Abstract
The comorbidity among balance disorders, anxiety disorders and migraine has been studied extensively from clinical and basic research perspectives. From a neurological perspective, the comorbid symptoms are viewed as the product of sensorimotor, interoceptive and cognitive adaptations that are produced by afferent interoceptive information processing, a vestibulo–parabrachial nucleus network, a cerebral cortical network (including the insula, orbitofrontal cortex, prefrontal cortex and anterior cingulate cortex), a raphe nuclear–vestibular network, a coeruleo–vestibular network and a raphe–locus coeruleus loop. As these pathways overlap extensively with pathways implicated in the generation, perception and regulation of emotions and affective states, the comorbid disorders and effective treatment modalities can be viewed within the contexts of neurological and psychopharmacological sites of action of current therapies.
Keywords: anxiety disorders, balance disorders, migraine, vestibular rehabilitation, vestibular system
The most commonly recognized neurologic signs and symptoms of balance dysfunction include dizziness and autonomic symptoms, accompanied by quantifiable eye movement, head movement and postural abnormalities. However, there is widespread recognition of the comorbidity of balance disorders (both neuro-otologic disease and chronic subjective dizziness) with psychiatric disorders [1–5] and with migraine [6–8], as well as of a significant comorbidity of migraine with phobic disorders and panic disorder [9]. Migraine is associated with vertigo [10–12], motion sickness [8,13–15], which is attenuated by triptan treatment [16,17], and anxiety [6,7]. These clinical observations have prompted explorations of neurological linkages between vestibular pathways, pain pathways and the control of emotion and affect.
The current clinical classification for patients presenting with comorbid aspects of balance disorders, migraine and anxiety disorders is shown in Table 1. These terms reflect different diagnostic tools and symptom interpretations from the neuro-otologic and psychiatric clinic (e.g., [1,3,6,11,18–20]). table 1 contains symptoms, signs, dimensional constructs, syndromes and diagnoses. Dizziness, visual sensitivity and motion sensitivity are symptoms. Clinical signs include the results of static and dynamic balance performance (e.g., Romberg test and dynamic posturography), caloric testing and vestibulo-ocular reflex testing. A dimensional construct [21,22], such as space and motion discomfort (SMD) [1,23,24], displays an ordered set of values along a continuum, rather than simply present or absent. Normal levels of a manifestation of a dimensional construct may be benign or even protective. For example, moderate SMD can lead normal individuals with a perception of postural instability to avoid potentially dangerous situations. Recent studies document clear relationships among the SMD dimensional construct and metrics of vestibular function and balance control, which include an association of specific vestibulo-ocular findings (e.g., decreased horizontal vestibulo-ocular reflex time constant and increased reflex gain) with anxiety disorder and SMD status [25], and an association of visual and somatosensory dependence for postural control with SMD status [23,26]. Hence, SMD will be discussed in association with these other signs of altered balance control.
Table 1.
Terminology for comorbid balance disorders, anxiety and migraine.
| Term | Taxonomic status | Visual flow and motion sensitivity (symptoms) | Vestibular/balance disorder (signs) | Role of anxious temperament | Initial clinical appearance | Original purpose | Ref. |
|---|---|---|---|---|---|---|---|
| Space and motion discomfort | Dimension | Necessary | Likely | Low | Anxiety clinic | Identify balance disorders among anxiety patients | [1,23,24] |
| Space and motion phobia | Syndrome | Necessary | Likely | High | Anxiety clinic | Identify and treat ‘excessive space and motion discomfort’ | [1] |
| Visual vertigo | Syndrome | Necessary | Possible | High | Otoneurology clinic | Identify new balance disorder | [28] |
| Chronic subjective dizziness | Syndrome | Associated | Not necessary | High | Otoneurology clinic | Identify balance patients in need of psychiatric treatment | [20,29] |
| Primary somatoform vertigo | Syndrome | Associated | None | High | Otoneurology clinic | Identify balance patients in need of psychiatric treatment, primary gain | [19,30] |
| Secondary somatoform vertigo | Syndrome | Augmentative | Not necessary | High | Otoneurology clinic | Identify cause for some balance symptoms | [19,30] |
| Phobic postural vertigo | Diagnosis | Common | Assumed none | Low | Otoneurology clinic | Identify balance patients in need of psychiatric treatment | [27] |
| Migrainous vertigo/vestibular migraine | Diagnosis | Prominent | By definition | Likely | Otoneurology clinic | Identify cause for some balance symptoms | [7,11,18] |
| Psychiatric dizziness | Symptom | None | None | High | Otoneurology or psychiatric clinic | Provide Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition diagnosis | [1] |
| Psychiatric overlay | Interactive factor | Augmentative | By definition | High | Psychiatric clinic | Identify factors contributing to poor adjustment to dizziness | [1] |
| Migraine– anxiety related dizziness | Diagnosis | Prominent | By definition | High | Otoneurology clinic | Account for comorbidity | [6] |
A syndrome can be defined as a symptom complex, without a requirement for objective signs. Phobic postural vertigo is an example of a syndrome. Patients with phobic postural vertigo report vestibular symptoms and symptoms of compulsivity [27]. Space and motion phobia is a syndrome that ensues when excessive behavioral reactions to SMD produce situationally inappropriate avoidance and anxiety, including panic responses [1]. Visual vertigo is a syndrome characterized by dizziness (a symptom) that is elicited particularly by visual sensitivity of SMD, such as moving scenes in motion picture scenes, crowd motions or open roads [28]. The chronic subjective dizziness syndrome [20,29] is defined by subjective, persistent complaints of nonvertiginous dizziness or imbalance, without specific or localizing signs of vestibular or balance dysfunction. The syndrome primary somatoform vertigo [19,30] and the symptom of psychiatric dizziness [1] both designate individuals with an anxious temperament and no association with objective evidence of a balance disorder; secondary somatoform vertigo [19,30] is differentiated from primary somatoform vertigo by reported vertigo (symptom) and/or balance disorder signs.
A diagnosis differs from a syndrome because it is a differentiated attribution based upon both signs and symptoms. For example, migrainous vertigo [11] is a diagnosis defined by signs of a balance disorder and symptoms that meet International Headache Society migraine criteria. Migraine- and anxiety-associated dizziness [6] designates migrainous vertigo patients with a comorbid anxiety disorder. This article summarizes our current understanding of the bases for neurotherapeutic approaches to balance disorders associated with migraine and/or anxiety.
A neurobiological perspective for neurotherapeutics
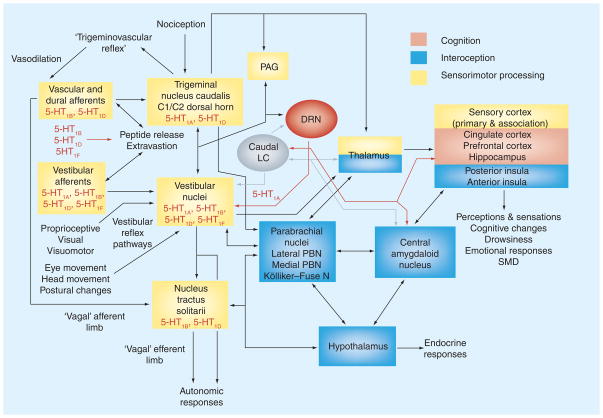
Figure 1 illustrates pathways that contribute to the feature of comorbid clinical features of migraine, anxiety disorders and balance dysfunction. The balance–anxiety linkages appear to involve integrated activity of at least six neural components:
Figure 1. Neurological bases for the comorbidity between signs and symptoms of balance disorders, space and motion discomfort and migraine.
Components related to sensorimotor (yellow), interoceptive (blue) and cognitive (light red) networks are color-coded. The boxes that represent brainstem sensorimotor structures include peripheral parallels between vestibular pathways and migraine mechanisms [6,8]. The cross-cutting modulatory noradrenergic contributions from LC and serotonergic contributions from the DRN are shown in gray and red, respectively. See text for further discussion.
5-HT1A,B,D or F : Serotonin receptors 1A, 1B, 1D or 1F; C1/C2: Cervical spinal cord segments C1 and C2; DRN: Dorsal raphe nucleus; LC: Locus coeruleus; N: Nucleus; PAG: Periaqueductal gray; PBN: Parabrachial nucleus; SMD: Space and motion discomfort.
Afferent interoceptive information processing
A vestibulo–parabrachial nucleus network
Cerebral cortical vestibular and interoceptive processing
A raphe nuclear–vestibular network
A coeruleo–vestibular network
A raphe–locus coeruleus loop
In addition, a linkage of these pathways to pain pathways, including migraine-related pathways, is provided by the trigemino–vascular reflex loop and parallel neurochemical mechanisms in the periphery.
These pathways can be divided conceptually into cognitive–behavioral (light red box), neurologic sensorimotor performance (yellow boxes) and interoceptive (blue boxes) domain components. This parcellation reflects concepts of the neural bases for the comorbidity of balance disorders, migraine and anxiety disorders [6,31,32], as well as mechanisms for controlling the emotional components of psychiatric [33] and pain [34] syndromes. The cognitive–behavioral domain includes: first, pathways for involuntary or homeostatic regulation of affect by the ventral lateral prefrontal cortex, orbitofrontal cortex and the ventral aspect of the cingulate cortex; and second, pathways for the effortful regulation of affect in the dorsal lateral prefrontal cortex, dorsal medial prefrontal cortex, dorsal anterior cingulate cortex and hippocampus [33]. The sensorimotor performance component includes pathways for both the sensory perceptual responses to vestibular, visual, proprioceptive and somatosensory information and the somatic and visceral motor response mechanisms (e,.g., trigeminovascular, vestibulo-ocular and vestibulospinal reflexes) [32]. Finally, the interoceptive component of the model is mediated by ascending vestibular, visceral and nociceptive projections to the insula and amygdala, via the parabrachial nucleus and thalamus. These interoceptive pathways integrate information regarding ongoing sensory processes relative to the current physiological condition of the body [35], and their translation into the ‘sentient self’ [34] as subjective awareness and feelings.
Afferent interoceptive & vestibular pathways: shared neurochemical properties
Shared organizational and neurochemical features of central vestibular and nociceptive projections to the same structures may explain some aspects of the comorbidity and interactions among anxiety, migraine and balance disorders. The different pain pathways show specific neurochemical characteristics. Fine polymodal afferents that express calcitonin gene-related peptide (CGRP) and/or substance P terminate on neurons in lamina I of the spinal cord, which both project directly to the parabrachial nucleus, to medullary pathways that influence preganglionic sympathetic outflow, to thalamic ‘pain’ sites and to sites in the hypothalamus (reviewed by [35]). Further studies have demonstrated that smaller ganglion cells (trigeminal and dorsal root ganglia) and their mainly unmyelinated peptidergic afferents express 5-hydroxy-tryptamine (5-HT)1D [36], and that many of the postsynaptic lamina I neurons express μ opioid receptor 1 [37]. By contrast, non-peptidergic (but nociceptive) afferents (purinergic P2X3 positive and IB4-binding cells) appear to project to lamina II dorsal horn neurons that give rise to projections to lamina V neurons that are connected directly with the hypothalamus, amygdala, bed nucleus of the stria terminalis and globus pallidus. Both peptid-ergic and nonpeptidergic dorsal root and trigeminal ganglion cells also express the so-called ‘capsaicin receptor’, TRPV1 [38,39]. Large ganglion cells that do not contribute to these pathways express calretinin [40].
