Summary
Background
Chemotherapy has historically proven ineffective in advanced differentiated thyroid cancers, but the realisation that various tyrosine kinases are activated in the disease suggested a potential therapeutic role for tyrosine-kinase inhibitors. We investigated the safety and efficacy of pazopanib.
Methods
This phase 2 trial was done from Feb 22, 2008, to Jan 31, 2009, in patients with metastatic, rapidly progressive, radioiodine-refractory differentiated thyroid cancers. Each patient received 800 mg continuous pazopanib daily in 4-week cycles until disease progression, drug intolerance, or both occurred. Up to two previous therapies were allowed, and measurable disease with radiographic progression in the 6-month period before enrolment was a requirement for inclusion. The primary endpoint was any tumour response, according to the Response Evaluation Criteria in Solid Tumors 1.0. This study is registered with ClinicalTrials.gov, number NCT00625846.
Findings
39 patients were enrolled. One patient had received no previous radioiodine therapy and another withdrew consent before treatment. Clinical outcomes could, therefore, be assessed in 37 patients (19 [51%] men, median age 63 years). The study is closed to accrual of new patients, but several enrolled patients are still being treated. Patients received a median of 12 cycles (range 1 to >23, total >383). Confirmed partial responses were recorded in 18 patients (response rate 49%, 95% CI 35–68), with likelihood of response lasting longer than 1 year calculated to be 66%. Maximum concentration of pazopanib in plasma during cycle one was significantly correlated with radiographic response (r=−0·40, p=0·021). 16 (43%) patients required dose reductions owing to adverse events, the most frequent of which (any grade) were fatigue (29 patients), skin and hair hypopigmentation (28), diarrhoea (27), and nausea (27). Two patients who died during treatment had pre-existing contributory disorders.
Interpretation
Pazopanib seems to represent a promising therapeutic option for patients with advanced differentiated thyroid cancers. The correlation of the patient’s response and pazopanib concentration during the first cycle might indicate that treatment can be individualised to achieve optimum outcomes. Assessment of pazopanib in an expanded cohort of patients with differentiated thyroid cancer, as well as in cohorts of patients with medullary and anaplastic thyroid cancers, is presently being done.
Funding
National Cancer Institute, supported in part by NCI CA15083 and CM62205.
Introduction
Despite progress in the treatment of cancers and a consequent decline in overall mortality in the USA, the incidence of thyroid cancer has doubled in the past decade, in association with an increase in mortality of more than 33%.1–4 Of particular concern is the striking rise in the frequency of differentiated thyroid cancers among women in the USA;1,4 with more than 37 000 new cases annually, this cancer has the seventh highest incidence and is the tenth most frequently diagnosed, ahead of ovarian, gastric, or oesophageal cancers.5 Moreover, the incidence of thyroid cancer among women is rising worldwide. Differentiated thyroid cancer is now the second most frequently diagnosed cancer among women in the Middle East, behind only breast cancer, and accounting for more than 10% of all cancers among women in Saudi Arabia.6
Fortunately, most patients with differentiated thyroid cancers have an excellent outlook with use of traditional therapies, including surgery, suppression of thyroid-stimulating hormone (TSH) secretion with levothyroxine, and therapeutic radioiodine, and sometimes also radiation therapy. Nevertheless, about 5% of patients with advanced thyroid cancer develop life-threatening progressive disease, which led to about 1600 deaths in 2009 in the USA alone.1 Few therapeutic options are available for patients with aggressive and life-threatening, radioiodine-insensitive disease, with no clinically meaningful benefit yet demonstrated with traditional cytotoxic chemotherapy.7
Improved understanding of the molecular alterations in differentiated thyroid cancers, however, has led to the realisation that a variety of kinases, including the vascular endothelial growth factor (VEGF) receptor tyrosine kinases, are frequently activated in differentiated thyroid cancer lesions and surrounding stroma. This finding has provided a rationale for the development of targeted treatments for these cancers that can be tailored to the individual patient.7,8 Consequently, we did a phase 2 therapeutic clinical trial to assess the efficacy and safety of pazopanib (Votrient, GlaxoSmithKline, Brentford, UK), a tyrosine-kinase inhibitor (TKI) targeting VEGF receptors, platelet-derived growth factor, and c-KIT, among other kinases,9,10 in patients with metastatic, rapidly progressive, radioiodine-refractory differentiated thyroid cancers.
