Abstract
Clozapine is an antipsychotic medication with superior efficacy in treatment-refractory schizophrenia. The molecular basis of clozapine’s therapeutic profile is not well understood. We studied behavioral effects of clozapine in Caenorhabditis elegans to identify novel pathways that modulate clozapine’s biological effects. Clozapine stimulated egg laying in C. elegans in a dose-dependent manner. This effect was clozapine-specific, as it was not observed with exposure to a typical antipsychotic, haloperidol or an atypical antipsychotic, olanzapine. A candidate gene screen of biogenic amine neurotransmitter systems identified signaling pathways that mediate this clozapine-specific effect on egg laying. Specifically, we found that clozapine-induced increase in egg laying requires tyramine biosynthesis. To test the implications of this finding across species, we explored whether trace amine systems modulate clozapine’s behavioral effects in mammals by studying trace amine-associated receptor 1 (TAAR1) knockout mice. Clozapine increased pre-pulse inhibition (PPI) in wild-type mice. This increase in PPI was abrogated in TAAR1 knockout mice, implicating TAAR1 in clozapine-induced PPI enhancement. In transfected mammalian cell lines, we found no TAAR activation by antipsychotics, suggesting that modulation of trace amine signaling in mice does not occur directly at the receptor itself. In summary, we report a heretofore-unknown role for trace amine systems in clozapine-mediated effects across two species: C. elegans and mice.
Keywords: clozapine, antipsychotics, schizophrenia, tyramine, trace amine, TAAR1
1. Introduction
Clozapine is an atypical antipsychotic medication that has superior therapeutic efficacy in treatment-refractory schizophrenia (Kane et al., 1988; Kumra et al., 2008). It has multiple side effects, including the development of metabolic syndrome and an elevated risk of agranulocytosis (Krupp and Barnes, 1992; Lamberti et al. 2006). Though clozapine modulates various neurotransmitter receptors with inhibition constants (Ki) in the nanomolar range, the specific molecular mechanisms underlying clozapine’s superior therapeutic actions and its particular side effect profile are not known (Brunello et al., 1995; Schotte et al., 1996).
C. elegans provides a tractable system that can be used to characterize targets and pathways affected by psychoactive drugs (Weinshenker et al., 1996; Bettinger et al., 2004). Pathways originally described in C. elegans have been shown to underlie cellular mechanisms in mammalian biology (Horvitz and Sternberg, 1991; Hengartner and Horvitz, 1994; Fire et al., 1998; Horvitz, 1999; Ruvkun, 2001). Many behaviors under the control of the nervous system can be studied in C. elegans (Bargmann, 1998; Girard et al., 2007). Studies in C. elegans have led to the identification of novel targets affected by the antidepressant fluoxetine (Choy and Thomas, 1999), the antipsychotic clozapine (Karmacharya et al., 2009) and ethanol (Davies et al., 2003). C. elegans is increasingly being used to study the biology underlying psychiatric neuroscience (Donohoe et al., 2008a, 2008b; Zubenko et al., 2009; Weeks et al., 2010). Here, we investigated clozapine’s effects on C. elegans behavior and systematically tested mutants with defects in biogenic amine neurotransmitter systems to identify novel pathways involved in clozapine’s behavioral effects.
Effects observed in worms must subsequently be tested in mammalian systems to document their possible relevance in humans. Prepulse inhibition of acoustic startle (PPI) is an assay used in mammalian behavioral pharmacology to identify antipsychotics with therapeutic benefit (Geyer and Dulawa, 2003). PPI is a reduction of whole-body startle magnitude occurring when the startling stimulus is preceded by a non-startling pre-stimulus. Clozapine is known to enhance PPI across species, in mice and healthy humans, as well as in the special case of people with schizophrenia (Geyer et al., 2001). We extended our findings from C. elegans to investigate whether pathways identified in C. elegans mediate clozapine-induced PPI enhancement in mice. If not, the actions might not be relevant to humans and might not deserve further study in efforts to understand the clinical effects of clozapine.
We report the discovery of interactions between clozapine and trace amine signaling in both C. elegans and mouse. Trace amines refer to the compounds tyramine, tryptamine, octopamine and β-phenylethylamine (β-PEA) that are present in 1000-fold lower concentrations than the classical biogenic amines, dopamine, norepinephrine and serotonin, in the mammalian brain (Branchek and Blackburn, 2003; Berry, 2004, 2007). Trace amines were previously thought to be byproducts of monoamines in the mammalian brain, but the discovery of specific trace amine receptors expressed in the human nervous system suggests that trace amines are signaling molecules in human brain (Borowsky et al., 2001). Although trace amines have been implicated in the pathophysiology of mental illness (reviewed by Branchek and Blackburn, 2003), no previous genetic evidence has linked trace amines to the biological effects of antipsychotic drugs. Therefore, our finding of interaction between clozapine and trace amine signaling may have implications for the therapeutic actions of clozapine and other antipsychotic drugs in human brain.
2. Results
2.1 Studies in C. elegans
2.1.1 Effect of clozapine on egg laying in C. elegans
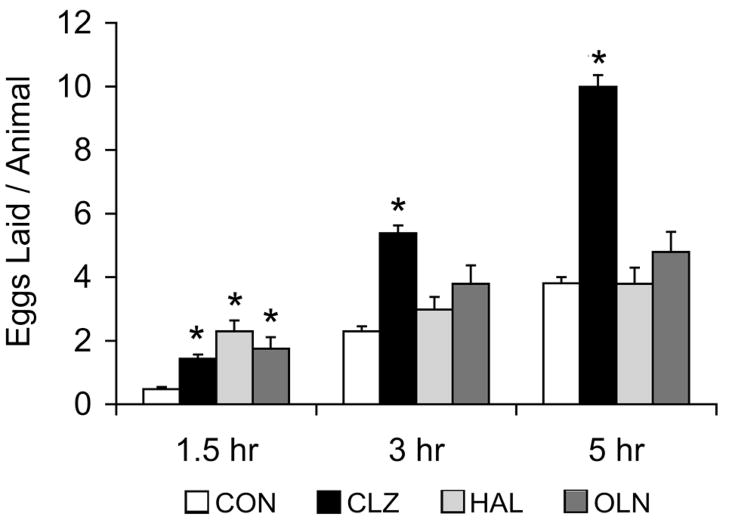
Egg laying is a phenotype that has been extensively studied in C. elegans (Trent et al., 1983; Weinshenker et al., 1995; Schafer, 2006). The neuropsychiatric drugs fluoxetine and imipramine, both antidepressants that modulate serotonin signaling, affect egg laying by a serotonergic mechanism (Dempsey et al., 2005). We tested the effects of clozapine on egg laying at various time points (Fig. 1). At 1.5 hours, exposure to clozapine led to more than a two-fold increase in egg laying compared to controls. Over time, the effect of clozapine became more pronounced. After 5 hours, animals exposed to clozapine displayed sustained increases in egg laying compared to animals under control conditions (Fig. 1). We carried out a dose-response study and found that clozapine increased egg laying in a dose-dependent manner (Fig. 2).
