Abstract
Background
Ipsilateral breast tumor recurrence (IBTR) is the most common failure event after lumpectomy for ductal carcinoma in situ (DCIS). We evaluated invasive IBTR (I-IBTR) and its influence on survival among participants in two National Surgical Adjuvant Breast and Bowel Project (NSABP) randomized trials for DCIS.
Methods
In the NSABP B-17 trial (accrual period: October 1, 1985, to December 31, 1990), patients with localized DCIS were randomly assigned to the lumpectomy only (LO, n = 403) group or to the lumpectomy followed by radiotherapy (LRT, n = 410) group. In the NSABP B-24 double-blinded, placebo-controlled trial (accrual period: May 9, 1991, to April 13, 1994), all accrued patients were randomly assigned to LRT+ placebo, (n=900) or LRT + tamoxifen (LRT + TAM, n = 899). Endpoints included I-IBTR, DCIS-IBTR, contralateral breast cancers (CBC), overall and breast cancer–specific survival, and survival after I-IBTR. Median follow-up was 207 months for the B-17 trial (N = 813 patients) and 163 months for the B-24 trial (N = 1799 patients).
Results
Of 490 IBTR events, 263 (53.7%) were invasive. Radiation reduced I-IBTR by 52% in the LRT group compared with LO (B-17, hazard ratio [HR] of risk of I-IBTR = 0.48, 95% confidence interval [CI] = 0.33 to 0.69, P < .001). LRT + TAM reduced I-IBTR by 32% compared with LRT + placebo (B-24, HR of risk of I-IBTR = 0.68, 95% CI = 0.49 to 0.95, P = .025). The 15-year cumulative incidence of I-IBTR was 19.4% for LO, 8.9% for LRT (B-17), 10.0% for LRT + placebo (B-24), and 8.5% for LRT + TAM. The 15-year cumulative incidence of all contralateral breast cancers was 10.3% for LO, 10.2% for LRT (B-17), 10.8% for LRT + placebo (B-24), and 7.3% for LRT + TAM. I-IBTR was associated with increased mortality risk (HR of death = 1.75, 95% CI = 1.45 to 2.96, P < .001), whereas recurrence of DCIS was not. Twenty-two of 39 deaths after I-IBTR were attributed to breast cancer. Among all patients (with or without I-IBTR), the 15-year cumulative incidence of breast cancer death was 3.1% for LO, 4.7% for LRT (B-17), 2.7% for LRT + placebo (B-24), and 2.3% for LRT + TAM.
Conclusions
Although I-IBTR increased the risk for breast cancer–related death, radiation therapy and tamoxifen reduced I-IBTR, and long-term prognosis remained excellent after breast-conserving surgery for DCIS.
CONTEXT AND CAVEATS
Prior knowledge
Lumpectomy performed to treat ductal carcinoma in situ (DCIS) is associated with the risk of ipsilateral breast tumor recurrence (IBTR), particularly with invasive IBTR (I-IBTR).
Study design
Two prospective randomized trials by the National Surgical Adjuvant Breast and Bowel Project (NSABP) compared the risk of IBTR in patients with localized DCIS who were treated by lumpectomy only (LO) or lumpectomy followed by radiation therapy (LRT) in the B-17 trial, and with 5 years of placebo (LRT + placebo) or tamoxifen (LRT + TAM) added to LRT in the B-24 trial. The long-term findings of these trials showing the most risk reduction in LRT + TAM group are published. In the current update of the B-17 and B-24 trials, the 15-year cumulative incidence of the primary failure event I-IBTR and its impact on survival have been investigated in LO, LRT, LRT + placebo, and LRT + TAM groups, along with recurrence of DCIS and other failure events.
Contribution
Results showed that after 15 years I-IBTR developed in 19.4% of patients who received LO for treatment of DCIS compared with 8.5% of patients who received LRT + TAM. I-IBTR was associated with an increase in mortality risk, but recurrence of DCIS was not. Overall mortality was low in patients who underwent lumpectomy.
Implications
Although I-IBTR increases the risk of breast cancer–related death, adjuvant therapies like radiation and tamoxifen remain highly effective in treatment of DCIS after lumpectomy.
Limitations
The trials were designed over 20 years ago when breast imaging technologies were inferior. There was no evaluation of hormone receptor and HER2 status in these trials, as their role in DCIS was not known.
From the Editors
The prevalence of clinically occult ductal carcinoma in situ (DCIS) has increased dramatically over the last two decades largely as a result of detection by the increased use of mammographic screening, accounting for approximately 25% of all new breast cancers today (1,2). The implementation of clinical trials to study breast-conserving techniques in this type of breast cancer was temporally preceded by trials comparing mastectomy to lumpectomy for invasive breast cancer (3,4). By the mid-1980s, single-institution nonrandomized studies of DCIS treated by breast-conserving surgery began to emerge (5–9). Satisfactory local control and similar survival after lumpectomy as compared with mastectomy were reported (8,10,11). However, concerns over the risk of subsequent ipsilateral breast tumor recurrences (IBTRs) with lumpectomy, particularly the occurrence of invasive IBTR (I-IBTR) persisted (9).
In 1985, the National Surgical Adjuvant Breast and Bowel Project (NSABP) initiated a groundbreaking prospective randomized trial (NSABP B-17) comparing IBTR after lumpectomy only (LO) to lumpectomy followed by radiation therapy (LRT) in patients with localized DCIS (9). Five-year outcomes were first reported for the B-17 trial in 1993 (12). Women treated by LRT had a 60% lower risk of IBTR compared with those treated by LO. Subsequent updates continue to demonstrate a large benefit for LRT compared with LO (12,13). A second prospective randomized trial (NSABP B-24) investigated the addition of tamoxifen (TAM) to LRT (LRT + TAM) (14). Updated findings of this trial showed that women treated with LRT + TAM experienced a 31% reduction in risk of IBTR compared with those treated by LRT, as well as a 53% reduction in risk of contralateral breast tumors (15).
All invasive breast cancers have an associated risk of metastasis. Therefore, it is important to investigate the impact of I-IBTR on the long-term prognosis of patients receiving breast-conserving treatments for DCIS (16,17). The B-17 and B-24 trials were closed to further follow-up, providing the impetus for an update on the incidence of I-IBTRs and its effect on mortality.
Participants and Methods
Trial Characteristics
Previous publications have provided detailed descriptions of study designs, patient eligibility, and treatments in NSABP B-17 and B-24 trials (12–14). Trials were approved by institutional review boards of participating institutions, and patients were required to sign informed consent to participate. The NSABP B-17 trial enrolled 818 patients with DCIS treated by lumpectomy that achieved tumor-free margins between October 1, 1985, and December 31, 1990 (the trial also included a registry of patients diagnosed with lobular carcinoma in situ) (18). Participants were randomly assigned to receive either radiation to the affected breast (LRT) or no further therapy (LO). A subsequent trial, NSABP B-24, enrolled 1804 patients between May 9, 1991, and April 13, 1994. Patients in the B-24 trial underwent LRT and were randomly assigned (double blind) to receive either 5 years of tamoxifen (AstraZeneca, Wilmington, DE), or placebo, shown in a CONSORT trial flow diagram (Figure 1). In contrast to B-17, B-24 allowed entry of women whose tumor margins were involved with DCIS and women whose mammograms contained foci of calcifications that were not excised, as long as their radiological appearance was not suggestive of invasive cancer (14). With respect to margin status, patients for whom margin status was reported as uncertain or was unknown were considered to be margin positive. Initially, axillary dissections were required in B-17, but the requirement was dropped in 1987, and excluded altogether in B-24.
