupoints alternated bilaterally between positive and negative. To minimize discomfort, stimulation intensity was gradually increased over a period of two minutes to 2 mA, which we have found to be the maximum level that can be tolerated by unrestrained rats. Mild muscle twitching was observed. During EA treatment, each rat was placed under an inverted clear plastic chamber (approximately 5”× 8”×11”) but was neither restrained nor given anesthetic. The animals remained awake and still during treatment and gave no observable signs of distress. For sham control, acupuncture needles were inserted bilaterally into GB30 without electrical or manual stimulation. Sham EA showed little anti-hyperalgesia in our previous study (Lao et al. 2004), making it an appropriate control for non-specific needling effects. Sham-treated and EA-treated animals were handled identically.
Fig. 1.
Showing the needle in hind legs. Arrow points to GB 30.
2.5 Reverse transcription-polymerase chain reaction
RT-PCR was used to determine effects of EA on PPD mRNA expression (Zhang et al. 2005a). The lumbar4-5 spinal cord (n=4 per group) was removed and separated into ipsilateral and contralateral sections. Total RNAs were extracted using the RNeasy Mini kit (QIAGEN sciences, Germantown, MD). The RT-PCR reaction mixture (50 μl) contained 250 ng of total RNA as template, 5 × QIAGEN OneStep RT-PCR buffer providing a final concentration of 2.5 mM MgCl2, 4 deoxynucleoside triphosphates (0.4 mM each), enzyme mixture (Omnizcript and Sensiscript reverse transcriptase, HotStarTaq DNA ploymerase), 1 μM of each of the 5′ and 3′ PPD sequence-specific target primers (5′-TGATGAATGATGAAGCCGCAC-3′/5′-ACCGAGTCACCACCTTGAACTG-3′), and 0.6 μM of each of the 5′ and 3′GAPDH sequence-specific target primers (5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′/5′CATGTAGGCCATGAGGTCCACCAC-3′). The primers were synthesized by the Biopolymer/Genomics Core Facility at the University of Maryland. PCR was performed after reverse transcription at 50 °C for 30 minutes and initial denaturation at 95 °C for 15 minutes. The temperature cycles (Roche, Branchburg, NJ) were 94 °C/30 s (denaturing), 55 °C/40 s (annealing), and 72 °C/1 min (extension). A total of 26 cycles and a final 10-minute extension at 72 °C were conducted. The PCR amplified fragments, 282 bp for PPD and 983bp for GAPDH, were separated on 3% ethidium bromide-stained agarose gel with 1Kb plus DNA ladder (cat no. 10787-018, Invitrogen, Carlsbad, CA). The PCR gel image was captured and analyzed by a gel documentation system (DigiGenius Syst. DG1T, SYNGENE, Frederick, MD). The positive PCR bands were purified (Wizard DNA Clean-Up kit, Promega, Madison WI) and sequenced, and the resulting sequences were identical to the targeted cDNA sequences. The raw data from 4 individual RT-PCR analyses of each group were used for statistical analysis. Mock RT-PCR reaction controls included the omission of reverse transcriptase, primers, or template. No specific PCR product was found in these reactions.
2.6. Immunohistochemistry
After the behavioral test, rats (n=6 per group) were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and perfused transcardially with 4% paraformaldehyde (Sigma) in 0.1 M phosphate buffer (PB) at pH 7.4. The lumbar 4-5 spinal cord was cut at 30 μm and stained as before (Zhang et al. 2005c). The stained sections were analyzed under a Nikon microscope for distribution of prodynorphin-immunoreactive cells within the spinal dorsal horn. Five sections were randomly selected from each animal for cell counting. Prodynorphin-immunoreactive cells were counted individually under a Nikon microscope using a 20X objective lens in laminae I-II and V-VI of each selected section, averaged separately for sections of each rat, and then averaged for the group.
2.7. Statistical analyses
Data from the thermal and mechanical hyperalgesia tests were analyzed using analysis of variance (ANOVA) with repeated measures followed by post-hoc Scheffé's multiple comparisons (Statistical Analysis System). Data from the immunohistochemistry and RT-PCR studies were analyzed using between-subject ANOVA followed by Scheffé's multiple comparisons. P<0.05 was set as the level of statistical significance.
