Abstract
Introduction
There remains an unmet need for safe and effective antiarrhythmic drugs, especially for the treatment of atrial fibrillation. Vanoxerine is a drug that is free of adverse cardiac events in normal volunteers, yet is a potent blocker of the hERG (hKv11.1) cardiac potassium channel. Consequently we hypothesized that vanoxerine might also be a potent blocker of cardiac calcium and sodium currents, and would not affect transmural dispersion of repolarization.
Methods
The whole cell patch clamp technique was used to measure currents from cloned ion channels overexpressed in stable cell lines and single ventricular myocytes. We measured intracellular action potentials from canine ventricular wedges and Purkinje fibers using sharp microelectrode technique.
Results
We found that vanoxerine was a potent hKv11.1 blocker, and at submicromolar concentrations, it blocked calcium and sodium currents in a strongly frequency-dependent manner. In the canine ventricular wedge preparation vanoxerine did not significantly affect transmural action potential waveforms, QT interval or transmural dispersion of repolarization.
Conclusions
Vanoxerine 1) is a potent blocker of cardiac hERG, sodium and calcium channels; 2) block is strongly frequency-dependent especially for sodium and calcium channels; and 3) transmural dispersion of ventricular repolarization is unaffected. The multichannel block and repolarization uniformity resemble the effects of amiodarone, the exemplar atrial fibrillation drug. Vanoxerine is a completely different chemical and has none of amiodarone's toxic effects. Vanoxerine has characteristics of a potentially effective and safe antiarrhythmic.
Keywords: antiarrhythmic drug, patch clamp, vanoxerine, GBR-12909, cardiac electrophysiology, ventricular wedge, Purkinje fiber action potential
Introduction
The need for safer and more effective antiarrhythmic drugs, especially for the treatment of atrial fibrillation (AF), is well recognized.1 Class IA, IC and III antiarrhythmic drugs are widely used but have only about 50% efficacy, and have potentially lethal adverse effects.1 Amiodarone2 is the most efficacious and widely used drug for treatment of AF.2 Amiodarone is unlike Class I or Class III drugs that target primarily a single class of channels because it is nonselective and blocks IK, INa and ICa.3,4 However, its adverse effect profile is long and daunting, ultimately limiting its usefulness. An alternative to amiodarone that had a similar electrophysiologic profile, was clinically as or more effective, and had fewer serious, if any, potential adverse effects would be quite desirable.
Vanoxerine (Vx) {1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-[3-phenylpropyl] piperazine dihydrochloride} (synonym GBR-12909) is a dopamine transporter (DAT1) antagonist.5 It was developed for treatment of Parkinson's disease and depression,6 but lacked efficacy. During a preclinical safety screen for another potential clinical indication, we observed that Vx was a potent blocker of the hKv11.17 channel at concentrations known to be safe in man.6 Despite potent hKv11.1 block, Vx did not produce adverse cardiac events in normal healthy volunteers8-10, leading to our hypothesis that Vx also blocked cardiac sodium and calcium currents.11,12 In this paper we demonstrated that Vx was a potent blocker of cardiac sodium and calcium currents in addition to hKv11.1, and did not prolong action potential duration (APD). Block was strongly frequency-dependent, and was unaccompanied by a change in transmural dispersion of repolarization. Poly-ionic block effects also occurred with amiodarone and our results lead us to propose that vanoxerine might be antiarrhythmic. We subsequently tested and proved this hypothesis in a sterile pericarditis animal model of atrial fibrillation.13 A part of this work has appeared in abstract form.14
Methods
Animal Use and Care
All animal experiments were conducted in accordance with the guidelines of the American Heart Association on Research Animal Use and the Public Health Service Policy on Use of Laboratory Animals. Animals were housed in AAALAC accredited facilities at Case Western Reserve University (Cleveland, OH) and Main Line Health Heart Center and Lankenau Institute for Medical Research (Wynnewood, PA). Tissues were isolated following Institutional Animal Care and Use Committee approved protocols at each institution.
Statistical Analysis
Changes in measured parameters relative to control were evaluated using ANOVA and Dunnett's multiple comparison test. Data were presented as mean ± standard error of the mean (SEM); (n) indicates number of replicates; a P value < 0.05 was considered statistically significant.
Patch Clamp Concentration-Response Measurements
Cell Preparation
Human HEK-293 (ATCC, Manassas, VA) cells were stably transfected with hKv11.1 or hNav1.5 cDNA and mouse L cells were stably transfected with hKv1.5 or rKv4.3 cDNA.15 L-type calcium currents were recorded from acutely isolated, enzymatically dispersed adult guinea pig ventricular myocytes.16 hCav3.2 was stably expressed in HEK-293 cells.
Solutions
Except for hNav1.5 and ICa,L recordings, extracellular Vx concentrations were prepared by diluting aqueous stock solutions into a HEPES buffered physiological solution (HB-PS; composition in mM): NaCl, 137; KCl, 4.0; CaCl2, 1.8; MgCl2, 1; HEPES, 10; Glucose, 10; pH adjusted to 7.4 with NaOH. For hNav1.5 recordings, extracellular Vx concentrations were prepared by diluting stock solutions into a low-Na solution (NaCh-PS) with 40 mM NaCl and 97 mM L-aspartic acid substituted for NaCl in HB-PS (pH adjusted with N-methyl-D-glucamine). Guinea pig myocytes were superfused with a Ca-channel isolating external solution (CaCh-PS) with 5.4 mM CsCl substituted for KCl in HB-PS to record ICa,L.
Intracellular pipette solution for whole cell recordings (except for hNav1.5, ICa,L and hCav3.2) was (composition in mM): K-aspartate, 130; MgCl2, 5; EGTA, 5; ATP, 4; HEPES, 10; pH adjusted to 7.2 with KOH. Internal solutions for hNav1.5, ICa,L and hCav3.2 measurements were designed to reduce overlapping currents through other channels. The intracellular pipette solution for hNav1.5 and hCav3.2 was (composition in mM): cesium-aspartate, 130; MgCl2, 5; EGTA, 5; Na2ATP, 4; GTP, 0.1; HEPES, 10; pH adjusted to 7.2 with aspartic acid. The internal solution for ICa,L measurements in adult guinea pig myocytes had the following composition (in mM): Cs-methanesulfonate, 130; tetraethylammonium chloride, 20; MgCl2, 1; EGTA, 10; HEPES, 10 adjusted to pH 7.2 with methanesulfonic acid. Internal solutions were supplemented with (composition in mM): Mg-ATP, 4; GTP, 0.3; tris-phosphocreatine, 14; and creatine phosphokinase, 50U/ml to prevent rundown of L-type calcium channels17 and hKv7.1/hKCNE1 channels. All pipette solutions were prepared in batches, aliquoted and stored frozen at -20°C (-80°C for solutions containing enzyme), and were freshly thawed each day of use. Measurements were made at room temperature (20 to 25 °C).
Voltage Clamp Protocols
Methods for voltage clamp of cells, drug application, data acquisition and analysis were performed essentially as previously described.18 hCav3.2 currents were measured with a commercial automated patch clamp assay (FASTPatch®) developed at ChanTest Corporation (Cleveland, OH) using a PatchXpress 7000A (MDS Analytical Technologies, Sunnyvale, CA).