Figure 1.
CONSORT diagram for National Surgical Adjuvant Breast and Bowel Project (NSABP) B-17 and NSABP B-24 trials. Patients with no follow-up information after random assignment were excluded from the analysis. LO = lumpectomy only; LRT = lumpectomy followed by radiation therapy; LRT + placebo = LRT plus 5 years of placebo; LRT + TAM = LRT plus 5 years of tamoxifen.
Radiation Treatment
In both B-17 and B-24, radiation to the whole breast began within 8 weeks of definitive surgery. The treatment occurred over 5 weeks to a dose of 50 Gy given at 10 Gy/wk. In B-24, investigators were permitted to modify the radiation technique by adding a boost of 10 Gy (range 0.1–20 Gy) to the lumpectomy cavity (19).
Follow-up and Event Determination
Follow-up clinical examinations were conducted semiannually, and mammography was performed annually. An IBTR was defined as recurrent DCIS (DCIS-IBTR) or invasive carcinoma (I-IBTR) occurring after lumpectomy in either the skin or parenchyma of the ipsilateral breast. Tumor in the non-breast skin of the ipsilateral chest wall was defined as other local failure. Recurrences in the ipsilateral internal mammary, supraclavicular, infraclavicular, and axillary nodes were classified as regional recurrences. Local or regional failures were verified by tissue biopsy or fine needle aspiration cytology. Distant metastases were confirmed by clinical, radiographic, or pathological findings. Second primary cancers and deaths without evidence of recurrence or second primary cancer were also verified by NSABP central medical review.
These findings reflect information reported to the NSABP Biostatistical Center through June 30, 2007, following closure of follow-up on May 1, 2007. For B-17, median follow-up time was 207 months (minimum = 1 month; 25th percentile, Q1 = 194 months to 75th percentile, Q3 = 222 months; maximum = 257 months). For B-24, median follow-up time was 163 months (minimum = 2 months; 25th percentile, Q1 = 153 months to 75th percentile, Q3 = 173 months; maximum = 191 months).
Statistical Methods
The primary endpoint in the B-17 and B-24 trials was event-free survival, defined as time from surgery to any of the following events: recurrence (I-IBTR or DCIS-IBTR, and other local, regional, distant), contralateral breast invasive or DCIS tumors, second primary cancer, or death before any of these events. For the analyses presented in this report, I-IBTR was the primary event of interest. We also present treatment comparisons for DCIS-IBTR and contralateral breast cancers (CBC). Analyses were based on first failure events after study entry except in specified instances in which we also examined time to occurrence of certain event types (CBC, endometrial cancer), whether or not the event was preceded by IBTR.
We also analyzed all-cause mortality and breast cancer–specific mortality, with attributed cause of death based on the clinical event history. Specifically, patients reported as 1) having died of breast cancer or 2) having confirmed distant metastatic disease with the cause of death unspecified were classified as breast cancer deaths. The remainder (those deaths without any breast cancer event subsequent to the initial DCIS and those with breast cancer events but without any supportive information indicating likely breast cancer death) were classified as deaths from other causes.
For all events comprising event-free survival, frequencies and average annual cause-specific hazards (number of events divided by person-time at risk) were calculated. For the effects of treatment on I-IBTR, DCIS-IBTR and deaths, cause-specific hazard ratios (HRs) with 95% confidence intervals (CIs) were computed using the Cox proportional hazards model (20). The proportional hazards assumption was evaluated graphically using Schoenfeld residual plots (21) and although there was some evidence of nonproportionality over time, time-invariant hazard ratios were found to be a suitable summary of treatment effects. The influence of patient and disease characteristics (age, tumor size, mode of detection, comedonecrosis, and margin status) on the hazard of I-IBTR were also evaluated using the Cox proportional hazards model. The influence of I-IBTR on mortality risk was evaluated using an indicator variable that equals zero before the time of occurrence of I-IBTR and equals one after the event (i.e., a time-varying covariate) in the hazard regression model for mortality. Because of the similarity in outcomes in the LRT group (LRT in B-17 and LRT + placebo in B-24) that was common to both trials, results for some covariate effects have been presented for patients from the two trials combined. Cumulative incidence functions were used to estimate cumulative probabilities over time of the occurrence of I-IBTR and other events comprising event-free survival, contralateral breast tumors, and cause-specific deaths (22). All P values presented are two-sided, and those less than .05 were considered statistically significant. Analyses were performed using SAS (SAS Institute, Cary, NC) and Stata (Statacorp, College Station, TX) statistical software.
Consistent with earlier reports of primary trial results (12–14), this analysis included all patients for whom follow-up information was available (total 2612 patients; 813 patients from B-17 and 1799 patients from B-24) (Figure 1). Several previous NSABP reports on pathological features of DCIS in relation to IBTR focused on subsets of trial participants for whom central pathology review was performed (23–25). In contrast, this analysis used information from tumor pathology reports submitted by the NSABP investigators.
Results
Patient and Disease Characteristics of the NSABP DCIS Trials
As detailed in Table 1, patient and disease characteristics were quite similar between the B-17 and B-24 trials. Overall, approximately 50% of the women were 55 years or older, and 22% were 65 years or older at diagnosis. Greater than 75% of tumors measured less than 1 cm in diameter based on pathological measurements or size of radiological abnormality. Cancers were detected mammographically in more than 80% of patients. Based on the trial inclusion criteria, patients in B-17 were required to have tumor-free lumpectomy margins; in B-24, approximately 25% of lumpectomy margins were reported as either involved or of uncertain status.
Table 1.