The molecular phenotypes of vestibular ganglion cells show strong parallel features with trigeminal and dorsal root ganglion cells. The vestibular ganglion (Scarpa’s ganglion) contains a population of small caliber cell bodies that express substance P [41], a population of small-to-medium-sized cell bodies that express P2X3 [42] and a large-sized population (related to calyceal endings on hair cells) that expresses calretinin. Vestibular ganglion cells also express TRPV1 [43]. Recent data also demonstrate that a large percentage of rat and monkey vestibular ganglion cells display 5-HT1B-receptor immunoreactivity and 5-HT1D-receptor subunit immunoreactivity, with colocalization in at least 80% of the cells [44]. However, unlike some trigeminal ganglion cells, the vestibular ganglion cells do not express CGRP; rather, it is expressed by vestibular efferents [45,46].
Vestibulo–parabrachial network
Due to its important role in the formation of conditioned fear and anxiety responses, the parabrachial nucleus has been widely cited as a substrate for panic and anxiety disorders [47–50]. In addition, studies of spinal cord lamina I nociceptors resulted in the intriguing suggestion that nociceptive pathways through the parabrachial nucleus mediate the autonomic, affective and emotional aspects of pain [35,51,52]. Hence, the neurochemical similarities between nociceptive and vestibular afferents suggest: first, that convergent, neurochemically similar interoceptive, pain and vestibular pathways through the parabrachial nucleus may contribute to interoceptive representations of body status; and second, that a loss of predictability of sensations during balance control or predictable visceral sensations may contribute to psychiatric disorders.
Two vestibular nuclear regions contribute to the vestibulo–parabrachial pathway. The caudal medial vestibular nucleus and the inferior vestibular nucleus contribute: first, light descending projections to the nucleus of the solitary tract, dorsal motor vagal nucleus, nucleus ambiguus/parambiguus, the ventrolateral medullary reticular formation, nucleus raphe magnus and the lateral medullary tegmentum; second, an ascending projection to preganglionic parasympathetic neurons in the Edinger–Westphal and anteromedian nuclei [53]; and third, relatively dense ascending projections to the parabrachial nucleus [54,55]. The superior vestibular nucleus and the rostral pole of the medial vestibular nucleus contribute only dense ascending projections to the caudal aspect of the parabrachial region. The parabrachial nucleus has descending connections to both rostral and caudal aspects of the vestibular nuclei, which are distributed more extensively than the sites of origin of the vestibuloparabrachial pathway [56]. The para-brachial nucleus also has reciprocal connections with the central amygdaloid nucleus, infralimbic cortex and hypothalamus [57–59]. The caudal parabrachial nucleus (including the vestibulo-recipient region) also projects to several midline and thalamic intralaminar nuclei, including the centromedian nucleus, ventromedial nucleus and ventroposterior nucleus [60], providing potential integrative input with visceral pathways to the insular, anterior cingulate and medial prefrontal cortex.
Cerebral cortical vestibular & interoceptive processing
Vestibulo–thalamo–cortical projections [61–64] have been regarded as a gateway for vestibular information to interact with conscious sensory and cognitive processes. Dieterich and Brandt have reviewed imaging results with regard to the potential roles of telencephalic regions in perceptual phenomena, navigation and limbic manifestations [65]. Functional imaging studies during caloric irrigation or galvanic stimulation have demonstrated patterns indicating increased blood flow in the regions corresponding to vestibular sensorimotor processing regions (areas 3a, area 2/7 and the parietal operculum [area 44 and ventral area 6]), interoceptive regions of the insula (including parietal-posterior insular vestibular cortex and the anterior insula) and cognition-related regions that include the posterior and anterior cingulate gyri, orbitofrontal cortex and several prefrontal fields [65–72]. The fields associated with sensorimotor processing and the interoceptive insula regions were activated consistently by either caloric or galvanic stimulation; the other regions were activated less consistently. The parahippocampal gyrus and hippocampus are also included because they have an important role in navigation and spatial orientation [73–75], show changes in functional imaging studies during caloric irrigation of the ear [66,71,72] and show signs of atrophy in patients with bilateral vestibular loss [76].
The vestibular activation of the insula is significant in light of recent evidence that the insula serves an important role for interoceptive representations and their incorporation into motivational, attentional and cognitive contexts [34]. Craig has proposed that the posterior insula participates in the formation of interoceptive representations [34]. The posterior insula information is then relayed to the mid-insula for association with homeostatic functions and hedonistic conditions, via a network that includes the amygdala, ventral striatum and orbitofrontal cortex. The interoceptive state from the mid-insula is then proposed to be associated with motivational, social and cognitive contexts in the anterior insula, via interactions with dorsal and ventral prefrontal and anterior cingulate cortices. The activation of the anterior insula during the expression of elicited and recalled emotion in imaging studies [33,77–79] is consistent with this role in mapping affective and emotional components onto interoceptive representations. Hence, the anterior insula has figured prominently in models of interoceptive aspects of visceral awareness and conscious pain [33,35,80,81]. Of particular interest are findings that the anterior insula also shows functional MRI activation with pain, and that cognitive aspects of pain may be associated with activation in the mid-cingulate gyrus region (for a review, see [82]). These findings are consistent with the influence of vestibular information on our body perception of well-being [2,6,83].
The existence of a right hemispheric predominance for the emotional and affective consequences of pain was supported strongly in 1996 by the demonstration of a right dominance for cortical activation of the anterior cingulate cortex, orbitofrontal cortex and insula during nitrogylcerine-induced cluster headache [84]. More recently, Craig has reviewed subsequent evidence supporting a right predominance in anterior insula activation that is associated with visceral or somatic interoception (including heart rate awareness) [34]. In an earlier article, it was suggested that this right predominance corresponds to a right predominance for homeostatic (autonomic) control [85]. Activation of left and right cortical regions by vestibular stimulation is asymmetric, but without a similar right dominance: activation tends to be stronger on the side ipsilateral to caloric vestibular stimulation, in the nondominant hemisphere (for handedness), and in the hemisphere ipsilateral to the slow-phase vestibular nystagmus direction (for a review, see [65]). However, these acute findings do not preclude the recent suggestion [86] that the negative emotional and affective consequences of dizziness reflect the more general right hemispheric dominance for interoceptive effects.
Raphe–vestibular network
Serotonergic innervation in the CNS arises from neurons in the mesencephalic and rhombencephalic raphe nuclei and adjacent neurons in the brainstem reticular formation [87,88]. These nuclei contain both serotonergic and nonserotonergic neurons, which appear to project in parallel to efferent targets. The non-serotonergic neurons utilize a variety of transmitters. For example, 5-HT-negative cells in the dorsal raphe nucleus (DRN) include populations that express GABA [89,90], dopamine [91,92], excitatory amino acids [93] and neuropeptides [94,95]. The vestibular nuclei receive major serotonergic and nonserotonergic projections from the DRN and minor serotonergic and nonserotonergic projections from the nuclei raphe pallidus and obscurus [96].
In addition to the vestibular nuclei, the DRN projects to the amygdala. In fact, approximately 25% of both serotonergic and nonserotonergic DRN-vestibular cells also send collaterals to the central amygdaloid nucleus [97], a site of dense termination of DRN afferents [98]. Significantly though, there appears to be no corresponding dorsal raphe projection system to the spinal trigeminal nucleus (e.g., [99]). Anterograde tracing studies [100,101] demonstrated that DRN-vestibular pathways include: fine caliber serotonergic and nonserotonergic pathways through the ventricular plexus; and a large caliber serotonergic pathway via the medial longitudinal fasciculus. Immunoreactivity for 5-HT2A [102], 5-HT1B and 5-HT1D receptors [103] are expressed fairly widely by vestibular nucleus neurons, but 5-HT1A receptors in the vestibular nuclei are expressed heavily by ventricular plexus axons. More recently, it has been reported that 5-HT1F receptors and glutamate immunoreactivity are colocalized extensively within the vestibular nuclei [104], with prominent staining in the lateral vestibular nucleus. These findings suggest that circuits for vestibular sensorimotor pathways receive regionally-specialized serotonergic inputs and include potential sites of action for triptans and selective serotonin reuptake inhibitors (SSRIs). Functional imaging studies have revealed prominent increases in regional cerebral blood flow in the dorsal and dorso-lateral pons during either spontaneous or glyceryl trinitrate-induced migraine [105–108]. Although the spatial resolution does not permit localization of specific nuclear regions, these regional perfusion changes seem to include portions of the vestibular nuclei, medial parabrachial nucleus, locus coeruleus and raphe nuclei (including the dorsal raphe nuclei). These brainstem effects were suggested to be an indication of the primary migraine disorder because they were not resolved after sumatriptan controlled the migraine pain.
In addition to innervating the amygdala and the vestibular nuclei, the DRN also has relatively dense projections, via the ventral ascending pathway, to the cortical regions (for a review, see [109]) that are believed to mediate interoception and provide input for cognitive processes in conditions such as migraine and vestibular dysfunction. The insular and dorsal frontal cortices receive the densest innervation, while moderately dense projections terminate in orbitofrontal, entorhinal, anterior cingulate and infralimbic cortices; a hippocampal projection has also been described. These terminal regions are additional potential sites of a coordinated influence of SSRIs and triptans in comorbid aspects of balance disorders, migraine and panic disorder.
Coeruleo–vestibular network
The noradrenergic coeruleo-vestibular pathway originates from the caudal pole of locus coeruleus and the adjacent nucleus subcoeruleus [110,111]; these locus coeruleus regions also provide innervation to the cerebellum, neocortex, hypothalamus and hippocampus [111,112]. The superior and lateral vestibular nuclei receive a relatively dense coeruleo–vestibular innervation [111]. The medial vestibular nucleus receives a more moderate density of noradrenergic innervation. The inferior vestibular nucleus receives minimal innervation. The terminals may affect vestibular nucleus neurons via postsynaptic α- and β-adrenergic mechanisms (reviewed in [111]). The regionally differentiated pattern of noradrenergic innervation in vestibular nuclei contrasts sharply with the relatively sparse, undifferentiated pattern of noradrenergic innervations of neocortex [113].
Locus coeruleus and related central noradrenergic transmission have long been implicated in the pathophysiology of panic disorder [47,49,114–116]. The recent description of dense projections from the anterior cingulate and orbitofrontal cortices to locus coeruleus [117] provides a substrate for regulation of affective states in parallel with modulation of postural control. In particular, these descending projections have the potential to participate in the strong association between the degree of SMD and postural sway in anxiety patients [23,26] via the dense coeruleovestibular innervation of the lateral vestibular nucleus. Finally, it is also of interest that locus coeruleus-elicited neurogenic oligemia via diffuse cortical projections has been suggested as a potential mechanism for cortical hypofusion in active migraine [106].
Raphe–coeruleus interactions
Interactions between closely coupled locus coeruleus and DRN networks are a potential substrate for coregulation of noradrenergic, serotonergic and nonserotonergic influences on the sensorimotor, interoceptive and cognitive components of comorbid balance, migraine and anxiety signs and symptoms. The first level of interaction is mediated by direct connections between the locus coeruleus and the DRN, which may be termed the raphe–coeruleus coregulatory loop. The second level of interaction is provided by their differential relationships with brainstem and telencephalic substrates for sensorimotor, interoceptive and affective responses. For example, the DRN provides dense innervation to the anterior cingulate and orbitofrontal cortices [109], which in turn provides heavy innervation of the locus coeruleus [117].