3. Results
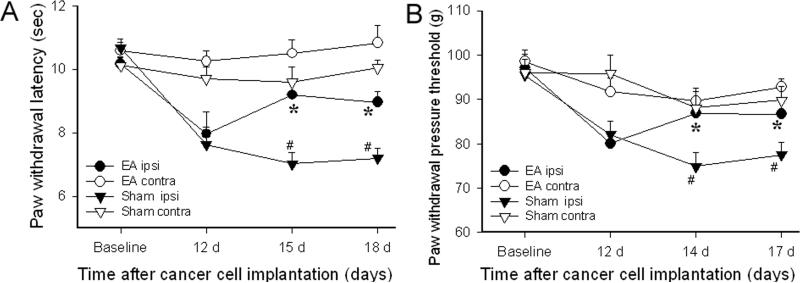
Figure 2A shows the effect of EA on PWL in bone cancer rats. Before the inoculation of the tibia with prostate cancer cells, there were no significant differences in overall mean baseline PWL to noxious thermal stimuli between the two groups of rats (10.57 ± 0.38 vs 10.11 ± 0.24 seconds). Statistical analysis revealed that cancer cell inoculation of the tibia induced a significant (P<0.05) decrease in PWL on days 15 and 18 after inoculation in ipsilateral hind paws. PWL of contralateral hind paws remained at the pre-injection level. EA treatment significantly (P<0.05) increased PWL of ipsilateral hind paws on days 15 and 18 compared to sham EA. These data indicate that bone cancer induced significant ipsilateral thermal hyperalgesia and that EA significantly alleviated this hyperalgesia. EA did not significantly increase the PWL of contralateral hind paws compared to baseline. Figure 2B shows the effect of EA on PWPT in bone cancer rats. Before prostate cancer cell inoculation of the tibia, there were no significant differences in the overall mean baseline PWPT to noxious mechanical stimuli among the groups of rats or in PWPT between left and right hind paws. Statistical analysis revealed that cancer cell inoculation of the tibia induced a significant (P<0.05) decrease of PWPT on days 14 and 17 after inoculation in ipsilateral hind paws compared to contralateral hind paws, which did not show any significant changes. The EA treatment significantly (P<0.05) increased the PWPT of ipsilateral, but not contralateral, hind paws compared to sham EA. These data demonstrated that bone cancer induces significant and progressive mechanical hyperalgesia which EA treatment significantly alleviated ipsilaterally, but that EA did not raise the mechanical pain threshold of the contralateral hind paws (Fig. 2B).
Fig. 2.
Effects of EA treatment on bone cancer-induced thermal and mechanical hyperalgesia (n=7 per group). Baseline signifies the PWL value before cancer cell implantation. EA at 10 Hz, 2 mA and 30 min was given on days 14-18. EA significantly increased PWL and PWPT of the hind paw ipsilateral to the cancer cell inoculation compared to sham EA, but it did not induce any significant changes contralaterally. *P<0.05 compared to sham EA; # P<0.05 compared to contralateral values; ipsi: ipsilateral; contra: contralateral.
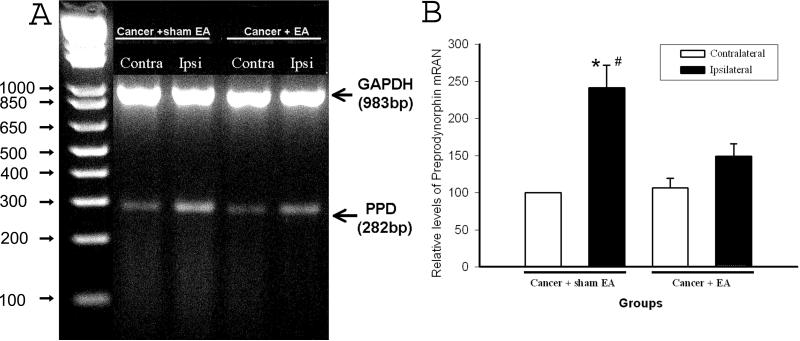
Ipsilateral PPD mRNA levels were significantly higher than contralateral levels in spinal cords of cancer rats given sham EA treatment (P<0.05). PPD mRNA levels in the contralateral spinal cords of sham EA-treated cancer rats showed no change compared to levels in sham cancer rats (data not shown). This suggests that bone cancer induces PPD mRNA up-regulation. Levels in the ipsilateral spinal cord were significantly lower in EA-treated cancer rats than in those given sham EA (P<0.05), while levels in the contralateral spinal cord were the same in both groups, indicating that EA significantly inhibited bone cancer-induced PPD mRNA transcription compared to sham EA (P<0.05). See Fig. 3.