Cells stably expressing cardiac ion channel cDNAs were held at −80 mV and block was measured using pulse patterns with fixed amplitudes. hKv1.5 current was measured at the end of a 300 ms step to +20 mV repeated at 10 s intervals. HKv7.1/hKCNE1 peak current at -40 mV was evaluated with pulses (depolarization: +20 mV for 2 s; repolarization: -40 mV for 0.5 s) repeated at 15 s intervals. rKv4.3 currents at peak and after 70 ms were evaluated during 300 ms pulses to +20 mV repeated at 15 s intervals. HCav3.2 peak inward current during a 50 ms step to -30 mV was measured after a hyperpolarizing conditioning step (-100 mV amplitude, 250 ms duration) repeated at 10 s intervals. HKv11.1 peak tail current was measured during a 2 s step to -50 mV following a 2 s step to +20 mV repeated at 10 s intervals. HNav1.5 peak inward current was measured during a 10 ms step to -15 mV following a 20 ms conditioning voltage step to −120 mV repeated at 10 s intervals. Guinea pig single ventricular myocyte L-type calcium channel (ICa,L) currents were measured by holding cells at −40 mV to inactivate Na channels and peak inward ICa,L was measured during a 300 ms step to 0 mV repeated at 20 s intervals.
Frequency-Dependence of Drug Block
The increase in drug block produced by repetitive stimulation (use-dependence) was measured relative to control at selected pulse frequencies (1 and 3 Hz). After holding the cell for a period of at least 1 minute at rest, the time course of peak or isochronal currents during a train of pulses was measured. Measured current magnitudes from each pulse in the train were normalized to the first pulse magnitude. Vx-induced increases in block were expressed as the ratio of normalized steady state current in the presence of Vx to the normalized steady state current in the absence of Vx measured at the end of the train.
Purkinje Fiber Action Potential Concentration-Response Measurements
Purkinje Fiber Solutions
Standard Purkinje fiber Tyrode's (PFT) solution was prepared fresh weekly and refrigerated (composition in mM): NaCl, 131; KCl, 4.0; CaCl2, 2.0; MgCl2, 0.5; NaHCO3, 18.0; NaH2PO4, 1.8; Glucose, 5.5. Before and during use, solutions were aerated with a mixture of 95% O2 and 5% CO2 (pH 7.2 at room temperature) and warmed to 37 °C by the superfusion system.
Electrophysiological Recording
Purkinje fibers were excised from adult canine ventricles by standard methods.17 Purkinje fibers were mounted in a glass-bottomed Plexiglas chamber (approximate volume, 1 ml) affixed to a heated platform, and superfused with PFT solutions warmed to 37 ± 1 °C at approximately 4 ml/minute. Intracellular membrane potentials were recorded using conventional intracellular microelectrodes filled with 3 M KCl solution and connected to an intracellular electrometer amplifier (Warner Instruments IE 210; Hamden, CT).
Action potentials (APs) were evoked by a commercial electronic stimulator. Analog signals were low-pass filtered at 20 kHz before digitization with a Notocord-Hem 3.5 system (Notocord Systems SA, Croissy sur Seine, France). Acceptable fibers were stimulated continuously at a basic cycle length (BCL) of 2 s for 20 minutes to establish a control response following a stabilization period of at least 25 minutes. At the end of the control period, baseline AP rate-dependence was measured at 2, 1 and 0.5 s BCLs. Responses were measured from means of the last 5 recorded APs at each BCL.
Arterially-Perfused Canine Ventricular Wedge Preparation
Details of the methods used have been published previously.19 Transmembrane action potentials in wedge preparations were recorded simultaneously from epicardial (Epi), subepicardial (M cells) and endocardial (Endo) sites in canine left ventricular wedge preparations by use of 3 separate intracellular floating microelectrodes. A transmural ECG was recorded concurrently in all experiments. Transmural dispersion of repolarization (TDR) was defined as the difference between the longest and shortest repolarization times across the left ventricular wall.
Plasma Protein Binding of Vx in Dogs and Humans
Binding to dog and human plasma proteins of Vx was measured in a commercial assay by equilibrium dialysis at concentrations of 0.1, 0.4 and 1 μM at Ricerca Biosciences, LLC (Concord, OH). Pooled human plasma, prepared by blood collection from healthy paid donors into sodium EDTA in FDA licensed and inspected donor blood centers, was purchased by Ricerca Biosciences from Bioreclamation, Hicksville, NY. The concentration of test article in the plasma and buffer compartments was determined by LC-MS/MS analysis and the % binding reported.
Results
Block of Cardiac Ion Channels
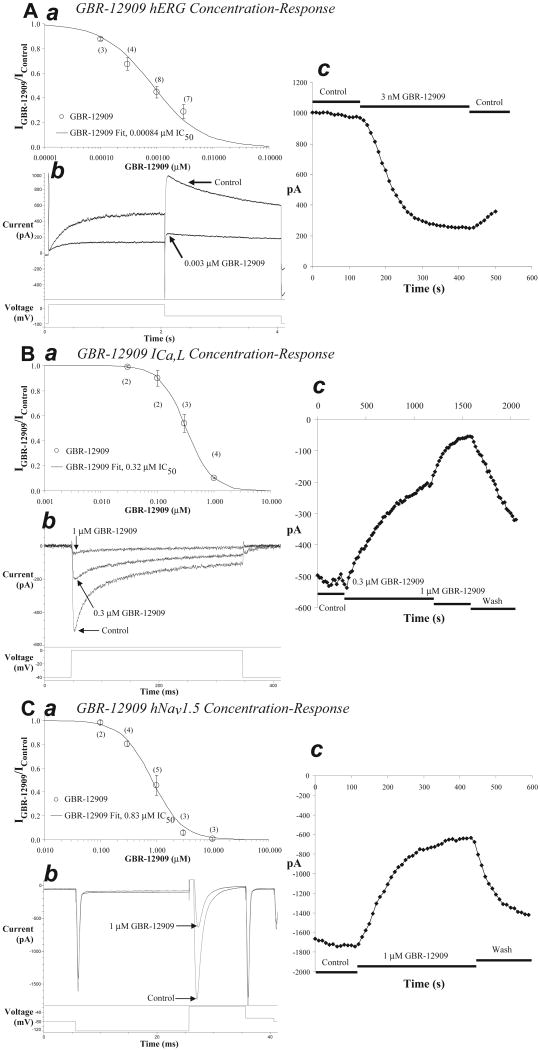
Vx is a potent blocker of hKv11.1 current with an IC50 of 0.00084 μM (Figure 1 Panel A:abc), and of ICa,L (Figure 1, Panel B:abc) and hNav1.5 (Figure 1, Panel C:abc) channel currents as well. The rank order of potency was hKv11.1 > ICa,L > hNav1.5 with IC50 values of 0.84, 320 and 830 nM, respectively (Table 1). IC50 values for Vx block of hKv7.1/hKCNE1, rKv4.3 and hKv1.5, the channels thought to underlie the cardiac currents IKs, Ito, and IKur, respectively, fell in the range of 1 to 10 μM with a rank order of potency of hKv1.5 > delayed rKv4.3 > hKv7.1/hKCNE1 >peak rKv4.3 (Table 1). The low-voltage-activated or T-type Ca channel was blocked 83% by 10 μM Vx (Table 1) when evaluated in a commercial automated patch clamp assay, similar to amiodarone.20
Figure 1. Block of hKv11.1, ICa,L and hNav1.5 current by Vx.