Characteristics at diagnosis for National Surgical Adjuvant Breast and Bowel Project (NSABP) ductal carcinoma in situ (DCIS) trial participants*
| Characteristic | NSABP B-17 |
NSABP B-24 |
||||||
| LO (n = 403) |
LRT (n = 410) |
LRT + placebo (n = 900) |
LRT + TAM (n = 899) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Age, y | ||||||||
| <45 | 65 | 16.1 | 72 | 17.6 | 161 | 17.9 | 150 | 16.7 |
| 45–54 | 124 | 30.8 | 127 | 31.0 | 305 | 33.9 | 289 | 32.1 |
| 55–64 | 131 | 32.5 | 135 | 32.9 | 228 | 25.3 | 252 | 28.0 |
| ≥65 | 83 | 20.6 | 76 | 18.5 | 206 | 22.9 | 208 | 23.1 |
| Menopausal status | ||||||||
| Pre- or perimenopausal | 112 | 27.8 | 109 | 26.6 | 324 | 36.0 | 322 | 35.8 |
| Postmenopausal | 291 | 72.2 | 301 | 73.4 | 576 | 64.0 | 577 | 64.2 |
| Race | ||||||||
| White | 354 | 87.8 | 359 | 87.6 | 764 | 84.9 | 777 | 86.4 |
| Black | 24 | 6.0 | 26 | 6.3 | 68 | 7.6 | 57 | 6.3 |
| Other | 25 | 6.6 | 25 | 6.1 | 68 | 7.6 | 65 | 7.2 |
| Tumor size, cm† | ||||||||
| Unknown | 14 | 3.5 | 14 | 3.4 | 16 | 1.8 | 10 | 1.1 |
| ≤1.0 | 297 | 73.7 | 307 | 74.9 | 743 | 82.6 | 765 | 85.1 |
| 1.1–2.0 | 60 | 14.9 | 54 | 13.2 | 104 | 11.6 | 83 | 9.2 |
| ≥2.1 | 32 | 7.9 | 35 | 8.5 | 37 | 4.1 | 41 | 4.6 |
| Mode of DCIS detection | ||||||||
| Clinical examination | 33 | 8.2 | 33 | 8.0 | 72 | 8.0 | 84 | 9.3 |
| Mammography only | 325 | 80.6 | 329 | 80.2 | 756 | 84.0 | 733 | 81.5 |
| Clinical examination and mammography | 78 | 19.4 | 81 | 19.7 | 144 | 16.0 | 166 | 18.4 |
| Tumor margin status‡ | ||||||||
| Free | 403 | 100.0 | 410 | 100.0 | 676 | 75.1 | 667 | 74.2 |
| Involved or uncertain | 0 | 0 | 0 | 0 | 224 | 24.9 | 232 | 25.8 |
Patients in these trials had ductal carcinoma in situ (DCIS) treated by lumpectomy. The NSABP B-17 trial participants (N = 817), accrued between October 1, 1985, and December 31, 1990, were randomly assigned to the LO and LRT groups. NSABP B-24 trial participants (N = 1799), accrued between May 9, 1991, and April 13, 1994, underwent LRT and were randomly assigned to receive either 5 years of tamoxifen or placebo (double-blind). LO = lumpectomy only; LRT = lumpectomy followed by radiation therapy; LRT + placebo = LRT plus 5 years of placebo; LRT + TAM = LRT plus 5 years of tamoxifen.
Largest diameter on pathology report or size of radiological abnormality.
Margin evaluation per report from treating institution.
Summary of Events
Table 2 summarizes frequencies and annual rates for all events comprising event-free survival by trial and treatment group. IBTR constitutes the most common first failure event in all treatment groups. Of a total of 490 IBTR events among all treatment groups, 227 of 490 (46.3%) were noninvasive (DCIS-IBTR), and 263 of 490 (53.7%) were invasive (I-IBTR). Other local, regional, and distant recurrences in the absence of a documented previous I-IBTR were rare (34 events; 1.3% of 2612 patients). Contralateral breast cancers (CBC) occurred as first failure events in 7.2% of patients. The annual rate of CBC was similar in the LO (0.76) and LRT (B-17, 0.77; and B-24, 0.79) groups and lower in the LRT + TAM group (0.45). The frequency (7.6% of patients) and rate of second primary cancers were similar among treatment groups. The frequency and rate of deaths appeared higher in patients in the B-17 trial. Longer follow-up and greater current age of patients in this trial account for this phenomenon (Table 2).
Table 2.
Summary of first events by trial and treatment group*
| Failure event | NSABP B-17 LO (n = 403) | NSABP B-17 LRT (n = 410) | NSABP B-24 LRT + placebo (n = 900) | NSABP B-24 LRT + TAM (n = 899) | ||||||||
| No. of events | %† | Annual failure rate‡ | No. of events | %† | Annual failure rate‡ | No. of events | %† | Annual failure rate‡ | No. of events | %† | Annual failure rate‡ | |
| IBTR | 141 | 35.0 | 3.35 | 81 | 19.8 | 1.65 | 149 | 16.6 | 1.62 | 119 | 13.2 | 1.22 |
| Invasive§ | 79 | 19.6 | 1.88 | 44 | 10.7 | 0.90 | 81 | 9.0 | 0.88 | 59 | 6.6 | 0.60 |
| DCIS | 62 | 15.4 | 1.47 | 37 | 9.0 | 0.75 | 68 | 7.6 | 0.74 | 60 | 6.7 | 0.62 |
| Other breast cancer events | 7 | 1.7 | 0.18 | 10 | 2.4 | 0.20 | 10 | 1.1 | 0.11 | 7 | 0.8 | 0.07 |
| Local recurrence | 0 | 0 | 0 | 1 | 0.2 | 0.02 | 1 | 0.1 | 0.01 | 0 | 0 | 0 |
| Regional recurrence | 3 | 0.7 | 0.08 | 4 | 1.0 | 0.08 | 3 | 0.3 | 0.03 | 3 | 0.3 | 0.03 |
| Distant recurrence | 4 | 1.0 | 0.10 | 5 | 1.2 | 0.10 | 6 | 0.7 | 0.07 | 4 | 0.4 | 0.04 |
| Contralateral breast cancer | 32 | 7.9 | 0.76 | 38 | 9.3 | 0.77 | 73 | 8.1 | 0.79 | 44 | 4.9 | 0.45 |
| Invasive | 25 | 6.2 | 0.59 | 23 | 5.6 | 0.47 | 48 | 5.3 | 0.52 | 30 | 3.3 | 0.31 |
| DCIS | 7 | 1.7 | 0.17 | 15 | 3.7 | 0.31 | 25 | 2.8 | 0.27 | 14 | 1.6 | 0.14 |
| Second primary cancer | 28 | 6.9 | 0.67 | 35 | 8.5 | 0.71 | 64 | 7.1 | 0.69 | 71 | 7.5 | 0.73 |
| Death║ | 33 | 8.2 | 0.78 | 33 | 8.0 | 0.67 | 49 | 5.4 | 0.53 | 48 | 5.3 | 0.49 |
| All events | 241 | 59.8 | 5.73 | 197 | 48.0 | 4.02 | 345 | 38.0 | 3.74 | 289 | 32.1 | 2.96 |
Patients in these trials had DCIS treated by lumpectomy. The NSABP B-17 trial participants (N = 813) were randomly assigned to the LO and LRT groups. The NSABP B-24 trial participants (N = 1799) underwent LRT and were randomly assigned to receive either 5 years of tamoxifen or placebo (double-blind). The primary endpoint in the B-17 and B-24 trials was event-free survival (time from lumpectomy to first failure event). DCIS = ductal carcinoma in situ; I-IBTR = invasive ipsilateral breast tumor recurrence; LO = lumpectomy only; LRT = lumpectomy followed by radiation therapy; LRT + placebo = LRT plus 5 years of placebo; LRT + TAM = LRT plus 5 years of tamoxifen; NSABP = National Surgical Adjuvant Breast and Bowel Project.
Total frequency (events divided by N) in treatment group. B-17 percentages are larger regardless of annual failure rate because patients were followed for a longer period of time.
Events per100 patients per year.
I-IBTR was the primary event of interest.
Deaths before any of the events shown.
Treatment Effects
Effect of Radiotherapy and Tamoxifen on I-IBTR.