The caudal pole of locus coeruleus, which is the site of origin of the coeruleo–vestibular pathway, receives inputs from the DRN via both collateral projections of the large caliber serotonergic pathway to superior and lateral vestibular nuclei, and the small-caliber serotonerigic and nonserotonergic fibers that travel in the ventricular plexus [100,101]. The raphe–coeruleus connections appear to have reciprocal components [118]. There are several documented synaptic interactions between locus coeruleus and the DRN, reviewed by Blier and Szabo [119,120], which can potentially coregulate their influence on the vestibular nuclei, amygdala and the cerebral cortex. Serotonin release activates excitatory amino acid afferent terminals via presynaptic 5-HT1A receptors, which produces a subsequent depolarization of noradrenergic neurons in locus coeruleus. Conversely, presynaptic 5-HT2A receptor mechanisms depolarize GABAergic terminals in locus coeruleus, producing a depression of noradrenergic cell activity via actions at GABA A receptors. Finally, norepinephrine release from terminals in the DRN can affect serotonergic neurons via postsynaptic α1-adrenoreceptors.
This raphe–coeruleus coregulatory loop is likely to be affected profoundly by SSRI administration [119,120]. It has been proposed that SSRI treatment initially decreases 5-HT release via 5HT1A autoreceptor inhibition of raphe neurons [120]. Chronic SSRI administration results in a desensitization of 5HT1A and presynaptic 5HT1B receptors, which leads to increased serotonin release from the raphe neurons owing to decreased autoreceptor inhibition. The increased serotonin release then reduces locus coeruleus activity by a 5-HT2A receptor-mediated depolarization of GABAergic terminals.
Diagnostic & neurotherapeutic implications
Drugs that have been used in the treatment of balance- and anxiety-related clinical designations in Table 1 include 5-HT and noradrenaline reuptake inhibitors (e.g., SSRI and tricyclic anti-depressants), benzodiazepines and ion-gated channel blockers (e.g., nimodipine and flunarizine) [121]. For the migraine-associated disorders, the list of treatment options includes triptans, non-selective β-blockers (e.g., propranolol) and anticonvulsant medications such as topirimate (Topomax®), lamotrigine and valproate (Depakote®) [122]. The therapeutic actions of the anticonvulsant drugs are not well understood. Topiramate inhibits voltage-gated sodium and calcium channels, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainite excitatory amino acid receptor subtypes and carbonic anhydrase; the relevance of these actions to its empirical anticonvulsant activity is unknown [123]. The bases for therapeutic activity of lamotrigine [124] and valproate [125] are also not understood explicitly.
Selective serotonin reuptake inhibitors, tricyclic antidepressants and benzodiazepines are efficacious in the treatment of both balance disorders and anxiety disorders [126–129]. Different SSRIs and tricyclic antidepressants vary in their relative affinities for 5-HT and noradrenaline (NA) reuptake inhibition [130,131]. The SSRIs have a dominant effect on 5-HT reuptake. The tricyclic antide-pressants used in dizziness include drugs with a relatively weak 5-HT reuptake preference (imipramine), roughly equal 5-HT and NA reuptake inhibition (dothiepin), a relatively weak NA reuptake inhibition preference (amitriptyline) and more selective NA reuptake effect (nortryptyline). The tricyclic antidepressant dose ranges are typically 10–100 mg at bedtime, with effective doses usually less than 50 mg.
The integrative approach in this section is motivated by the parallel therapeutic responses reported in the literature for components of dizziness and anxiety in different patient groups (Table 1). For example, previous studies indicate that SSRIs have parallel effects on dizziness symptoms and anxiety in patients with high Subjective Depression Scale scores and clinical evidence of organic vestibular disease [132], and patients with chronic subjective dizziness [4,128]. It is noteworthy that the three symptom scales showing improvement in the former study [132] include the main components of SMD. A similar parallel effect of treatments on dizziness and migraine has been reported in a substantial proportion of patients with a diagnosis of migrainous vertigo [122,133]. These effects are the focus of the hypotheses that follow.
In our clinical experience, the fact that effective doses of clon-azepam, diazepam and lorazepam for balance disorders seem to be lower than for anxiety disorders suggests differences in sites of action that are sufficient for the resolution of the respective symptoms (Table 2). The seemingly synergistic effects of these benzodiazepines with SSRIs (e.g., sertraline or paroxetine plus clonazepam) in the treatment of these disorders further suggest that effects may reflect modulation of interactions between serotonergic and GABAergic mechanisms. For example, complex interactions between 5-HT1A, 5-HT1B and 5-HT1D autoreceptors and heteroceptors have been discussed extensively in relation to the actions of antidepressant drugs, including SSRIs, in the CNS [134]. These interactions have been implicated in phenomena as diverse as anxiety, stress responses, spatial memory, cognitive responses to complexity and migraine, and are often invoked in explaining the efficacy of SSRIs, tricyclic antidepressants and triptans. Potential sites of action in terms of simultaneous effects on comorbid balance disorders, migraine and anxiety disorders will now be examined.
Table 2.
Comparison of effective benzodiazepine doses for balance and anxiety disorders.
| Benzodiazepine | Balance disorder dose (typical) | Anxiety disorder dose | Route of administration |
|---|---|---|---|
| Clonazepam | 0.25–0.5 mg b.i.d. | 0.5 mg b.i.d. – 1 mg t.i.d. | Oral |
| Diazepam | 2–10 mg acutely, then1–2 mg b.i.d. | 2–10 mg t.i.d. | Oral |
| Lorazepam | 0.25–0.5 mg b.i.d. | 2–6 mg/day divided into 2–3 daily doses | Oral |
Doses are based upon the authors’ experience.
b.i.d.: Two-times daily; t.i.d.: Three-times daily.
Cerebral cortical targets for drug treatment
As reviewed earlier, functional imaging studies are consistent with the role of common forebrain sites in responses during caloric or galvanic vestibular stimulation, panic disorder and migraine. These interactions with sites of vestibular activation appear to be at the level of the anterior insula, prefrontal cortex, cingulate cortex and parahippocampal gyrus. Remarkably, these vestibular activation regions overlap with activation sites in functional imaging studies of spontaneous and evoked migraine in the anterior insula, anterior cingulate (right side), posterior cingulate cortex and prefrontal cortex [106–108]. The anterior insula also overlaps with a region of reduced GABA A-benzodiazepine receptor binding in individuals with panic disorder [135]. The posterior cingulate field that has been identified in vestibular stimulation experiments overlaps with a region that is both activated by anticipatory anxiety in CCK-4-induced panic attacks [136] and has reduced 5-HT1A receptor binding activity in panic disorder patients [137]. Finally, the anterior aspect of the parahippocampal gyrus also shows increased GABA A-benzodiazepine binding in panic disorder patients [138]. The effects of SSRIs and benzodiazepines on these sites of altered benzodiazepine and serotonin binding may provide a partial explanation for their efficacy in the treatment of interoceptive and cognitive aspects of comorbid balance, migraine disorders and anxiety disorders.
Brainstem & cerebellar targets
In addition to primary sensory and sen-sorimotor reflex processing, processes that mediate descending influences from the cerebral cortex, the brainstem and cerebellum may be important for understanding neurothera-peutic approaches to treating the comorbid aspects of balance and anxiety disorders. For example, the dense projections from the anterior cingulate and orbitofrontal cortices to locus coeruleus [117] provide a potential pathway for cortical modulation of noradrenergic effects on SMD, anxiety and height phobia on vestibulo-ocular and postural control. We suggest that the influence of raphe–coeruleus interactions on dorsal raphe–vestibular and coeruleo–vestibular pathways mediate both the association of visual and somatosensory dependence for postural control with SMD status and the association of vestibulo-ocular findings with anxiety disorders. One likely mechanism for these interactions is modulation of noradrenergic and serotonergic output to the vestibular nuclei and amygdala via the dorsal raphe–locus coeruleus loop. For example, GABA-A receptor mechanisms mediate the inhibitory effects of 5-HT1A receptor agonists on noradrenergic neurons in locus coeruleus [139]. Conversely, GABAergic inhibition of serotonergic dorsal raphe neurons is gated by activity of 5-HT2 family receptors [140]. It is tempting to hypothesize that the lower doses of benzodiazepines are influencing this circuit in treatment of acute vestibular disorders. The relative density of coeruleo–vestibular projections and raphe–vestibular projections to areas mediating vestibulo-ocular and vestibulo-spinal responses are summarized in Table 3.
Table 3.
Vestibular nuclear monoaminergic terminal regions in relation to association between space and motion discomfort, anxiety and balance function.
| Anatomical region | NE from LC | Large-caliber DRN (5-HT only via MLF) | Small-caliber DRN (5-HT and non-5-HT) via ventricular plexus | Influences on CNS (including VOR and postural control) |
|---|---|---|---|---|
| Deiters/dorsal LVN (LVST origin) | Heavy | Dense | Sparse | Postural sway |
| SVN-PBN path origin | Intermediate- to-heavy | Dense | Sparse | Interoceptive and autonomic |
| Nodulus terminal region in MVN and SVN | Intermediate | Sparse | Heavy medially, lighter laterally | VOR time constant |
| Nucleus prepositus hypoglossi | Intermediate- to-heavy | Sparse | Heavy medially, lighter laterally | VOR time constant |
| Flocculus terminal region in MVN and ventral LVN | Low-to-intermediate | Dense | MVN only | HVOR gain |
| Caudal MVN-PBN path origin | Minimal-to-low | Dense | Light | Interoceptive and autonomic |
| IVN-PBN path origin | Minimal-to-low | Sparse | Sparse | Interoceptive and autonomic |
The NE data are from monkeys [111], but the 5-HT data are from rats [97,100] because primate data are unavailable.
5-HT: Serotonin; DRN: Dorsal raphe nucleus; HVOR: Horizontal vestibuloocular reflex; IVN: Inferior vestibular nucleus; LC: Locus coeruleus; LVN: Lateral vestibular nucleus; LVST: Lateral vestibulospinal tract; MLF: Medial longitudinal fasiculus; MVN: Medial vestibular nucleus; NE: Norepinephrine; PBN: Parabrachial nucleus; SVN: Superior vestibular nucleus; VOR: Vestibulo-ocular reflex.
The concomitant balance and (afferent) interoceptive effects that are associated with SMD may be mediated by a convergence of a high density of noradrenergic innervation and the large-caliber DRN–vestibular serotonergic projections with the superior and rostral lateral vestibular nuclei. The intersection of the rostrodorsal dorsal raphe innervation field [100,101] and coeruleo–vestibular pathway [111] includes both the region of origin of the lateral vestibular spinal tract (Dieters region) and the region of the superior vestibular nucleus that gives rise to the parabrachial nucleus projection. As the increased visual and somatosensory dependence of postural sway is associated with high SMD levels and appears to be independent of the presence of the specific type of anxiety disorder or height phobia [23], we speculate that the large-caliber dorsal raphe–rostrodorsal vestibular terminal field pathway within the dorsal lateral vestibular nucleus (Deiters region) [100,101] is a mediator of the influence of SMD on postural control, while the superior vestibular nucleus is a mediator of the development of interoceptive representations leading to the expression of SMD in behavior. This proposed role of the large-caliber serotonergic raphe–vestibular pathway is a direct extension of the concept that activation of serotonergic dorsal raphe neurons facilitates motor output (e.g., behavior) and inhibits sensory processing (e.g., analysis) for execution of motor programs [141].
