Abstract
Resveratrol has been purported to modify risk factors for obesity and cardiovascular disease. We sought to examine the effects of resveratrol in a porcine model of metabolic syndrome and chronic myocardial ischemia. Yorkshire swine were fed either a normal diet (control), a high cholesterol diet (HCD), or a high cholesterol diet with supplemental resveratrol (HCD-R; 100mg/kg/day) for 11 weeks. After 4 weeks of diet modification a baseline cardiovascular MRI was performed and an ameroid constrictor was placed on the left circumflex coronary artery of each animal to induce chronic myocardial ischemia. At 7 weeks, a second cardiovascular MRI was performed and swine were sacrificed and myocardial tissue harvested. Resveratrol supplementation resulted in lower Body mass indices, serum cholesterol, and C-reactive protein levels, improved glucose tolerance and endothelial function, and favorably augmented signaling pathways associated with myocardial metabolism. Interestingly, serum tumor necrosis factor-α levels were not influenced by resveratrol treatment. Immunoblotting for markers of metabolism demonstrated that insulin receptor substrate-1, glucose transporters 1 and 4, and phospho-AMPK were increased in the HCD-R group. Peroxisome proliferator-activated receptor γ and retinol binding protein 4 were down-regulated in the HCD-R group as compared to the HCD group. Myocardial perfusion and function at rest as assessed with magnetic resonance imaging were not different between groups. By favorably influencing risk factors, resveratrol may decrease the burden of chronic metabolic disease and improve cardiovascular health.
Keywords: Resveratrol, Metabolic syndrome, Myocardial ischemia, Glucose metabolism, Myocardial infarction risk factor modification
1. Introduction
Obesity is a health problem of epidemic proportions throughout the developed world, in part, due to consumption of inexpensive, calorie-rich foods. Obesity is part of a quartet of risk factors for cardiovascular disease (central obesity, glucose intolerance, dyslipidemia, and hypertension) known as “metabolic syndrome” (Miranda et al., 2005). Approximately 50% of patients with coronary artery disease have metabolic syndrome (Milani and Lavie, 2003). Thus, modifying and decreasing the risk factors and prevalence of metabolic syndrome may improve cardiovascular health. A number of dietary supplements including vitamin E, omega-3 fatty acids, and resveratrol are being investigated for properties that may lead to decreased cardiovascular morbidity and mortality.
Resveratrol, found in abundance in red wine, has been shown to metabolically simulate calorie restriction (Lavu et al., 2008). Resveratrol is thought to activate sirtuin 1 (SIRT1), an NAD+-dependent deacetylase, which influences a diverse array of metabolic pathways. Studies in cultured cell and small animal models demonstrate that SIRT1 is involved in stress resistance, fat metabolism, cellular respiration (mitochondrial biogenesis), insulin production, inflammation, and glucose and lipid homeostasis (Bordone and Guarente, 2007; Yoshizaki et al., 2009). In previous studies we demonstrated the effect of resveratrol on perfusion and angiogenesis in the ischemic myocardium. Supplemental resveratrol resulted in improved flow reserve and upregulated the pro-angiogenic vasodilators vascular endothelial growth factor and endothelial nitric oxide synthase, but did not increase capillary density due to an upregulation of the anti-angiogenic protein angiostatin (Robich et al., 2010; Robich et al., 2010). Currently there are a paucity of data on the impact of resveratrol in human and large animal models of metabolic syndrome and chronic myocardial ischemia. The aim of this work was to examine the effects of resveratrol on risk factors that lead to the development of coronary artery disease.
Our hypothesis was that supplemental resveratrol would improve the burden of common chronic diseases and positively influence coronary artery disease risk factors in swine with diet-induced metabolic syndrome and chronic myocardial ischemia.
2. Materials and Methods
2.1 Animal Model
Yorkshire miniswine (Parsons Research, Amherst, MA) were fed one of three diets throughout the 11 week experiment. The first group was given 500 grams of a hypercholesterolemic diet daily (HCD, n=7) (2,248 kcal/day) composed of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow. A second group was fed the same hypercholesterolemic diet supplemented with 100mg/kg/day of resveratrol (HCD-R, n=7) (ChromaDex, Irvine, CA). The third group of swine was fed regular chow (control, n=7, 1824 kcal/day) and served as the control. All animals were housed singly, and were observed during feeding. All animals consumed the entire portion of feed each day. There have been concerns about the bioavailablity of orally delivered resveratrol, and some studies have shown low systemic concentrations. In one study, 25 mg of resveratrol was given to healthy volunteers and showed approximately 70% absorption, with peak plasma levels of resveratrol and metabolites of 491 ± 90 ng/ml and a plasma half-life of 9.2 ± 0.6 hours (Walle et al., 2004). Early studies in animal models and humans demonstrated no adverse effects at doses up to 1 gm/kg/day and 5 grams, respectively (Elliott and Jirousek, 2008). Thus we chose a higher dose in an attempt to achieve adequate cardiac tissue levels, and though these doses were higher than some previous work, they are well below the doses that were tolerated in other studies.