Fig. 3.
Effect of EA treatment on bone cancer-induced PPD mRNA up-regulation in the spinal cord (n=4 per group). A: An example of agarose gel electrophoresis of PCR products. GAPDH PCR (a specific 983-bp segment of cDNA) was used as an internal control. B: Quantification of relative levels of spinal PPD mRNA expression. Each bar is expressed as a percentage (mean ± S.E.M.) of the levels of the contralateral spinal cord in cancer rats given sham EA, which is set arbitrarily as 100%. The PPD mRNA levels of the ipsilateral spinal cord were markedly higher than those of contralateral spinal cord in rats with cancer plus sham EA. EA suppressed up-regulated PPD mRNA compared to sham EA. # P<0.05 compared to contralateral spinal cord; *P<0.05 compared to EA-treated cancer rats.
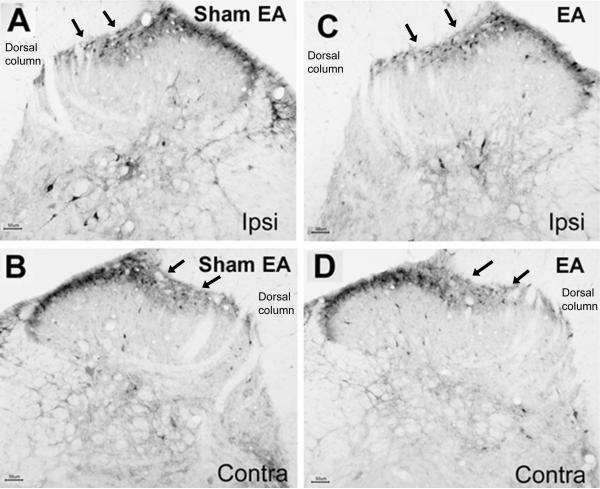
At the protein level, there were significantly more prodynorphin-labeled cells (P<0.05) in the ipsilateral spinal cord than in the contralateral spinal cord of cancer rats with sham EA in the laminae V-VI (8.9 ± 2.0 vs 3.7 ± 1.7/per section) and the medial half of the superficial laminae (23.4 ± 2.7 vs 14.1 ± 2.1; Fig. 4A & 4B). The prodynorphin-immunoreactive cells in laminae V-VI were darkly labeled ipsilaterally (Figs. 4A & 4C) and lightly labeled contralaterally (Figs. 4B & 4D). The results indicate bone cancer-induced dynorphin up-regulation.
Fig. 4.
Dynorphin staining of the L4–5 spinal cord 19 days after prostate cancer cell inoculation of the tibia (n=3). A-B: Dorsal horn of the ipsilateral (A) and contralateral (B) spinal cord from sham EA group. C-D: Dorsal horn of the ipsilateral (C) and contralateral (D) spinal cord from EA group. Note that there are many dynorphin immunostained cells on laminae I-II and V-VI. EA (C) markedly inhibited dynorphin expression in ipsilateral laminae V-VI compared to sham EA (A). Arrows point to medial laminae I-II. Scale bars are 50 μm; Ipsi: ipsilateral; Contra: contralateral.