Methods
Study design and patients
Patients for this study were enrolled from Feb 22, 2008, to Jan 31, 2009. Eligibility requirements were pathologically confirmed differentiated thyroid cancer (papillary, Hürthle cell, or follicular) resistant to therapeutic radioiodine and radiographically confirmed disease progression, according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 (development of new metastatic lesions, progression of pre-existing metastatic lesions [≥20% increase in the sums of unidimensional tumour measurements], or both) on successive scans within the 6 months immediately before enrolment. Further require ments for enrolment included age of at least 18 years, Eastern Cooperative Oncology Group performance status of 2 or less, controlled blood pressure (<140/90 mm Hg), platelet count 100×109/L or more, neutrophil count 1·5×109/L or more, international normalised ratio 1·2 times the upper limit of normal or less, serum creatinine and bilirubin concentrations at the upper limit of normal or lower, proteinuria of grade 1 or less, aminotransferase concentrations less than 2·5 times the upper limit of normal, and haemoglobin concentration 90 g/L or greater (≥9 g/dL). Enrolment of patients with brain metastases was permitted if disease had been radiographically stable for more than 1 year. Patients with active diverticulitis or inflammatory bowel disease were excluded, as were patients with heart failure of New York Heart Association class II or higher, QTc intervals longer than 500 ms, or both. Patients with known bleeding disorders and those receiving warfarin were excluded, but continued use of low-molecular-weight heparin, antiplatelet medications, or both was allowed. Concurrent use of octreotide or bisphosphonates was prohibited unless disease progression was demonstrated while receiving these agents, as was concurrent use of medications that interacted with cytochrome P450 or combination HIV antiretroviral therapy. Patients with serious infections or who had undergone radiotherapy or surgery within the 4 weeks before registration were excluded, as were pregnant or lactating women and patients who had undergone external irradiation of more than 25% of bone-marrow reserve. TSH suppression with levothyroxine was not a requirement for enrolment, but study guidelines specified that TSH concentrations had to be maintained below 0·1 mIU/L by adjustment of levothyroxine dose during treatment with pazopanib. Up to two previous systemic therapies in addition to therapeutic radioiodine were allowed. Patients receiving antihypertensive therapy and narcotics at enrolment were allowed to continue these treatments during the study.
The trial principal investigator, KCB, adjudicated any cases in which eligibility was uncertain. Final decisions were based on the extent of disease progression, assessed by repeat biopsy in case of uncertainty, and the presence of metastatic deposits larger than 1 cm in diameter to preclude the enrolment of patients with indolent disease or minimum disease burden not immediately needing systemic or immediate therapy. Resistance to therapeutic radioiodine was defined as non-response, disease progression, or both within 6 months of the latest administration. We did not require central pathology review, but the pathology was reviewed by an endocrine pathologist at the Mayo Clinic in almost all cases. Likewise, we did not require repeat tumour biopsy immediately before study enrolment in clearly eligible cases. This study was approved by the local institutional review boards of the Mayo Clinics in Rochester, MN, and Jacksonville, FL, each participating site, and the Center for Translational Experimental Therapeutics. Consent was obtained in writing within 14 days before the first cycle of pazopanib administration was started.
Procedures
All patients were started on 800 mg pazopanib daily, administered continuously in 4-week cycles, until disease progression, drug intolerance, or both occurred; formal tumour re-staging was required at least every 8 weeks, but was allowed at any timepoint at the discretion of the investigators, and more frequent assessments were encouraged if clinical tumour progression was suspected. Intolerance was defined as a need to lower the dose to less than 200 mg daily. Data are still being collected in all patients who are still receiving the study drug and data were updated during revision of this report. Follow-up continued until discontinuation of study drug or for at least 18 months. This dosing schedule was selected on the basis of the manufacturer’s recommendation, which was guided by experience with pazopanib in other tumour types.10 The primary endpoint was tumour response rate, which was defined as the number of eligible patients who fulfilled RECIST 1.0 for a complete or partial response at two consecutive assessments at least 8 weeks apart, divided by the total number of eligible patients who started treatment. The regimen was to be deemed promising if the response rate was at least 20%, not promising if the rate was 5% or less, and of unclear promise if the rate was 5–20%. For patients with a confirmed response, duration of response was defined as the time from the assessment in which a complete or partial response was first noted to disease progression. All patients treated at the two Mayo primary enrolment sites, but not the other study sites, underwent formal independent audit by the sponsor.
A secondary endpoint of this trial involved assessment of whether pazopanib therapy might lead to a decline in thyroglobulin concentrations in patients negative for antibodies to thyroglobulin.
The Mayo Clinic Cancer Center Data and Safety Monitoring Board reviewed all adverse events, irrespective of attribution, every 6 months during the treatment phase of this trial and, when completed, reviewed the interim efficacy analysis results.
While receiving pazopanib, patients were assessed every 4 weeks for the first year of study participation, when history was recorded and physical examinations and laboratory tests were done, including measurement of TSH and thyroglobulin concentrations, chemistry tests, and haematological studies. Thereafter the frequency of assessments could be lessened to every 8 weeks at the investigator’s discretion. Plasma samples were prospectively collected before treatment, weekly during the first cycle, at the time of first evaluation (4 weeks after treatment was started) and when treatment was stopped for the assessment of pazopanib concentrations. If toxic effects of grade 3 or higher occurred, pazopanib was withheld until they resolved or notably improved, after which treatment was restarted with a proscribed dose reduction in 200 mg increments; pazopanib was discontinued if deemed appropriate by treating physicians, in the cases of severe toxic effects.