After 4 weeks of dietary modification, all animals underwent ameroid constrictor placement on the proximal left circumflex coronary artery. Anesthesia was induced with ketamine (10 mg/kg i.m) and thiopental 2.5%, and maintained with a gas mixture of oxygen at 1.5-2 l/min and 3.0% isoflurane. Animals were weighed and measured while under anesthesia to allow accurate measurements. Body mass index was calculated by using the weight of the animal and the length (from tip of the snout to the base of the tail along the back). During the first procedure, the pericardium was opened through a left mini-thoracotomy, and a titanium ameroid constrictor (1.75mm) was placed around the proximal left circumflex coronary artery. This device is designed to produce myocardial ischemia without causing infarction. Post operatively, the animals received a single dose of buprenorphine (0.03 mg/kg) and fentanyl (4 mcg/kg transdermal for 72 hours).
Seven weeks after ameroid placement, swine were again anesthetized and a functional cardiovascular magnetic resonance imaging (MRI) study was completed (please see details below). The heart was exposed through a median sternotomy and physiologic measurements were taken, followed by euthanasia. Blood pressure was measured using an intra-aortic pressure transducer. Myocardial tissue was harvested for study.
All experiments were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Animals were cared for in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee and in accordance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ (NIH publication no. 5377-3 1996).
2.2 Serum Cholesterol and Intravenous Glucose Tolerance Test
An intravenous glucose tolerance test was performed prior to placement of the ameroid and again prior to euthanasia. A fasting baseline blood glucose level was measured and dextrose, 0.5 gm/kg, was infused. Blood glucose levels were measured at 30 minutes and 60 minutes post-infusion, and are reported as mg/dl. Homeostatic model assessment of insulin resistance (HOMA-IR), a calculation to quantify insulin resistance and beta-cell function, was measured using fasting glucose and insulin levels. Serum C-reactive protein, total cholesterol, direct low-density lipoprotein (LDL) and high-density lipoprotein (HDL) measurements were performed by AccelLAB, Boisbriand, Quebec, Canada.
2.3 Serum Insulin and Tumor Necrosis Factor-α (TNFα) Levels
Serum insulin and TNFα levels were measured using commercially available swine insulin ELISA kits (ALPCO Diagnostics, Salem, NH). Undiluted serum, along with standards and controls were added to a 96-well plate pre-coated with specific anti-insulin or anti-TNFα monoclonal antibody.
2.4 Measurement of Global and Regional Myocardial Function
Indices of global and regional left ventricular function were obtained prior to animal sacrifice for 10 sequential beats via a catheter in the femoral artery using a Sonometrics system (Sonometrics Corp. London, ON, Canada).
2.5 Cardiovascular Magnetic Resonance Imaging
Animals underwent a functional cardiovascular magnetic resonance imaging study prior to sacrifice. A 1.5T Philips Achieva (Philips Healthcare, Best, Netherlands) with a five-element cardiac phased-array receiver coil was used. Left ventricular systolic function was assessed by cine short axis steady state free processing (SSFP) slices covering the entire left ventricle during suspended respiration (10mm thickness, 0mm gap). Myocardial perfusion at rest was assessed by first-pass turbo field echo-echo planar imaging in three short axis 10mm-thick slices (basal, mid, and distal ventricle) after injection of 0.05mmol/kg gadolinium-diethylenetriamine penta-acetic acid (Gd-DTPA) (Magnevist, Berlex/Schering AG, Berlin, Germany). Fifteen minutes after an additional 0.15mmol/kg Gd-DTPA, a late gadolinium enhancement- cardiovascular magnetic resonance imaging was performed in the 2 chamber, 4 chamber, and short axis orientations corresponding to SSFP cine slices (Kim et al., 1999).
2.6 Microvascular Responses
After cardiac harvest, coronary arterioles (80-180μm in diameter) from the left circumflex territory were placed in isolated microvessel chambers. Vessels were preconstricted by 25-50% of the baseline diameter with the thromboxane A2 analog, U46619 (10-6 M). The microvascular responses to sodium nitroprusside (SNP, 10-9 to 10-4 mol/l, an endothelium-independent vasodilator) and adenosine diphosphate (ADP, 10-12 to 10-7 mol/l, an endothelium-dependent vasodilator) were evaluated. Control microvessels were also subjected to ADP pretreated with a nitric oxide synthase (NOS) inhibitor, L-NG-Nitroarginine methyl ester. All reagents were obtained from Sigma-Aldrich (St Louis, MO).
2.7 Immunoblotting Studies
Whole-cell lysates were isolated from homogenized myocardial samples from the ischemic myocardium with radio-immunoprecipitation assay buffer (Boston Bioproducts, Worcester, MA). Sixty micrograms of total protein were fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (Invitrogen, San Diego, CA) and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). Each membrane was incubated with the following specific primary antibodies involved in glucose metabolism: glucose transporter 1 (GLUT 1)(Abcam, Cambridge, MA), GLUT 4 (Abcam), insulin receptor substrate-1 (IRS-1)(Cell Signaling, Beverly, MA), peroxisome proliferator-activated receptor gamma (PPARγ)(Cell Signaling), retinol binding protein 4 (RBP 4)(Cell Signaling), total and phosporylated AMP-activated protein kinase (AMPK)(Cell Signaling), and total and phosphorylaed mammalian target of rapamycin (mTOR)(Cell Signaling). Immune complexes were visualized with enhanced chemiluminescence (Amersham, Piscataway, NJ). Bands were quantified by densitometry of autoradiograph films. Ponceau staining was used to ensure equal protein loading.