There were also significantly more prodynorphin-labeled cells in the ipsilateral spinal dorsal horn in sham EA cancer rats than in EA-treated cancer rats in laminae V-VI (8.9 ± 2.0 vs 4.7 ± 1.7/per section) but not in the medial part of laminae I-II (23.4 ± 2.7 vs 21.2 ± 2.1/per section; Figs. 4A & 4C). This indicates that EA inhibited dynorphin up-regulation in deep laminae. Equal numbers of prodynorphin-labeled cells were observed in the contralateral spinal cords of EA and sham EA rats both in laminae I-II (26.6 ± 3.3 vs 28.6 ± 2.8) and V-VI (4.7 ± 1.2 vs 4.3 ± 1.9; Figs 4B & 4D). This suggests that EA did not affect dynorphin expression in the contralateral spinal cord. The number of prodynorphin-labeled cells of sham cancer rats was equal to that in the contralateral spinal cord of cancer rats (data not shown). Control sections showed no labeling.
Figure 5A shows the effect of dynorphin A (1-17) antiserum on PWL in bone cancer rats. Before prostate cancer cell inoculation of the tibia, there were no significant differences between the two groups of rats in overall mean baseline PWL to noxious heat stimuli or in PWL of the left and right hind paws. Statistical analysis revealed that antiserum treatment significantly (P<0.05) attenuated ipsilateral hyperalgesia compared to vehicle control, reducing the changes of the ipsilateral PWL from -39 ± 4.75% to -18.3 ± 5.4% on day 15 and from -37.3 ± 2.58% to -15.9 ± 6.65 seconds on day 18. It had no effect on PWL of contralateral hind paws (data not shown) (Fig. 5A).
Fig. 5.
Effects of dynorphin A1-17 antiserum on bone cancer-induced thermal (A) and mechanical (B) hyperalgesia (n=6 per group). Although data are presented as percent changes from the baseline level: (post-CFA - baseline)/ baseline × 100%, the actual PWL and PWPT data were used for statistical analysis. Antiserum administration (50 μg/5 μl/rat, i.t.) was started 14 days post-cancer cell inoculation and continued daily until day 18. The antisereum significantly increased PWL (A) and PWPT (B) of the hind paw ipsilateral to cancer cell inoculation compared to vehicle control. *P<0.05 compared to vehicle rats.
Figure 5B shows the effect of dynorphin A1-17 antiserum on PWPT in bone cancer rats. Statistical analysis revealed that the antiserum significantly (P<0.05) increased PWPT of the ipsilateral hind paws compared to vehicle saline. It had no effect on the PWPT of contralateral hind paws (data not shown).
These data demonstrated that dynorphin A1-17 antiserum significantly alleviates the mechanical hyperalgesia of the ipsilateral paws (Fig. 5B).
4. Discussion
EA significantly attenuates bone cancer-induced hyperalgesia as shown by the fact that EA treatment significantly increased PWL and PWPT ipsilaterally to the cancer cell inoculation compared to sham control. This is consistent with previous reports that EA inhibits pain in inflammatory, neuropathic and cutaneous cancer pain models (Dai et al. 2001; Koo et al. 2002; Zhang et al. 2004a; Mao-Ying et al. 2006). Although previous studies have commonly used acupoint ST36, our current study with a bone cancer pain model and previous study with an inflammatory pain model (Zhang et al. 2004a) demonstrate that EA treatment at GB 30 also significantly attenuates hyperalgesia. These data provide the rationale for using multiple points clinically to treat inflammation-, nerve injury- and cancer-caused pain. It should be mentioned that EA treatment in the present and previous (Zhang et al. 2004a) animal studies only partially inhibited pain. This may be due to the fact that EA treatment in animal studies only involves stimulation of two acupoints. This also supports the clinical usage of multiple acupoints.
Further, EA had no significant effect on the nociceptive threshold of the contralateral hind paw although it significantly inhibited ipsilateral hyperalgesia. The data indicate that EA at 10Hz, 3 mA, which is much lower than the EA intensity used in previous studies (Romita et al. 1997), had little anti-nociceptive effect in the normal paw but had a significant anti-hyperalgesic effect in the inflamed hind paw. Previous studies demonstrated that the receptive field size of ipsilateral dorsal horn nociceptive-specific and wide-dynamic-range neurons expanded in rats receiving a complete Freund's adjuvant injection in the hind paw when compared to control rats. MK-801 significantly reduced the receptive field size in rats with peripheral inflammation but had no significant effect on the receptive field size of dorsal horn neurons in rats without Freund's adjuvant-induced inflammation (Ren et al. 1992). These data demonstrate that the spinal cord ipsilateral to the inflamed paw exhibits hyper-excitability and hyper-responsiveness compared to the contralateral spinal cord. This may be why the relatively low EA current intensity (i.e. 3 mA) used in the present study was sufficient to induce a therapeutic effect ipsilaterally but not contralaterally.