Plasma samples were also used for exploratory analyses to assess the usefulness of interleukins 6 and 8, hepatocyte growth factor, E-selectin, VEGF, and platelet-derived growth factor as biomarkers of treatment response. These potential biomarkers were selected on the basis of the known molecular targets of pazopanib and results of analogous studies in renal cell carcinoma. Plasma thyroglobulin concentrations were measured prospectively at each treatment evaluation.
Adherence with study therapy was assessed by review of diaries recorded daily by patients and by pill counts. All patients also recorded twice daily pulse rate and blood pressure, which were monitored with devices provided for use at home. Patients were asked to report expeditiously blood pressure greater than 150 mm Hg systolic at any time. Blood pressures greater than 150 mm Hg systolic, greater than 90 mm Hg diastolic, or both for longer than 48 h, or higher than 180/105 mm Hg at any time led to pazopanib being withheld until blood pressure could be lowered to below 140/90 mm Hg. Induced hypertension was promptly treated, most frequently with calcium-channel blockers.
Electrocardiographic assessment was done once at enrolment. Serial electrocardiograms or assessment of cardiac ejection fraction were not required thereafter, although patients could electively undergo these tests.
Adverse events were assessed according to the Common Terminology Criteria for Adverse Events, version 3.0.
Statistical analysis
The study was designed to provide a 90% chance of detecting a tumour response rate of at least 20% at a significance level of α=0·1, when the true response rate was at least 5%. We calculated that 33 eligible patients needed to be recruited and a response would need to be seen in at least four patients for the study to be deemed promising, in two or three patients to be of unclear promise, and in no or one patient to have no promise. Thus, if none of the first 14 patients enrolled had a confirmed complete or partial response, enrolment would be terminated.
Wilcoxon rank sum test was used to assess whether baseline or maximum percentage change in biomarker concentrations differed between patients who required dose reductions and/or discontinued pazopanib due to adverse events and patients who did not, as well as patients whose tumours responded to treatment and those who did not. Spearman’s rank correlation coefficient was used to assess the strength of the association between maximum plasma pazopanib concentrations during the first treatment cycle and the maximum change in tumour size during treatment. Fisher’s exact test was used to assess whether response rates differed with respect to histological subtype. Results of all analyses were deemed significant if p<0·05. The trial is registered on ClinicalTrials.gov, number NCT00625846.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all trial data. KCB had the final responsibility to submit for publication.
Results
As four of the first 14 eligible patients enrolled had confirmed partial responses, the trial continued to enrol. The total number of patients considered for the study was 39. One patient was found to be ineligible, having had no previous radioiodine treatment, and one patient withdrew consent before the start of treatment. Thus, the study cohort consisted of 37 patients (19 men, 18 women) aged 23–79 years (median 63 years). Patient characteristics are presented in table 1. Of note, we enrolled patients with well differentiated (grades 1 and 2) and poorly differentiated (grade 3) cancers if disease was rapidly progressive; patients with anaplastic tumours were excluded and instead enrolled in a separate pazopanib trial specifically designed for this histotype. Previous pathology findings that were unclear were verified by a Mayo Clinic endocrine pathologist in 31 (84%) patients. 26 (70%) of 37 patients had TSH concentrations lower than 0·1 mIU/L at enrolment; two of the remaining patients had concentrations higher than the institutional upper limit of normal (>5·0 mIU/L). No patients had brain metastases. All patients began treatment and adhered to the required imaging schedule until treatment discontinuation.
Table 1.
Selected demographic and baseline characteristics of patients
| Patients (n=37) | |
|---|---|
| Median age (range) | 63 (23–79) |
|
| |
| Male | 19 |
|
| |
| Female | 18 |
|
| |
| Radiation therapy | 18 |
|
| |
| No previous systemic therapies other than radioiodine and TSH suppression | 27 |
|
| |
| ECOG performance status | |
| 0 | 19 |
| 1 | 16 |
| 2 | 2 |
|
| |
| Pre-existing signs and symptoms | |
| Grade 2 anorexia | 1 |
| Grade 2 anaemia | 1 |
| Grade 2 fatigue | 3 |
|
| |
| Most frequent metastatic sites* | |
| Lung | 37 |
| Nodal | 19 |
| Bone | 13 |
|
| |
| Baseline medications | |
| Narcotics | 10 |
| Hypertensive medications | 17 |
| Octreotide | 0 |
|
| |
| Histological subtype | |
| Follicular | 11 |
| Hürthle cell | 11 |
| Papillary | 15 |
TSH=thyroid-stimulating hormone. ECOG=Eastern Cooperative Oncology Group.
Several patients had disease at more than one metastatic site.