2.8 Non-esterified fatty acids
Serum levels of non-esterified, “free”, fatty acids were measured by an enzymatic colorimetric assay (NEFA-HR, Wako Diagnostics, Richmond, VA). Coenzyme A (CoA) was acylated by non-esterified fatty acids in serum in the presence of acyl-CoA synthetase. Acyl-CoA was then oxidized by the addition of acyl-CoA oxidase, which produced hydrogen peroxide. Peroxidase was added the colorimetric product was measured at 550nm.
2.9 Protein oxidative stress
Dinitrophenylhydrazine-derivatized myocardial tissue homogenates samples were separated by 10% PAGE and transferred to PVFD membranes (Chemicon International, Inc. Temecula, CA). Membranes were incubated with a primary antibody specific for the 2,4-dinitrophenyl moiety, followed by incubation with secondary antibody. Immune complexes were visualized with the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ, USA).
2.10 Immunoflorescence
Frozen sections of ischemic myocardium were fixed in acetone and blocked. Anti-GLUT-4 primary antibody was applied (Epitomics, Burlingame, CA). Slides were washed and secondary antibody applied (Jackson Immunoresearch). Slides were mounted with 4′,6-diamidino-2-phenylindole (DAPI) and viewed with a confocal microscope. Photomicrographs were taken with a Zeiss Axiolab microscope (Carl Zeiss Inc, Thornwood, NY) equipped with a digital camera (Photodoc, Upland, CA).
2.11 Data Analysis
All results are expressed as mean ± standard error of the mean (S.E.M.). Microvessel responses are expressed as percent relaxation of the preconstricted diameter and were analyzed using a two-way, repeated-measures analysis of variance (ANOVA) with Bonferroni correction. Western blots were analyzed after digitalization of x-ray films using a flatbed scanner (ScanJet 4c; Hewlett-Packard, Palo Alto, CA) and NIH ImageJ 1.40g software (National Institute of Health, Bethesda, MD). For data analysis, levels of phosphorylated proteins were normalized to total levels. Comparisons between groups were analyzed by a one-way ANOVA with a Newman-Keuls Multiple Comparison post-hoc test.
3. Results
3.1 Experimental model
All animals survived the entire procedure. The swine had similar body mass indices (BMI) at the time of the ameroid placement (baseline, P=0.11). Immediately prior to sacrifice animals in the HCD group were significantly larger (Fig. 1A). The ameroid caused one-hundred percent occlusion of the left circumflex coronary artery based on an angiogram completed prior to sacrifice. Core body temperature at sacrifice was similar among the groups (P=0.68). There was no gross evidence of myocardial infarction in any of the animals.
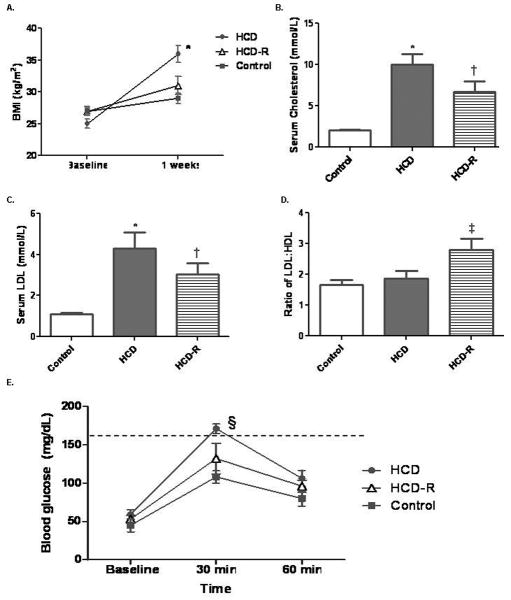
Fig. 1. Clinical results.
A) The BMI of animals in the HCD group were significantly higher than the control and HCD-R animals (*P< 0.001). B) Total cholesterol in the HCD group was significantly increased as compared to the control and HCD-R groups (*P<0.001). Though total cholesterol in the HCD-R group was about 30% lower than the HCD group it was still significantly increased when compared to the control group (†P<0.05). C) LDL was significantly increased in the HCD group as compared to the control and HCD-R groups (*P<0.001 and †P<0.05). D) The ratio of LDL:HDL demonstrated a significant increase in the HCD-R group (‡P=0.01). E) Blood glucose levels were significantly increased 30 minutes after dextrose infusion (§P=0.002). Dashed line indicates blood glucose of 160, above which is considered glucose intolerance.
3.2 Serum Cholesterol Measurements
The HCD group had significantly higher levels of total cholesterol as compared to the control and HCD-R groups. While total cholesterol in HCD-R was approximately 30 percent lower than HCD, it was still significantly higher than the control. Serum LDL demonstrated a similar pattern, with the HCD group significantly greater than control and HCD-R, but HCD-R still elevated compared to the control group. The ratio of LDL:HDL cholesterol was significantly higher in the HCD-R group as compared to the HCD and control groups, while there was no difference between the control and HCD groups (Fig. 1A-D).
3.3 Intravenous Glucose Challenge Testing
Pre-euthanasia intravenous glucose challenge testing showed no difference between groups at baseline measurement (P=0.4). Thirty minutes post-dextrose infusion, the HCD group had blood glucose levels significantly higher than the control and HCD-R groups. There was no statistical difference between the HCD-R group and the control group at 30 minutes. One hour post-glucose infusion there was no difference between any of the groups (P=0.2, Fig. 1E).