The data also showed that spinal cord PPD mRNA and dynorphin were significantly up-regulated during bone cancer pain. This accords with a previous study demonstrating a large increase of dynorphin in spinal cord in tumor-implanted mice (Schwei et al. 1999). Notably, previous studies demonstrated that dynorphin is pronociceptive. For instance, neuropathic and inflammatory pain states are associated with increased spinal dynorphin expression (Ruda et al. 1988; Kajander et al. 1990; Zhang et al. 2005a). Intrathecal dynorphin induces pain behavior (Laughlin et al. 1997; Lai et al. 2006). Intrathecal administration of an antiserum to dynorphin A (1-17) reverses neuropathic pain (Malan et al. 2000) and enhances morphine-produced anti-allodynia in sciatic nerve ligated mice (Wu et al. 2005). These studies suggest that dynorphin may facilitate bone cancer-caused hyperalgesia. This is confirmed by our dynorphin antiserum intervention study, which demonstrates that intrathecal administration of an antiserum against dynorphin A (1-17) alleviates bone cancer-caused hyperalgesia. The data demonstrate that endogenous dynorphin is involved in the spinal transmission and processing of noxious inputs from the peripheral cancer area and that it facilitates bone cancer-induced hyperalgesia.
Moreover, we demonstrate that EA significantly inhibits bone cancer-induced PPD mRNA and dynorphin up-regulation in the ipsilateral spinal cord. The data suggest that EA is associated with decreased up-regulation of spinal dynorphin. Regarding the mechanisms of EA inhibition of dynorphin up-regulation, previous studies demonstrate that dynorphin is up-regulated to a greater extent in rats with spinal cord transections than in rats with intact spinal cords (MacArthur et al. 1999). This indicates that the descending inhibitory system may suppress dynorphin expression. Further, intrathecal MK-801 inhibits peripheral inflammation-induced PPD mRNA expression (Zhang et al. 1998). Therefore, we hypothesize that EA may activate the descending inhibitory system, which in turn suppresses excitatory amino acid activities and dynorphin expression. This warrants further study. However, we do not exclude the possibility that EA may modulate activities of other peptides or neurotransmitters. Actually, previous studies demonstrated that EA down-regulated NK1 expression (Zhang et al. 2005b) and up-regulated nociceptin/orphanin FQ (Fu et al. 2007). It is suggested that EA may modulate the activities of a group of complex chemicals to inhibit pain.
In conclusion, the present study demonstrates that EA attenuates bone cancer-induced hyperalgesia and that this effect may be due, at least in part, to EA inhibition of spinal dynorphin.
Acknowledgements
We would like to thank Dr. Lyn Lowry for her editorial support. This work was funded by NIH grant R21 CA102383 and P01 AT002605.