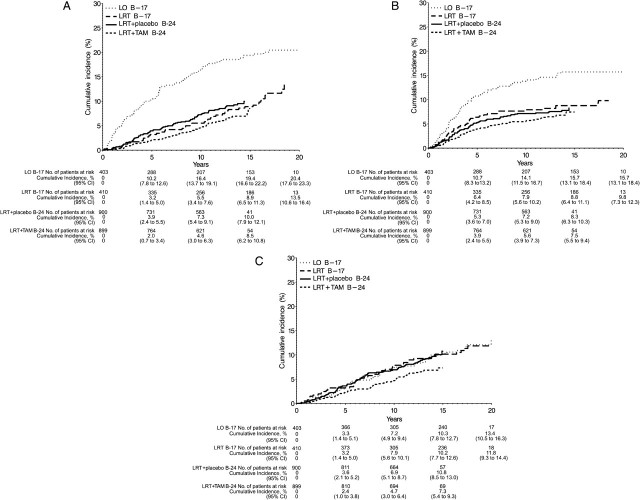
Consistent with earlier reports for the B-17 and B-24 trials, both radiotherapy and tamoxifen reduced the risk of I-IBTR (12–15). In NSABP B-17, the radiation therapy (LRT) group showed a 52% reduction in the risk of I-IBTR compared with the LO group (HR = 0.48, 95% CI = 0.33 to 0.69, P < .001). In NSABP B-24, tamoxifen in combination with radiation therapy (LRT + TAM) showed a 32% reduction in the risk of I-IBTR compared with the LRT + placebo group (HR = 0.68, 95% CI = 0.49 to 0.95, P = .025). Comparing across trials, the LRT + TAM group showed a relative risk reduction of I-IBTR by approximately 70% compared with the LO group (HR = 0.30, 95% CI = 0.21 to 0.42, P < .001). Radiation therapy decreased the cumulative incidence of I-IBTR at 15 years from 19.4% in LO to 8.9% in the B-17 LRT group and to 10% in the B-24 LRT + placebo group (Figure 2, A). The cumulative incidence of I-IBTR was lower in the LRT + TAM group but reached 8.5% at 15 years (Figure 2, A).
Figure 2.
Effects of radiation and tamoxifen on cumulative incidence of breast cancer events. A) Invasive ipsilateral breast tumor recurrences (I-IBTR), B) ductal carcinoma in situ–ipsilateral breast tumor recurrences (DCIS-IBTR), and C) all contralateral breast cancers (CBC). Data are shown by trial (National Surgical Adjuvant Breast and Bowel Project [NSABP] B-17 and NSABP B-24) and treatment group—lumpectomy only (LO), lumpectomy followed by radiation therapy (LRT), and lumpectomy with radiation therapy plus 5 years of placebo (LRT + placebo) or tamoxifen (LRT+TAM). CI = confidence interval.
Effect of Radiation and Tamoxifen on DCIS-IBTR.
The effects of radiation and tamoxifen on noninvasive breast tumor recurrence (DCIS-IBTR) were similar to those observed in earlier analyses of these trials. Compared with LO, LRT in B-17 resulted in a 47% relative reduction in the risk of DCIS-IBTR (HR = 0.53, 95% CI = 0.35 to 0.80, P < .001). In the B-24 trial, the addition of tamoxifen resulted in a non-statistically significant risk reduction of 16% compared with LRT + placebo (HR = 0.84, 95% CI = 0.60 to 1.19, P = .33). The cumulative incidence of DCIS-IBTR was similar to that of I-IBTR in the LO group through 5 years and slightly greater than that of I-IBTR in the LRT and LRT + TAM groups (Figure 2, B). After 5 years, the rate of DCIS-IBTR appeared to diminish, whereas the rate of invasive recurrences was more constant over time.
Effect of Radiation and Tamoxifen on CBC.
In B-24, there was a 32% reduction in CBC for patients who received LRT + TAM vs LRT + placebo (HR = 0.68, 95% CI = 0.48 to 0.95, P = .023). The 15-year cumulative incidence of CBC, either as first failure events or subsequent to IBTR, was 7.3% for the LRT + TAM group and 10.8% for the LRT + placebo group. In the B-17 trial, 15-year cumulative incidence was 10.2% for the LRT group and 10.3% for the LO group (Figure 2, C). Approximately 67% of these tumors were invasive.
Effect of Radiation and Tamoxifen on Other Events.
There were no statistically significant differences in the hazard of local, regional, or distant disease as a first failure event (eg, without previous IBTR) between the LRT and LO groups (HR = 1.23, 95% CI = 0.47 to 3.23), as observed in the B-17 trial. The LRT + TAM group also showed no statistically significant differences in the hazard of these events compared with the LRT + placebo group (HR = 0.66, 95% CI = 0.25 to 1.73), as observed in the B-24 trial.
The three treatment modalities did not differ with respect to hazard of death as a first event or occurrence of second primary cancers (data not shown). In B-24, we specifically examined the risk of endometrial cancer. There were 22 endometrial cancers (seven in the LRT + placebo group and 15 in the LRT + TAM group) and an approximate twofold increased risk in the LRT + TAM group compared with the LRT + placebo group (HR = 2.09, 95% CI = 0.85 to 5.13).
Patient and Tumor Characteristics in Relation to I-IBTR
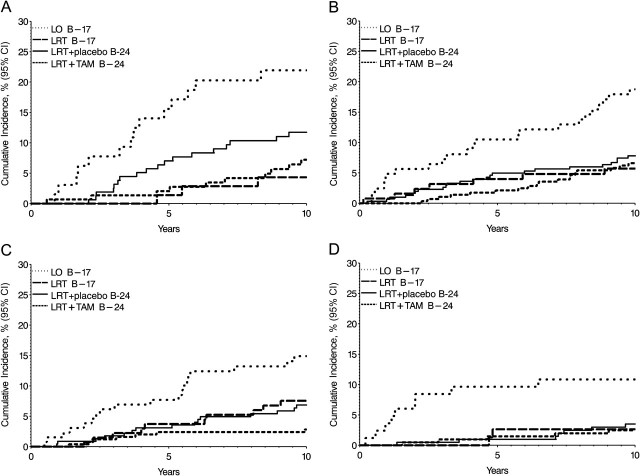
We examined whether patient or tumor characteristics influenced the risk of I-IBTR (Table 3). Compared with women aged 65 years and older at diagnosis, women younger than 45 years showed a 2.1-fold increased risk of I-IBTR. Women aged 45–64 years also showed an increased risk of I-IBTR relative to women aged 65 years and older. Cumulative incidence of I-IBTR by age group and treatment is shown in Figure 3. Although there were differences in absolute risk of I-IBTR by age, there was no statistical evidence of differential treatment effects; benefit from radiation in all age groups is demonstrated in Figure 3.
Table 3.