The concentration-response relation for Vx (GBR-12909) block of hKv11.1 currents was fit with an IC50 value of 0.00084 μM (panel A:a). Numbers in parenthesis indicate measurements at each concentration. Sample current records during application of control and 0.003 μM Vx solutions (panel A:b). Time course of peak tail current values during control, application of 0.003 μM Vx and partial washout (panel A:c). The concentration-response relation for Vx block of ICa,L currents was fit with an IC50 value of 0.32 μM (panel B:a). Sample current records during application of control, 0.3 and 1 μM Vx solutions (panel B:b). Time course of peak current values during application of Vx (panel B:c). The concentration-response relation for Vx block of hNav1.5 currents was fit with an IC50 value of 0.83 μM (panel C:a). Sample current records during application of control and 1 μM GBR-12909 solutions (panel C:b). Time course of peak current values during application of GBR-12909 (panel C:c). A steady state was maintained for at least 30 s before and after applying Vx. Peak current was measured until a new steady state was achieved.
Table 1. Patch clamp IC50 values for block of cardiac ion channels by Vx a.
| Vx Concentration-Response Relationship | |||||
|---|---|---|---|---|---|
| Channel | Hz | Test Pulses / Minute | Cell Line | IC50 (μM) | |
|
| |||||
| hKv11.1 (IKr) | 0.1 | 6 | HEK-293 | 0.00084 (n=3-8) |
|
|
| |||||
| ICa,L | 0.05 | 3 | ventricular myocyte | 0.32 (n=2-4) |
|
|
| |||||
| hCav3.2 | 0.1 | 6 | HEK-293 | 2.0 b | |
|
| |||||
| hNav1.5 (INa) | 0.1 | 6 | HEK-293 | 0.83 (n=2-5) |
|
|
| |||||
| hKv1.5 (IKur) | 0.1 | 6 | HEK-293 | 1.0 (n=2-3) |
|
|
| |||||
| rKv4.3 | (Ito peak) | 0.067 | 4 | L | 9.8 |
| (Ito 70 ms) | 0.067 | 4 | L | 2.0 (n=2-3) |
|
|
| |||||
| hKv7.1/hKCNE1 (IKs) | 0.067 | 4 | CHO | 2.9 (n=3-5) |
|
Data from 2 or more cells were pooled for IC50 measurements; n indicates the range of replicates from all concentrations used to construct the IC50. See Figure 1 for n at each concentration for hKv11.1, ICa,L and hNav1.5.
Estimated IC50 from 83% block at 10 μM (n=2).
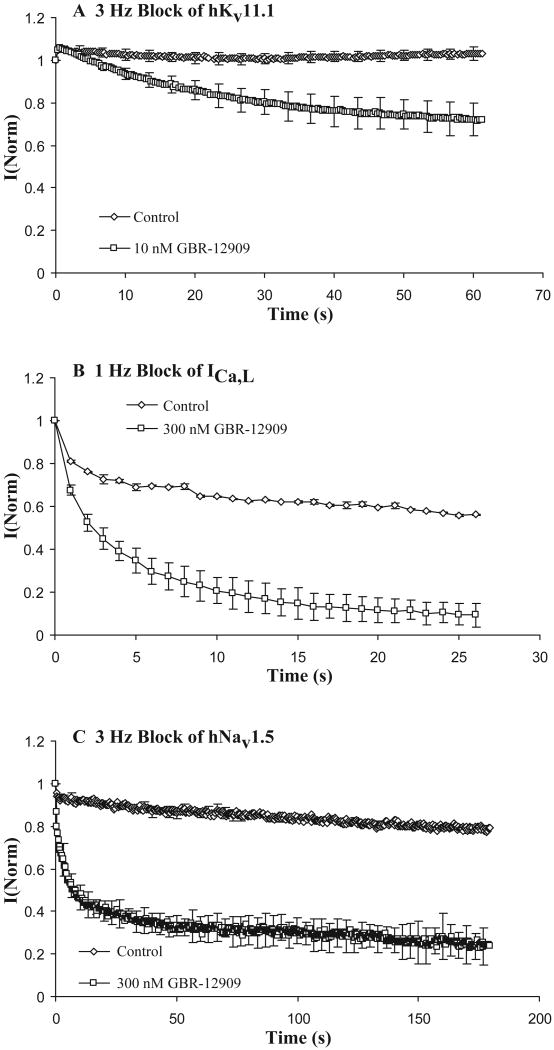
Use- or frequency-dependent block is a desirable property for an antiarrhythmic drug and we evaluated Vx for this characteristic. Block of hKv11.1, ICa,L and hNav1.5 channels at frequencies from 0.3 to 3 Hz showed that Vx's potency increased with rate, especially for Na and Ca channels, so that block values converge at higher frequencies (Figure 2 and Table 2). Block of hKv11.1 at 10 nM and 3 Hz was increased 30% (Figure 2A), Ca channel block at 300 nM and 1 Hz was increased by 83% (Figure 2B) and Na channel block at 300 nM and 3 Hz was increased by 66% (Figure 2C), relative to control. Data from a compound without frequency-dependent block would superimpose the control time course data.
Figure 2. Use-dependent block of hKv11.1, ICa,L and hNav1.5 currents.
Use-dependent block was measured relative to control. Steady state block of Vx measured at 0.1 to 0.05 Hz was established before measurement of Vx's rate-dependence at higher frequencies. Data in the presence and absence of Vx at the indicated concentrations were normalized to the first pulse amplitude after a rest at the holding potential of at least one minute duration. Rapid stimulation alone can cause a decline of current over time in the absence of drug, especially for Ca2+ channels. In all cases Vx enhanced the rate and extent of current decay relative to control. If rate did not enhance block, the control and drug time courses would overlay in this analysis. Upper panel A shows use-dependent block of hKv11.1 at 3 Hz before and after equilibration of the cell with Vx at 10 nM (30% increase, n=3). Panel B shows use-dependent block of ICa,L at 1 Hz and 300 nM concentration (83% increase, n=2) and Panel C shows the same for Vx block of hNav1.5 at 3 Hz and 300 nM Vx (66% increase, n=2).
Table 2. Vx ion channel block use-dependence.
| Channel | BCL (s) |
Vx (nM) |
Increase (Δ%) |
|---|---|---|---|
| ICa,L | 1 | 30 | 11±1 (3) |
| 1 | 100 | 23±19 (2) | |
| 1 | 300 | 83±10 (2)* | |
| 0.3 | 30 | 10±7 (2) | |
| 0.3 | 100 | 60±13 (2)* | |
|
| |||
| hNav1.5 | 3 | 300 | 5±5 (2) |
| 0.3 | 300 | 70±11 (2)* | |
|
| |||
| hKv11.1 | 3 | 10 | 3±3 (3) |
| 0.3 | 10 | 30±8 (3)* | |
Statistically significant change from control (P<0.05) using ANOVA and Dunnett's multiple comparison test.