References
- Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of Complementary and Alternative Medicine Therapies in Relieving Cancer Pain: A Systematic Review. Journal of Clinical Oncology. 2006;24(34):5457–5464. doi: 10.1200/JCO.2006.08.3725. [DOI] [PubMed] [Google Scholar]
- Berman BM, Lao L, Langenberg P, Lee WL, Gilpin AMK, Hochberg MC. Effectiveness of Acupuncture as Adjunctive Therapy in Osteoarthritis of the Knee: A Randomized, Controlled Trial. Ann Intern Med. 2004;141(12):901–910. doi: 10.7326/0003-4819-141-12-200412210-00006. [DOI] [PubMed] [Google Scholar]
- Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Human Pathology. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Dubner R. Ifenprodil blocks the excitatory effects of the opioid peptide dynorphin 1-17 on NMDA receptor-mediated currents in the CA3 region of the guinea pig hippocampus. Neuropeptides. 1998;32(1):87–95. doi: 10.1016/s0143-4179(98)90022-1. [DOI] [PubMed] [Google Scholar]
- Cheng X. Chinese Acupuncture and Moxibustion. Foreign Languages Press; Beijing: 1999. [Google Scholar]
- Civelli O, Douglass J, Goldstein A, Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985;82(12):4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JF. Cancer pain management. Cancer Control. 2000;7(2):120–131. doi: 10.1177/107327480000700202. [DOI] [PubMed] [Google Scholar]
- Dai Y, Kondo E, Fukuoka T, Tokunaga A, Miki K, Noguchi K. The effect of electroacupuncture on pain behaviors and noxious stimulus-evoked Fos expression in a rat model of neuropathic pain. Journal of Pain. 2001;2(3):151–159. doi: 10.1054/jpai.2001.19964. [DOI] [PubMed] [Google Scholar]
- Fu X, Wang Y-Q, Wang J, Yu J, Wu G-C. Changes in expression of nociceptin/orphanin FQ and its receptor in spinal dorsal horn during electroacupuncture treatment for peripheral inflammatory pain in rats. Peptides. 2007;28(6):1220–1228. doi: 10.1016/j.peptides.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ. Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides. 1990;11(4):719–728. doi: 10.1016/0196-9781(90)90187-a. [DOI] [PubMed] [Google Scholar]
- Koo ST, Park YI, Lim KS, Chung K, Chung JM. Acupuncture analgesia in a new rat model of ankle sprain pain. Pain. 2002;99(3):423–431. doi: 10.1016/S0304-3959(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Lai J, Luo M, Chen Q, Ma S, Gardell L, Ossipov M, Porreca F. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nature Neuroscience. 2006;9(12):1534–1540. doi: 10.1038/nn1804. [DOI] [PubMed] [Google Scholar]
- Lao L, Zhang G, Wei F, Berman BM, Ren K. Electroacupuncture attenuates behavioral hyperalgesia and selectively reduces spinal Fos protein expression in rats with persistent inflammation. Journal of Pain. 2001;2:111–117. doi: 10.1054/jpai.2001.19575. [DOI] [PubMed] [Google Scholar]
- Lao L, Zhang R-X, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Research. 2004;1020(1-2):18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- Laughlin TM, Larson AA, Wilcox GL. Mechanisms of Induction of Persistent Nociception by Dynorphin. The Journal of pharmacology and experimental therapeutics. 2001;299(1):6–11. [PubMed] [Google Scholar]
- Laughlin TM, Vanderah TW, Lashbrook J, Nichols ML, Ossipov M, Porreca F, Wilcox GL. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain. 1997;72(1-2):253–260. doi: 10.1016/s0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Lee H, Schmidt K, Ernst E. Acupuncture for the relief of cancer-related pain--a systematic review. Eur J Pain. 2005;9(4):437–444. doi: 10.1016/j.ejpain.2004.10.004. [DOI] [PubMed] [Google Scholar]
- MacArthur L, Ren K, Pfaffenroth E, Franklin E, Ruda MA. Descending modulation of opioid-containing nociceptive neurons in rats with peripheral inflammation and hyperalgesia. Neuroscience. 1999;88(2):499–506. doi: 10.1016/s0306-4522(98)00204-8. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain. 2000;86(1-2):185–194. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Mao-Ying QL, Cui KM, Liu Q, Dong ZQ, Wang W, Wang J, Sha H, Wu GC, Wang YQ. Stage-dependent analgesia of electro-acupuncture in a mouse model of cutaneous cancer pain. European Journal of Pain. 2006;10(8):689–694. doi: 10.1016/j.ejpain.2005.11.001. [DOI] [PubMed] [Google Scholar]
- McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, Lau J, Carr D, Americal Pain S. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. Journal of Pain. 2003;4(5):231–256. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. Journal Of Clinical Oncology. 1991;9(3):509–524. doi: 10.1200/JCO.1991.9.3.509. [DOI] [PubMed] [Google Scholar]
- O'Connor J, Bensky D. Acupuncture: A Comprehensive Text. Eastland Press; Chicago: 1981. [Google Scholar]
- Pasternak GW. Multiple morphine and enkephalin receptors and the relief of pain. Jama. 1988;259(9):1362–1367. [PubMed] [Google Scholar]
- Rana A, Chisholm GD, Khan M, Sekharjit SS, Merrick MV, Elton RA. Patterns of bone metastasis and their prognostic significance in patients with carcinoma of the prostate. British Journal of Urology. 1993;72(6):933–936. doi: 10.1111/j.1464-410x.1993.tb16301.x. [DOI] [PubMed] [Google Scholar]
- Ren K, Hylden JLK, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50(3):331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Romita VV, Suk A, Henry JL. Parametric Studies on Electroacupuncture-Like Stimulation in a Rat Model: Effects of Intensity, Frequency, and Duration of Stimulation on Evoked Antinociception. Brain Research Bulletin. 1997;42(4):289–296. doi: 10.1016/s0361-9230(96)00264-x. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Iadarola MJ, Cohen LV, Young WSr. In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc Natl Acad Sci U S A. 1988;85(2):622–626. doi: 10.1073/pnas.85.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. Journal of Neuroscience. 1999;19(24):10886–10897. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang P. Cancer pain--a provoker of emotional, social and existential distress. Acta Oncologica. 1998;37(7-8):641–644. doi: 10.1080/028418698429973. [DOI] [PubMed] [Google Scholar]
- Tan-No K, Esashi A, Nakagawasai O, Niijima F, Tadano T, Sakurada C, Sakurada T, Bakalkin G, Terenius L, Kisara K. Intrathecally administered big dynorphin, a prodynorphin-derived peptide, produces nociceptive behavior through an N-methyl-aspartate receptor mechanism. Brain Research. 2002;952(1):7–14. doi: 10.1016/s0006-8993(02)03180-3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Jr, Lai J, Porreca F. Pronociceptive Actions of Dynorphin Maintain Chronic Neuropathic Pain. J Neurosci. 2001;21(5):1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollemann M, Benyhe S. Non-opioid actions of opioid peptides. Life Sciences. 2004;75(3):257–270. doi: 10.1016/j.lfs.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Wu H-E, Schwasinger ET, Hong J-S, Tseng LF. Pretreatment with antiserum against dynorphin, substance P, or cholecystokinin enhances the morphine-produced anti-allodynia in the sciatic nerve ligated mice. Neuroscience Letters. 2005;386(1):46–51. doi: 10.1016/j.neulet.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Xu R, Guan X, Wang C. Influence of capsaicin treating sciatic nerve on the pain threshold and the effect of acupuncture analgesia of rats. Acupuncture Research. 1993;18:280–284. [PubMed] [Google Scholar]
- Zhang R-X, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Research. 2004a;1020(1-2):12–17. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Zhang R-X, Liu B, Lao L, Qiao J-T, Ruda MA. Spinal preprodynorphin mRNA expression in neonatal rats following peripheral inflammation. Brain Research. 2005a;1038(2):238–242. doi: 10.1016/j.brainres.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Zhang R-X, Liu B, Qiao J-T, Wang L, Ren K, Berman BM, Lao L. Electroacupuncture suppresses spinal expression of neurokinin-1 receptors induced by persistent inflammation in rats. Neuroscience Letters. 2005b;384(3):339–343. doi: 10.1016/j.neulet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Zhang R-X, Liu B, Wang L, Ren K, Qiao J-T, Berman BM, Lao L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005c;118(1-2):125–136. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Lao L, Qiao JT, Ruda MA. Effects of aging on hyperalgesia and spinal dynorphin expression in rats with peripheral inflammation. Brain Research. 2004b;999(1):135–141. doi: 10.1016/j.brainres.2003.11.042. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Ruda MA, Qiao JT. Pre-emptive intrathecal Mk-801, a non-competitive N-methyl-D-aspartate receptor antagonist, inhibits the up-regulation of spinal dynorphin mRNA and hyperalgesia in a rat model of chronic inflammation. Neuroscience Letters. 1998;241(1):57–60. doi: 10.1016/s0304-3940(97)00969-5. [DOI] [PubMed] [Google Scholar]