Figure 1.
Effects of different antipsychotics on egg laying. Mean number of eggs laid by gravid adult worms at different time-points during exposure to a control condition of 1% ethanol, clozapine, haloperidol, and olanzapine. On average, control animals (n=290) laid 2.3±0.2 eggs at 3 hours, whereas clozapine-exposed animals (n=285) laid 5.6±0.3 eggs at 3 hours (p<0.0001, unpaired t-test). On average, control animals (n=290) laid 3.7±0.2 eggs at 5 hours, whereas clozapine-exposed animals (n=285) laid 10.1±0.4 eggs at 5 hours (p<0.0001, unpaired t-test). Haloperidol (n=48) and olanzapine (n=24) produced no effect at 3 and 5 hours, even at the highest soluble doses that we could test, when compared to control. Error bars represent 1 SEM. * indicates p < 0.0001.
Figure 2.
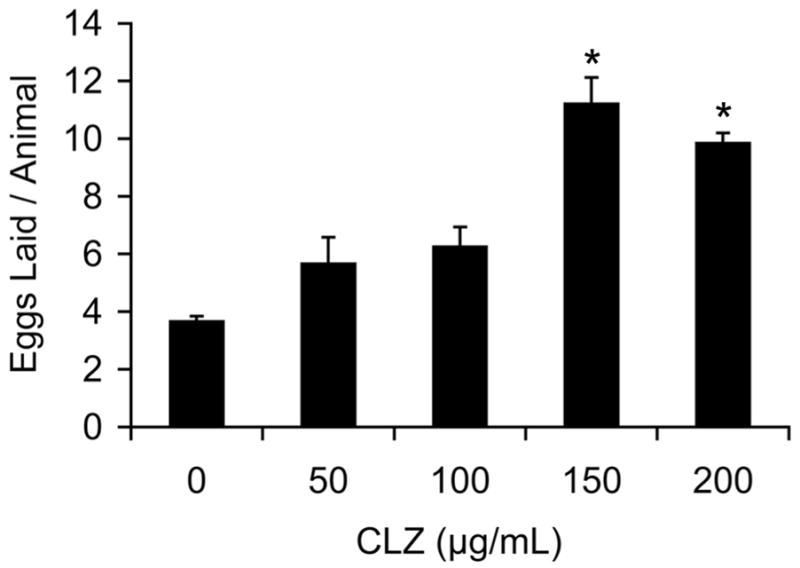
Dose-related effects on clozapine-induced egg laying. Mean number of eggs laid by gravid adult worms with exposure to different doses of clozapine at 5 hours. On average, control animals (n=290) laid 3.7±0.2eggs, compared to 5.7±0.9 eggs at 50 μg/ml (n=20), 6.3±0.7 eggs at 100 μg/ml (n=20), 11.2±0.9 eggs at 150 μg/ml (n=20), and 10.1±0.4 eggs at 200 μg/ml (n=285). Error bars represent 1 SEM. * indicates p < 0.0001 (unpaired t-test).
While clozapine is clinically unique in its superiority to other antipsychotics, it shares some biological effects with other antipsychotics. In order to determine whether clozapine’s effect on egg laying behavior is recapitulated by other antipsychotic medications, we tested the first-generation, typical antipsychotic haloperidol and an atypical antipsychotic olanzapine. Although haloperidol and olanzapine also increased egg laying after an exposure of 1.5 hours, the sustained increase in egg laying in response to clozapine was not observed with exposure to either haloperidol or olanzapine. There was no statistically significant difference in egg laying between haloperidol-exposed animals, olanzapine-exposed animals and control animals at the 3 hour and 5 hour timepoints (Fig. 1). We also found that the clozapine metabolite N-desmethyl-clozapine phenocopied the clozapine-induced increase in egg laying after 5 hours (data not shown).
While noting the unique effect of clozapine on egg laying, we add a caveat that the concentrations of the three drugs are different due to the different solubility profiles of the drugs. Animals were exposed to the highest soluble level of the drugs in 1% ethanol – clozapine was used at 200 μg/ml, olanzapine at 100 μg/ml and haloperidol at 50 μg/ml. We note that, clinically, both haloperidol and olanzapine are used in human patients at doses (~10–20 mg/day) that are more than an order of magnitude lower than clozapine (~400 mg/day). Nonetheless, we found that clozapine’s effect on egg laying was still observed at lower doses (Fig. 2).
2.1.2 Effect of mutations in biogenic amine pathways on clozapine-induced egg laying
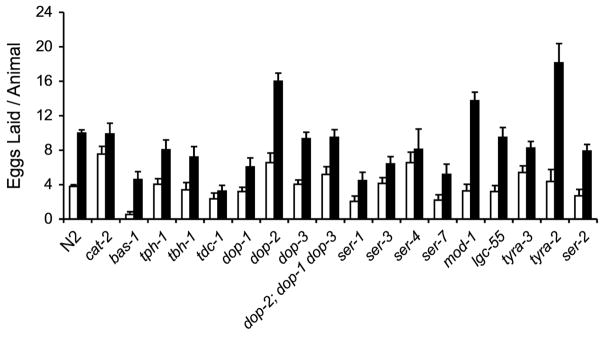
Since antipsychotics are known to affect signaling through multiple monoamines, including dopaminergic and serotonergic systems, we carried out a candidate gene screen in animals with mutations in genes required for these neurotransmitter systems (Table 1). We tested whether any of these mutants suppressed clozapine-induced egg laying. The cat-2 gene encodes a tyrosine hydroxylase involved in the synthesis of dopamine (Lints and Emmons, 1999). The tm2261 allele contains an out-of-frame deletion early in the coding sequence of cat-2 and therefore is likely to be a null. No dopamine is detectable in this strain by FIF assay (Sulston et al., 1975; National BioResource Project). bas-1 encodes an aromatic amino acid decarboxylase, and mutations in this gene yield worms deficient in serotonin and dopamine (Loer and Kenyon, 1993). The ad446 and tm351 alleles contain deletions in the coding sequence of bas-1 and are deficient in serotonin immunoreactivity (Hare and Loer, 2004). tph-1 encodes tryptophan hydroxylase, which is necessary for serotonin biosynthesis. The tph-1(mg280) deletion mutant does not synthesize serotonin (Sze et al., 2000). Clozapine-induced increases in egg laying were present in cat-2, bas-1 and tph-1 mutants (Fig. 3). Failure of these mutants to block the clozapine-induced increase in egg laying suggests that neither serotonin nor dopamine is required for clozapine’s effect on egg laying.