Purkinje Fiber Action Potential Concentration-Response
Intracellular recordings from canine Purkinje fibers were used to evaluate proarrhythmia arising from the complex ion channel effects of Vx. Action potentials were recorded from canine Purkinje fibers at BCL's of 2, 1 and 0.5 seconds. Measurements from 3 fibers of action potential duration at 60% (APD60) and 90% (APD90) repolarization, the action potential amplitude (APA), and the resting membrane potential (RMP) at each cycle length in vehicle and 0.01 μM, 0.1 μM and 1 μM Vx were analyzed. No statistically significant differences were observed between control and all Vx concentrations tested (Figure 3 and Table 3).
Figure 3. Effect of Vx on Purkinje fiber action potential waveforms.
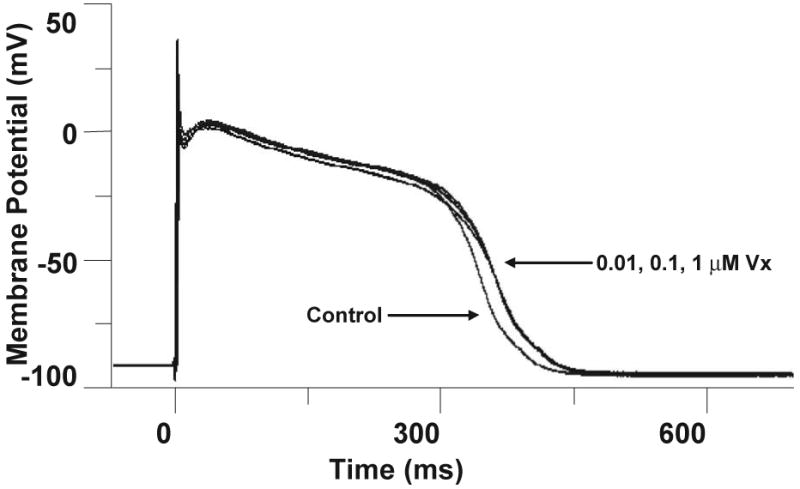
Superimposed records before (control) and after sequential 20 minute equilibration periods with Vx at the indicated concentrations (0.01, 0.1 and 1 μM). Temperature = 37 ± 1 °C, BCL = 2 s. Vx did not cause significant changes in any of the measured action potential parameters (n=3).
Table 3. Vx modulation of canine Purkinje fiber AP parameters a.
| Vx (μM) |
BCL (s) |
APD60 (Δ%) |
APD90 (Δ%) |
RMP (ΔmV) |
APA (ΔmV) |
|---|---|---|---|---|---|
| 0.5 | 2.7±1.6 | 1.8±1.9 | 0.1±3.7 | 5.9±6.2 | |
| 0.01 | 1 | 4.3±0.8 | 3.6±1.6 | 0.6±3.9 | 6.3±6.5 |
| 2 | 7.5±1.9 | 7.0±2.7 | 0.4±3.8 | 6.7±6.5 | |
| 0.5 | 1.8±3.3 | 4.9±1.8 | 2.0±5.0 | -3.1±14.6 | |
| 0.1 | 1 | 2.7±2.9 | 10.1±2.5 | 2.5±5.9 | -2.7±15.0 |
| 2 | 2.7±3.9 | 0.1±6.1 | 3.9±6.4 | -1.0±13.4 | |
| 0.5 | -5.3±5.8 | 2.1±3.2 | -0.4±2.7 | 4.3±8.5 | |
| 1 | 1 | -5.0±7.0 | 2.6±4.5 | -0.1±3.2 | 5.5±8.3 |
| 2 | -4.6±8.1 | 2.7±5.5 | 0.7±3.3 | 3.8±7.0 |
Data were obtained from 3 canine Purkinje fibers. Basic cycle length (BCL); action potential duration at 60% repolarization (APD60) and at 90% repolarization (APD90); resting membrane potential (RMP); action potential amplitude (APA).
No statistically significant changes from control (P<0.05) were observed using ANOVA and Dunnett's multiple comparison test.
Ventricular Wedge Recordings of Action Potentials, Transmural ECG and Transmural Dispersion of Repolarization
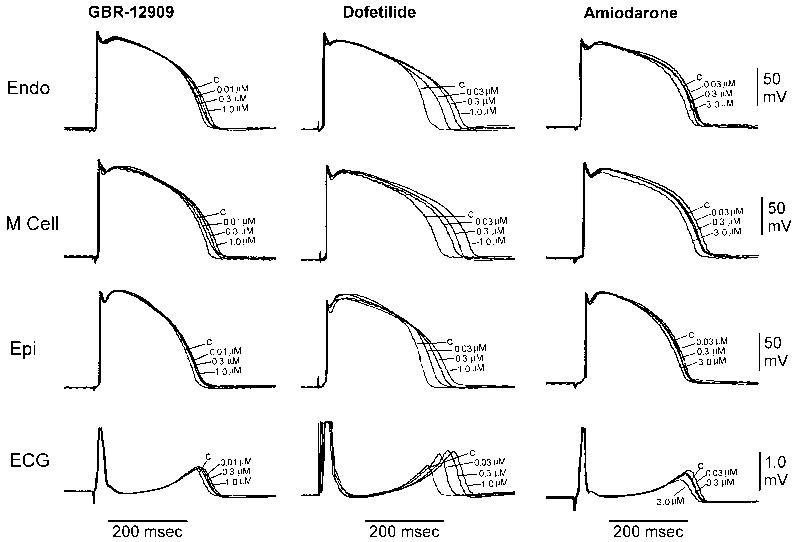
We used intracellular recordings from canine ventricular wedge preparations to search for any proarrhythmic signals in the transmural ECG and action potentials measured simultaneously from myocytes in the epicardium, midmyocardium, and endocardium. Vx's effects on the perfused canine ventricular wedge preparation were compared to those of dofetilide and amiodarone. Figure 4 and Tables 4 and 5 show that Vx acts similarly to amiodarone and is distinctly different from dofetilide in this assay. Dofetilide significantly increased the APD90 and QT of all ventricular cardiomyocytes. Vx rarely prolonged APD90 significantly and produced no significant changes in QT or TDR. Amiodarone significantly decreased APD90 only at the highest concentration tested in all ventricular myocytes and decreased QT at 2 and 1 s BCL. Vx did not produce any phase 2 early afterdepolarizations (EADs), R-on-T xtrasystoles or torsades de pointes arrhythmias over the concentration range tested (0.01 to 3.0 μM).
Figure 4. Effect of Vx on ventricular wedge action potential waveforms and transmural ECG.
Transmural dispersion of repolarization was measured in the canine ventricular wedge preparation (n=8). Recordings from endocardial, midmyocardial and epicardial layers of the ventricular wall and the transmural ECG are shown. The effects of Vx are similar to amiodarone and distinct from those of dofetilide, which has a proarrhythmic risk.