It is important to note that the large-caliber dorsal raphe pathway to the rostrodorsal field appears to be almost exclusively serotonergic. Furthermore, the serotonergic contributions to activity in this network may be coordinated by the approximately 25% of dorsal raphe–vestibular projection neurons that send collateralized projections to the central amygdaloid nucleus [97]. The collateral innervation of locus coeruleus by the rostrodorsal DRN pathway [100,101] also suggests that the coregulatory raphe–coeruleus loop may exert a strong influence on these SMD-related phenomena. Consistent with this hypothesis, one expects that SSRI treatment will either ameliorate both SMD and increased postural sway or, at very least, attenuate the association of increased visually- and somatosensory-induced postural sway with the degree of SMD.
A parallel analysis leads to the inference that the small-caliber DRN pathway may be an important mediator of the shortened horizontal vestibulo-ocular reflex time constant in anxiety disorder patients [25]. The relatively large reduction in the horizontal vestibulo-ocular reflex time constant in anxiety disorder is likely to be associated with information processing in the nodulus terminal region within the vestibular nuclei and the nucleus prepositus hypoglossi [142]. These regions receive substantial densities of both serotonergic and nonserotonergic small-caliber DRN fibers from the ventricular plexus and an intermediate-to-heavy density of noradrenergic innervation, and are connected reciprocally with the DRN [143].
The flocculus terminal regions that affect vestibulo-ocular reflex gain include the ventral aspect of the rostral medial vestibular nucleus and the ventral lateral vestibular nucleus (also termed either pars-β or magnocellular medial vestibular nucleus) [144]. This region is included in the caudoventral projection field of the large-caliber serotonergic pathway from the DRN [100,101] and a light-to-intermediate density of noradrenergic innervation from the locus coeruleus [111]. The rostral medial vestibular nucleus component of this region also receives appreciable input from the small-caliber serotonergic and nonserotonergic raphe projections in the ventricular plexus [100,101]. This mixed, lighter pattern of innervation may contribute to both the small magnitude of these eye movement’s effects and the more complex relationship between vestibulo-ocular performance, anxiety, SMD and height phobia [23,25]. For example, the overall effect of anxiety on horizontal vestibulo-ocular reflex gain and the magnitude of the modulation response during off-vertical axis rotation (an otolith-ocular response) [25] may reflect involvement of both the noradrenergic locus coeruleus and small-caliber serotonergic and nonserotonergic dorsal raphe afferents. Furthermore, the finding that horizontal vestibulo-ocular reflex gain is elevated in anxiety disorder patients with normal SMD, but normal in anxiety disorder patients with excessive SMD [25], may reflect an interactive SMD-related contribution from the large caliber serotonergic projection to the caudoventral field. The cerebellar posterior lobe is another potential site where benzodiazepines could have a simultaneous effect on the vestibular system and activity engaged in panic attacks. There is a significant enhancement of blood oxygenation level-dependent signals from the cerebellar posterior lobe and culmen during precipitation of a panic attack with CCK-4 [136]. The Purkinje cells from these cerebellar sites project to both vestibulo-ocular and vestibulo-spinal pathways [145], and could contribute to postural instability. Hence, benzodiazepines could inhibit the development of a panic attack by direct actions at the level of both Purkinje cells (which receive GABAergic inputs from basket cells) and GABAA receptors on postsynaptic targets of those Purkinje cells in the vestibular nuclei.
Brainstem targets affecting migraine & balance disorder linkages
Migrainous vertigo has recently been cited as the second most frequent cause of recurrent vertigo after benign paroxysmal positional vertigo [18]. We propose that the comorbidity of migraine with balance disorders [7,11] and the increased susceptibility to motion sickness and SMD in migraineurs [8,14] can be understood as additive effects in preparabrachial and prethalamic processing of the afferent information to vestibular and pain pathways. These mechanisms can include both descending cortical projections and intrinsic parallel neurochemical features of brainstem vestibular and spinal trigeminal nuclei. For example, the activation of the anterior insula, anterior cingulate (right side), posterior cingulate cortex and prefrontal cortex [106–108] in migraine is likely to produce the same effects as SMD on vestibular processing through descending projections from the orbitofrontal, frontal and anterior cingulate cortex to the locus coeruleus. Koo and Balaban demonstrated parallel plasma extravasation in the vestibular periphery and meninges in a murine model of neurogenic migraine, and sites of action of triptans are present in both the vestibular and trigeminal ganglia and the vestibular and trigeminal nuclei (Figure 1). Finally, either spontaneous or glyceryl trinitrate-induced migraine are accompanied by increased cerebral blood flow in a region of the dorsal and dorsolateral pons [96–99,124] that appears to include portions of the vestibular nuclei, medial parabrachial nucleus, locus coeruleus and raphe nuclei. These findings support the concept of parallel activation of vestibular and cranial nociceptive pathways as an explanation for the otologic features of migrainous vertigo.
Phenomenological additivity of multisensory information has been demonstrated clearly. Drummond reported that trigeminal pain, pain sensitivity in the fingers and photophobia are augmented in migraineurs by simultaneous optokinetic stimulation motion [13]. They have also reported that motion sickness is augmented by simultaneous painful trigeminal stimulation in migraineurs [15]. This effect of trigeminal pain is blocked by rizatriptan [16]. In a similar vein, Marano et al. reported that painful trigeminal stimulation has significant effects on nystagmus in migraine patients [146]. The efficacy of triptans against motion sensitivity in migraineurs is consistent with both peripheral effects and central effects at the level of ganglion cell processes and early processing of afferent information in the vestibular and trigeminal nuclei and the dorsal horn.
Although SSRIs are effective for comorbid balance and anxiety disorders, a systematic literature review indicates that a 2-month course of treatment is no more effective than placebo in migraine headache prophylaxis [147]. Thus, the most likely reason that SSRIs have not become popular for migraine is the fact that the vast majority of migraine patients complain of headache, not dizziness. In addition, in our clinical experience, the relatively common undesirable side effects of SSRIs, especially sexual dysfunction, have been a major limiting factor. An even higher prevalence of side effects has also limited the popularity of tricyclic antidepressants in migraine treatment, despite evidence that they show greater efficicacy than SSRIs in preventing migraine headache [148]. The high prevalence of side effects with SSRIs and tricyclic antidepressants probably reflects the fact that they affect serotonin and norepinephrine turnover [130,131], rather than the classes of receptors involved in migraine headache generation.
Periphery & ganglion cells as sites of action
Vestibular ganglion cells and nociceptors in the trigeminal ganglia show remarkable similarities in distribution of functional markers for potential therapeutic targets for comorbid headache (migraine) and neuro-otological complaints. For example, the vast majority of vestibular and spiral ganglion cells are immunopositive for targets of triptans [44,149], as are small-to-medium-sized trigeminal and dorsal root ganglion cells [27,127–130], where they are believed to be primary sites of triptan-related antinociceptive activity in migraine. Furthermore, the high proportion of cells expressing these receptors raises the likelihood that many of the TRPV1-immunopositive vestibular ganglion cells [43,150] also express 5-HT1B, 5-HT1D, 5-HT1F and P2X3 receptors [44,149]. However, the known differences in the spontaneous activity and occurrence of the physiological drive to trigeminal versus vestibular ganglion cells may provide a partial explanation for differential responses of headache and vertigo to antimigraine medications. First, the spontaneous activity of trigeminal nocicecptive afferents innervating dura mater [151] is approximately an order of magnitude lower than the spontaneous activity vestibular ganglion cells [152]. Second, the vestibular afferents are activated by any head movement; nociceptive afferents are not. We suggest that these factors can plausibly contribute to differences in efficacy of particular drug regimens for headache and dizziness in migrainous vertigo. Within the inner ear, the serotonin receptor subunits are expressed in association with blood vessels in regions that show extensive protein extravasation in an intravenous serotonin infusion model of migraine [153], as well as blood vessels associated with the vestibular ganglion and vestibular nerve and the margin of the spiral ganglion. This localization of protein is consistent with a previous report of mRNA for 5-HT1B receptors in the lateral wall of mouse cochlea [154]. Because the trigeminal ganglion provides sensory innervation to the vertebrobasilar, anterior inferior cerebellar and labyrinthine arteries [155,156], it seems likely that vascular innervation is associated with axons originating in 5-HT1B and 5-HT1D receptor immunopositive cell bodies in the trigeminal ganglion [127–130].
Triptans have the greatest affinity for 5-HT1B and 5-HT1D receptors, but also have a reasonably strong affinity for 5-HT1A receptors [157,158] and 5-HT1F receptors [159]. The accepted peripheral antimigraine effects of triptans include selective constriction of pain-producing intracranial extracerebral blood vessels, reduction of trigeminal sensory nerve activation, and inhibition of vasoactive neuropeptide release and inhibition of neurotransmitter release from activated trigeminal nerves in the brainstem and upper cervical spinal column [160,161]. Parallel effects in the ear might be expected to contribute to concomitant reduction in the vertigo symptoms in patients with migrainous vertigo.
The membranous labyrinth of the inner ear is a specialized endolymph-filled structure that is surrounded by a perilymph-filled extension of the subarachnoid space. The perilymph communicates freely with the cerebrospinal fluid in the sub-arachnoid space through the cochlear aqueduct [162], and bathes the interstitial spaces around the sensory hair cells and the ganglion cells. This relationship is analogous to the location of the trigeminal ganglion in an extension of the subarachnoid space within Meckel’s cave [163], in the sense that ganglion cells can be exposed to molecules in communicating fluid compartment. The blood supply of these ‘subarachnoid’ inner ear structures is derived from the cerebral circulation, similar to the blood supply of the trigeminal ganglion. The similarities between trigeminal, vestibular and spiral ganglion cells may be particularly germane to understanding acute and delayed effects of subarachnoid hemorrhage [164,165] and deleterious effects of subarachnoid hemorrhage in the inner ear [166]. Stated simply, both the neurons and blood vessels of the trigeminal ganglion and the inner ear are very likely to be influenced directly by molecules in the surrounding cerebrospinal fluid. For example, subarachnoid hemorrhage produces acute increases in concentrations of both serotonin and 20-hydroxyeiscosatetraenoic acid (20-HETE; an arachidonic acid metabolite and TRPV1 agonist) in the cerebrospinal fluid, which produce a drop in regional cerebral blood flow by synergistic vasoconstrictor actions involving 5-HT1B receptors and direct responses to 20-HETE [167].
We have suggested recently that the enhanced motion sickness susceptibility in migraineurs may be a function of the net vestibular nuclear and solitary nucleus effects of trigeminal nucleus activity associated with trigeminovascular reflex sensitivity and parallel effects in the vestibular periphery [153]. In this context, a triptan (e.g., rizatriptan) can act on ganglion cell and brainstem trigeminal, solitary nucleus and vestibular nuclear neuronal 5-HT1B and 5-HT1D receptors to reduce nociceptive activity [168–170]. The peripheral vascular effects of the triptan would also be expected to simultaneously impact on migraine and motion sickness sensitivity via the trigeminal nerve innervation of the inner ear vascular supply [155,156]. Thus, parallel activation of vestibular and cranial nociceptive pathways may contribute to the otoneurologic features of migrainous vertigo, reinforcing the view that motion sickness may be a form of interoceptive discomfort akin to pain [32,171].