Table 1.
C. elegans strains used in behavioral assays
| Strain | Gene(allele) | Function |
|---|---|---|
| CB1112 | cat-2(e1112) | tyrosine hydroxylase |
| FX02261 | cat-2(tm2261) | tyrosine hydroxylase |
| MT7988 | bas-1(ad446) | aromatic amino acid decarboxylase |
| LC33 | bas-1(tm351) | aromatic amino acid decarboxylase |
| GR1321 | tph-1(mg280) | tryptophan hydroxylase |
| MT13113 | tdc-1(n3419) | tyrosine decarboxylase |
| RB993 | tdc-1(ok914) | tyrosine decarboxylase |
| MT9455 | tbh-1(n3427) | tyramine beta-hydroxylase |
| RB1161 | tbh-1(ok1196) | tyramine beta-hydroxylase |
| LX645 | dop-1(vs100) | D1-like dopamine receptor |
| LX702 | dop-2(vs105) | D2-like dopamine receptor |
| FX01062 | dop-2(tm1062) | D2-like dopamine receptor |
| LX703 | dop-3(vs106) | D2-like dopamine receptor |
| LX734 |
dop-2(vs105); dop-1(vs100) dop-3(vs106) |
|
| DA1814 | ser-1(ok345) | G-coupled serotonin receptor |
| RB1622 | ser-3(ok1995) | G-coupled serotonin receptor |
| AQ866 | ser-4(ok512) | G-coupled serotonin receptor |
| RB1585 | ser-7(ok1944) | G-coupled serotonin receptor |
| DA2109 |
ser-1(ok345); ser-7(tm1325) |
|
| MT9668 | mod-1(ok103) | serotonin-gated chloride channel |
| QW89 | lgc-55(tm2913) | tyramine-gated chloride channel |
| VC125 | tyra-3(ok325) | tyramine receptor |
| FX01815 | tyra-2(tm1815) | tyramine receptor |
| FX01846 | tyra-2(tm1846) | tyramine receptor |
| OH313 | ser-2(pk1357) | tyramine receptor |
Figure 3.
Effect of amine neurotransmitter mutants on clozapine-induced egg laying. The mean number of eggs laid by adult mutant worms, counted after 5 hours of exposure to 200 μg/ml of clozapine (black bars) and under control conditions of 1% ethanol (white bars). Error bars represent 1 SEM.
In the candidate gene screen, we were intrigued to find that a tdc-1 mutant showed no statistically significant increase in egg laying in response to clozapine, compared to control conditions (Fig. 3). tdc-1 worms contain a mutation in the tyrosine decarboxylase gene, which is required for the biosynthesis of tyramine and octopamine (Alkema et al., 2005). TDC-1 is co-expressed with TBH-1 in RIC neurons and in gonadal sheath cells. The RICs are a pair of head interneurons in the lateral ganglion. Their processes enter the ventral cord and run anteriorly into the nerve ring where they form gap junctions with the ASH and AVK neurons. Synaptic inputs to the RICs come from the CEP, OLQ, URB, and URX neurons, while the principal synaptic outputs of the RICs are the AVA, SMD, and SMBD neurons (White et al., 1986). TDC-1 is also expressed in the RIM neurons and uv1 cells that do not express TBH-1 (Alkema et al., 2005). Tyramine may be released from uterine uv1 cells onto the vulva (Schafer, 2006). The RIMs are a pair of motor neurons that modulate reversal behavior and that are required for suppression of head oscillations. The ALM/AVM sensory neurons are thought to stimulate tyramine release from the RIMs through activation of the AVA and AVD backward locomotion command neurons in response to anterior touch (Alkema et al., 2005).
We repeated the egg laying study with a different allele of tdc-1 to confirm our original observation. Both tdc-1(ok914), a strain with a 629 bp deletion removing part of exon 4, all of exons 5 and 6, and part of exon 7, and tdc-1(n3149), with a 578 bp deletion removing part of exon 6 and all of exon 7 suppressed clozapine-induced egg laying (Fig. 4). tdc-1, not bas-1, encodes the tyrosine decarboxylase required for the synthesis of both tyramine and octopamine, while tbh-1 encodes a tyramine β-hydroxylase gene that is required for octopamine biosynthesis (Alkema et al., 2005). tdc-1 mutants block clozapine-induced egg laying (Fig. 3, 4), but tbh-1 mutants do not (Fig. 3), suggesting that this phenotype is mediated by tyramine, not octopamine. We tested a number of tyramine receptor mutants, including tyra-2, tyra-3, lgc-55, and ser-2 (Rex and Komuniecki, 2002; Tsalik et al., 2003; Rex et al., 2005; Wragg et al., 2007; Pirri et al., 2009; Ringstad et al., 2009) and observed no effects of these mutations on clozapine-induced egg laying (Fig. 3). These results suggest that a currently unidentified tyramine receptor may mediate the effect of clozapine on egg laying. Alternatively, parallel pathways that employ different or multiple known receptors may mediate the effect of clozapine on egg laying.
Figure 4.
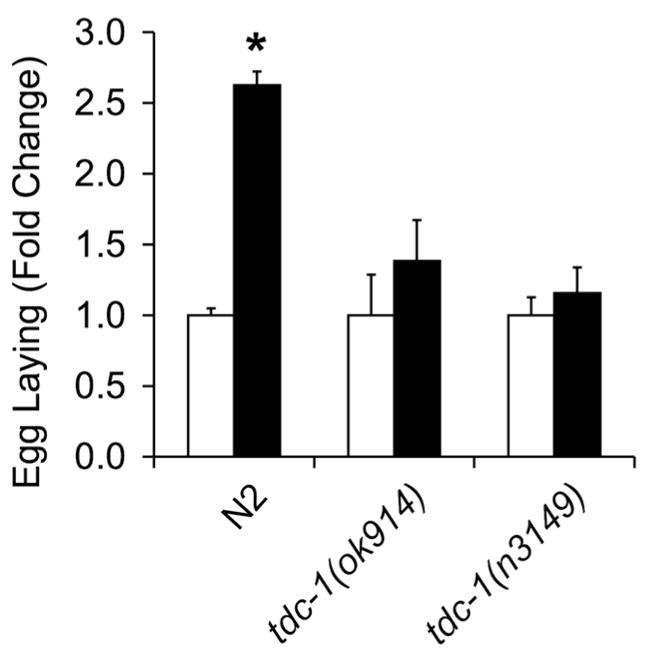
Suppression of clozapine-induced egg laying by tdc-1 mutants. Mean fold-change (black bars) in egg laying over baseline (white bars) in wild-type (N2) worms and in two strains of tdc-1 mutants. Error bars represent 1 SEM. * indicates p < 0.0001.