Table 4. Vx, dofetilide and amiodarone modulation of APD90 in the canine left ventricular wedge a.
| APD90 | Epicardial | Midmyocardial | Endocardial | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BCL(s) | 0.5 | 1 | 2 | 0.5 | 1 | 2 | 0.5 | 1 | 2 |
| Vx (μM) | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% |
| 0.01 | -0.1 ± 0.7 (7) | 1.1 ± 0.7 (7) | 1.4 ± 0.6 (7) | 1.2 ± 1.0 (7) | 1.6 ± 0.4 (7) | 0.6 ± 0.8 (7) | 0.7 ± 0.5 (7) | 2.6 ± 0.9 (7) | 2.0 ± 1.5 (7) |
| 0.03 | 0.0 ± 0.8 (8) | 1.1 ± 0.8 (8) | 2.8 ± 0.8 (8) | 1.3 ± 0.7 (8) | 2.2 ± 0.5 (8) | 1.5 ± 0.8 (8) | 0.9 ± 0.8 (8) | 3.3 ± 0.8 (8) | 2.8 ± 1.2 (8) |
| 0.1 | 0.5 ± 1.1 (8) | 2.0 ± 1.3 (8) | 3.5 ± 0.5 (8) | 2.3 ± 1.2 (8) | 3.5 ± 0.5 (8) | 2.8 ± 1.3 (8) | -0.7 ± 1.4 (8) | 4.2 ± 0.6 (8) | 4.5 ± 1.9 (8) |
| 0.3 | 2.1 ± 0.9 (8) | 3.4 ± 1.2 (8) | 4.2 ± 0.8 (8) | 4.0 ± 0.7 (8) | 5.5 ± 1.0 (8) | 5.6 ± 1.2 (8) | 0.4 ± 2.0 (8) | 5.2 ± 0.9 (8) | 6.9 ± 1.4 (8) |
| 1 | 4.2 ± 1.2 (8) | 4.8 ± 1.0 (8) | 5.6 ± 0.8 (8) | 4.4 ± 0.7 (8) | 7.0 ± 0.9 (8)* | 7.4 ± 1.1 (8) | 3.4 ± 0.6 (8) | 6.4 ± 0.7 (8) | 8.7 ± 1.0 (8) |
| 3 | 6.1 ± 1.8 (5)* | 6.0 ± 1.2 (5) | 5.1 ± 0.7 (5) | 6.0 ± 0.9 (5) | 6.9 ± 1.0 (5) | 7.8 ± 1.3 (5) | 4.8 ± 0.6 (5) | 6.8 ± 0.7 (5) | 9.8 ± 1.2 (5) |
| Dof (μM) | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% |
| 0.01 | 11.7 ± 2.1 (6)* | 8.9 ± 1.3 (6)* | 7.4 ± 0.9 (6) | 10.9 ± 1.9 (6)* | 10.5 ± 1.8 (6)* | 8.8 ± 1.3 (6) | 11.9 ± 1.5 (6) | 10.5 ± 2.0 (6) | 10.7 ± 2.3 (6) |
| 0.03 | 14.7 ± 1.4 (6)* | 13.5 ± 1.8 (6)* | 15.4 ± 1.3 (6)* | 13.4 ± 2.0 (6)* | 15.0 ± 1.3 (6)* | 17.3 ± 1.4 (6)* | 13.3 ± 2.9 (6)* | 16.1 ± 3.7 (6) | 18.3 ± 1.4 (6)* |
| 0.1 | 17.8 ± 2.0 (6)* | 17.8 ± 1.7 (6)* | 18.3 ± 1.6 (6)* | 17.6 ± 2.6 (6)* | 20.7 ± 1.8 (6)* | 22.5 ± 1.8 (6)* | 15.3 ± 2.4 (6)* | 20.6 ± 3.5 (6)* | 23.4 ± 1.6 (6)* |
| 0.3 | 19.0 ± 2.0 (6)* | 20.2 ± 2.0 (6)* | 20.7 ± 1.8 (6)* | 21.5 ± 2.6 (6)* | 22.3 ± 1.8 (6)* | 24.7 ± 1.3 (6)* | 18.1 ± 3.0 (6)* | 23.2 ± 2.9 (6)* | 26.2 ± 2.4 (6)* |
| 1 | 19.9 ± 1.9 (6)* | 23.3 ± 1.9 (6)* | 23.4 ± 1.5 (6)* | 21.6 ± 2.8 (6)* | 25.1 ± 2.1 (6)* | 29.5 ± 1.9 (6)* | 19.8 ± 1.6 (6)* | 24.9 ± 3.2 (6)* | 27.0 ± 2.7 (6)* |
| Amio (μM) | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% |
| 0.01 | 1.2 ± 0.5 (4) | 2.0 ± 0.8 (4) | 1.8 ± 0.5 (4) | 0.7 ± 0.3 (4) | 0.8 ± 0.3 (4) | 1.0 ± 0.9 (4) | 0.0 ± 1.0 (4) | 1.7 ± 0.8 (4) | 0.7 ± 0.8 (4) |
| 0.03 | 3.1 ± 0.7 (4) | 3.1 ± 1.4 (4) | 2.0 ± 0.4 (4) | 0.1 ± 0.8 (4) | 2.1 ± 0.5 (4) | 1.9 ± 0.5 (4) | -0.2 ± 0.8 (4) | 2.4 ± 1.2 (4) | 2.6 ± 1.0 (4) |
| 0.1 | 1.2 ± 0.5 (4) | 1.4 ± 0.6 (4) | 2.7 ± 1.3 (4) | 0.9 ± 1.0 (4) | 1.9 ± 0.3 (4) | 1.8 ± 0.7 (4) | -1.3 ± 1.5 (4) | 1.1 ± 1.0 (4) | 2.2 ± 0.6 (4) |
| 0.3 | -2.1 ± 1.3 (4) | -0.7 ± 1.4 (4) | 0.7 ± 1.4 (4) | -2.8 ± 2.3 (4) | -2.6 ± 1.1 (4) | -2.1 ± 1.2 (4) | -4.7 ± 2.6 (4) | -3.1 ± 3.7 (4) | -0.9 ± 1.3 (4) |
| 1 | -2.3 ± 2.3 (4) | -3.2 ± 2.7 (4) | -2.8 ± 1.9 (4) | -7.1 ± 4.1 (4) | -5.5 ± 2.3 (4) | -3.1 ± 1.6 (4) | -5.8 ± 3.5 (4) | -4.6 ± 4.1 (4) | -2.8 ± 1.2 (4) |
| 3 | -3.9 ± 2.6 (4) | -5.3 ± 3.8 (4) | -4.2 ± 3.1 (4) | -4.6 ± 3.7 (4) | -4.9 ± 1.8 (4) | -4.2 ± 2.1 (4) | -9.1 ± 4.8 (4) | -9.4 ± 5.4 (4) | -4.3 ± 1.2 (4) |
| 10 | -6.4 ± 2.0 (4)* | -8.9 ± 2.5 (4)* | -10.8 ± 5.2 (4)* | -36.0 ± 21.4 (4)* | -13.1 ± 4.3 (4)* | -12.0 ± 4.9 (4)* | -9.7 ± 4.4 (4) | -14.2 ± 3.9 (4) | -10.6 ± 4.0 (4)* |
Statistically significant change from control (P<0.05) using ANOVA and Dunnett's multiple comparison test.