Expert commentary
Over the previous 20 years, clinical observations and basic research have provided evidence that the prevalent comorbidity of balance disorders, motion sickness, migraine and anxiety disorders is not a chance occurrence. Many comorbid combinations have been reported. Balance disorders (both neuro-otologic disease and chronic subjective dizziness) are often comorbid with psychiatric disorders [1–5] and with migraine [6–8]. Migraine is often associated with vertigo [10–12]. The increased motion sickness susceptibility in migraineurs [8,13–15] is attenuated by triptan treatment [16,17]. Migraine is also associated with phobic disorders and panic disorder [9]. Migraine and balance disorders are comorbid with anxiety disorders [6,7]. We suggest that three mechanisms may contribute to the comorbidity and overlap in treatment regimens. First, the parallel patterns of serotonin, TRPV1 and purinergic receptor expression in trigeminal, vestibular and spiral ganglion cells are consistent with parallel therapeutic effects at the level of primary afferents. Second, parallel behavior of protein extravasation in inner ear, meningeal and peripheral tissues and activation of central trigeminal and vestibular pathways may contribute to comorbid migraine, vestibular disorders and hyperacusis or tinnitus. Since these migraine and audiovestibular symptoms share common peripheral mechanisms, they are expected to respond in parallel to treatment that reduces extravasation. Finally, the parallel organization of vestibular and nociceptive pathways through the parabrachial nucleus and thalamus to the amygdala and cerebral cortex is consistent with a common central representation of interoceptive well-being, which influences control of affect. The clinical picture that emerges is a balance disorder–migraine–anxiety syndrome that can manifest differentially in different patients, with comorbid components that can respond to similar treatment regimens.
Five-year view
Both basic and clinical studies will continue to probe the hypothesis that parallel neural mechanisms are a substrate for comorbid balance disorders, migraine and anxiety disorders. From the basic science perspective, there are likely to be more detailed examinations of phenomena that include parallel molecular phenotypes of trigeminal, vestibular and spiral ganglion cells, parallel organization of vestibular and nociceptive pathways through the parabrachial nucleus and thalamus and parallel activation of central trigeminal and vestibular pathways in an animal model of neurogenic migraine. From a therapeutic perspective, intranasal ergotamine is worth testing as an alternative for triptans in the prevention of motion sickness in migraineurs.
Because recent clinical studies indicate that the CGRP antagonists BIBN4096BS [172] and MK-0974 (telcagepant) [173,174] are very efficacious in the acute treatment of migraine, clinical studies of the efficacy of these agents against comorbid balance disorders will be of great interest. Trigeminal ganglion cells that express 5-HT1B/1D receptors also express CGRP, such that triptans can inhibit CGRP release from both central and peripheral terminals of these afferents [175]. It is believed that the antimigraine activity of CGRP antagonists reflect effects at both peripheral and central release sites. Although CGRP is present in nerve terminals in the vestibular periphery, termed vestibular efferents [45,46], it appears to be absent in vestibular ganglion cells. The actions of CGRP on cells are mediated by an unusual class of G-protein-coupled receptor, formed as a heterodimer of calcitonin receptor-like protein (CLR or CRLR) and an accessory transmembrane protein from the receptor activity modifying protein (RAMP) family. The original type 1 CGRP receptor is formed by CRLR and RAMP1, but CGRP also has significant binding to adrenomedullin formed by heterodimers of CRLR and either RAMP2 or RAMP 3. The distribution of these CGRP receptor forms will need to be mapped in the inner ear tissues in basic studies to elucidate potential sites of action against neuro-otologic findings in clinical studies.
Another area of evolution of the field will probably be the systematic exploration of whether there is a migrainous tinnitus, which would be predicted by the similar receptor repertoire between trigeminal nociceptive neurons and spiral ganglion cells. The clinical explorations of this issue will likely include testing the hypothesis that the subjective and objective findings of tinnitus will improve concomitantly with migraine symptoms during antimigraine therapy.
Finally, comorbid signs and symptoms of balance disorders, migraine and anxiety are also components of mild traumatic brain injury. This is defined operationally by the appearance of temporary symptoms, such as headache, nausea, vomiting, dizziness/balance problems, fatigue, sleep disturbances, difficulty remembering and difficulty concentrating following exposure to blunt or blast trauma [176,201]. Dizziness and headache are reported in more than 70% of cases presenting acutely (<72 h), subacutely (4–30 days) and chronically (30–360 days) postexposure [177]; vertigo emerges subacutely. Other significant sequelae are hearing loss and emergent and delayed post-traumatic balance disorders, and chronic migrainous disorders in the absence of clinical radiological evidence of damage. These mild traumatic brain injury symptoms are often accompanied by ringing in the ears, sensitivity to light and sensitivity to sound [176]. Recent animal studies have reported inner ear hemorrhage after mild blast exposure [202], which could contribute to these signs and symptoms. It will obviously be an area for more intensive investigation over the next 5 years.
Key issues
The current clinical terms for patients presenting with comorbid aspects of balance disorders, migraine and anxiety disorders reflect different diagnostic tools and symptom interpretations from the neuro-otologic and psychiatric clinic.
The comorbid aspects of balance, migraine and anxiety disorders reflect shared mechanisms in afferent interoceptive information processing, central processing in pathways involving the amygdala and neocortex, serotonergic raphe nuclear-vestibular and noradrenergic coeruleo-vestibular networks, and interactions between the vestibular and trigeminal systems. These pathways can be divided conceptually into cognitive-behavioral, neurologic sensorimotor performance and interoceptive components.
The parallel organization of vestibular and nociceptive pathways through the parabrachial nucleus and thalamus to the amygdala and cerebral cortex is consistent with the concept that these pathways maintain a common central representation of interoceptive well-being that influences effect.
The synergistic effects of low doses of benzodiazepines and selective serotonin reuptake inhibitors on interoceptive and cognitive aspects of comorbid balance and anxiety disorders may involve sites of benzodiazepine and serotonin binding in the prefrontal cortex, insula, cingulate cortex and parahippocampal gyrus.
We hypothesize that direct effects and interactions between dorsal raphe-vestibular and coeruleo-vestibular pathways may contribute to the parallel effects of selective serotonin reuptake inhibitors on dizziness symptoms and anxiety in some patient groups, the association of visual and somatosensory dependence for postural control with space and motion discomfort status and the association of vestibulo-ocular findings with anxiety disorders.
Vestibular ganglion cells and nociceptors in the trigeminal ganglia show remarkable similarities in distribution of transmitter receptors (e.g., 5-HT1B, 5-HT1D, 5-HT1F, TRPV1 and P2X3) that are potential shared therapeutic targets for comorbid headache (migraine) and neurotologic complaints.
Parallel activation of vestibular and cranial nociceptive pathways may contribute to the otoneurologic features of migrainous vertigo.
Acknowledgments
Carey Balaban gratefully acknowledges the long-term encouragement of these efforts by Dr Daniel Sklare and Dr Christopher Platt of the National Institute on Deafness and Other Communication Disorders (NIDCD).
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Carey Balaban gratefully acknowledges the support of this area of translational research by R01 DC000739 from the NIDCD. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurologic setting. J Anxiety Disord. 2001;15:9–26. doi: 10.1016/s0887-6185(00)00040-2. [DOI] [PubMed] [Google Scholar]
- 2.Jacob RG, Furman JM, Balaban CD. Psychiatric aspects of vestibular disorders. In: Baloh RW, Halmagyi GM, editors. Handbook of Neurotology/Vestibular System. Oxford University Press; Oxford, UK: 1996. pp. 509–528. [Google Scholar]
- 3.Staab JP. Chronic dizziness: the interface between psychiatry and neuro-otology. Curr Opin Neurol. 2006;19(1):41–48. doi: 10.1097/01.wco.0000198102.95294.1f. [DOI] [PubMed] [Google Scholar]
- 4.Staab JP, Ruckenstein MJ. Chronic dizziness and anxiety: effect of course of illness on treatment outcome. Arch Otolaryngol Head Neck Surg. 2005;131(8):675–679. doi: 10.1001/archotol.131.8.675. [DOI] [PubMed] [Google Scholar]
- 5.Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170–176. doi: 10.1001/archotol.133.2.170. [DOI] [PubMed] [Google Scholar]
- 6.Furman JM, Balaban CD, Jacob RG, Marcus DA. Migraine-anxiety associated dizziness (MARD): a new disorder? J Neurol Neurosurg Psychiatry. 2005;76:1–8. doi: 10.1136/jnnp.2004.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furman JM, Marcus DA, Balaban CD. Migrainous vertigo: development of a pathogenetic model and structured diagnostic interview. Curr Opin Neurology. 2003;16:5–13. doi: 10.1097/01.wco.0000053582.70044.e2. [DOI] [PubMed] [Google Scholar]
- 8.Marcus DA, Furman JM, Balaban CD. Motion sickness in migraine sufferers. Expert Opin Pharmacotherapy. 2005;6(15):2691–2697. doi: 10.1517/14656566.6.15.2691. [DOI] [PubMed] [Google Scholar]
- 9.Radat F, Swendsen J. Psychiatric morbidity in migraine: a review. Cephalagia. 2004;25:165–178. doi: 10.1111/j.1468-2982.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 10.Cutrer FM, Baloh RW. Migraine-associated dizziness. Headache. 1992;32:300–304. doi: 10.1111/j.1526-4610.1992.hed3206300.x. [DOI] [PubMed] [Google Scholar]
- 11••.Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56(4):436–441. doi: 10.1212/wnl.56.4.436. Provides the seminal clinical description of migrainous vertigo. [DOI] [PubMed] [Google Scholar]
- 12.Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol. 2009;256:333–338. doi: 10.1007/s00415-009-0149-2. [DOI] [PubMed] [Google Scholar]
- 13.Drummond PD. Motion sickness and migraine: optokinetic stimulation increases scalp tenderness, pain sensitivity in the fingers and photophobia. Cephalagia. 2002;22:117–124. doi: 10.1046/j.1468-2982.2002.00332.x. [DOI] [PubMed] [Google Scholar]