Hazard ratios for invasive ipsilateral breast tumor recurrence (I-IBTR) or ductal carcinoma in situ (DCIS)-IBTR according to patient and disease characteristics*
| Characteristic | Ipsilateral breast tumor events |
|||
| I-IBTR |
DCIS-IBTR |
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age at diagnosis, y | ||||
| ≥65 | 1.00 (reference) | .003 | 1.00 (reference) | <.001 |
| <45 | 2.14 (1.40 to 3.26) | 2.90 (1.84 to 4.56) | ||
| 45–54 | 1.80 (1.22 to 2.66) | 1.81 (1.16 to 2.81) | ||
| 55–64 | 1.50 (1.00 to 2.26) | 1.72 (1.10 to 2.70) | ||
| Tumor size, cm | ||||
| ≤1.0 | 1.00 (reference) | 1.00 (reference) | ||
| >1.0 | 0.94 (0.68 to 1.30) | .70 | 1.03 (0.73 to 1.44) | .89 |
| Mode of detection | ||||
| Mammography only | 1.00 (reference) | 1.00 (reference) | ||
| Clinically detected | 1.37 (1.03 to 1.84) | .03 | 1.48 (1.09 to 2.01) | .01 |
| Comedonecrosis†,‡ | ||||
| Absent | 1.00 (reference) | 1.00 (reference) | ||
| Present | 0.87 (0.62 to 1.21) | .41 | 2.21 (1.52 to 3.20) | <.001 |
| Treatment group, tumor margin status†,§ | ||||
| LRT, margin-free | 1.00 (reference) | 1.00 (reference) | ||
| LRT, involved/uncertain | 2.61 (1.68 to 4.05) | <.001 | 1.65 (1.00 to 2.73) | .05 |
| LRT + TAM, free | 1.00 (reference) | 1.00 (reference) | ||
| LRT + TAM, involved/uncertain | 1.27 (0.73 to 2.20) | .40 | 1.32 (0.77 to 2.28) | .31 |
Two-sided Wald test for model coefficient(s) representing the covariate. CI = confidence interval; HR = hazard ratio; LRT = lumpectomy followed by radiation therapy; LRT + TAM = LRT plus 5 years of tamoxifen.
Patients from B-24 trial only, based on institutional reports.
For 36 patients (2.0%), comedonecrosis was unknown.
A statistically significant interaction between treatment group and tumor margin status was noted (two-sided Wald test, P = .04).
Figure 3.
Effects of radiation and tamoxifen on cumulative incidence of invasive ipsilateral breast tumor recurrences (I-IBTR) by age at diagnosis. A) Patients younger than 45 years, B) patients aged 45–54 years, C) patients aged 55–64 years, and D) patients 65 years and older. Data are shown by trial (National Surgical Adjuvant Breast and Bowel Project [NSABP] B-17 and NSABP B-24) and treatment group—lumpectomy only (LO), lumpectomy with radiation therapy (LRT), and lumpectomy with radiation therapy plus 5 years of placebo (LRT + placebo) or tamoxifen (LRT + TAM).
DCIS detected by clinical examination was associated with increased risk of I-IBTR. Tumor size was not associated with risk of I-IBTR. The presence of comedonecrosis in tumors from the B-24 trial was not associated with an increased risk of I-IBTR. None of these factors showed a statistically significant variation in association with I-IBTR by type of treatment received (ie, there were no statistically significant interactions or evidence of a differential effect in the estimates by treatment type). Covariate effects in relation to DCIS-IBTR were similar to those for I-IBTR, with the exception of comedonecrosis (HR = 2.21, 95% CI = 1.52 to 3.20, P < .001) (Table 3).
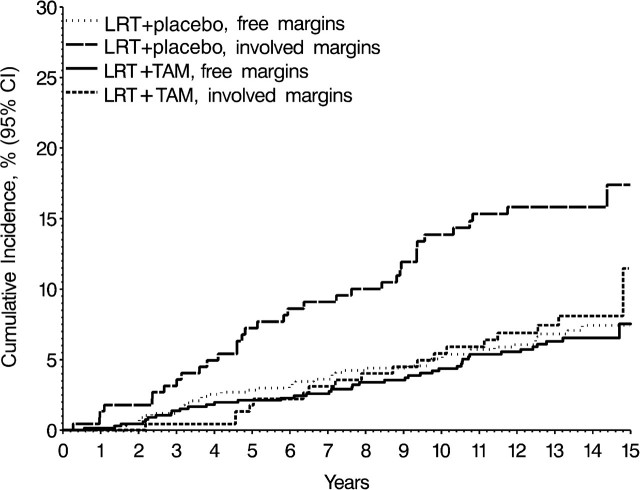
The margin status in patients from the B-24 trial was associated with an approximate twofold overall increased risk of I-IBTR, but there was also evidence of a differential effect by treatment group (interaction P = .04). When examined by treatment group, the prognostic effect of positive margins was greater among patients in the LRT + placebo group, whereas among patients in the LRT + TAM group, there was a smaller statistically nonsignificant increased risk for margin-positive patients (Table 3). In the LRT + placebo group, the 15-year cumulative incidence of I-IBTR was 17.4% in patients with positive margins compared with 7.4% in those with tumor-free margins. In the LRT + TAM group, cumulative incidence was 11.5% in those with positive margins compared with 7.5% in those with free margins (Figure 4).
Figure 4.
Cumulative incidence of invasive ipsilateral breast tumor recurrences within categories of margin status and treatment in the National Surgical Adjuvant Breast and Bowel Project B-24 trial. Treatment groups were lumpectomy with radiation therapy plus 5 years of placebo (LRT + placebo) or tamoxifen (LRT + TAM), without tumor at margins, or with involved or unknown tumor at margins as defined in the B-24 trial.
Mortality Outcomes
Overall Survival by Treatment Group.
Of the 2612 patients in this analysis, 385 (14.7%) have died. Cumulative all-cause mortality through 15 years was 15.8% in the LO group, 17.1% in the LRT group of the B-17 trial, 17.1% in the LRT + placebo group of the B-24 trial, and 14.4% in the LRT + TAM group. In the B-17 trial, radiotherapy was not associated with any overall mortality reduction compared with LO (HR = 1.08, 95% CI = 0.79 to 1.48). Among patients in the B-24 trial, the addition of tamoxifen in the LRT + TAM group did not result in a statistically significant reduction in mortality risk compared with LRT (HR = 0.86, 95% CI = 0.66 to 1.11). In a nonrandomized comparison across trials, similar results were seen comparing LRT + TAM to all patients who did not receive tamoxifen (LO and LRT combined, HR = 0.82, 95% CI = 0.65 to 1.03).
Mortality by Probable Cause.
As described earlier, deaths were classified into those that were breast cancer–related and those from other causes. Of the 385 deaths, 72 (18.7%) were attributable to breast cancer, with a 15-year cumulative incidence of 3.1% for LO, 4.7% for LRT in the B-17 trial, 2.7% for LRT + placebo in the B-24 trial, and 2.3% for LRT + TAM (Figure 5, A). Breast cancer mortality did not differ for the LRT group compared with the LO group in the B-17 trial (32 events, HR = 1.44, 95% CI = 0.71 to 2.92) or for the LRT + placebo group compared with the LRT + TAM group in the B-24 trial (40 events, HR = 0.81, 95% CI = 0.43 to 1.50). In a nonrandomized comparison across trials, patients who received LRT + TAM had a statistically nonsignificant 32% mortality reduction compared with all patients who did not receive tamoxifen (HR = 0.68, 95% CI = 0.40 to 1.16). Deaths attributed to other causes (313 of 385, 81.3% of deaths) did not differ among treatment groups; the 15-year cumulative mortality incidence of other-cause deaths was 12.6% for the LO group, 12.4% for the LRT group in B-17, 14.4% for the LRT + placebo group in B-24, and 12.0% for the LRT + TAM group (Figure 5, B).
Figure 5.