The median number of 4-week cycles administered was 12 (range 1 to >23, total >383) and the median number of follow-up assessments was 12 (range 1 to >18; until discontinuation or for at least 18 months). 16 (43%) patients required dose reductions. Daily doses were reduced to 600 mg in 12 patients for the following reasons: raised aminotransferase concentrations (n=2), hyper tension (n=1), proteinuria (n=1), peripheral neurological difficulties (n=1), weight loss and mucositis (n=1), radiation recall tracheitis (n=1), diarrhoea with intermittent phlebitis (n=1), fatigue with weight loss (n=1), cough (n=1), abdominal pain (n=1), and at the request of the patient (n=1). A further four patients had their daily dose reduced to 400 mg for the following reasons: oral mucositis, bleeding, and mouth pain (n=1). nausea and colonic inflammation (n=1), raised aminotransferase concentrations (n=1), and raised aminotransferase concentrations followed by peripheral neurological difficulties (n=1). In addition, two patients discontinued treatment owing to serious haemorrhagic events (one grade 3 lower-gastrointestinal haemorrhage and one grade 4 intracranial haemorrhage in the absence of hypertension or brain metastases but in the presence of concomitant ibuprofen use). Both events were self-limiting and the two patients recovered fully on discontinuation of the study drug. Two (5%) patients died during the study. The first was a man aged 62 years with known atherosclerotic heart disease who presented as an emergency with tachycardia, hypotension, and dehydration and who died during his first cycle of treatment from an acute massive myocardial infarction. The second, a man aged 63 years, developed acute cholecystitis after two cycles of treatment; surgery was required and was later complicated by a bowel volvulus with perforation at the site of a remote surgical anastomosis. No known clinically apparent or relevant arrhythmic events were encountered in any study patient and no patients developed heart failure. All other adverse events deemed to be possibly, probably, or definitely related to treatment are presented in table 2.
Table 2.
Adverse events deemed to be possibly, probably, or definitely related to treatment
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
|
Cardiovascular
| |||
| Atrial fibrillation | 0 | 2 | 0 |
| Hypertension | 5 | 13 | 1 |
| Hypotension | 1 | 0 | 0 |
|
| |||
|
Constitutional symptoms
| |||
| Chills | 1 | 0 | 0 |
| Fatigue | 21 | 8 | 0 |
| Fever | 2 | 0 | 0 |
| Sweating | 1 | 0 | 0 |
| Weight loss | 2 | 7 | 1 |
|
| |||
|
Dermatology/skin
| |||
| Alopecia | 13 | 0 | 0 |
| Bruising | 0 | 1 | 0 |
| Burn | 1 | 0 | 0 |
| Dry skin | 1 | 0 | 0 |
| Flushing | 2 | 0 | 0 |
| Nail changes | 2 | 0 | 0 |
| Pruritus | 1 | 0 | 0 |
| Rash | 5 | 0 | 0 |
| Skin hyperpigmentation | 1 | 0 | 0 |
| Skin and hair hypopigmentation | 22 | 6 | 0 |
| Skin reaction, hand/foot | 2 | 0 | 0 |
|
| |||
|
Endocrine
| |||
| Adrenal insuffciency | 0 | 1 | 0 |
| Hot flushes | 2 | 0 | 0 |
|
| |||
|
Gastrointestinal
| |||
| Abdominal distension | 2 | 0 | 0 |
| Anorexia | 14 | 8 | 0 |
| Colonic perforation | 0 | 0 | 1 |
| Constipation | 3 | 0 | 0 |
| Diarrhoea | 16 | 10 | 1 |
| Dry mouth | 2 | 0 | 0 |
| Dysphagia | 1 | 0 | 0 |
| Flatulence | 2 | 0 | 0 |
| Nausea | 19 | 8 | 0 |
| Oral cavity examination abnormal | 1 | 0 | 0 |
| Oral mucositis | 2 | 1 | 1 |
| Pharyngeal mucositis | 1 | 0 | 0 |
| Small intestine mucositis | 1 | 0 | 0 |
| Taste | 20 | 1 | 0 |
| Vomiting | 15 | 0 | 0 |
|
| |||
|
Haematology
| |||
| Anaemia | 13 | 0 | 0 |
| Leucopenia | 11 | 3 | 0 |
| Lymphopenia | 1 | 0 | 0 |
| Neutropenia | 7 | 4 | 0 |
| Thrombocytopenia | 11 | 0 | 0 |
|
| |||
|
Haemorrhage
| |||
| Epistaxis | 6 | 0 | 0 |
| Intracranial haemorrhage* | 0 | 0 | 0 |
| Oral haemorrhage | 2 | 0 | 0 |
| Petechiae | 1 | 0 | 0 |
| Vaginal haemorrhage | 1 | 0 | 0 |
| Lower-gastrointestinal haemorrhage | 0 | 0 | 3 |
| Upper-gastrointestinal haemorrhage | 1 | 0 | 0 |
|
| |||
|
Hepatic
| |||
| Raised ALT concentration | 7 | 3 | 4 |
| Raised alkaline phosphatase concentration | 5 | 0 | 0 |
| Raised AST concentration | 9 | 5 | 1 |
| Bilirubin | 5 | 4 | 0 |
| GGT | 0 | 0 | 1 |
| Hypoalbuminaemia | 0 | 0 | 1 |
|
| |||
|
Infection/febrile neutropenia
| |||
| Pneumonia with grade 3/4 ANC | 0 | 1 | 0 |
|
| |||
|
Lymphatics
| |||
| Limb oedema | 1 | 0 | 0 |