- 14.Drummond PD. Triggers of motion sickness in migraine sufferers. Headache. 2005;45(6):653–656. doi: 10.1111/j.1526-4610.2005.05132.x. [DOI] [PubMed] [Google Scholar]
- 15.Drummond PD, Granston A. Facial pain increases nausea and headache during motion sickness in migraine sufferers. Brain. 2004;127(3):526–534. doi: 10.1093/brain/awh061. [DOI] [PubMed] [Google Scholar]
- 16.Furman JM, Marcus DA. A pilot study of rizatriptan and visually-induced motion sickness in migraineurs. Int J Med Sci. 2009;6:212–217. doi: 10.7150/ijms.6.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furman JM, Marcus DA, Balaban CD. Rizatriptan reduces vestibular-induced motion sickness in migraineurs. J Headache Pain. 2010 doi: 10.1007/s10194-010-0250-z. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuhauser HK. Epidemiology of vertigo. Curr Opin Neurology. 2007;20:40–46. doi: 10.1097/WCO.0b013e328013f432. [DOI] [PubMed] [Google Scholar]
- 19.Eckhardt-Henn A, Tschan R, Best C, Dieterich M. [Somatoform vertigo syndrome] Nervenarzt. 2009;80:909–917. doi: 10.1007/s00115-009-2736-y. [DOI] [PubMed] [Google Scholar]
- 20••.Staab JP. Psychological aspects of vestibular disorders. In: Eggers SDZ, Zee DS, editors. Vertigo and Imbalance: Clinical Neurophysiology of the Vestibular System. Elsevier; MA, USA: 2010. pp. 502–522. Provides a comprehensive review of psychological factors associated with balance disorders. [Google Scholar]
- 21.Helzer JE, Kraemer HC, Krueger RF. The feasibility and need for dimensional psychiatric diagnoses. Psycholog Med. 2006;36:1671–1680. doi: 10.1017/S003329170600821X. [DOI] [PubMed] [Google Scholar]
- 22.Helzer JE, Kraemer HC, Krueger RF, et al. Dimensional Approaches in Diagnostic Classification: Refining the Research Agenda for DSM-V. American Psychiatric Association; VA, USA: 2008. [Google Scholar]
- 23.Jacob RG, Redfern MS, Furman JM. Space and motion discomfort and abnormal balance control in patients with anxiety disorders. J Neurol Neurosurg Psychiatry. 2009;80:74–78. doi: 10.1136/jnnp.2007.136432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob RG, Woody S, Clark DB, et al. Discomfort with space and motion: a possible marker of vestibular dysfunction assessed by the situational characteristics questionnaire. J Psychopathol Behav Assess. 1993;15:299–324. [Google Scholar]
- 25••.Furman JM, Redfern MS, Jacob RG. Vestibulo-ocular function in anxiety disorders. J Vestib Res. 2006;16:209–215. Provides new insights into the interactions between space and motion discomfort, anxiety and vestibulo-ocular reflex performance. [PubMed] [Google Scholar]
- 26.Redfern MS, Furman JM, Jacob RG. Visually induced postural sway in anxiety disorders. J Anxiety Disord. 2007;21:704–716. doi: 10.1016/j.janxdis.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieterich M, Krafczyk S, Querner V, Brandt T. Somatoform phobic postural vertigo and psychogenic disorders of stance and gait. Adv Neurol. 2001;87:225–233. [PubMed] [Google Scholar]
- 28.Bronstein AM. Visual vertigo syndrome: clinical and posturographic findings. J Neurol Neurosurg Psychiatry. 1995;59:472–476. doi: 10.1136/jnnp.59.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin N Am. 2009;42:71–77. doi: 10.1016/j.otc.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Eckhardt-Henn A, Best C, Bense S, et al. Psychiatric comorbidity in different organic vertigo syndromes. J Neurol. 2008;255:420–428. doi: 10.1007/s00415-008-0697-x. [DOI] [PubMed] [Google Scholar]
- 31.Balaban CD, Jacob RG. Background and history of the interface between anxiety and vertigo. J Anxiety Disord. 2001;15:27–51. doi: 10.1016/s0887-6185(00)00041-4. [DOI] [PubMed] [Google Scholar]
- 32.Balaban CD, Yates BJ. Vestibulo-autonomic interactions: a teleologic perspective. In: Highstein SN, Fay RR, Popper AN, editors. Springer Handbook of Auditory Research: The Vestibular System. Springer-Verlag; NY, USA: 2004. pp. 286–342. [Google Scholar]
- 33.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 34••.Craig AD. How do you feel – now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. Presents a systematic overview of neural bases for interoception and its relationship to awareness. [DOI] [PubMed] [Google Scholar]
- 35.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 36.Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: inplications for the selective antimigraine action of triptans. J Neurosci. 2003;23:10988–10997. doi: 10.1523/JNEUROSCI.23-34-10988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell JL, Silverman MB, Aicher SA. Rat trigeminal lamina I neurons that project to the thalamic or parabrachial nuclei contain the μ-opioid receptor. Neuroscience. 2004;128:571–582. doi: 10.1016/j.neuroscience.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 39.Caterina MJ, Leffler A, Schumacher MA, et al. The capsaicin receptor: a heat activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 40.Ichikawa H, Jin HW, Terayama R, et al. Calretinin-containing neurons which co-express parvalbumin and calbindin D-28k in the rat spinal and cranial sensory ganglia; triple immunofluorescence study. Brain Res. 2005;1061:118–123. doi: 10.1016/j.brainres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Kevetter GA, Leonard RB. Molecular probes of the vestibular nerve II. Characterization of neurons in Scarpa’s ganglion to determine separate populations. Brain Res. 2002;928:18–29. doi: 10.1016/s0006-8993(01)03264-4. [DOI] [PubMed] [Google Scholar]
- 42.Xiang Z, Bo X, Burnstock G. P2X receptor immunoreactivity in the rat cochlea, vestibular ganglion and cochlear nucleus. Hear Res. 1999;128:190–196. doi: 10.1016/s0378-5955(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 43.Balaban CD, Zhou J, Li HS. Type1 vanilloid receptor expression by mammalian inner ear ganglion cells. Hear Res. 2003;175(1–2):165–170. doi: 10.1016/s0378-5955(02)00734-7. [DOI] [PubMed] [Google Scholar]
- 44.Ahn SK, Balaban CD. Distribution of receptors in the inner 5-HT1B and 5-HT1D ear. Brain Res. 2010;1346:92–101. doi: 10.1016/j.brainres.2010.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong WJ, Scholtz AW, Kammen-Jolly K, et al. Ultrastructural evaluation of calcitonin gene-related peptide immunoreactivity in the human cochlea and vestibular endorgans. Eur J Neurosci. 2002;15:487–497. doi: 10.1046/j.0953-816x.2001.01880.x. [DOI] [PubMed] [Google Scholar]
- 46.Popper P, Ishiyama A, Lopez I, Wackym PA. Calcitonin gene-related peptide and choline acetyltransferase colocalization in the human vestibular periphery. Audiol Neurootol. 2002;7:298–302. doi: 10.1159/000064445. [DOI] [PubMed] [Google Scholar]
- 47.Charney DS, Deutsch A. A functional neuroanatomy of anziety and fear: implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol. 1996;10:419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 48.Goddard AW, Charney DS. Toward an integrated neurobiology of panic disorder. J Clin Psychiatry. 1997;58(Suppl 2):4–12. [PubMed] [Google Scholar]
- 49.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- 50.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 51.Bernard JF, Bester H, Besson JM. Involvement of the spino–parabrachio–amygdaloid and –hypothalmic systems in the autonomic and emotional aspects of pain. In: Hostege G, Bandler R, Saper CB, editors. The Emotional Motor System. Elsevier; Amsterdam, The Netherlands: 1996. pp. 243–255. [DOI] [PubMed] [Google Scholar]
- 52.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87(2):251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 53.Balaban CD. Vestibular projections to the Edinger-Westphal and anteromedian nuclei of rabbits. Brain Res. 2003;963:121–131. doi: 10.1016/s0006-8993(02)03955-0. [DOI] [PubMed] [Google Scholar]
- 54.Balaban CD. Vestibular nucleus projections to the parabrachial nucleus in rabbits: Implications for vestibular influences on autonomic function. Exp Brain Res. 1996;108:367–381. doi: 10.1007/BF00227260. [DOI] [PubMed] [Google Scholar]
- 55.Porter JD, Balaban CD. Connections between the vestibular nuclei and regions that mediate autonomic function in the rat. J Vestib Res. 1997;7:63–76. [PubMed] [Google Scholar]
- 56.Balaban CD. Projections from the parabrachial nucleus to the vestibular nuclei: potential substrates for autonomic and limbic influences on vestibular responses. Brain Res. 2004;996:126–137. doi: 10.1016/j.brainres.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 57.Moga MM, Herbert H, Hurley KM, et al. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- 58.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 59.Fulweiler CE, Saper C. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Rev. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 60.Krout KE, Loewy AD. Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2000;428:475–494. doi: 10.1002/1096-9861(20001218)428:3<475::aid-cne6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 61.Lang W, Büttner-Ennever JA, Büttner U. Vestibular projections to the monkey thalamus: an autoradiographic study. Brain Res. 1979;177:3–17. doi: 10.1016/0006-8993(79)90914-4. [DOI] [PubMed] [Google Scholar]
- 62.Maciewicz R, Phipps BS, Bry J, Highstein SM. The vestibulothalamic pathway: contribution of the ascending tract of Deiters. Brain Res. 1982;252:1–11. doi: 10.1016/0006-8993(82)90973-8. [DOI] [PubMed] [Google Scholar]
- 63.Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K. The vestibular nuclei of the rat project to the lateral part of the thalamic parafascicular nucleus (centromedian nucleus in primates) Brain Res. 1995;704:130–134. doi: 10.1016/0006-8993(95)01194-3. [DOI] [PubMed] [Google Scholar]
- 64.Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K. Projections of the vestibular nuclei to the thalamus in the rat: a Phaseolus vulgaris leucoagglutinin study. J Comparative Neurology. 1999;407:318–332. [PubMed] [Google Scholar]
- 65•.Dieterich M, Brandt T. Functional brain imaging of peripheral and central vestibular disorders. Brain. 2008;131:2538–2552. doi: 10.1093/brain/awn042. Provides a comprehensive overview of functional imaging in vestibular disorders. [DOI] [PubMed] [Google Scholar]
- 66.Dieterich M, Bense S, Lutz S, et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003;13(9):994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- 67.Bucher S, Dieterich M, Wiesmann M, et al. Cerebral functional magnetic resonance imaging of vestibular, auditory, and nociceptive areas during galvanic stimulation. Ann Neurol. 1998;44:120–125. doi: 10.1002/ana.410440118. [DOI] [PubMed] [Google Scholar]