3.4 Functional Measurments
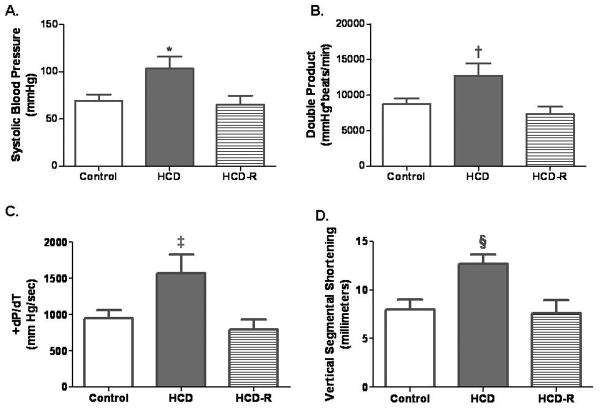
Systolic blood pressure was higher in the HCD group than either the control or HCD-R groups (Fig. 2A). There was no difference in heart rate (control 122.7±10.5 beats/min, HCD 129.4±15.7 beats/min and HCD-R 115.3±16.7 beats/min, P=0.8). The HCD group demonstrated an increased resting double product, a measure of myocardial work that is proportional to myocardial oxygen consumption (Fig. 2B). Measurement of +dP/dt and vertical segmental shortening in the ischemic territory demonstrated higher values in the HCD group (Fig. 2C and D).
Fig. 2. Myocardial function results.
A) Mean systolic blood pressure was significantly increased in the HCD group (*P=0.03). There was not a difference between the control and HCD-R groups. B) The double product was significantly increased in the HCD group (†P=0.02). C) The +dP/dt was increased in the HCD group, and there was no difference between the control and HCD-R groups (‡P=0.04). D) Vertical segmental shortening in the ischemic territory was increased in the HCD group (§P=0.01).
3.5 Cardiac Magnetic Resonance Imaging
There was normal perfusion at rest to the territories subtended by the left circumflex in all animals. Global left ventricular systolic function was also similar between groups. Cardiac index, Stroke-volume index, and left ventricular wall mass index were not different between groups. There was no evidence of mitral regurgitation in any of the animals (Table 1).
Table 1. Cardiovascular magnetic resonance imaging data.
| Control | HCD | HCD-R | p-value | |
|---|---|---|---|---|
| Left ventricular ejection fraction (%) | 39.4±5.4 | 45.9±4.4 | 48.5±6.9 | 0.6 |
| Cardiac index (mL/min/m) | 3.4±0.1 | 4.1±0.3 | 4.2±0.4 | 0.43 |
| Stroke volume index (L /(beat × m2) | 42.2±3.4 | 39.5±3.8 | 42.6±6.8 | 0.86 |
| Aortic flow (mL/min) | 38±1 | 38.4±2.6 | 38.6±6.3 | 0.99 |
| Left ventricular mass index (g/m2) | 96±3.4 | 80.5±4.9 | 91.1±1.5 | 0.16 |
| Mitral regurgitation (mL/beat) | 1.4±0.2 | 3.7±0.9 | 2.5±1.0 | 0.38 |
3.6 Microvascular Responses
There was no difference in the baseline diameter of the microvessels between groups (control 157 ± 12 μm, HCD 139 ± 10 μm, and HCD-R 137 ± 7 μm, P=0.37), or the preconstricted diameter of the microvessels when the responses to ADP were examined (control 99 ± 7 μm, HCD 92 ± 8 μm, and HCD-R 85 ± 6 μm, P= 0.43). There was no difference in the percent vessel preconstriction between the groups with U46619 (control -37 ± 2%, HCD -34 ± 2%, and HCD-R -39 ± 3%, P= 0.42).
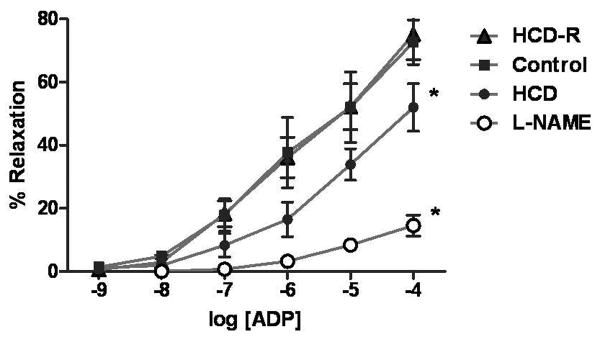
Response of microvessels from the collateral dependent territory to the endothelial-independent drug SNP demonstrated no difference between groups (P=0.95). Endothelial-dependent microvascular relaxation response to ADP (10-4 M) was diminished in HCD swine but was normalized in the HCD-R group. Addition of L-NG-Nitroarginine methyl ester led to a marked decrease in the vasorelaxation response of the control coronary microvessels as compared to ADP alone (Fig. 3).
Fig. 3. Myocardial microvascular response to adenosine diphosphate (ADP).
The microvascular relaxation response to the endothelial-dependent vasorelaxing agent ADP is dysfunctional in the HCD group and normalized to the control in the HCD-R group (*P<0.001). Addition of L-NG-Nitroarginine methyl ester, a specific nitric oxide inhibitor, with ADP to control vessels demonstrates a significant decrease in microvascular vasorelaxation as compared to ADP alone (*P<0.001).