Data are shown as mean ± SEM (n). Vanoxerine (Vx); dofetilide (Dof); amiodarone (Amio).
Table 5. Vx, dofetilide and amiodarone modulation of the QT and TDR intervals in the canine left ventricular wedge a.
| QT | TDR | |||||
|---|---|---|---|---|---|---|
| BCL(s) | 0.5 | 1 | 2 | 0.5 | 1 | 2 |
| Vx (μM) | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% |
| 0.01 | 0.7 ± 1.0 (6) | 1.5 ± 1.4 (8) | 0.8 ± 0.7 (8) | 3.2 ± 13.2 (6) | 1.6 ± 5.9 (7) | 1.7 ± 3.2 (8) |
| 0.03 | 2.4 ± 2.1 (7) | 1.9 ± 1.8 (8) | 1.3 ± 1.1 (8) | -3.3 ± 16.6 (7) | 1.6 ± 5.2 (8) | 4.5 ± 3.5 (8) |
| 0.1 | 3.0 ± 0.6 (7) | 2.3 ± 1.6 (8) | 2.8 ± 1.2 (8) | 16.1 ± 16.6 (7) | 3.0 ± 5.8 (8) | 10.0 ± 4.4 (8) |
| 0.3 | 4.8 ± 1.1 (8) | 4.5 ± 1.5 (8) | 5.0 ± 0.8 (8) | 9.2 ± 18.2 (8) | 4.4 ± 6.4 (8) | 12.4 ± 3.3 (8) |
| 1 | 3.6 ± 1.9 (7) | 6.5 ± 0.9 (8) | 7.3 ± 0.7 (8) | 6.8 ± 18.2 (8) | 5.5 ± 6.3 (8) | 18.5 ± 3.2 (8) |
| 3 | 4.1 ± 7.1 (2) | 6.8 ± 1.1 (5) | 6.7 ± 0.5 (5) | 8.7 ± 31.3 (5) | 7.0 ± 6.9 (5) | 15.8 ± 4.3 (5) |
| Dof (μM) | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% |
| 0.01 | 9.2 ± 1.0 (6)* | 10.5 ± 1.0 (6)* | 9.2 ± 1.2 (6)* | 20.4 ± 9.3 (6) | 9.9 ± 14.6 (6) | 8.8 ± 4.7 (6) |
| 0.03 | 11.5 ± 0.9 (6)* | 15.0 ± 1.2 (6)* | 15.4 ± 1.8 (6)* | 14.7 ± 11.6 (6) | 17.0 ± 17.2 (6) | 12.5 ± 4.7 (6) |
| 0.1 | 16.4 ± 1.2 (6)* | 20.5 ± 1.4 (6)* | 20.3 ± 1.8 (6)* | 32.2 ± 19.2 (6) | 27.4 ± 18.9 (6) | 23.3 ± 6.4 (6) |
| 0.3 | 16.8 ± 1.2 (6)* | 22.4 ± 0.6 (6)* | 22.9 ± 1.8 (6)* | 19.8 ± 15.7 (6) | 30.7 ± 13.2 (6) | 28.1 ± 3.1 (6) |
| 1 | 18.2 ± 1.9 (6)* | 24.8 ± 1.1 (6)* | 28.0 ± 1.8 (6)* | 19.9 ± 11.7 (6) | 41.0 ± 14.1 (6)* | 44.3 ± 2.6 (6)* |
| Amio (μM) | Δ% | Δ% | Δ% | Δ% | Δ% | Δ% |
| 0.01 | 1.1 ± 0.8 (4) | 0.3 ± 0.2 (4) | 1.6 ± 0.8 (4) | 3.4 ± 2.6 (4) | 1.3 ± 1.0 (4) | 5.5 ± 2.8 (4) |
| 0.03 | 1.5 ± 1.1 (4) | 1.6 ± 0.5 (4) | 2.3 ± 0.8 (4) | 4.6 ± 5.6 (4) | 5.3 ± 4.5 (4) | 10.8 ± 2.2 (4) |
| 0.1 | -0.5 ± 1.6 (4) | 0.4 ± 0.6 (4) | 1.8 ± 1.0 (4) | -1.6 ± 8.1 (4) | 0.5 ± 6.4 (4) | 7.5 ± 1.6 (4) |
| 0.3 | -1.1 ± 0.8 (4) | -2.6 ± 1.1 (4) | -2.2 ± 1.3 (4) | -3.1 ± 8.6 (4) | -13.3 ± 11.3 (4) | 0.3 ± 5.9 (4) |
| 1 | -2.7 ± 1.1 (4) | -4.5 ± 1.8 (4) | -3.9 ± 2.2 (4) | -1.0 ± 12.3 (4) | -12.9 ± 11.4 (4) | -4.1 ± 7.8 (4) |
| 3 | -2.3 ± 1.9 (4) | -6.9 ± 1.5 (4)* | -5.6 ± 2.4 (4) | 0.7 ± 18.7 (4) | -28.7 ± 10.6 (4) | -8.4 ± 5.4 (4) |
| 10 | -6.8 ± 2.1 (2) | -13.5 ± 0.3 (3)* | -10.3 ± 2.9 (4)* | -21.3 ± 44.4 (2) | -31.2 ± 11.7 (3) | -14.0 ± 8.4 (4) |
Statistically significant change from control (P<0.05) using ANOVA and Dunnett's multiple comparison test.
Data are shown as mean ± SEM (n). Vanoxerine (Vx); dofetilide (Dof); amiodarone (Amio).
Plasma Protein Binding of Vx
Protein binding for Vx was greater than 99% for both dog and human plasma at all 3 concentrations tested: 0.1, 0.4, and 1 μM. The results demonstrated a high propensity for Vx to bind plasma proteins. Cytochrome P450 enzymes generate a single Vx metabolite in vitro, primarily due to CYP3A4 isoform activity, with lesser contributions by the CYP2C8 and CYP2E1 isoforms.21 The Vx metabolite has not been characterized chemically or pharmacologically.
Discussion
Using in vitro techniques we initially demonstrated that, unexpectedly, Vx was a potent hKv11.1 channel blocker. Since previous studies in man and mammals indicated no cardiovascular risk for Vx6, we evaluated Vx's interactions with other cardiac ion channels for offsetting effects. Subsequently, we demonstrated that Vx was also a potent blocker of cardiac sodium and calcium currents, activities that can offset hKv11.1 block, and did not significantly prolong Purkinje fiber APD60 and APD90. Vx had no significant effect on QT or TDR. Block of hNav1.5 and ICa,L was strongly frequency-dependent. Vx appears to have similar effects to amiodarone. Based on Vx's use in previous clinical trials to treat Parkinson's disease and depression, its adverse effect profile is much less than amiodarone's. The available clinical trial data suggests that concentrations of Vx that are effective in nonclinical data are well tolerated and safe in man as opposed to amiodarone's toxic effects on pulmonary and corneal function. Vx terminated AF in a preclinical in vivo animal model.13 An ongoing clinical trial to assess oral Vx's efficacy as an antiarrhythmic for treatment of AF showed promising efficacy for acute termination of AF without proarrhythmia and significant adverse effects (unpublished observations from ChanTest Corporation).