The increase in egg laying with clozapine was also not seen in egl-1(n487) or egl-1(n4065) mutants. On average (mean), egl-1(n4065) worms exposed to clozapine laid only 6.0±0.8 eggs (n=21), which is similar to the 6.5±0.8 eggs (n=24) that egl-1(n4065) worms laid under control conditions. In contrast, N2 worms exposed to clozapine laid an average of 10.1±0.4 eggs (n=285) compared to 3.7±0.2 eggs under control conditions (n=290). egl-1(gf) causes death of the HSN, the hermaphrodite-specific neuron that drives egg laying. This result suggests that clozapine stimulates egg laying through the nervous system, rather than by stimulating the vulval muscle directly.
That tdc-1(lf) suppresses clozapine-induced egg laying indicates that the presence of tyramine is required for this phenotype and that clozapine may trigger egg laying by directly or indirectly stimulating tyramine release. We therefore performed q-RT-PCR experiments to test the effect of clozapine on tdc-1 expression. Clozapine induced a dramatic reduction of tdc-1 expression suggesting a possible inhibitory feedback effect of clozapine-stimulated tyramine release on the expression of tdc-1 (Supplemental Fig. S1).
2.2 Studies in mammals
2.2.1 Effect of clozapine on activity of mammalian TAARs
TAAR1 in mice is a G protein-coupled receptor that activates adenylate cyclase. Therefore, we used a highly sensitive cAMP response element (CRE)-luciferase (Luc) assay to test whether clozapine as well as other antipsychotics can directly interact with mammalian TAARs. In our CRE-Luc assay, 1 μM clozapine did not change activity in TAAR1, TAAR3, TAAR5 or TAAR6 relative to baseline alone or when activated by forskolin (Supplemental Fig. S2). Nor was TAAR1 activity changed by 1 μM clozapine when activated by the TAAR1 agonists β-phenylethylamine (β-PEA) or methamphetamine (Xie and Miller, 2008, 2009; data not shown). These results are congruent with and extend those of Bunzow et al. (2001), who showed no antipsychotic-induced change in cAMP activation in cells transfected with rat TAAR1. Thus, we found no evidence that clozapine was a simple agonist or antagonist at the TAARs tested.
2.2.2 Studies of Clozapine-induced PPI enhancement in wild type and TAAR1 knockout mice
Using TAAR1 knockout mice, we tested the possibility that clozapine might have effects on a behavior mediated in part through TAAR1 (Wolinksy et al., 2007). Results of our mouse behavioral studies implicate TAAR1 in the therapeutic enhancement of PPI by clozapine (Fig. 5). An omnibus ANOVA across all conditions (2 strains × 2 treatments × 2 repeated-measures of prepulse intensity) of the pre- vs. post-injection difference in percent PPI showed a significant strain x treatment interaction (F1,45=4.4, p≤0.04). Post hoc analyses within each strain showed that clozapine-treated wild-type mice exhibited significantly enhanced PPI after three days of clozapine exposure compared to vehicle-treated controls (F1,23=13.8, p <0.001), while TAAR1 knockout mice receiving clozapine failed to exhibit elevated %PPI after three days of treatment compared to vehicle-treated controls (F1,22=0.3, p >0.6).
Figure 5.
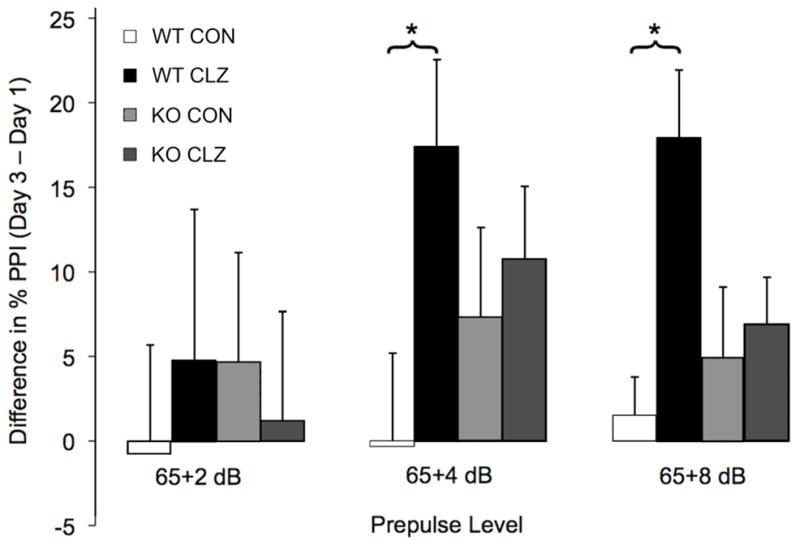
Effect of TAAR1 mutations on clozapine-induced PPI enhancement. Mean of the within-subject before- and after-treatment differences in startle amplitude is plotted over three prepulse levels for TAAR1 knockout and wild-type mice. Error bars represent 1 SEM. * denotes p < 0.001.
Knockout mice displayed somewhat elevated %PPI with respect to wild-type mice regardless of clozapine vs. vehicle treatment, suggesting that somewhat elevated PPI may be inherent to the knockout strain. Though the difference was not statistically significant, the failure of knockouts receiving clozapine to show elevated %PPI could conceivably have been due to a failure to elevate PPI above already high “baseline” levels. However, absolute %PPI values (Supplemental Fig. S3) show the typical monotonic increase in %PPI with prepulse level, indicating no such ceiling effect.
3. Discussion
The key mechanisms underlying clozapine’s superior therapeutic effects and specific side effect profile remain unclear. A central challenge in advancing the treatment of schizophrenia is the identification of signaling pathways relevant to both the unique therapeutic and detrimental effects of clozapine. Characterizing these pathways might enable the identification of new compounds that possess clozapine’s unique benefits but have a more favorable side effect profile. We undertook a study to identify novel pathways modulating clozapine-induced behavioral effects in C. elegans with subsequent follow-up of suggestive results in a mammalian system. We report heretofore-unrecognized interactions between antipsychotic medications and trace amine pathways in C. elegans and mice.