3.7 Myocardial Protein Oxidation
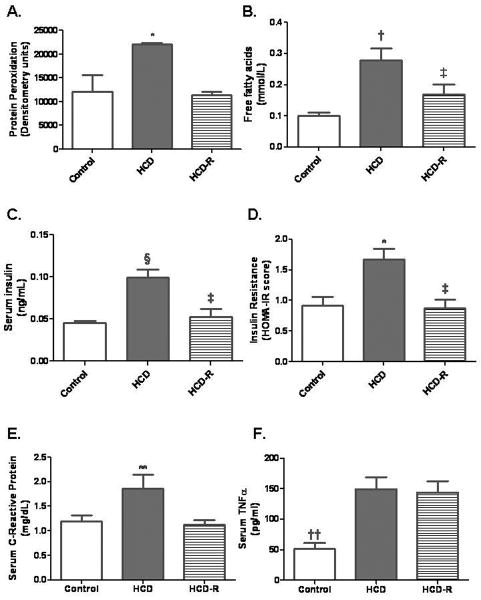
Measurement of the levels of protein peroxidation demonstrated significantly increased stress in the HCD and this was attenuated in the HCD-R group; HCD increased 1.8 fold v. control and 2.0 fold v. HCD-R (Fig. 4A).
Fig. 4. Assessment of protein oxidative stress, serum free fatty acids, serum insulin levels, and HOMA-IR.
A) Protein peroxidation in the ischemic territory was significantly higher in the HCD group (*P=0.03). B) The concentration of free fatty acids was increased significantly in the HCD group compared to the control and HCD-R groups. The concentration in the HCD-R group was significantly lower than in the HCD group (†P<0.001, ‡P<0.05). C) Fasting serum levels of insulin were significantly elevated in the HCD group, and decreased in the HCD-R group (§P=0.002, ‡P<0.05). D) HOMA-IR scores were higher in the HCD group and significantly lower in the HCD-R group as compared to the HCD group (**P=0.02, ‡P<0.5). E) Levels of C-reactive protein were increased in the HCD group and significantly lowered in the HCD-R group (**P=0.04). F) Serum concentration of TNFα were significantly lower in the control group as compared to the HCD and HCD-R groups (††P=0.002).
3.8 Serum Non-Esterified Fatty Acid Level
Serum levels of free fatty acids were significantly increased in the HCD group as compared to the control, and were significantly lower in the HCD-R group (Fig. 4B).
3.9 Serum Insulin Levels
Serum insulin levels were significantly elevated in the HCD group, but were normalized in the HCD-R group as compared to the control group (Fig. 4C).
3.10 Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)
HOMA-IR, an index of insulin resistance, was increased in the HCD group and significantly decreased in the HCD-R group as compared to HCD. There was no difference in HOMA-IR between HCD-R and control groups (Fig. 4D).
3.11 Markers of Inflammation
Serum levels of C-reactive protein were significantly increased in the HCD group and were normalized in the HCD-R group as compared to the control group (Fig. 4E). Conversely, serum levels of TNFα were not affected by resveratrol supplementation, and were significantly increased in both the HCD and HCD-R groups as compared to the control group (Fig. 4F). Local expression of TNFα in ischemic myocardial tissue was not different between the groups (P=0.36).
3.12 Immunoblotting
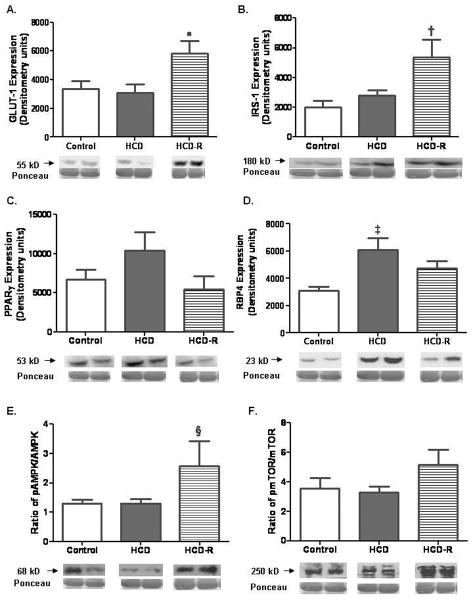
Glucose transporter-1 (GLUT 1) was increased in the HCD-R group, as was insulin receptor substrate-1 (IRS-1). Two proteins demonstrated increased expression in the HCD group, peroxisome proliferator-activated receptor-γ (PPARγ) and retinol binding protein-4 (RBP4).
Expression of phospho-AMPK (thr 132) was significantly increased in the HCD-R group. The expression of phospho-p38 mitogen activated protein kinase (MAPK) (thr180/tyr182) was not different between the groups (P=0.58). Levels of phosphorylated mammalian target of rapamycin (mTOR) (ser2448) were not different between groups. Rictor expression was equal in the HCD-R and control groups, and they were 1.4 fold elevated as compared to HCD; however, this did not reach statistical significance (P=0.18). p70S6K also was not significantly different between groups (P=0.38) (Fig. 5A-F).
Fig. 5. Protein immunoblotting studies.
A) Expression of GLUT 1 was significantly increased in the HCD-R group (*P=0.02). B) Insulin receptor substrate 1 (IRS-1) was increased in the HCD-R group (†P=0.03). There was no significant difference between the control and HCD-R groups (P>0.05). C) PPARγ had a trend toward increased expression in the HCD group (P=0.18). D) RBP4, an inhibitor of GLUT 1 and 4, was increased in the HCD group (‡P=0.009). E) The ratio of phospho-AMPK (thr172) to total AMPK expression was significantly increased in the HCD-R group (§P=0.03). F) The ratio of phospho-mTOR (ser2244) to total mTOR was not different between groups, P=0.28.