|
| |||
|
Metabolic/laboratory
| |||
| Hyperglycaemia | 3 | 0 | 0 |
| Hypercholesterolaemia | 2 | 0 | 0 |
| Hypophosphataemia | 0 | 1 | 0 |
| Hypokalaemia | 1 | 0 | 1 |
| Hypertriglyceridaemia | 5 | 0 | 0 |
|
| |||
|
Musculoskeletal
| |||
| Arthritis | 1 | 0 | 0 |
| Joint disorder NOS | 1 | 0 | 0 |
| Muscle weakness | 1 | 0 | 0 |
|
| |||
|
Neurology
| |||
| Dizziness | 2 | 0 | 0 |
| Extrapyramidal disorder | 1 | 0 | 0 |
| Insomnia | 4 | 0 | 0 |
| Peripheral sensory neuropathy | 6 | 1 | 0 |
| Olfactory nerve disorder | 1 | 0 | 0 |
|
| |||
|
Ocular/visual
| |||
| Blurred vision | 2 | 0 | 0 |
| Double vision | 1 | 0 | 0 |
| Photopsia | 1 | 0 | 0 |
| Photophobia | 1 | 0 | 0 |
| Watering eyes | 1 | 0 | 0 |
|
| |||
|
Pain
| |||
| Abdominal pain | 2 | 0 | 1 |
| Arthralgia | 3 | 0 | 0 |
| Back pain | 1 | 0 | 0 |
| Chest pain | 1 | 0 | 1 |
| Headache | 3 | 1 | 1 |
| Myalgia | 6 | 0 | 0 |
| Neuralgia | 1 | 0 | 0 |
| Oral pain | 2 | 1 | 0 |
| Pharyngolaryngeal pain | 2 | 0 | 0 |
| Pleuritic pain | 0 | 1 | 0 |
| Pain NOS | 3 | 0 | 0 |
| Skin pain | 1 | 0 | 0 |
|
| |||
|
Pulmonary
| |||
| Cough | 1 | 1 | 1 |
| Voice alteration | 3 | 0 | 0 |
|
| |||
|
Renal/genitourinary
| |||
| Raised creatinine concentration | 2 | 0 | 1 |
| Proteinuria | 5 | 3 | 0 |
| Urinary frequency | 3 | 0 | 0 |
|
| |||
|
Sexual/reproductive function
| |||
| Vaginal mucositis | 1 | 0 | 0 |
ALT=alanine aminotransferase. AST=asparate aminotransferase. ANC=absolute neutrophil count. GGT=gamma glutamyl transpeptidase. NOS=not otherwise specified.
One patient had grade 4 intracranial haemorrhage.
Of the 20 patients who had not required antihypertensive treatment before enrolment, one developed grade 3 hypertension during the first cycle of treatment. The patient received antihypertensive treatment and the dose of study drug was lowered. Another nine of these 20 patients required antihypertensive medication for two or more cycles of study treatment. Among the 17 patients who were already taking antihypertensive treatments at enrolment, one developed grade 3 hypertension and the dose of the study drug was lowered, and four patients developed grade 2 hypertension that required modifications to their antihypertensive regimens. Three (11%) of the 27 patients who were not taking narcotics before the start of study treatment received narcotics with two or more cycles of study treatment.
Other frequently seen minor side-effects that did not lead to lowering of the study drug dose were slight fatigue, altered taste perception, nausea, and notable hypopigmentation of the skin, hair, or both if patients received treatment for longer than 6 months.
No patient had a complete response, but 18 had confirmed partial responses (response rate 49% [95% CI 35–68]). Responses were seen in eight (73%) of 11 patients with follicular tumours, five (45%) of 11 patients with Hürthle cell tumours, and five (33%) of 15 patients with papillary tumours. The proportion of responses in patients with follicular histology did not differ significantly from that of patients with Hürthle cell (p=0·387) or papillary histologies (p=0·111) but these analyses were not prespecified and the trial was not powered to assess such differences statistically. The Center for Translational Experimental Therapeutics confirmed all 15 audited responses. Moreover, responses were sustained, with the likelihood of a response lasting for more than 1 year calculated to be 66%.
17 (46%) of 37 patients took pazopanib for 12 months or longer (eight [73%] of 11 patients with follicular, five [45%] of 11 with Hürthle cell, and four [27%] of 15 with papillary histologies). Median overall survival at 1 year was 81% (95% CI 69–95), with median overall survival not yet attained. Progression-free survival (PFS) at 1 year was 47% (35–68; figure 1). The median duration of PFS was 11·7 months (range 1 to >23). Tumour size decreased substantially from before treatment in most patients (figure 2). Thyroglobulin concentrations decreased by at least 30% from before treatment in 28 (88%) of 32 patients with complete data (figure 2). Complete serial tumour measurement data, according to RECIST, from before enrolment and during treatment were assessable retrospectively for nine (50%) responders (figure 3). In these patients, pazopanib therapy seemed to modify the natural history of thyroid cancer.