Vernakalant is a promising antiarrhythmic for treatment of recent-onset AF,22 but fails to terminate atrial flutter.23 Vx terminated both AF and atrial flutter in the canine sterile pericarditis model.13 Dronedarone (derived from amiodarone) is another recent antiarrhythmic targeting multiple ion channels with efficacy similar to amiodarone but with reduced toxicity,24 like Vx. However, intravenous Vx is effective at terminating recent-onset AF in minutes,13 while dronedarone is likely to share the delayed onset of cardioversion seen with amiodarone.25
Relationship between pharmacokinetics of Vx in man and AF/AFL termination
Phase I clinical trials of Vx indicated no significant effects on ECG, heart rate or blood pressure.6 In a dose-escalation Phase I study of Vx, 4 healthy males aged 20-45 years received 100, 200 and 300 mg oral doses of Vx.8 At the 300 mg dose, the peak plasma concentration (Cmax) of Vx ranged up to 831 nM (mean Cmax was 533 ± 109 nM). This mean value is greater than the mean plasma concentration measured at termination of sustained AF/AFL in the canine sterile pericarditis model (399 ± 69 nM, n=19).13 No adverse cardiac events (AEs) were reported for any of the doses. No significant effects on heart rate (HR) or blood pressure (BP) occurred. At the 100 and 200 mg doses there was one occurrence of a slight reduction in the T-wave of the ECG. At the 300 mg dose all subjects had a reduction in T-wave amplitude; however, none were flat or negative. The Bazett's corrected QT, QTc(B), was within the normal range (< 0.47 s).
In a Phase I study on relative oral bioavailability in 12 healthy men aged 22-34 years, a 100 mg single oral dose was well tolerated.10 In a 14-day repeat oral dosing Phase I study in 14 healthy men aged 19-33 years administered doses up to 125 mg of Vx, noncardiac adverse events were mild and generally of short duration.9 In these Phase I trials, Cmax values exceeded the minimum plasma concentrations measured at termination of AF/AFL in the canine sterile pericarditis model.
Preclinical Studies
Vx was compared to dofetilide and amiodarone in the arterially perfused canine ventricular wedge preparation. Vx was similar to amiodarone in its small effects on action potentials from the endocardial, midmyocardial, and epicardial ventricular myocytes, transmural ECG and transmural dispersion of repolarization (TDR), and distinct from the large changes associated with dofetilide. Increased TDR is associated with proarrhythmia,19 and Vx's lack of effect on TDR, similar to amiodarone, suggests that Vx is also not likely to be proarrhythmic. Independent studies with telemetered conscious dogs found no adverse effects of oral Vx at doses up to 30 mg/kg.26
Predominantly, Class III hKv11.1 blockers like sotalol and dofetilide always carry the concern of proarrhythmia associated with QT prolongation and hKv11.1 block.27 However, other potent hKv11.1 blockers like verapamil do not have a QT prolongation risk due to compensatory block of Ca channels at similar potency. We expected this would explain the lack of QT prolongation risk for Vx, although the potencies at low frequencies for the Ca and Na compensatory currents were lower. However, Vx potency increased with rate, especially for Na and Ca channels, and the IC50 values for all 3 target channels became closer at fast physiological frequencies. We believe Vx will demonstrate antiarrhythmic efficacy because of its 1) potent block of hKv11.1 combined with strongly frequency-dependent block of cardiac calcium and sodium currents; and 2) maintenance of TDR. Confirmation of Vx antiarrhythmic activity in the canine sterile pericarditis model is presented in detail in a companion paper.13
Limitations
We showed that Vx was safe and effective for acute pharmacological cardioversion of AF/AFL in an animal model,13 and initial clinical trial data show that Vx is safe for this indication in man (ChanTest Corporation, unpublished data). Although the longest repeat dosing period used in Vx clinical trials is 14 days and amiodarone pulmonary toxicity typically develops over a longer period, amiodarone pulmonary toxicity can appear in a few days and in a third of cases in weeks.28 Corneal microdeposits appear in weeks and occur in 76-100% of patients.29 We have not evaluated Vx's potential to prevent AF/AFL experimentally, although our initial experience with maintained intravenous dosing at 1 mg/kg throughout the electrophysiology parameter measurement period following termination prevented induction of AF/AFL on the following day. Stopping Vx infusion at termination reduced the total dose of Vx and allowed initiation of sustained AF/AFL on the following experimental days.13 Our oral dosing experiments were consistent with the possibility that Vx might provide effective maintenance therapy.30
Vanoxerine is a substrate for CYP3A4, and drug interactions will need to be considered with its use.21 Metabolism of Vx by human liver microsomes, human hepatocytes, and microsomes containing cDNA-expressed human P450 isoforms identified a single primary metabolite by HPLC.21 The chemical identity, toxicity, pharmacokinetics and pharmacological activity of this metabolite remain unknown.
Acknowledgments
We want to thank William Giroski and Sharon Beringuel for preparation of cell cultures.
The research described in this report was supported in part by NIH grant R44 HL067503 and ChanTest Corporation.
Footnotes
Antonio E. Lacerda and Yuri A. Kuryshev are employed at ChanTest Corporation, which holds a use patent on vanoxerine. Dr. Brown is co-inventor listed on the patent and majority owner of ChanTest. Dr. Waldo reports participation in research grants supported by CV Therapeutics & Sequel Pharmaceuticals; compensation for participation in a speaker's bureau relevant to this topic from GlaxoSmithKline; honoraria from Sonofi-Aventis & participation on the advisory board for dronederone.