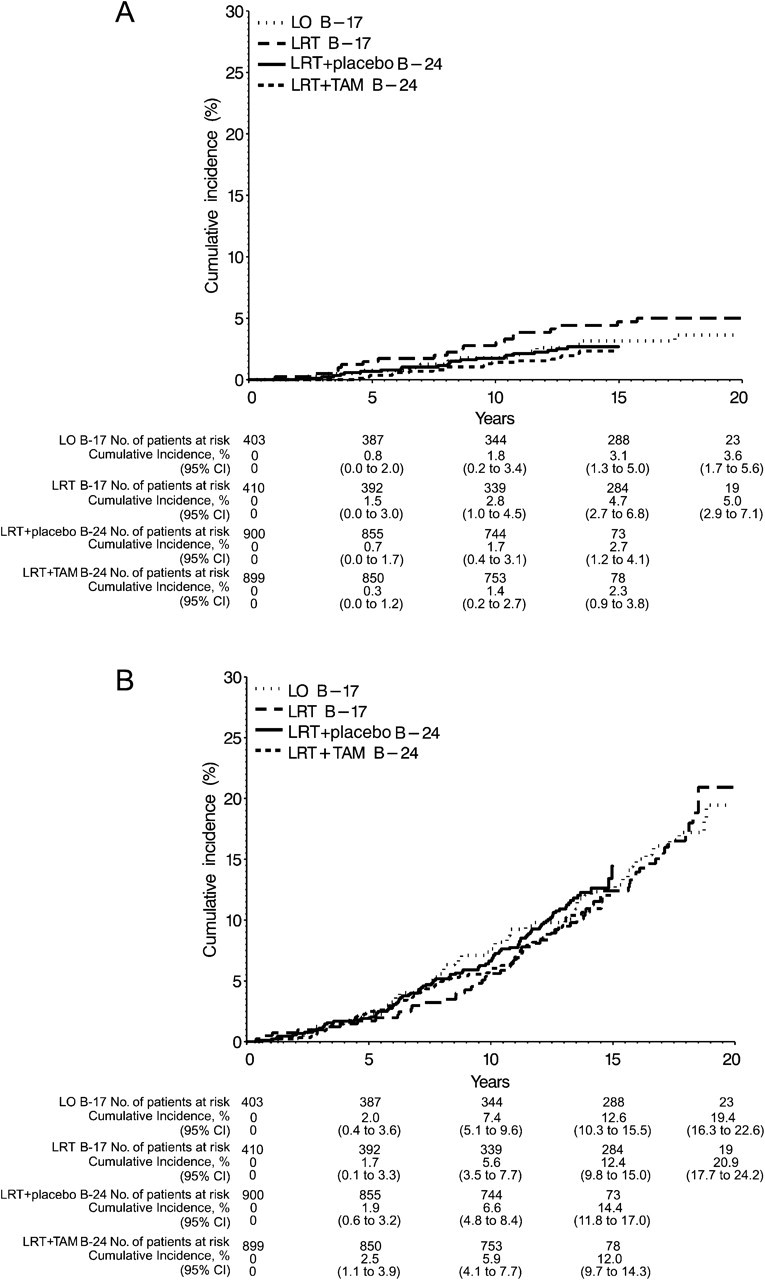
Cumulative incidence of deaths. A) Deaths attributed to breast cancer. B) Deaths attributed to other causes. Data are shown by trial (National Surgical Adjuvant Breast and Bowel Project [NSABP] B-17 and NSABP B-24) and treatment group—lumpectomy only (LO), lumpectomy followed by radiation therapy (LRT), and lumpectomy with radiation therapy plus 5 years of placebo (LRT + placebo) or tamoxifen (LRT + TAM). CI = confidence interval.
I-IBTR and Mortality Risk.
Women who developed an I-IBTR had a greater risk of all-cause death compared with those without I-IBTR (HR = 1.75, 95% CI = 1.24 to 2.45). If only breast cancer–related deaths were considered, the effect was larger (HR = 7.06, 95% CI = 4.14 to 12.03). In contrast, there was no statistically significant increase in overall mortality risk (HR = 0.81, 95% CI = 0.51 to 1.27) or breast cancer mortality risk (HR = 1.49, 95% CI = 0.71 to 3.15) for those who had DCIS-IBTR compared with those who did not. Interestingly, women who developed an invasive CBC had a similar increase in mortality risk (HR = 2.62, 95% CI = 1.82 to 3.77) as those who developed an I-IBTR.
There were 39 deaths among the 263 women with I-IBTR as a first failure; 22 were attributed to breast cancer. The cumulative probability of breast cancer-related death was 7.3% at 5 years and 10.4% at 10 years after occurrence of the I-IBTR (Figure 6). By comparison, the cumulative breast cancer-related death after DCIS-IBTR was 2.7% at 10 years. Breast cancer deaths (8) among these patients were due to additional invasive breast cancer recurrence events after DCIS-IBTR (ie, a second IBTR that was invasive) or CBC. Twelve additional deaths after DCIS-IBTR were attributed to other causes. Findings were similar for patients with CBC as a first failure, with 14 of 32 deaths among those with invasive CBC being breast cancer related, and two of eight deaths among those with DCIS CBC being breast cancer related.
Figure 6.
Cumulative incidence of breast cancer deaths after invasive ipsilateral breast tumor recurrences (I-IBTR) or ductal carcinoma in situ (DCIS)-IBTR. The patient cohort includes 263 patients with a prior I-IBTR and 227 patients with a prior DCIS-IBTR as a first failure event.
Discussion
The NSABP B-17 and B-24 trials represent the largest prospective evaluation of breast-conserving therapies for DCIS to date (12,14). Long-term findings in these studies demonstrate the effectiveness of this approach in the management of DCIS. The most common first failure event is an IBTR. In this update of the trials, we showed that 19.4% of patients who received LO for treatment of DCIS had developed an I-IBTR after 15 years compared with 8.5% of patients who received LRT + TAM. I-IBTR was associated with increased mortality risk, whereas there was no association with recurrence of DCIS, and neither overall nor breast cancer–specific survival differed between the LO, LRT, and LRT + TAM treatment groups.
In light of the fact that about 60% of operations performed in the United States and Canada for DCIS are lumpectomies (26,27), it is reassuring that mortality in the NSABP trials was comparable across treatment groups and similar to that reported for mastectomy-treated patients (28). There has never been a randomized comparison of overall survival between mastectomy and lumpectomy in patients with DCIS except among a cohort of 78 patients inadvertently enrolled as invasive cancers in the NSABP B-06 trial (4,11). IBTR remains the most common first failure event in lumpectomy-treated DCIS irrespective of the type of adjuvant treatment. The benefits of adjuvant radiation in reducing ipsilateral breast events have been sustained since the first results were reported for NSABP B-17 in 1993 (12).