Figure 1.
Progression-free and overall survival in patients receiving pazopanib
Figure 2. Maximum changes in tumour size or thyroglobulin concentrations from before treatment in response to pazopanib therapy for differentiated thyroid cancers.
(A) Changes in tumour size, according to the Response Evaluation Criteria in Solid Tumors 1.0 (n=37). (B) Changes in thyroglobulin concentrations, measured in patients negative for antibodies to thyroglobulin (n=32). Circles indicate patients with incomplete data.
Figure 3. Effects of pazopanib on rates of disease progression in nine patients who responded to therapy.
Data for tumour size, according to the Response Evaluation Criteria in Solid Tumors 1.0, are shown for the time periods immediately before and during pazopanib therapy for nine of 18 patients who attained partial responses. The data only indicate changes in tumour size of target lesions selected at study entry.
Disease progression or clinical deterioration was ultimately seen in 27 (73%) of 37 patients (table 3). In 18 (67%) of these 27 patients, progression was based on disease in new sites, with or without involvement of the target lesions.
Table 3.
Sites of disease progression triggering discontinuation of pazopanib
| Primary sites of progression | Clinical deterioration | |||
|---|---|---|---|---|
| Target only | Target and non-target | New and non-target | ||
| Partial response | 3 | 1 | 8 | 0 |
| Stable disease | 3 | 0 | 3 | 1 |
| Progression | 1 | 0 | 6 | 1 |
| Total | 7 | 1 | 17 | 2 |
Treatment was discontinued in 27 patients because of progression of disease (n=22), death (n=2), adverse events (n=2), and a request for withdrawal of treatment (n=1). At the latest follow-up, 12 patients were alive without disease progression, 15 patients were alive with progressive disease, and ten patients had died.
Neither of the two patients with TSH concentrations higher than 5·0 mIU/L at enrolment attained a response to therapy, despite achieving TSH suppression. Moreover, TSH concentrations increased by more than two times during treatment with pazopanib in 23 (62%) of 37 patients. Among these patients 13 (57%) responded to therapy compared with four (29%) of 14 patients with no increase in TSH concentration during the study; data were incomplete for one patient. Consistent TSH suppression did not, therefore, seem to play a part in tumour response.
Plasma concentrations of interleukins 6 and 8, hepatocyte growth factor, E-selectin, VEGF and platelet-derived growth factor before and after one cycle of treatment were available in 24 patients, and values from before therapy alone were available for 30 patients. Baseline and maximum percentage change in biomarker concentrations did not differ between patients who required dose reductions and/or discontinued pazopanib due to adverse events and patients who did not, nor between patients whose tumours responded to treatment and those who did not.
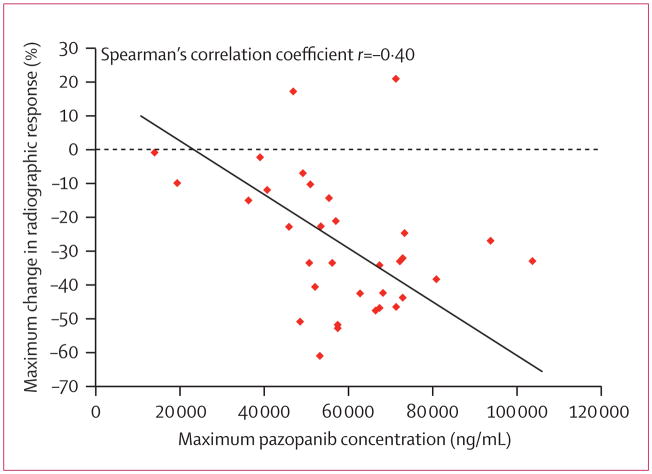
The highest plasma pazopanib concentrations during the first cycle of treatment were about seven times more than the lowest achieved concentrations among the 36 patients with complete data. The maximum plasma pazopanib concentration during treatment cycle one correlated with the maximum change in tumour size (r=−0·40, p=0·021; figure 4) and was significantly higher in the 18 patients who achieved confirmed RECIST partial responses than in those who did not (p=0·009). The maximum plasma pazopanib concentration, however, was not found to differ with respect to the requirement for pazopanib dose reduction. Moreover, we found no significant association between the need for a dose reduction and achieving a response or time to progression (p>0·05).
Figure 4. Correlation between radiographic response pazopanib concentrations in plasma during the first cycle of pazopanib treatment.
Maximum values are used.
Discussion
The present trial suggests that pazopanib is clinically efficacious in patients with metastatic, rapidly progressive, and radioiodine-refractory differentiated thyroid cancers. In particular, pazopanib induced RECIST partial responses in a substantial proportion of patients, with an estimated 66% likelihood of having responses longer than 1 year’s duration.