3.13 Glucose Transporter-4 (GLUT 4) Protein Expression and Localization within the Myocyte
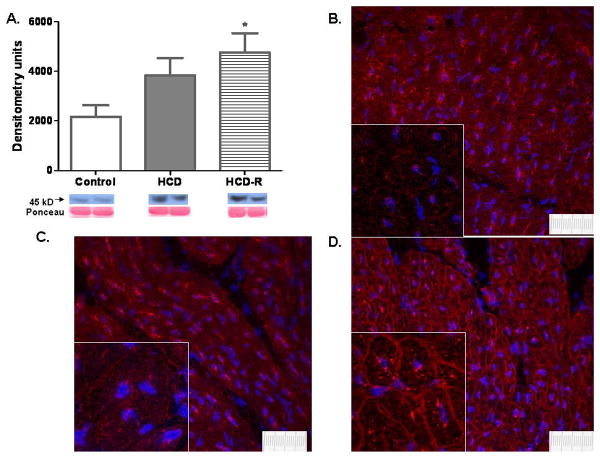
GLUT 4 protein expression was significantly increased only in the HCD-R group as compared to the control. Localization of GLUT 4 by immunofluorescence demonstrated a diffuse cytoplasmic and sarcolemmal distribution in the ischemic myocardium of the control group. In the HCD group, GLUT 4 protein was confined to the cytoplasm. In the HCD-R group the protein was heavily localized to the sarcolemmal membrane (Fig. 6A-D).
Fig. 6. Glucose transporter 4 (GLUT 4) protein expression and localization within the cell.
A) Protein expression of GLUT 4 was significantly increased in the HRCV group, though the HCD group had a trend to increased translation (*P=0.04). B) Immunofluorescence of GLUT 4 in the control group demonstrates diffuse and rather equal distribution of GLUT 4 in the cytoplasm and sarcolemmal membrane. C) GLUT 4 distribution in the HCD group demonstrates areas of high concentration in the cytoplasm. The protein is thought to be inactive in this location. D) In the HCD-R group GLUT 4 is heavily concentrated in the cell membrane. In this location it is active and likely at least partially responsible for improved glucose metabolism (40×, inset 180×, scale bar represents 10μm).
4. Discussion
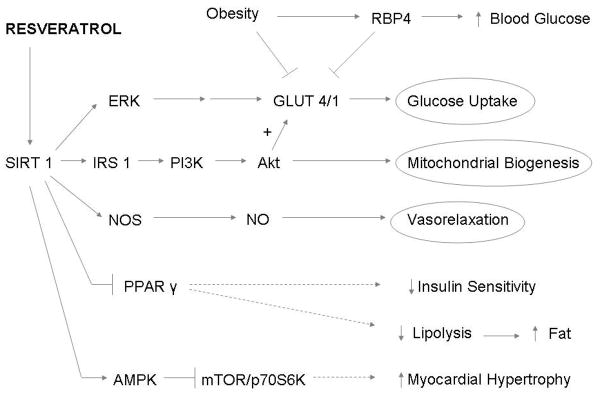
In this study, we examined the metabolic effects of resveratrol in a clinically relevant large animal model of metabolic syndrome and chronic myocardial ischemia. Our results demonstrated that orally supplemented resveratrol considerably modified major risk factors for cardiovascular disease. Many molecular pathways which regulate cardiac metabolism were altered in the ischemic myocardium (Fig. 7).
Fig. 7. Summary figure.
Schematic figure describing the metabolic molecular pathways influenced by resveratrol (→ indicates activation, ┤ indicates inhibition).
Our results show that a high fat, high cholesterol diet significantly increases BMI, and that resveratrol treatment alleviates the HCD-associated increase in weight. Calorie restriction has been shown in experimental studies to be an effective means of reducing many obesity-related diseases, including heart disease and cancer. SIRT1, stimulated by caloric restriction and resveratrol, is thought to upregulate a number of molecular pathways that lead to decreased weight. Resveratrol leads to enhanced lipolysis, which has been linked to improving the diseases associated with metabolic syndrome (Lavu et al., 2008). In addition, SIRT1 inhibits PPARγ which leads to decreased lipogenesis (Picard et al., 2004).
Resveratrol supplementation resulted in decreased total cholesterol and LDL levels by 33% and 30% respectively in the current study. These results are similar to those found in patients taking HMG CoA reductase inhibitors (statins) (Illingworth and Bacon, 1987). Possible mechanisms involve upregulation of enzymes involved with lipid metabolism and excretion in the liver. In particular, resveratrol stimulates scavenger receptor-B1, involved in reverse cholesterol transport which returns cholesteryl esters to the liver for excretion, and Cyp7A1, the rate limiting enzyme in bile acid synthesis and cholesterol degradation (Rodgers and Puigserver, 2007). Interestingly, levels of HDL were also decreased and the LDL:HDL ratio increased in the HCD-R animals. Possible explanations include decreased HDL production and increased metabolism. It has been shown that HDL production is linked to PPARα expression (Shah et al., 2009). If PPARα is downregulated this may lead to decreased HDL levels. Additionally, cholesteryl esters are returned to the liver by HDL and the entire complex is brought into the hepatocyte (Chen et al., 2000). The fate of HDL after endocytosis in vivo is not entirely clear, though it may be metabolized.