In spite of the fact that approximately 80% of the cancers in these trials were small clinically occult lesions, treatment by lumpectomy alone was associated with a relatively high incidence of IBTR, reaching 35% by 15 years. Our findings are in agreement with long-term findings in other randomized trials such as the European Organization for Research and Treatment of Cancer Trial 10853 and the Swedish Trial (29,30), both showing that radiation therapy is necessary for local control after breast-conserving therapy. The B-24 trial continues to show benefits in terms of I-IBTR reduction, as well as contralateral breast tumor reduction, with the addition of tamoxifen to radiation. Contrary to our experience, the United Kingdom, Australia, and New Zealand (UK/ANZ) DCIS Trial reported in their first analysis of 1694 DCIS patients (some with and some without radiation by choice) that tamoxifen did not reduce the incidence of I-IBTR (31). In a recent update, tamoxifen continued to have a minimal effect on I-IBTR but did reduce DCIS-IBTR, especially among those with low- and/or intermediate-grade tumors (32). With respect to survival prognosis, women diagnosed with in situ breast disease have greater probability of death from other causes than that from breast cancer (33), and all-cause mortality is low in DCIS-treated patients. It is speculated that these women may represent a healthier group than the population at large, based on associations between breast cancer screening and other health behaviors (34). In our trials, 15-year overall survival exceeded 85% and the incidence of breast cancer death was 4.7% or less in all treatment groups. These findings are similar to ones from the Surveillance, Epidemiology, and End Results program, in which the 10-year breast cancer death rate was 2.3% for women diagnosed from 1978 to 1989 (34). Although survival was not a primary endpoint in the NSABP DCIS trials, the observed mortality reduction for those receiving tamoxifen is consistent with early follow-up results of tamoxifen-treated node-negative breast cancer (35). The occurrence of an I-IBTR after breast-conserving surgery for DCIS, as with breast tumor recurrence after invasive cancer (36,37), alters subsequent prognosis. In this study, there was an approximate twofold increase in mortality risk for patients with I-IBTR compared with those who did not develop an I-IBTR. Despite a higher incidence of I-IBTR in the LO group compared with LRT, survival was similar. However, the death rate in all patients is low, rendering emergent survival differences unlikely. Also, we speculate that I-IBTR after radiation therapy may be biologically more aggressive and that many of the I-IBTR in the LO group are biologically indolent. This is similar to what we have observed in invasive cancer treated by lumpectomy (36,37). We note that I-IBTR is the dominant but not the sole pathway by which a breast cancer–related death may occur after DCIS. It may be preceded by an invasive contralateral breast tumor, an event that confers nearly the same mortality risk increase as the occurrence of I-IBTR. Moreover, among the small number of patients whose first documented failure event was local (non-breast), regional, or distant disease without prior I-IBTR, mortality was also increased (not shown). These observations suggest strong caution in considering prophylactic surgery as a means to prevent breast cancer mortality among DCIS patients. In these mature trials, the 21 breast cancer deaths occurring following an I-IBTR represent 8.4% of patients who developed an I-IBTR and only 0.8% of all trial participants.
With respect to limitations, these prospective clinical trials were designed more than 20 years ago. Because the breast imaging quality of that era was inferior to the diagnostic technologies currently available, these cases might present differently if diagnosed today. However, as we discuss below, most tumors were small. Secondly, the inadvertent (B-17) or intentional (B-24) inclusion of cases with involved or uncertain surgical margins can be viewed as introducing heterogeneity that obscures treatment effects. Finally, tumor hormone receptor and HER2 receptor assessment was not performed at study entry, and both of these factors have since become more important in DCIS.
Tumor size, tumor grade, and lumpectomy margins have been the subject of much discussion in relation to DCIS treatment. Investigators have proposed that adjuvant radiation could be omitted for small- or low-grade tumors. Based on the predominance of relatively small DCIS in our trials and the observed benefit for radiation, tumor size was neither a significant prognostic factor nor an indicator for patients in whom radiotherapy could be omitted. Readers may be concerned about the applicability of the findings of NSABP B-17 and B-24 to clinical practices today, given this reported small size of tumors. Tumor size in NSABP DCIS trial participants was addressed extensively in the second update of B-17 (13). Clinical, mammographic, and pathological assessments confirmed that indeed the great majority of tumors would be considered 1 cm or less by modern standards. The allowance of microscopically involved margins and scattered calcifications in B-24 does not detract, in our opinion, from the importance of the findings in this trial, but rather provides a perspective on the effects of potential residual disease on recurrence. In previous analyses of these trials, tumor grade and associated features were found to predict IBTR risk, but radiation still benefited low-risk patients (23,24). With respect to margins, previous analyses of NSABP B-17 based on central pathological review found that tumor-positive margins imparted a roughly twofold risk increase for IBTR relative to negative margins (23), but this effect attenuated with longer follow-up (24). The use of wider excision margins has also been proposed as a means to omit radiation. Wong et al. (38) performed a single-arm prospective trial of wide excision alone (margins ≥1 cm) for low- to intermediate-grade DCIS (measuring ≤2.5 cm) and observed an unacceptably high 5-year rate of IBTR of 12%, prompting early closure of the trial. An update of the Eastern Cooperative Oncology Group 5104 study reported increasing rates of ipsilateral recurrence from 6.1% at 5 years to 10.5% at 7 years (39). The design of NSABP B-24 afforded a greater opportunity to examine the effect of positive margins on IBTR. The relative risk of IBTR more than doubled when margins were involved. However, the use of tamoxifen appears to largely offset the added risk from involved margins. The B-24 trial was not designed with consideration of the estrogen receptor status; however, a separate report summarizing the effects of tamoxifen in a subset of patients with estrogen receptor information will appear shortly (40).
The incidence of DCIS has risen over the past decade and may be continuing to do so (41). Therefore, it is increasingly important to advance our understanding of the biological behavior of this disease in parallel with testing of diagnostic and therapeutic strategies (1). This long-term follow-up analysis of NSABP B-17 and B-24 clinical trials confirms earlier findings (15). Regardless of treatment, these women have an excellent overall prognosis, with an incidence of death due to breast cancer of less than 5% at 15 years, despite persistent risks of breast cancer in the same or contralateral breast. Additional insights will be forthcoming from three ongoing trials: NSABP B-35, comparing adjuvant tamoxifen to anastrozole after LRT in estrogen receptor–positive DCIS (42); NSABP B-39, comparing whole-breast irradiation to partial breast irradiation in both invasive cancer and DCIS (43); and NSABP B-43, comparing whole-breast irradiation with or without two doses of trastuzumab in HER2-positive DCIS (44).
In conclusion, we believe that these long-term findings of NSABP B-17 and B-24 demonstrate that lumpectomy and adjuvant therapies are effective modalities for the treatment of DCIS. It is highly likely that current breast imaging practices, improvements in margin assessments, and advances in adjuvant treatments will continue to reduce the incidence of invasive recurrences after DCIS.
Funding
Public Health Service (U10-CA-69651, U10-CA-12027, U10-CA-37377, U10-CA-69974, and NCI P30-CA-14599) from the U.S. National Cancer Institute (to the NSABP). Tamoxifen for the B-24 trial was provided by AstraZeneca, Wilmington, DE.
Footnotes
Clinical Trial Registration: PDQ: NSABP B17, NSABP B-24. J. J. Dignam had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. B. Fisher was responsible for the concept, design, conduct, and initial publication of findings from the B-17 and B-24 trials, which provided the opportunity for conducting this study. All authors have declared no potential conflicts of interest. We are grateful to Dr Barbara Good and Wendy L. Rea for editorial assistance. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the article; and the decision to submit the article for publication.