Searches at the time of writing this report yielded results from ten peer-reviewed therapeutic clinical trials in differentiated thyroid cancers (table 4, panel).11–20 Confirmed RECIST partial response rates in these trials ranged from 0 with vorinostat19 and gefitinib20 to 31% with axitinib.11 Among the TKIs, multitargeted drugs, such as pazopanib, sorafenib,13,14 and axitinib11 have generally shown the greatest activity in relation to RECIST in differentiated thyroid cancers. [90Yttrium-DOTA]-TOC has also produced a high proportion of biochemical responses, as assessed by decreases in thyroglobulin concentrations; RECIST outcomes, however, were not reported, making interpretation of results within the context of the other trials challenging.12
Table 4.
Summary of results of published clinical trials in differentiated thyroid cancers
| Agent and regimen | Radioiodine-resistance required | Threshold for progression | Patients with any RECIST response (n/total [%]) | Median survival (months) | ||
|---|---|---|---|---|---|---|
| Progression-free | Overall | |||||
| Cohen et al11 | Axitinib 5 mg twice daily | Yes | None | 14/45 (31%) | 18·1* | NR |
| Iten et al12 | [90Yttrium-DOTA]-TOC median cumulative administered activity 12·6 GBq (range 1·7–29·6 GBq) | Yes | <12 months | NR | NR | 16·8 |
| Gupta-Abramson et al13 | Sorafenib 400 mg twice daily | Yes | <12 months | 7/27 (26%) | 19·75 | NR |
| Kloos et al14 | Sorafenib 400 mg twice daily | No | None | 6/52 (12%) | 12·6 | 25·5 |
| Aim et al15 | Thalidomide 800 mg daily | Yes | <12 months | 5/28 (18%)† | 4† | 17† |
| Sherman et al16 | Motesanib 125 mg daily | Yes | <6 months | 13/93 (14%) | 40 | NR |
| Argiris et al17 | Interferon alfa 2b, 12 million units/m2 subcutaneously on days 1–5, and doxorubicin, 40 mg/m2 intravenously on day 3; 28-day cycles | Yes | None | 1/14 (7%) | 5·9 | 26·4 |
| Mrozek et al18 | Celecoxib 400 mg twice daily | Yes | <12 months | 1/32 (3%) | NR | NR |
| Woyach et al19 | Vorinostat 200 mg twice daily (2 weeks on, 1 week off) | Yes | None | 0/16 | NR | NR |
| Pennell et al20 | Gefitinib 250 mg daily | Yes | None | 0/17 | 3·7* | 17·5* |
NR=not reported. RECIST=Response Evaluation Criteria in Solid Tumors.
Includes patients with anaplastic and medullary thyroid cancers.
Includes patients with medullary thyroid cancers.
Akin to our analysis of pazopanib on rates of disease progression in patients who responded to treatment (figure 3), Cabanillas and co-workers21 have presented data on tumour trajectory before and during treatment with TKIs for eight of 33 patients with differentiated thyroid cancers, who were studied retrospectively. Their findings suggested effects of sunitinib or sorafenib on the natural disease course. Moreover, pooled data on responses seen in patients with thyroid cancer treated in selected phase 1 clinical trials at one institution indicated a confirmed overall RECIST partial response rate of 18%.22 The responses were, however, confined to patients with medullary and anaplastic tumours; no partial responses were seen among the 17 enrolled patients with differentiated thyroid cancers.22
The partial response rate for pazopanib in our study seems very favourable in the light of results from previous trials (table 4) and, to our knowledge, represents the highest response rate yet reported in patients with differentiated thyroid cancers. Moreover, pazopanib seems to modify the disease course in responders. Heterogeneity among patients enrolled in various trials of differentiated thyroid cancer trials, however, hinders comparison of data related to time to progression, overall survival, or both.
Assessment of data from previous studies and this one suggests that TKIs targeting VEGF receptor have some class effects in differentiated thyroid cancers. Furthermore, two other antiangiogenic agents, thalidomide and lenalidomide, also have some degree of clinical activity in these cancers.15,23 Therefore, agents targeting attenuation of tumour angiogenesis seem collectively to be promising treatments in differentiated thyroid cancers, but several critical questions remain. In particular, it is still unclear whether the clinical activity of multitargeted TKIs is due exclusively or even primarily to inhibition of VEGF receptor kinase. VEGF seems to have an important role in differentiated thyroid cancers. First, expression of this growth factor is increased in thyroid cancer lesions and surrounding stroma relative to that in normal thyroid tissue and to benign thyroid lesions.24–26 In addition, VEGF expression is highest in tumours from patients with metastases,24–26 which suggests that its increased expression is associated with aggressive, metastatic, or both phenotypes. Consistent with this observation, treatment of mice that have xenografted follicular thyroid cancers with a VEGF-directed monoclonal antibody substantially attenuated tumour growth.27
The question of why pazopanib seems to have better clinical activity than other VEGF receptor kinase inhibitors tested so far in differentiated thyroid cancers remains unanswered. No comparative clinical trials of TKIs have yet been undertaken in this population. Because each TKI has a distinct profile of kinase inhibition, kinases other than VEGF receptor might be targeted more effectively by pazopanib than by other agents in differentiated thyroid cancers. This issue is important, since studies in this area could provide insights into additional and perhaps as yet under-appreciated candidate therapeutic molecular targets. In this study, however, we found no correlations between plasma biomarker concentrations and response to treatment.