The cloning of a family of G-protein-coupled trace amine-associated receptors (TAARs) found in the mammalian brain has led to resurgent interest in trace amines as neuromodulators and neurotransmitters (Borowsky et al., 2001; Premont et al., 2001; Grandy, 2007; Liberles and Buck, 2008; Sotnikova et al,. 2009). Abnormal levels of trace amines have been reported in schizophrenia and TAAR genes have been associated with susceptibility for schizophrenia and bipolar disorder in some reports (Potkin et al., 1979, 1980; Branchek and Blackburn, 2003; Vladimirov et al., 2007; Pae et al., 2008a, 2008b). Some TAARs, including TAAR1, localize to rodent and primate amygdala (Xie et al., 2007; Lindemann et al., 2008), a region shown to activate anomalously in mood and psychotic disorders. In addition, TAARs map to chromosome 6 in humans near a susceptibility locus for schizophrenia (Bunzow et al., 2001).
We found that clozapine-induced increase in egg laying is suppressed in tdc-1 animals, which have a mutation in a tyrosine decarboxylase required for the synthesis of octopamine and tyramine (Alkema et al.. 2005). We followed the leads from our C. elegans studies to gain insight into the potential role played by signaling pathways activated by a trace amine receptor in clozapine’s effects in mice. We assayed prepulse inhibition of acoustic startle because of its frequent use in mouse-based behavioral pharmacology of antipsychotics and its association with therapeutic benefit in patients with schizophrenia. Startle reactivity and the prepulse induced attenuation/inhibition of the startle response (PPI) are commonly used behavioral assays believed to reflect, in part, dopaminergic sensory-motor gating of extraneous sensory information by forebrain structures in support of normal cognitive and motor function (Braff and Geyer, 1990; Swerdlow and Geyer, 1999). Patients with schizophrenia exhibit weak PPI (Braff et al., 2001) and show significant association of severity of psychotic symptoms with PPI impairment (Perry et al., 1999). PPI improves in step with other symptoms in patients on antipsychotic treatment and is a routinely used assay to screen new drugs for possible antipsychotic benefit (Geyer and Dulawa, 2003). We used PPI to examine the role of trace amine signaling in the therapeutic action of clozapine and discovered that TAAR1 knockout mice do not exhibit clozapine-induced enhancement of PPI. While this abnormality may be due to developmental or compensatory changes in the knockout mice, our findings nevertheless suggest that clozapine may enhance PPI in mice through modulation of TAAR1-mediated signaling pathways.
Our results indicate that clozapine interacts with trace amine-mediated pathways to affect behaviors in C. elegans and that the TAAR1 receptor many be involved in the enhanced PPI response to clozapine in mice. What is common in the results from both nematode and mouse is that functional trace amine signaling must be present for clozapine to produce the behavioral effects we measured. Since our data show minimal effects of clozapine in the activity of TAAR reporter lines, we speculate that clozapine’s modulation of trace amine signaling in mice does not occur directly at the receptor itself. It is possible, of course, that clozapine interacts with TAAR1 in a fashion that is not observed with the assays commonly used to detect agonists or antagonists.
Our data suggest an intriguing mechanism of clozapine action using behavioral-genetic screening in a nematode followed up in mice. However, there are limitations to generalization of results from C. elegans and rodents to humans. While many genes are conserved between nematodes and mammals, the similarities and differences in the binding sites for neurotransmitters and small molecules across species are not known in detail. Moreover, at this time we cannot distinguish whether clozapine-induced behaviors are a direct result of clozapine’s effects on trace amine signaling or whether other events occurring during clozapine exposure affect these behaviors, with trace amine signaling playing a permissive role. We also note that clear TAAR orthologs have not been identified in C. elegans based on overall sequence homology, though this does not rule out the possibility that such orthologs exist (Gloriam et al., 2005; Lindemann et al., 2005; Hashiguchi and Nishida, 2007; Hussain et al., 2009). For example, conservation of a small number of key residues can be sufficient for orthology, as in the case of cep-1, the C. elegans p53 ortholog (Derry et al., 2001). Similarly, it remains unclear whether mammalian homologs for invertebrate trace amine receptors exist, but this field is in rapid flux (Branicky and Schafer, 2009; Pirri et al., 2009; Ringstad et al., 2009). Another caveat in these studies is that exogenous application of drugs to C. elegans makes accurate control of tissue levels difficult. However, we have measured levels of clozapine in the tissue of worms exposed to clozapine and have found that the tissue level in C. elegans is in the same range (~18 mg/ml) as expected in the brains of humans patients (~11 mg/ml) (Karmacharya et al., 2009). Another important limitation in our approach is that the TAAR1 knockout mouse strain is not a conditional knockout. TAAR1 knockout mice exhibit potentially important differences relative to wild-type besides the lack of the target receptor. For example, D2 receptor density in the striatum is 2.6 times higher in TAAR1 knockout mice than in wild-type mice (Wolinsky et al., 2007). Though clozapine binds the D2 receptor weakly, our results must be interpreted in the context of these differences, inherent to the use of knockout technologies (Kapur et al., 1999; Gardner et al., 2005). Future studies using a recently discovered TAAR1 antagonist (Bradaia et al., 2009) could resolve this question.
With these notes of caution, we raise the possibility that antipsychotic drugs may exert some of their therapeutic effects in the human brain through modulation of trace amine systems. While there is some evidence linking trace amine signaling to the pathogenesis of schizophrenia, we believe that our report is the first to link the biological effects of antipsychotic drugs to trace amine signaling. Further exploration of the role of the trace amine systems in the pathophysiology and treatment of schizophrenia could lead to new and improved approaches to therapeutic intervention.
4. Experimental Procedures
4.1 C. elegans strains
Most of the strains used in this study were obtained from the Caenorhabditis Genetics Center (CGC) in Minneapolis, MN (Table 1). C. elegans Bristol strain N2 was used as the wild-type parent of our mutant strains. MT9455 tbh-1(n3247), GR1321 tph-1(mg280), DA1814 ser-1(ok345), FX01815 tyra-2(tm1815) and QW89 lgc-55(tm2913) were gifts from Dr. Mark Alkema (University of Massachusetts Medical Center, Worcester, MA). FX01062 dop-2(tm1062), FX02261 cat-2(tm2261) and FX01846 tyra-2(tm1846) were contributed by Dr. Shohei Mitani (National BioResource Project, Tokyo, Japan). The backcrossed tyra-3 strain was contributed by Dr. Richard Komuniecki (University of Toledo, Toledo, OH). We backcrossed the tdc-1 (ok914) strain to the N2 strain seven times and backcrossed the dop-2(tm1062) strain to the N2 strain six times. We obtained clozapine and haloperidol from Sigma-Aldrich and olanzapine from Waterstone Technology. Nematodes were grown and studied under standard culture conditions at 20°C unless otherwise noted.