- 68.Eikhoff SB, Weiss PH, Amunts K, Fink GR, Zilles K. Identifying human parieto-insular vestibular cortex using fMRI and cytoarchitectonic mapping. Hum Brain Mapp. 2006;27:611–621. doi: 10.1002/hbm.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fasold O, von Brevern M, Kuhberg M, et al. Human vestibular cortex identified with caloric stimulation in functional magnetic resonance imaging. NeuroImage. 2002;17:1384–1392. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- 70.Lobel E, Kleine JF, Le Bihan D, Leroy-Willig A, Berthoz A. Functional MRI of galvanic vestibular stimulation. J Neurophysiol. 1998;80:2699–2709. doi: 10.1152/jn.1998.80.5.2699. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki M, Kitano H, Ito R, et al. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Cogn Brain Res. 2001;12:441–449. doi: 10.1016/s0926-6410(01)00080-5. [DOI] [PubMed] [Google Scholar]
- 72.Bottini G, Karnath HO, Vallar G, et al. Cerebral representations for egocentric space: functional-anatomical evidence from caloric vestibular stimulation and neck vibration. Brain. 2001;124:1182–1196. doi: 10.1093/brain/124.6.1182. [DOI] [PubMed] [Google Scholar]
- 73.Karnath HO, Dieterich M. Spatial neglect – a vestibular disorder? Brain. 2006;129:293–305. doi: 10.1093/brain/awh698. [DOI] [PubMed] [Google Scholar]
- 74.Russell N, Horii A, Smith PF, Darlington CL, Bilkey DK. Bilateral peripheral vestibular lesions produce long-term changes in spatial learning in the rat. J Vestib Res. 2003;13:9–16. [PubMed] [Google Scholar]
- 75.Schautzer F, Hamilton D, Kalla R, Strupp M, Brandt T. Spatial memory deficits in patients with chronic bilateral vestibular failure. Ann NY Acad Sci. 2003;1004:316–324. doi: 10.1196/annals.1303.029. [DOI] [PubMed] [Google Scholar]
- 76.Brandt T, Schautzer F, Hamilton D, et al. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain. 2005;128:2732–2741. doi: 10.1093/brain/awh617. [DOI] [PubMed] [Google Scholar]
- 77.Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson R. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiat. 2000;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 78.Reiman EM, Lane RD, Ahern GL, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiat. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- 79.Shin LM, Dougherty DD, Orr SP, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biol Psychiat. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- 80.Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26(6):303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 81.Wiens S. Interoception in emotional experience. Curr Opin Neurol. 2005;18:442–447. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- 82.Schnitzler A, Ploner M. Neurophysiology and functional neuroanatomy of pain perception. J Clin Neurophysiol. 2000;17:592–603. doi: 10.1097/00004691-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 83.Jacob RGJ, Furman MR, Clark DB, Durrant JD, Balaban CD. Psychogenic dizziness. In: Sharpe JA, Barber HO, editors. The Vestibulo-Ocular Reflex and Vertigo. Raven Press; NY, USA: 1993. pp. 305–317. [Google Scholar]
- 84.Hsieh JC, Hannerz J, Ingvar M. Right-lateralised central processing for pain of nitroglycerine-induced cluster headache. Pain. 1996;67:59–68. doi: 10.1016/0304-3959(96)03066-7. [DOI] [PubMed] [Google Scholar]
- 85.Craig AD. Forebrain emotional asymmety: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 86.Carmona JE, Holland AK, Harrison DW. Extending the functional cerebral systems theory of emotion to the vestibular modality: a systematic and integrative approach. Psychol Bull. 2009;135:286–302. doi: 10.1037/a0014825. [DOI] [PubMed] [Google Scholar]
- 87.Baumgarten HG, Grozdanovic Z. Anatomy of central serotonergic projection systems. In: Baumgarten HG, Gothert M, editors. Serotonergic Neurons and 5-HT Receptors in the CNS. Springer; Berlin, Germany: 1997. pp. 41–89. [Google Scholar]
- 88.Jacobs BL, Azmitia E. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 89.Bagdy E, Kiraly I, Harsing LG., Jr Reciprocal innervation between serotonergic and GABAergic neurons in the raphe nuclei of the rat. Neurochem Res. 2000;25:1465–1473. doi: 10.1023/a:1007672008297. [DOI] [PubMed] [Google Scholar]
- 90.Stamp JA, Semba K. Extent of colocalization of serotonin and GABA in the neurons of the rat raphe nuclei. Brain Res. 1995;677:39–49. doi: 10.1016/0006-8993(95)00119-b. [DOI] [PubMed] [Google Scholar]
- 91.Stratford TR, Wirtshafter D. Ascending dopaminergic projections from the dorsal raphe nucleus in the rat. Brain Res. 1990;511:173–176. doi: 10.1016/0006-8993(90)90239-8. [DOI] [PubMed] [Google Scholar]
- 92.Yoshida M, Shiriuza M, Tanaka M, Semba K, Fibiger HC. Dopaminergic neurons in the nucleus raphe dorsalis innervate the prefrontal cortex in the rat: a combined retrograde tracing and immunohistochemical study using anti-dopamine serum. Brain Res. 1989;496:373–376. doi: 10.1016/0006-8993(89)91091-3. [DOI] [PubMed] [Google Scholar]
- 93.Clements JR, Madl JE, Johnson RL, Larson AA, Beitz AJ. Localization of glutamate, glutaminase, aspartate and aspartate aminotransferase in the rat. Exp Brain Res. 1987;67:594–602. doi: 10.1007/BF00247290. [DOI] [PubMed] [Google Scholar]
- 94.Lechner J, Leah JD, Zimmermann M. Brainstem peptidergic neurons projecting the the medial and lateral thalamus and zona incerta in the rat. Brain Res. 1993;603:47–56. doi: 10.1016/0006-8993(93)91298-7. [DOI] [PubMed] [Google Scholar]
- 95.Petit JM, Luppi PH, Peyron C, Rampon C, Jouvet M. VIP-like immunoreactive projections from the dorsal raphe and linear raphe nuclei to the bed nucleus of the stria terminalis demonstrated by a double immunohistochemical method in the rat. Neurosci Lett. 1995;193:77–80. doi: 10.1016/0304-3940(95)11669-n. [DOI] [PubMed] [Google Scholar]
- 96.Halberstadt AL, Balaban CD. Organization of projections from the raphe nuclei to the vestibular nuclei in rats. Neuroscience. 2003;120:571–592. doi: 10.1016/s0306-4522(02)00952-1. [DOI] [PubMed] [Google Scholar]
- 97.Halberstadt AL, Balaban CD. Serotonergic and nonserotonergic neurons in the dorsal raphe nucleus send collateralized projections to both the vestibular nuclei and the central amygdaloid nucleus. Neuroscience. 2006;140(3):1067–1077. doi: 10.1016/j.neuroscience.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 98.Vertes R. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 99.Vertes RP, Kocsis B. Projections of the dorsal raphe nucleus to the brain stem: PHA-L analysis in the rat. J Comp Neurol. 1994;340:11–26. doi: 10.1002/cne.903400103. [DOI] [PubMed] [Google Scholar]
- 100.Halberstadt AL, Balaban CD. Anterograde tracing of projections from the dorsal raphe nucleus to the vestibular nuclei. Neuroscience. 2006;143:641–654. doi: 10.1016/j.neuroscience.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Halberstadt AL, Balaban CD. Selective anterograde tracing of the individual serotonergic and nonserotonergic components of the dorsal raphe nucleus projection to the vestibular nuclei. Neuroscience. 2007;147:207–223. doi: 10.1016/j.neuroscience.2007.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balaban CD. Neural substrates linking balance control and anxiety. Physiol Behav. 2002;77:469–475. doi: 10.1016/s0031-9384(02)00935-6. [DOI] [PubMed] [Google Scholar]
- 103.Ahn SK, Balaban CD. Regional receptors distribution of 5-HT1A, 1B, and 1D in rat vestibular nuclei (VN) Soc Neurosci Abstracts. 2007;37:180.5. [Google Scholar]
- 104.Ahn SK, Khalmuratova R, Jeon SY, et al. receptor and Colocalization of 5-HT1F glutamate in neurons of the vestibular nuclei in rats. NeuroReport. 2009;20:111–115. doi: 10.1097/WNR.0b013e328320795e. [DOI] [PubMed] [Google Scholar]
- 105•.Afridi SK, Giffin NJ, Kaube H, et al. A positron emission tomography study in spontaneous migraine. Arch Neurol. 2005;62:1270–1275. doi: 10.1001/archneur.62.8.1270. Provides functional imaging information on pathways active in spontaneous migraine. [DOI] [PubMed] [Google Scholar]
- 106.Afridi SK, Goadsby PJ. Neuroimaging of migraine. Curr Pain Headache Rep. 2006;10:221–224. doi: 10.1007/s11916-006-0049-4. [DOI] [PubMed] [Google Scholar]
- 107.May A. A review of diagnostic and functional imaging in headache. J Headache Pain. 2006;7:174–184. doi: 10.1007/s10194-006-0307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.May A, Matharu M. New insights into migraine: application of functional and structural imaging. Curr Opin Neurol. 2007;20:306–309. doi: 10.1097/WCO.0b013e328136c20b. [DOI] [PubMed] [Google Scholar]
- 109.Michelsen K, Schmitz C, Steinbusch HW. The dorsal raphe nucleus – from silver stainings to a role in depression. Brain Res Rev. 2007;55:329–342. doi: 10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Schuerger RJ, Balaban CD. Immunohistochemical demonstration of regionally-selective projections from locus coeruleus to the vestibular nuclei in rats. Exp Brain Res. 1993;92:351–359. doi: 10.1007/BF00229022. [DOI] [PubMed] [Google Scholar]
- 111.Schuerger RJ, Balaban CD. Organization of the coeruleo-vestibular pathway in rats, rabbits and monkeys. Brain Res Rev. 1999;30:189–217. doi: 10.1016/s0165-0173(99)00015-6. [DOI] [PubMed] [Google Scholar]
- 112.Loughlin SE, Foote SL, Bloom FE. Efferent projections of locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- 113.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 114.Gorman JM, Liebowitz MR, Fyer AJ, Stein J. A neuroanatomical hypothesis for panic disorder. Am J Psychiatry. 1989;146:148–161. doi: 10.1176/ajp.146.2.148. [DOI] [PubMed] [Google Scholar]
- 115.Ninan PT. The functional anatomy, neurochemistry and pharmacology of anxiety. J Clin Psychiatry. 1999;60(Suppl 22):12–17. [PubMed] [Google Scholar]
- 116.Pratt JA. The neuroanatomical basis of anxiety. Pharmacol Ther. 1992;55:149–181. doi: 10.1016/0163-7258(92)90014-q. [DOI] [PubMed] [Google Scholar]
- 117.Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus–norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- 118.Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and nuclear core of the locus coeruleus. Brain Res. 2004;1026:56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 119.Blier P, Szabo ST. Potential mechanisms of action of atypical antipsychotic medications in treatment-resistant depression and anxiety. J Clin Psychiatry. 2005;66(Suppl 8):30–40. [PubMed] [Google Scholar]
- 120.Blier P, Tremblay P. Physiologic mechanisms underlying the antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(Suppl 4):8–13. [PubMed] [Google Scholar]
- 121.Tusa RJ. Dizziness. Med Clin N Am. 2009;93:263–271. doi: 10.1016/j.mcna.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 122•.Fotuhi M, Glaun B, Quan SY, Sofare T. Vestibular migraine: a critical review of treatment trials. J Neurol. 2009;256:711–716. doi: 10.1007/s00415-009-5050-5. Provides a succinct and balanced review of treatment trials. [DOI] [PubMed] [Google Scholar]
- 123.Shank RP, Maryanoff BE. Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate. CNS Neurosci Ther. 2008;14:120–142. doi: 10.1111/j.1527-3458.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eisenberg E, River Y, Shifrin A, Krivoy N. Antiepileptic drugs in the treatment of neuropathic pain. Drugs. 2007;67:1265–1289. doi: 10.2165/00003495-200767090-00003. [DOI] [PubMed] [Google Scholar]