References
- 1.Waldo AL. A perspective on antiarrhythmic drug therapy to treat atrial fibrillation: there remains an unmet need. Am Heart J. 2006;151:771–8. doi: 10.1016/j.ahj.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 2.AFFIRM First Antiarrhythmic Drug Substudy Investigators. Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J Am Coll Cardiol. 2003;42:20–9. doi: 10.1016/s0735-1097(03)00559-x. [DOI] [PubMed] [Google Scholar]
- 3.Nattel S. Drugs to promote sinus rhythm reversion and maintenance in atrial fibrillation--why amiodarone is better. Cardiovasc Drugs Ther. 2003;17:5–6. doi: 10.1023/a:1024295422944. [DOI] [PubMed] [Google Scholar]
- 4.Manios EG, Mavrakis HE, Kanoupakis EM, Kallergis EM, Dermitzaki DN, Kambouraki DC, Vardas PE. Effects of amiodarone and diltiazem on persistent atrial fibrillation conversion and recurrence rates: a randomized controlled study. Cardiovasc Drugs Ther. 2003;17:31–9. doi: 10.1023/a:1024203824761. [DOI] [PubMed] [Google Scholar]
- 5.Giros B, el Mestikawy S, Godinot N, Zheng K, Han H, Yang-Feng T, Caron MG. Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. Mol Pharmacol. 1992;42:383–90. [PubMed] [Google Scholar]
- 6.Preti A. Vanoxerine National Institute on Drug Abuse. Curr Opin Investig Drugs. 2000;1:241–51. [PubMed] [Google Scholar]
- 7.Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sogaard U, Michalow J, Butler B, Lund LA, Ingersen SH, Skrumsager BK, Rafaelsen OJ. A tolerance study of single and multiple dosing of the selective dopamine uptake inhibitor GBR 12909 in healthy subjects. Int Clin Psychopharmacol. 1990;5:237–51. doi: 10.1097/00004850-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Ingwersen SH, Snel S, Mant TG, Edwards D. Nonlinear multiple-dose pharmacokinetics of the dopamine reuptake inhibitor vanoxerine. J Pharm Sci. 1993;82:1164–6. doi: 10.1002/jps.2600821120. [DOI] [PubMed] [Google Scholar]
- 10.Ingwersen SH, Mant TG, Larsen JJ. Food intake increases the relative oral bioavailability of vanoxerine. Br J Clin Pharmacol. 1993;35:308–10. doi: 10.1111/j.1365-2125.1993.tb05699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AM, Rampe D. Drug-induced long QT syndrome: is HERG the root of all evil? Pharmaceutical News. 2000;7:15–20. [Google Scholar]
- 12.Clancy CE, Kass RS. Inherited and acquired vulnerability to ventricular arrhythmias: cardiac Na+ and K+ channels. Physiol Rev. 2005;85:33–47. doi: 10.1152/physrev.00005.2004. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto N, Khrestian C, Ryu K, Lacerda A, Brown A, Waldo AL. Vanoxerine, a new drug for terminating atrial fibrillation and flutter. J Cardiovasc Electrophysiol. doi: 10.1111/j.1540-8167.2009.01622.x. in press. [DOI] [PubMed] [Google Scholar]
- 14.Lacerda A, Matsumoto N, Khrestian C, Ryu K, Yan GX, Waldo A, Brown A. Effects of vanoxerine on cardiac ion channels. 7th Annual NIH SBIR/STTR Conference; 2005. p. B-5. [Google Scholar]
- 15.Kuryshev YA, Wible BA, Gudz TI, Ramirez AN, Brown AM. KChAP/Kvbeta1.2 interactions and their effects on cardiac Kv channel expression. Am J Physiol Cell Physiol. 2001;281:C290–C299. doi: 10.1152/ajpcell.2001.281.1.C290. [DOI] [PubMed] [Google Scholar]
- 16.Kuryshev YA, Ficker E, Wang L, Hawryluk P, Dennis AT, Wible BA, Brown AM, Kang J, Chen XL, Sawamura K, Reynolds W, Rampe D. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther. 2005;312:316–23. doi: 10.1124/jpet.104.073692. [DOI] [PubMed] [Google Scholar]
- 17.Lacerda AE, Kuryshev YA, Chen Y, Renganathan M, Eng H, Danthi SJ, Kramer JW, Yang T, Brown AM. Alfuzosin delays cardiac repolarization by a novel mechanism. J Pharmacol Exp Ther. 2008;324:427–33. doi: 10.1124/jpet.107.128405. [DOI] [PubMed] [Google Scholar]
- 18.Kirsch GE, Trepakova ES, Brimecombe JC, Sidach SS, Erickson HD, Kochan MC, Shyjka LM, Lacerda AE, Brown AM. Variability in the measurement of hERG potassium channel inhibition: effects of temperature and stimulus pattern. J Pharmacol Toxicol Methods. 2004;50:93–101. doi: 10.1016/j.vascn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially perfused canine left ventricular wedge preparations. Circulation. 1998;98:1921–7. doi: 10.1161/01.cir.98.18.1921. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita N, Kaku T, Uchino T, Isomoto S, Yoshimatsu H, Ono K. Short- and long-term amiodarone treatments regulate Cav3.2 low-voltage-activated T-type Ca2+ channel through distinct mechanisms. Mol Pharmacol. 2006;69:1684–91. doi: 10.1124/mol.105.021253. [DOI] [PubMed] [Google Scholar]
- 21.Cherstniakova SA, Bi D, Fuller DR, Mojsiak JZ, Collins JM, Cantilena LR. Metabolism of vanoxerine, 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine, by human cytochrome P450 enzymes. Drug Metab Dispos. 2001;29:1216–20. [PubMed] [Google Scholar]
- 22.Roy D, Pratt CM, Torp-Pedersen C, Wyse DG, Toft E, Juul-Moller S, Nielsen T, Rasmussen SL, Stiell IG, Coutu B, Ip JH, Pritchett EL, Camm AJ. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation. 2008;117:1518–25. doi: 10.1161/CIRCULATIONAHA.107.723866. [DOI] [PubMed] [Google Scholar]
- 23.Pratt CM, Roy D, Juul-Moller S, Torp-Pedersen C, Toft E, Wyse DG, Nielsen T, Rasmussen SL. Efficacy and tolerance of RSD1235 in the treatment of atrial fibrillation or atrial flutter: results of a phase III, randomized, placebo-controlled, multicenter trial. J Am Coll Cardiol. 2006;47:10A. abstract. [Google Scholar]
- 24.Laughlin JC, Kowey PR. Dronedarone: a new treatment for atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:1220–6. doi: 10.1111/j.1540-8167.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- 25.Chevalier P, Durand-Dubief A, Burri H, Cucherat M, Kirkorian G, Touboul P. Amiodarone versus placebo and class Ic drugs for cardioversion of recent-onset atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2003;41:255–62. doi: 10.1016/s0735-1097(02)02705-5. [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith P, Golder Z, Hunt J, Berghmans S, Jones D, Stables JP, Murphree L, Howden D, Newton PE, Richards FM. GBR12909 possesses anticonvulsant activity in zebrafish and rodent models of generalized epilepsy but cardiac ion channel effects limit its clinical utility. Pharmacology. 2007;79:250–8. doi: 10.1159/000102061. [DOI] [PubMed] [Google Scholar]
- 27.Waldo AL, Camm AJ, deRuyter H, Freidman PL, MacNeil DJ, Pitt B, Pratt CM, Rodda BE, Schwartz PJ. Survival with oral d-sotalol in patients with left ventricular dysfunction after myocardial infarction: rationale, design, and methods (the SWORD trial) Am J Cardiol. 1995;75:1023–7. doi: 10.1016/s0002-9149(99)80717-6. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein I, Topilsky M, Segev D, Isakov A, Heller I. Very early onset of acute amiodarone pulmonary toxicity presenting with hemoptysis. Chest. 1997;111:1446–7. doi: 10.1378/chest.111.5.1446. [DOI] [PubMed] [Google Scholar]
- 29.Pollak PT. Clinical organ toxicity of antiarrhythmic compounds: ocular and pulmonary manifestations. Am J Cardiol. 1999;84:37R–45R. doi: 10.1016/s0002-9149(99)00700-6. [DOI] [PubMed] [Google Scholar]
- 30.Cakulev I, Kyungmoo R, Celeen K, Lacerda AE, Brown AM, Waldo AL. Orally administered vanoxerine, a cardiac multichcannel blocker, terminates atrial flutter and prevents its reinducibility in the canine sterile pericarditis model. J Am Coll Cardiol. 2007;49:38A. [Google Scholar]