Resveratrol supplementation appears to have significantly reversed insulin resistance induced by HCD by normalizing insulin levels and the HOMA-IR index. Though the heart utilizes fatty acids as the main energy source, myocardial cells require glucose for proper function and glucose is especially important during ischemia (Liu et al., 1993). It has been postulated that the diabetic myocardium utilizes less glucose and preferentially increases free fatty acid uptake and oxidation (Vidavalur et al., 2006). The result is increased free radical production and decreased calcium dependent contractility. This situation makes the heart not only more susceptible to an ischemic event, but also increases the risk for a greater decline in ventricular function following an event (Barsotti et al., 2009).
A number of molecular pathways governing glucose metabolism in the ischemic myocardium were altered by resveratrol. We demonstrated an increase in expression of the GLUT-1 and -4 transmembrane proteins, which are involved in glucose transport into cardiomyocytes. They are translated within the cytoplasm of the cell and transported to the sarcolemmal membrane when activated (Vidavalur et al., 2006). It has been reported that AMPK plays an important role in activation and movement of GLUT 4 to the cell membrane (Weisova et al., 2009). AMPK activation is regulated by fatty acids and insulin, and results in improved glucose uptake into the cell and activation of energy producing pathways during stress (Fujii et al., 2006). Another important protein in glucose metabolism, IRS-1, was upregulated in the HCD-R group. This protein is involved in insulin binding and the signaling necessary for GLUT 4 translocation. Interestingly, GLUT 4 was localized in the cytoplasm in the HCD group, but was heavily localized to the sarcolemmal membrane in the HCD-R group. This supports the idea that GLUT 4 is stored in an inactive state without the signaling of AMPK and IRS-1 in the cardiomyocyte. However, signaling by insulin leads to increased AMPK and IRS-1 activation, which in turn stimulates the transition of GLUT 4 to the sarcolemmal membrane, as seen in the HCD-R group, where it is active.
Expression of RBP4 was increased in the HCD group and significantly decreased in the HCD-R group. RBP4 is an adipokine which inhibits glucose uptake and blocks insulin signaling in muscle (Muoio and Newgard, 2005). GLUT 4 and RBP4 protein expression levels are inversely proportional to one another, and this relationship is thought to be a cause of obesity-related diabetes (Graham et al., 2006). Thus with resveratrol decreasing free fatty acids available to the myocardium, and increasing glucose metabolism machinery, the detrimental energy switch to fatty acids may be mitigated, and theoretically the risk of fatal myocardial ischemic event lowered.
The altered substrate metabolism in dyslipidemia and diabetes (increased utilization of fatty acids) accelerates the oxidative stress burden (Stanley et al., 1997). Oxidative stress in the myocardium has been linked to global myocardial dysfunction, cellular injury and increased apoptosis, and this likely leads to ventricular remodeling (Barouch et al., 2003). Thus, reducing the amount of reactive oxygen species in the myocardium may act to improve function and minimize cardiomyopathy (Boudina and Abel, 2007). mTOR, a regulator of protein synthesis and cell growth, has been implicated in causing cellular senescence and pathologic cardiac hypertrophy (Wang et al., 2009). The proposed mechanism involves increased reactive oxygen species leading to production of reactive aldehydes which downregulate AMPK, an inhibitor of mTOR/p70S6K (Dolinsky et al., 2009). A study by Dolinsky et al. demonstrated that resveratrol prevented inhibition of AMPK which in turn inhibited mTOR/p70S6K leading to prevention of cardiac hypertrophy.
Animals in the HCD group had higher systolic blood pressure and double product, a measure of myocardial oxygen consumption. It has previously been demonstrated that SIRT1 can directly activate eNOS leading to increased nitric oxide (NO) production (Hung et al., 2000; Mattagajasingh et al., 2007). In the current study, endothelial dependent vasorelaxation was decreased in the ischemic myocardium of the HCD group and normalized in the HCD-R group. This may be a result of increased levels of NO in the endothelium leading to increased vasorelaxation. Addition of a NOS inhibitor to the microvessels led to significantly reduced vasorelaxation, and demonstrates the critical role of NO in microvascular endothelial function. Enhancement of similar pathways in systemic microvessels could lead to lower systemic blood pressures (Gracia-Sancho et al., 2010; Panza et al., 1993).
Serum C-reactive protein is a clinical indicator of inflammation and an independent marker for cardiovascular events (Kaptoge et al.). Our study demonstrated a significant reduction with supplemental resveratrol. The mechanism for this finding is not entirely clear. Some investigators have hypothesized that decreases in LDL and the burden of oxidative stress may lead to decreased C-reactive protein levels (Plenge et al., 2002). This is supported by our findings in the current study. Conversely, in a study performed in Hep3B cells it was shown that the molecular mechanism may involve decreased phosphorylation of p38 MAPK (Kaur et al., 2007). We did not demonstrate a reduction in phospho-p38 MAPK, and this would suggest the decrease in C-reactive protein was by a different mechanism.