- 125.Rosenberg G. The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? Cell Mol Life Sci. 2007;64:2090–2103. doi: 10.1007/s00018-007-7079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johnson GD. Medical management of migraine-related dizziness and vertigo. Laryngoscope. 1998;108(Suppl 85):1–28. doi: 10.1097/00005537-199801001-00001. [DOI] [PubMed] [Google Scholar]
- 127.Simon NM, Parker SW, Wernick-Robinson M, et al. Fluoxetine for vestibular dysfunction and anxiety: a prospective pilot study. Psychosomatics. 2005;46:334–339. doi: 10.1176/appi.psy.46.4.334. [DOI] [PubMed] [Google Scholar]
- 128.Staab JP, Ruckenstein MJ, Amsterdam JD. A prospective trial of sertraline for chronic subjective dizziness. Laryngoscope. 2004;114(9):1637–1641. doi: 10.1097/00005537-200409000-00025. [DOI] [PubMed] [Google Scholar]
- 129.Staab JP, Ruckenstein MJ, Solomon D, Shepard NT. Serotonin reuptake inhibitors for dizziness with psychiatric symptoms. Arch Otolaryngol Head Neck Surg. 2002;128(5):554–560. doi: 10.1001/archotol.128.5.554. [DOI] [PubMed] [Google Scholar]
- 130.Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151:737–748. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sanchez C, Hyttel J. Comparison of effects of antidepressants and their metabolites on reuptake of biogenic amine and on receptor binding. Cell Mol Neurobiol. 1999;19:467–489. doi: 10.1023/A:1006986824213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Horii A, Mitani K, Kitahara T, et al. Paroxetine, a selective serotonin reuptake inhibitor, reduces depressive symptoms and subjective handicaps in patients with dizziness. Otol Neurotol. 2004;25:536–543. doi: 10.1097/00129492-200407000-00022. [DOI] [PubMed] [Google Scholar]
- 133.Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol. 1997;18:350–354. [PubMed] [Google Scholar]
- 134.Stamford JA, Davidson C, McLaughlin DP, Hopwood SE. Control of dorsal raphe 5-HT function by multiple 5-HT1 autoreceptors: parallel purposes or plurality? Trends Neurosci. 2000;23:459–465. doi: 10.1016/s0166-2236(00)01631-3. [DOI] [PubMed] [Google Scholar]
- 135.Cameron OG, Huang GC, Nichols T, et al. Reduced γ-aminobutyric acidA-benzodiazepine binding sites in insular cortex of individuals with panic disorder. Arch Gen Psychiatry. 2008;64:793–800. doi: 10.1001/archpsyc.64.7.793. [DOI] [PubMed] [Google Scholar]
- 136.Eser D, Leicht G, Lutz J, et al. Functional neuroanatomy of CCK-4-induced panic attacks in healthy volunteers. Hum Brain Mapp. 2009;30:511–522. doi: 10.1002/hbm.20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Neumeister A, Bain E, Nugent AC, et al. receptor binding in Reduced serotonin 1A panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hasler G, Nugent AC, Carlson PJ, et al. Altered cerebral γ-aminobutyric acid type A-benzodiazepine receptor binding in panic disorder determined by [11C]flumazenil positron emission tomography. Arch Gen Psychiatry. 2008;65:1166–1175. doi: 10.1001/archpsyc.65.10.1166. [DOI] [PubMed] [Google Scholar]
- 139.Szabo ST, Blier P. Serotonin1A receptor ligands act on the norepinephrine neuron firing through excitatory amino acid and receptors: a microiontophoretic GABA A study in the rat locus coeruleus. Synapse. 2001;42:203–212. doi: 10.1002/syn.10009. [DOI] [PubMed] [Google Scholar]
- 140.Liu R, Jolas T, Aghajanian GK. Serotonin receptors activate local GABA 5-HT2 inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- 141.Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- 142.Cohen B, Raphan T. The physiology of the vestibuloocular reflex (VOR) In: Highstein SN, Fay RR, Popper AN, editors. Springer Handbook of Auditory Research: The Vestibular System. Springer-Verlag; NY, USA: 2004. pp. 235–285. [Google Scholar]
- 143.Cuccarazzu B, Halberstadt AL. Projections from the vestibular nuclei and nucleus prepositus hypoglossi to dorsal raphe nucleus in rats. Neuro Lett. 2008;439:70–74. doi: 10.1016/j.neulet.2008.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Balaban CD, Schuerger RJ, Porter JD. Zonal organization of flocculo-vestibular connections in rats. Neuroscience. 2000;99:669–682. doi: 10.1016/s0306-4522(00)00232-3. [DOI] [PubMed] [Google Scholar]
- 145.Ito M. The Cerebellum and Neural Control. 1. Raven Press; NY, USA: 1984. [Google Scholar]
- 146.Marano E, Marcelli V, Di Stasio E, et al. Trigeminal stimulation elicits a peripheral vestibular imbalance in migraine patients. Headache. 2005;45(4):325–331. doi: 10.1111/j.1526-4610.2005.05069.x. [DOI] [PubMed] [Google Scholar]
- 147.Moja L, Cussi C, Sterzi R, Canepari C. Selective serotonin reuptake inhibitors (SSRIs) for preventing migraine and tension-type headaches. Cochrane Database Syst Rev. 2005;3:CD002919. doi: 10.1002/14651858.CD002919.pub2. [DOI] [PubMed] [Google Scholar]
- 148.Jackson JL, Shimeall W, Sessums L, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010 doi: 10.1136/bmj.c522. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Balaban CD. Distribution of putative ‘nociceptive’ ganglion cell markers in the vestibular and spiral ganglia of rats and macaques. Soc Neurosci Abstracts. 2010 Abstract 583.4. [Google Scholar]
- 150.Kitahara T, Li HS, Balaban CD. Changes in transient receptor potential cation channel superfamily V (TRPV) mRNA expression in the mouse inner ear ganglia after kanamycin challenge. Hear Res. 2005;201(1–2):132–144. doi: 10.1016/j.heares.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 151.Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain. 2003;126:1801–1813. doi: 10.1093/brain/awg190. [DOI] [PubMed] [Google Scholar]
- 152.Wilson VJ, Melvill Jones G. Mammalian Vestibular Physiology. Plenum; NY, USA: 1974. [Google Scholar]
- 153.Koo JW, Balaban CD. Serotonin-induced plasma extravasation in the murine inner ear: possible mechanism of migraine-associated inner ear dysfunction. Cephalagia. 2006;26:1310–1319. doi: 10.1111/j.1468-2982.2006.01208.x. [DOI] [PubMed] [Google Scholar]
- 154.Oh CK, Drescher MJ, Hatfield JS, Drescher DG. Selective expression of serotonin receptor transcripts in the mammalian cochlea and its subdivisions. Hear Res. 1999;70:135–140. doi: 10.1016/s0169-328x(99)00110-2. [DOI] [PubMed] [Google Scholar]
- 155.Vass Z, Dai CF, Steyger PS, et al. Co-localization of the vanilloid capsaicin receptor and substance P in sensory nerve fibers innervating cochlear and vertebro-basilar arteries. Neuroscience. 2004;124:919–927. doi: 10.1016/j.neuroscience.2003.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vass Z, Shore SE, Nuttall AL, Miller JM. Direct evidence of trigeminal innervation of cochlear blood vessels. Neuroscience. 1998;84:559–567. doi: 10.1016/s0306-4522(97)00503-4. [DOI] [PubMed] [Google Scholar]
- 157.Napier C, Stewart M, Melrose H, et al. Characterization of the 5-HT receptor binding profile of eletriptan and kinetics of [3 H]eletriptan binding at human 5-HT1B and 5-HT1D receptors. Eur J Pharmacol. 1999;368:259–268. doi: 10.1016/s0014-2999(99)00026-6. [DOI] [PubMed] [Google Scholar]
- 158.Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs. 2000;60:1259–1287. doi: 10.2165/00003495-200060060-00003. [DOI] [PubMed] [Google Scholar]
- 159.Wood MD, Thomas DR, Watson JM. Therapeutic potential of serotonin antagonists in depressive disorders. Expert Opin Investig Drugs. 2002;11:457–467. doi: 10.1517/13543784.11.4.457. [DOI] [PubMed] [Google Scholar]
- 160.Goadsby PJ, Lipton RB, Ferrari MD. Drug therapy: migraine – current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 161.Silva SA, Marques FB, Fontes Ribero CA. Characterization of the human basilar artery contractile response to 5-HT and triptans. Fundam Clin Pharmacol. 2006;21:265–272. doi: 10.1111/j.1472-8206.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- 162.Gopen Q, Rosowski JJ, Merchant SN. Anatomy of the normal human cochlear aqueduct with functional implications. Hear Res. 1997;107:9–22. doi: 10.1016/s0378-5955(97)00017-8. [DOI] [PubMed] [Google Scholar]
- 163.Janjua R, Al-Mefty O, Densler DW, Shields CB. Dural relationships of Meckel cave and lateral wall of the cavernous sinus. Neurosurg Focus. 2008;25(6):E2. doi: 10.3171/FOC.2008.25.12.E2. [DOI] [PubMed] [Google Scholar]
- 164.Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- 165.Sehba FA, Bederson JB. Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:381–398. doi: 10.1179/016164106X114991. [DOI] [PubMed] [Google Scholar]
- 166.Schuknecht HF. Pathology of the Ear. Harvard University Press; MA, USA: 1974. p. 503. [Google Scholar]
- 167.Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke. 2003;34:1264–1275. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- 168.Donaldson C, Boers PM, Hoskin KL, Zagami AS, Lambert GA. The role of receptors in the 5-HT1B and 5-HT1D selective inhibitory effect of naratriptan on trigeminovascular neurons. Neurophamacology. 2002;42:374–385. doi: 10.1016/s0028-3908(01)00190-3. [DOI] [PubMed] [Google Scholar]
- 169.Goadsby PJ, Classey JD. Evidence for serotonin (5-HT)1B, 5-HT1D and 5-HT1F receptor inhibitory effects on trigeminal neurons with craniovascular input. Neuroscience. 2003;122:491–498. doi: 10.1016/s0306-4522(03)00570-0. [DOI] [PubMed] [Google Scholar]
- 170.Jeggo RD, Wang Y, Jordan D, Ramage AG. Activation of 5-HT1B receptors in the rat nucleus tractus 5-HT1D solitarius: opposing action on neurones that receive an excitatory vagal C-fibre input. Br J Pharmacol. 2007;150:987–995. doi: 10.1038/sj.bjp.0707169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Balaban CD. Vestibular autonomic regulation. Curr Opin Neurol. 1999;12:29–33. doi: 10.1097/00019052-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 172.Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 173.Ho TW, Mannix LK, Fan X, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- 174.Connor KM, Shapiro RE, Diener HC, et al. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology. 2009;73:970–977. doi: 10.1212/WNL.0b013e3181b87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Villalòn CM, Olesen J. The role of CGRP in the pathophysiology of migraine and the efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. 2009;124:309–324. doi: 10.1016/j.pharmthera.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 176.Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; GA, USA: 2003. p. 45. [Google Scholar]
- 177.Hoffer ME, Balaban CD, Gottschall KR, et al. Blast exposure: vestibular consequences and associated characteristics. Otol Neurotol. 2010;31:232–236. doi: 10.1097/MAO.0b013e3181c993c3. [DOI] [PubMed] [Google Scholar]
Websites
- 201.Facts for Physicians on mild TBI. 2010 www.cdc.gov/concussion/headsup/pdf/Facts_for_Physicians_booklet-a.pdf.
- 202.Hoffer ME, Balaban CD. Neurotologic consequences of blast injury. IBIA NeuroTrauma Letter. 2010 www.internationalbrain.org/?q=node/151.