4.2 Egg laying assay
We used staged worms 24 hours post-L4. Clozapine, olanzapine and haloperidol were dissolved in ethanol at the highest concentrations possible, 20 mg/ml, 10mg/ml and 5 mg/ml respectively, because the collagen cuticle of C. elegans forms a strong barrier to exogenously applied compounds. We conducted egg laying assays in 96-well microtiter plates. Each well contained 100 μl M9 solution with 200 μg/ml of clozapine, 100 μg/ml of olanzapine, 50 μg/ml of haloperidol or 1% ethanol (vehicle). One young adult worm was picked to an unseeded NGM plate and allowed to move away from the bacteria, then transferred to a well containing drug solution or control solution. The number of eggs laid was counted at 1.5, 3, and 5 hours.
4.3 Quantitative RT-PCR
Worms 24 hours post-L4 were placed on NGM plates containing 200 μg/ml clozapine or 1% DMSO plates. The worms were collected after five hours and RNA extracted by a standard Trizol-based protocol. After purification, cDNA was obtained from 1–2 μg mRNA by SuperScrpt® III Kit (Invitrogen, CA, USA). Real-time PCR were performed using these cDNAs as templates on MJ Research Opticon 2 System (BioRad, USA) with SYBR® Green qPCR reagents (Invitrogen, CA, USA). RT-PCR data were interpreted using the delta delta CT (2−;ΔΔCT) method to quantitate the relative change in tdc-1 expression, using actin-1 as a reference gene.
4.4 Cell-line studies
The HEK293 cell culture and transfection experiments were performed according to the procedures of Xie et al. (2007). HEK293 cells were transiently transfected with pcDNA 3.1 (+), rhesus monkey trace amine-associated receptor 1 (TAAR1), TAAR3, TAAR5 or TAAR6 along with CRE-Luc and pGL4.73 to generate cell lines: HEK, TAAR1, TAAR3, TAAR5 and TAAR6. Following transfection incubation (12 hours), the cells were exposed to 1 μM of each drug (clozapine, olanzapine, perphenazine, haloperidol, forskolin, or methamphetamine) for 18 hours in serum-free medium under growth conditions. To evaluate the influence of antipsychotics on activated TAARs, CRE-Luc expression was measured when these cell lines were exposed to vehicle or 1 μM of each drug in serum-free medium 5 minutes before and during 18 hour incubation with 1 μM forskolin, 1 μM β-PEA, or 1 μM methamphetamine. Three independent experiments were performed in triplicate for each condition.
4.5 Mice
A strain of mice engineered to lack the TAAR1 receptor has been developed and characterized (Wolinsky et al., 2007). TAAR1 knockout and wild-type mouse colonies were established at the New England Primate Research Center (Southborough, MA) from six pairs of heterozygous mice received as a gift from Lundbeck Research USA, Inc. (Paramus, NJ). The source strain is 129S1/SV, backcrossed two generations to C57BL/6. Verification of genotype and lack of expression of TAAR1 protein in the TAAR1 knockout mice was confirmed by PCR performed from genomic DNA of wild-type and TAAR1 knockout mice. Mice were kept on a 12 hour light/dark schedule at a room temperature of 22 °C with free access to food and water. Testing occurred during the light cycle. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, 1996) and all procedures were conducted in accordance with the Animal Experimentation Protocol approved by the Harvard Medical Area Standing Committee on Animals. Mice were approximately 34 g and 16 weeks old at testing. Only males were tested (n=24 KO mice, 12 received clozapine, 12 vehicle; n= 25 wild-type mice, 13 clozapine, 12 vehicle).
4.5.1 Drug dosing
Mice received once-daily IP injection of 4 mg/kg clozapine or vehicle for three days (after Zarate et al., 2004). Clozapine was dissolved in 2N HCl, brought to target volume with 0.9% saline, and to pH ~7 with 1N NaOH.
4.5.2 Prepulse inhibition
PPI experiments were conducted in a single SR-LAB mouse startle chamber (San Diego Instruments, San Diego, CA). We used PPI parameters used in the original study that characterized behavioral phenotypes of the TAAR1 knockout strain: 10 min acclimation to the startle chamber in the presence of 70 dB background noise; 20 ms prepulses 2, 4, or 8 dB above background preceding a 40 ms, 120 dB startle by 100 ms (Wolinsky et al., 2007). Fifty-six pseudo-randomized trials comprised prepulse-with-startle, prepulse-alone, startle-alone, and background-alone trials with a 15 sec variable inter-trial interval. We applied these parameters to a three-day clozapine administration protocol (Zarate et al., 2004) to examine within-subject change in PPI for clozapine vs. vehicle treated knockout and wild-type mice. On day 1, mice were tested prior to injection, on day 2 mice received injections only, and on day 3 mice were tested 1 hour post-injection. All groups were equated for mean startle-alone (F3,45=0.9, P>0.5) and day 1 PPI amplitudes (F3,45=0.2–0.5, P>0.7).
4.6 Statistical analysis
For the C. elegans studies, data are expressed as means±S.E.M. Statistical comparisons were performed using the unpaired, two-tailed Student’s t-test. For the cell-line studies, data were analyzed by ANOVA with Bonferroni adjustment. For the mouse PPI experiments, we used a mixed model ANOVA across conditions (2 strains × 2 treatments × 2 repeated-measures of prepulse intensity) to compare the pre- vs. post-injection difference in percent PPI.