References
- 1.Li CI, Daling JR, Malone KE. Age-specific incidence rates of in situ breast carcinomas by histologic type, 1980 to 2001. Cancer Epidemiol Biomarkers Prev. 2005;14(4):1008–1011. doi: 10.1158/1055-9965.EPI-04-0849. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Veronessi U, Sacozzi R, del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305(1):6–11. doi: 10.1056/NEJM198107023050102. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 5.Betsill WL Jr, Rosen PP, Lieberman PH, Robbins GF. Intraductal. carcinoma. Long term follow-up after treatment by biopsy alone. JAMA. 1978;239(18):1863–1867. doi: 10.1001/jama.239.18.1863. [DOI] [PubMed] [Google Scholar]
- 6.Page DL, Dupont WD, Rogers LW, Landenberger M. Intraductal carcinoma of the breast. Follow-up after biopsy only. Cancer. 1982;49(4):751–758. doi: 10.1002/1097-0142(19820215)49:4<751::aid-cncr2820490426>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Lagios MD, Westdahl PR, Margolin FR, Roses MR. Ductal carcinoma in situ. Relationship of extent of noninvasive disease to the frequency of occult invasion, multicentricity, lymph node metastases, and short-term treatment failures. Cancer. 1982;50(7):1309–1314. doi: 10.1002/1097-0142(19821001)50:7<1309::aid-cncr2820500716>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Montague ED. Conservation surgery and radiation therapy in the treatment of operable breast cancer. Cancer. 1984;53(3 suppl):700–704. doi: 10.1002/1097-0142(19840201)53:3+<700::aid-cncr2820531318>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Recht A, Danoff BS, Solin LJ, et al. Intraductal carcinoma of the breast: results of treatment with excisional biopsy and irradiation. J Clin Oncol. 1985;3(10):1339–1343. doi: 10.1200/JCO.1985.3.10.1339. [DOI] [PubMed] [Google Scholar]
- 10.Rosner D, Bedwani R, Vana J, Baker H, Murphy G. Noninvasive carcinoma breast, results of a national survey by the American College of Surgeons. Ann Surg. 1980;192(2):139–147. doi: 10.1097/00000658-198008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher ER, Sass R, Fisher B, Wickerham L, Paik SM. Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol 6).I. Intraductalcarcinoma (DCIS) Cancer. 1986;57(2):197–208. doi: 10.1002/1097-0142(19860115)57:2<197::aid-cncr2820570203>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328(22):1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16(2):441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28(4):400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 16.Romero L, Klein L, Ye W, et al. Outcome after invasive recurrence in patients with ductal carcinoma in situ of the breast. Am J Surg. 2004;188(4):371–376. doi: 10.1016/j.amjsurg.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Li CI, Malone KE, Saltzman BS, Daling JR. Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 1988–2001. Cancer. 2006;106(10):2104–2112. doi: 10.1002/cncr.21864. [DOI] [PubMed] [Google Scholar]
- 18.Fisher ER, Costantino JP, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Five-year observations concerning lobular carcinoma in situ. Cancer. 1996;78(7):1403–1416. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1403::AID-CNCR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Julian TB, Land SR, Wang Y, Vicini FA, Arthur DW, Wolmark N. National Surgical Adjuvant Breast and Bowel Project. Is boost therapy necessary in the treatment of DCIS? ASCO Breast Cancer Symposium. 2007 Abstract 146. [Google Scholar]
- 20.Prentice RL, Kalbfleisch JD, Peterson AV, Jr., Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–554. [PubMed] [Google Scholar]
- 21.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 22.Korn EL, Dorey FJ. Applications of crude incidence curves. Stat Med. 1992;11(6):813–829. doi: 10.1002/sim.4780110611. [DOI] [PubMed] [Google Scholar]
- 23.Fisher ER, Costantino J, Fisher B, Palekar AS, Redmond C, Mamounas E. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Intraductal carcinoma (ductal carcinoma in situ). The National Surgical Adjuvant Breast and Bowel Project Collaborating Investigators. Cancer. 1995;75(6):1310–1319. doi: 10.1002/1097-0142(19950315)75:6<1310::aid-cncr2820750613>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer. 1999;86(3):429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Fisher ER, Land SR, Saad RS, et al. Pathologic variables predictive of breast events in patients with ductal carcinoma in situ. Am J Clin Pathol. 2007;128(1):86–91. doi: 10.1309/WH9LA543NR76Y29J. p.7S. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim MN, Abdullah Z, Healy L, Murphy C, Yousif IY, Martin MJ. Comparison of survival rates in carcinoma in situ of the breast treated with total mastectomy to breast-conserving surgery and radiotherapy. ASCO Ann Meet Proc Part I. 2007;25(18 S) Suppl Abstract 519, p 7S. [Google Scholar]
- 27.Rakovitch E, Pignol JP, Chartier C, et al. The management of ductal carcinoma in situ of the breast: a screened population-based analysis. Breast Cancer Res Treat. 2007;101(3):335–347. doi: 10.1007/s10549-006-9302-0. [DOI] [PubMed] [Google Scholar]
- 28.Innos K, Horn-Ross PL. Risk of second primary breast cancers among women with ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2008;111(3):531–540. doi: 10.1007/s10549-007-9807-1. [DOI] [PubMed] [Google Scholar]
- 29.Bijker N, Meijnen P, Peterse JL, et al. Breast conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 30.Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26(8):1247–1252. doi: 10.1200/JCO.2007.12.7969. [DOI] [PubMed] [Google Scholar]
- 31.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362(9378):95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 32.Cuzick J, Sestak I, Pinder S, et al. Beneficial effect of tamoxifen for women with DCIS: long-term results from the UK/ANZ DCIS Trial in women with locally excised DCIS. Cancer Res. 2009;69(24 suppl) doi: 10.1016/S1470-2045(10)70266-7. Abstract 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96(17):1311–1321. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 34.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med. 2000;160(7):953–958. doi: 10.1001/archinte.160.7.953. [DOI] [PubMed] [Google Scholar]
- 35.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320(8):479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 36.Wapnir I, Anderson S, Mamounas E, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional failure in five National Surgical Adjuvant Breast and Bowl Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24(13):2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 37.Anderson S, Wapnir I, Dignam J, et al. Prognosis after IBTR and locoregional recurrences treated by breast-conserving surgery in five NSABP node-negative breast cancer protocols. J Clin Oncol. 2009;27(15):2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong JS, Kaelin CM, Troyan SL, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24(7):1031–1036. doi: 10.1200/JCO.2005.02.9975. [DOI] [PubMed] [Google Scholar]
- 39.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27(32):5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allred DC, Bryant J, Land S, et al. Estrogen receptor expression as a predictive marker of the effectiveness of tamoxifen in the treatment of DCIS: findings from NSABP protocol B-24. San Antonio Breast Cancer Symposium. Breast Cancer Res Treat. 2002;76(suppl 1):536. Abstract 30. [Google Scholar]
- 41.Altekruse SF, Kosary CL, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute: 2010. SEER Cancer Statistics Review, 1975–2007. http://seer.cancer.gov/csr/1975_2007/sections.html. Accessed February 4, 2011. [Google Scholar]
- 42.NSABP B-35. A clinical trial comparing anastrazole with tamoxifen in postmenopausal patients with DCIS undergoing lumpectomy with radiation therapy: http://www.clinicaltrials.gov/ct2/show/NCT00053898. Accessed February 4, 2011. [Google Scholar]
- 43.NSABP B-39. A randomized phase III study of conventional whole breast irradiation vs. partial breast irradiation for women with stage 0, I, or II breast cancer: http://www.clinicaltrials.gov/ct/show/NCT00103181. Accessed Feburary 4, 2011. [Google Scholar]
- 44.NSABP B-43. A phase III trial comparing trastuzumab given concurrently with radiation therapy and radiation therapy alone for women with HER-2 positive DCIS resected by lumpectomy: http://www.clinicaltrials.gov/ct2/show/NCT00769379. Accessed February 4, 2011. [Google Scholar]