Limitations
There are a number of limitations in this work that should be considered. First, it was performed in a porcine model of chronic myocardial ischemia. While in most situations, porcine coronary circulation closely mimics the physiology of the human coronary circulation, this may not be the case for resveratrol supplementation. While we did measure some baseline metrics (blood glucose and BMI), we did not assess blood pressure or myocardial glucose metabolism protein expression. These data would be helpful in assuring the animals were similar at baseline and providing data on changes over time. Second, the number of animals in each group was relatively small and our findings should be interpreted in this context. In addition, the length of the study was limited to 11 weeks of diet modification and resveratrol supplementation. A longer study period may have allowed for further changes to take place, such as myocardial hypertrophy in the setting of hypertension. Further, we did not include a time matched, non-ischemic control group. This would allow us to determine if the effects of resveratrol resulted in full reversal or attenuation of metabolic syndrome. Finally, although a number of significant changes were found in the HCD-R group, we did not demonstrate that these changes were wholly or in part secondary to SIRT1 activation.
Conclusion
In this swine study resveratrol favorably modified the risk factors of metabolic syndrome, and modified glucose metabolism in the ischemic myocardium to improve glucose metabolism and decrease levels of oxidative stress. Oral supplementation with resveratrol may provide a reasonable therapeutic option for patients with risk factors for cardiovascular disease.
Acknowledgments
Funding: This work was supported by the National Health Institute, National Heart, Lung, and Blood Institute (NHLBI RO1HL46716, RO1HL69024, and RO1HL85647), NIH T32-HL076130 (R.M.O.), NIH 5T32-HL0074 (M.P.R.) and the Irving Bard Memorial Fellowship (M.P.R., L.M.C., R.M.O.).
Footnotes
Disclosures: Dr. Frank W. Sellke has research support from Ikaria (Clinton, NJ) and Orthologic (Tempe, AZ), and is a consultant for Novo Nordisk (Princeton, NJ), Cubist Pharmaceuticals (Lexington, MA), and Pfizer (Princeton, NJ). There was no industry funding for this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barouch LA, Berkowitz DE, Harrison RW, O'Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- Barsotti A, Giannoni A, Di Napoli P, Emdin M. Energy metabolism in the normal and in the diabetic heart. Curr Pharm Des. 2009;15:836–840. doi: 10.2174/138161209787582066. [DOI] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Sirtuins and beta-cell function. Diabetes Obes Metab. 2007;9 2:23–27. doi: 10.1111/j.1463-1326.2007.00769.x. [DOI] [PubMed] [Google Scholar]
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- Chen W, Silver DL, Smith JD, Tall AR. Scavenger receptor-BI inhibits ATP-binding cassette transporter 1- mediated cholesterol efflux in macrophages. J Biol Chem. 2000;275:30794–30800. doi: 10.1074/jbc.M004552200. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Chan AYM, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JRB. Resveratrol Prevents the Prohypertrophic Effects of Oxidative Stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9:371–378. [PubMed] [Google Scholar]
- Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab. 2006;291:E867–877. doi: 10.1152/ajpendo.00207.2006. [DOI] [PubMed] [Google Scholar]
- Gracia-Sancho J, Villarreal G, Jr, Zhang Y, Garcia-Cardena G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res. 85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000;47:549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Illingworth DR, Bacon S. Hypolipidemic effects of HMG-CoA reductase inhibitors in patients with hypercholesterolemia. Am J Cardiol. 1987;60:33G–42G. doi: 10.1016/0002-9149(87)90589-3. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Rao LV, Agrawal A, Pendurthi UR. Effect of wine phenolics on cytokine-induced C-reactive protein expression. J Thromb Haemost. 2007;5:1309–1317. doi: 10.1111/j.1538-7836.2007.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- Liu Y, Thornton JD, Cohen MV, Downey JM, Schaffer SW. Streptozotocin-induced non-insulin-dependent diabetes protects the heart from infarction. Circulation. 1993;88:1273–1278. doi: 10.1161/01.cir.88.3.1273. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani RV, Lavie CJ. Prevalence and profile of metabolic syndrome in patients following acute coronary events and effects of therapeutic lifestyle change with cardiac rehabilitation. Am J Cardiol. 2003;92:50–54. doi: 10.1016/s0002-9149(03)00464-8. [DOI] [PubMed] [Google Scholar]
- Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: Definition, pathophysiology, and mechanisms. American Heart Journal. 2005;149:33–45. doi: 10.1016/j.ahj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB. Metabolism: A is for adipokine. Nature. 2005;436:337–338. doi: 10.1038/436337a. [DOI] [PubMed] [Google Scholar]
- Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenge JK, Hernandez TL, Weil KM, Poirier P, Grunwald GK, Marcovina SM, Eckel RH. Simvastatin lowers C-reactive protein within 14 days: an effect independent of low-density lipoprotein cholesterol reduction. Circulation. 2002;106:1447–1452. doi: 10.1161/01.cir.0000029743.68247.31. [DOI] [PubMed] [Google Scholar]
- Robich MP, Chu LM, Chaudray M, Nezafat R, Han Y, Clements RT, Laham RJ, Manning WJ, Coady MA, Sellke FW. Anti-angiogenic effect of high-dose resveratrol in a swine model of metabolic syndrome. Surgery. 148:453–462. doi: 10.1016/j.surg.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, Boodhwani M, Coady MA, Laham RJ, Sellke FW. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 122:S142–149. doi: 10.1161/CIRCULATIONAHA.109.920132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Rader DJ, Millar JS. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- Vidavalur R, Otani H, Singal PK, Maulik N. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp Clin Cardiol. 2006;11:217–225. [PMC free article] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, Walsh K, Moskowitz MA, Liao JK. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal. 2009;2:ra11. doi: 10.1126/scisignal.2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisova P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29:2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]