Supplementary Material
Acknowledgments
This work was supported by the NIH Clinical Scientist Development Award K08MH086846, the Harvard Medical School Maria Lorenz Pope Fellowship, the American Philosophical Society Daland Fellowship, a NARSAD Young Investigator Award and the Harvard-MIT Health Sciences and Technology CITP Fellowship awarded to Rakesh Karmacharya; a Stanley Medical Research Institute Center grant to Bruce M. Cohen; NIH grants DA016606, DA022323, DA025697, & MH077995 to Gregory M. Miller; and an NIH Clinical Scientist Development Award K08NS002083, a Shervert Frazier Research Institute Grant and a NARSAD Young Investigator Award to Edgar A. Buttner. Additional support for Drs. Lynn and Miller was provided by NIH/NCRR grant RR00168 (NEPRC Base Grant). We thank Lauraine Dalton for assistance with figures, Laurie Lynch and Helen Panas for technical assistance, and Theresa Stiernagle at the C. elegans Genetics Center and the National BioResource Project (Tokyo, Japan) for worm strains used in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- Berry MD. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials. 2007;2:3–19. doi: 10.2174/157488707779318107. [DOI] [PubMed] [Google Scholar]
- Bettinger JC, Carnell L, Davies AG, McIntire SL. The use of Caenorhabditis elegans in molecular neuropharmacology. Int Rev Neurobiol. 2004;62:195–212. doi: 10.1016/S0074-7742(04)62007-1. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, Pinard A, Buchy D, Gassmann M, Hoener MC, Bettler B. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci USA. 2009;106:20081–20086. doi: 10.1073/pnas.0906522106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer M, Swerdlow N. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharm. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Blackburn TP. Trace amine receptors as targets for novel therapeutics: legend, myth and fact. Curr Opin Pharmacol. 2003;3:90–97. doi: 10.1016/s1471-4892(02)00028-0. [DOI] [PubMed] [Google Scholar]
- Branicky R, Schafer WR. Tyramine: a new receptor and a new role at the synapse. Neuron. 2009;62:458–460. doi: 10.1016/j.neuron.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Brunello N, Masotto C, Steardo L, Markstein R, Racagni G. New insights into the biology of schizophrenia through the mechanism of action of clozapine. Neuropsychopharm. 1995;13:177–213. doi: 10.1016/0893-133X(95)00068-O. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharm. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nature Neurosci. 2004;7:1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- Choy RK, Thomas JH. Fluoxetine-resistant mutants in C. elegans define a novel family of transmembrane proteins. Mol Cell. 1999;4:143–152. doi: 10.1016/s1097-2765(00)80362-7. [DOI] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Dempsey CM, Mackenzie SM, Gargus A, Blanco G, Sze JY. Serotonin (5HT), fluoxetine, imipramine and dopamine target distinct 5HT receptor signaling to modulate Caenorhabditis elegans egg laying behavior. Genetics. 2005;169:1425–1436. doi: 10.1534/genetics.104.032540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry WB, Putzke AP, Rothman JH. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science. 2001;294:591–595. doi: 10.1126/science.1065486. [DOI] [PubMed] [Google Scholar]
- Donohoe DR, Phan T, Weeks K, Aamodt EJ, Dwyer DS. Antipsychotic drugs up-regulate tryptophan hydroxylase in ADF neurons of Caenorhabditis elegans: role of calcium-calmodulin-dependent protein kinase II and transient receptor potential vanilloid channel. J Neurosci Res. 2008a;86:2553–2563. doi: 10.1002/jnr.21684. [DOI] [PubMed] [Google Scholar]
- Donohoe DR, Weeks K, Aamodt EJ, Dwyer DS. Antipsychotic drugs alter neuronal development including ALM neuroblast migration and PLM axonal outgrowth in Caenorhabditis elegans. Int J Dev Neurosci. 2008b;26:371–380. doi: 10.1016/j.ijdevneu.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe DR, Jarvis RA, Weeks K, Aamodt EJ, Dwyer DS. Behavioral adaptation in C. elegans produced by antipsychotic drugs requires serotonin and is associated with calcium signaling and calcineurin inhibition. Neurosci Res. 2009;64:280–289. doi: 10.1016/j.neures.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by doublestranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. Can Med Assoc J. 2005;172:1703–1711. doi: 10.1503/cmaj.1041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Dulawa S. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr Protocols Neurosci. 2003:8.17.11–18.17.15. doi: 10.1002/0471142301.ns0817s24. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow N. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacol. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Girard LR, Fiedler TJ, Harris TW, Carvalho F, Antoshechkin I, Han M, Sternberg PW, Stein LD, Chalfie M. WormBook: the online review of Caenorhabditis elegans biology. Nucleic Acids Res. 2005;35:D472–D475. doi: 10.1093/nar/gkl894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloriam DE, Schiöth HB, Fredriksson R. Nine new human Rhodopsin family G-protein coupled receptors: identification, sequence characterization and evolutionary relationship. Biochim Biophys Acta. 2005;1722:235–246. doi: 10.1016/j.bbagen.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Grandy DK. Trace amine-associated receptor 1--Family archetype or iconoclast? Pharmacol & Ther. 2007;116:355–390. doi: 10.1016/j.pharmthera.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare EE, Loer CM. Function and evolution of the serotonin-synthetic bas-1 gene and other aromatic amino acid decarboxylase genes in Caenorhabditis. BMC Evol Biol. 2004;4:24. doi: 10.1186/1471-2148-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi Y, Nishida M. Evolution of trace amine associated receptor (TAAR) gene family in vertebrates: lineage-specific expansions and degradations of a second class of vertebrate chemosensory receptors expressed in the olfactory epithelium. Mol Biol Evol. 2007;24:2099–2107. doi: 10.1093/molbev/msm140. [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Horvitz HR. Programmed cell death in Caenorhabditis elegans. Curr Opin Genet Dev. 1994;4:581–586. doi: 10.1016/0959-437x(94)90076-f. [DOI] [PubMed] [Google Scholar]
- Horvitz HR. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59:1701s–1706s. [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- Horvitz HR, Sternberg PW. Multiple intercellular signaling systems control the development of the Caenorhabditis elegans vulva. Nature. 1991;351:535–541. doi: 10.1038/351535a0. [DOI] [PubMed] [Google Scholar]
- Hussain A, Saraiva LR, Korsching SI. Positive Darwinian selection and the birth of an olfactory receptor clade in teleosts. Proc Natl Acad Sci USA. 2009;106:4313–4318. doi: 10.1073/pnas.0803229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for treatment-resistant schizophrenia. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- Karmacharya R, Sliwoski GR, Lundy MY, Suckow RF, Cohen BM, Buttner EA. Clozapine Interaction with Phosphatidyl Inositol 3-Kinase (PI3K)/Insulin Signaling Pathway in Caenorhabditis elegans. Neuropsychopharm. 2009;34:1968–1978. doi: 10.1038/npp.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp P, Barnes P. Clozapine-associated agranulocytosis: risk and aetiology. Br J Psychiatry. 1992;17(Suppl):38–40. [PubMed] [Google Scholar]
- Kumra S, Kranzler H, Gerbino-Rosen G, Kester HM, De Thomas C, Kafantaris V, Correll CU, Kane JM. Clozapine and “high-dose” olanzapine in refractory early-onset schizophrenia: a 12-week randomized and double-blind comparison. Biol Psychiatry. 2008;63:524–529. doi: 10.1016/j.biopsych.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Lamberti JS, Olson D, Crilly JF, Olivares T, Williams GC, Tu X, Tang W, Wiener K, Dvorin S, Dietz MB. Prevalence of the metabolic syndrome among patients receiving clozapine. Am J Psychiatry. 2006;163:1273–1276. doi: 10.1176/ajp.2006.163.7.1273. [DOI] [PubMed] [Google Scholar]
- Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, Hoener MC. Trace amine-associated receptor 1 (TAAR1) modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324:948–956. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- Lints R, Emmons SW. Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFβ family signaling pathway and a Hox gene. Development. 1999;126:5819–5831. doi: 10.1242/dev.126.24.5819. [DOI] [PubMed] [Google Scholar]
- Loer CM, Kenyon CJ. Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans. J Neurosci. 1993;13:5407–5417. doi: 10.1523/JNEUROSCI.13-12-05407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Pae CU, Drago A, Kim JJ, Patkar AA, Jun TY, Lee C, Mandelli L, De Ronchi D, Paik IH, Serretti A. TAAR6 variation effect on clinic presentation and outcome in a sample of schizophrenic in-patients: an open label study. Eur Psychiatry. 2008;23:390–395. doi: 10.1016/j.eurpsy.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Pae CU, Yu HS, Amann D, Kim JJ, Lee CU, Lee SJ, Jun TY, Lee C, Paik IH, Patkar AA, Lerer B. Association of the trace amine associated receptor 6 (TAAR6) gene with schizophrenia and bipolar disorder in a Korean case control sample. J Psychiatr Res. 2008;42:35–40. doi: 10.1016/j.jpsychires.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Perry W, Geyer MA, Braff DL. Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Arch Gen Psychiatry. 1999;56:277–281. doi: 10.1001/archpsyc.56.3.277. [DOI] [PubMed] [Google Scholar]
- Pirri JK, McPherson AD, Donnelly JL, Francis MM, Alkema MJ. A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron. 2009;62:526–538. doi: 10.1016/j.neuron.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Karoum F, Chuang LW, Cannon-Spoor HE, Phillips I, Wyatt RJ. Phenylethylamine in paranoid chronic schizophrenia. Science. 1979;206:470–471. doi: 10.1126/science.504988. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Wyatt RJ, Karoum F. Phenylethylamine (PEA) and phenylacetic acid (PAA) in the urine of chronic schizophrenic patients and controls. Psychopharmacol Bull. 1980;16:52–54. [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR, Caron MG. Following the trace of elusive amines. Proc Natl Acad Sci USA. 2001;98:9474–9475. doi: 10.1073/pnas.181356198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex E, Hapiak V, Hobson R, Smith K, Xiao H, Komuniecki RW. TYRA-2 (F01E11.5): a Caenorhabditis elegans tyramine receptor expressed in the MC and NSM pharyngeal neurons. J Neurochem. 2005;94:181–191. doi: 10.1111/j.1471-4159.2005.03180.x. [DOI] [PubMed] [Google Scholar]
- Rex E, Komuniecki RW. Characterization of a tyramine receptor from Caenorhabditis elegans. J Neurochem. 2002;82:1352–1359. doi: 10.1046/j.1471-4159.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- Ringstad N, Abe N, Horvitz HR. Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science. 2009;325:96–100. doi: 10.1126/science.1169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. Glimpses of a tiny RNA world. Science. 2001;294:797–799. doi: 10.1126/science.1066315. [DOI] [PubMed] [Google Scholar]
- Schafer WR. Genetics of egg laying in worms. Ann Rev Genet. 2006;40:487–509. doi: 10.1146/annurev.genet.40.110405.090527. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharm (Berl) 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Sotnikova TD, Caron MG, Gainetdinov RR. Trace amine-associated receptors as emerging therapeutic targets. Mol Pharm. 2009;76:229–235. doi: 10.1124/mol.109.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J, Dew M, Brenner S. Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol. 1975;163(2):215–226. doi: 10.1002/cne.901630207. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Neurophysiology and neuropharmacology of short lead interval startle modification. In: Dawson M, Schell A, Bohmelt A, editors. Startle modification: Implications for neuroscience, cognitive science, and clinical science. New York: Cambridge University Press; 1999. pp. 114–133. [Google Scholar]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsuing N, Horvitz HR. Egg laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol. 2003;263:81–102. doi: 10.1016/s0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirov V, Thiselton DL, Kuo PH, McClay J, Fanous A, Wormley B, Vittum J, Ribble R, Moher B, van den Oord E, O’Neill FA, Walsh D, Kendler KS, Riley BP. A region of 35 kb containing the trace amine associate receptor 6 (TAAR6) gene is associated with schizophrenia in the Irish study of high-density schizophrenia families. Mol Psychiatry. 2007;12:842–853. doi: 10.1038/sj.mp.4001984. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci. 1995;15:6975–6985. doi: 10.1523/JNEUROSCI.15-10-06975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks K, Dwyer DS, Aamodt EJ. Antipsychotic Drugs Activate the C. elegans Akt Pathway via the DAF-2 Insulin/IGF-1 Receptor. ACS Chem Neurosci. 2010;1:463–473. doi: 10.1021/cn100010p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode C. elegans. Philos Trans R Soc Lond Series B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP. The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav. 2007;6:628–639. doi: 10.1111/j.1601-183X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- Wragg RT, Hapiak V, Miller SB, Harris GP, Gray J, Komuniecki PR, Komuniecki RW. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J Neurosci. 2007;27:13402–13412. doi: 10.1523/JNEUROSCI.3495-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, Yao WD, Madras BK, Miller GM. Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J Pharmacol Exp Ther. 2007;321:116–127. doi: 10.1124/jpet.106.116863. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM. β-phenylethylamine alters monoamine transporter function via trace amine-associated receptor 1: Implication for modulatory roles of trace amines in brain. J Pharmacol Exp Ther. 2008;325:617–628. doi: 10.1124/jpet.107.134247. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM. A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J Pharmacol Exp Ther. 2009;330:316–325. doi: 10.1124/jpet.109.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate J, Boksa P, Baptista T, Joober R. Effects of clozapine on behavioral and metabolic traits relevant for schizophrenia in two mouse strains. Psychopharm (Berl) 2004;171:162–172. doi: 10.1007/s00213-003-1553-4. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Jones ML, Estevez AO, Hughes HB, 3rd, Estevez M. Identification of a CREB-dependent serotonergic pathway and neuronal circuit regulating foraging behavior in Caenorhabditis elegans: a useful model for mental disorders and their treatments? Am J Med Genet B Neuropsychiatr Genet. 2009;150B:12–23. doi: 10.1002/ajmg.b.30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.