Although papillary thyroid cancer is much more frequent than the Hürthle cell or follicular forms in the general population, we recruited more patients than expected with the latter two cancer histological subtypes. We did not proscribe any particular ratio of histological types, and the distribution was not revealed to the study institutions during enrolment. The inclusion criterion of disease progression in the 6-month period before enrolment, however, led to patients with more-aggressive histological subtypes being included.
We noted that a higher percentage of patients with follicular thyroid cancer (73%) than with papillary thyroid cancer (33%) attained partial responses to pazopanib treatment; the difference was not significant (however, this analysis was not prespecified and our study was not powered to examine this issue definitively). All but one patient with follicular histology treated with pazopanib attained more than a 20% decrease in tumour size, but less than half of those with papillary histology attained similar levels of objective disease response (figure 2). In parallel, disease control was longer than 1 year in more patients with follicular than papillary tumours (73% vs 33%). These observations need to be assessed prospectively in a larger cohort of patients. If the findings are confirmed, however, they raise the critical question as to whether identifiable genetic or other differences between thyroid cancer histological subtypes can affect responsiveness to pazopanib and potentially to other TKIs. In particular, an area of active investigation in our laboratories relates to differences in BRAF V600E activating mutations among these different forms of differentiated thyroid cancers and whether these may be correlated, linearly or inversely, with patients’ response to pazopanib or other agents.28
Adverse events associated with the study drug were frequent but were generally mild enough to continue treatment at the intended dose in most patients. Nevertheless, pazopanib doses were lowered in 16 (43%) of 37 patients. Furthermore, two patients died while taking pazopanib. Although in both cases the patients had pre-existing disorders, we cannot exclude some contribution of pazopanib. In addition, two patients had serious but self-limiting bleeding events. Hypertension, a class effect caused by the inhibitory effect of pazopanib on the VEGF receptor, was also prevalent but was readily managed with standard antihypertensive therapy and did not result in additional complications. Also, the number of bleeding events in this study was similar to that seen with other oral VEGF-receptor inhibitors, such as axitinib in thyroid cancer11 and sorafenib in renal-cell carcinoma,29 which suggests a class effect that might not be avoidable.
Despite good clinical responses to TKIs in differentiated thyroid cancer patients, whether survival is improved by these agents remains uncertain. As a result, caution should be used when prescribing these agents to patients with differentiated thyroid cancers until the impact on overall survival is better clarified by controlled trials. In particular, although patients with life-threatening, rapidly progressive disease might seem good candidates for TKI therapy, most have disease with an indolent course for which the risks associated with TKI therapy might outweigh the benefits.
Panel: Research in context.
Systematic review
We aimed to identify all prospective therapeutic clinical trials undertaken in differentiated thyroid cancers and published in peer-reviewed journals. We used general search strategies to identify articles in online medical literature databases, primarily in PubMed, including the search terms “clinical trial AND thyroid cancer”. Articles were individually reviewed. We included all full-text reports of prospective studies that included cohorts of patients with differentiated thyroid cancers. The data were not combined or subjected to meta-analysis.
Interpretation
The observed high level of clinical activity of pazopanib suggests that this drug is a promising therapeutic option for advanced, progressive, radioiodine-refractory differentiated thyroid cancers. The correlation between response and pazopanib concentrations in the first cycle suggests that conventional doses will yield response in most patients. Our findings seem to support the hypothesis that angiogenic pathways collectively represent important therapeutic targets in differentiated thyroid cancers.
The wide range of achieved concentrations of pazopanib in plasma and the correlation with radiographic response outcomes were notable. The variation in achieved plasma concentrations raises the question of whether heterogeneity in pharmacogenomic parameters affects the pharmacokinetics of pazopanib. Crucially, these data also suggest the possibility of individualised dose escalation in patients with low plasma concentrations of pazopanib in the first cycle, particularly in the setting of acceptable tolerance of the agent. We are, therefore, presently examining alternative trial designs to assess critically this possibility.
Given the promising activity of pazopanib noted in this trial, we are currently undertaking further investigations including an expanded cohort of patients with differentiated thyroid cancers, as well as separate cohorts of patients with medullary or anaplastic thyroid cancers.
Acknowledgments
KCB, JRM, and CE were supported in part by the National Cancer Institute (grants CA15083 and CM62205).
Footnotes
Contributors
KCB was primarily responsible for overall trial design, accrual of patients, data collection and interpretation, and manuscript preparation with assistance from the study statisticians VJS and HT and from JRM, RCS, JKB, KPW, and CB. WJM, MEM, JR, and KS assisted with accrual of patients, data collection and interpretation, and manuscript preparation. JCM, BM, CE, and SPI assisted with trial design, data collection and interpretation, and manuscript preparation. AT, PJF, and BCG with accrual of patients and data collection and interpretation.
Conflicts of interest
The authors declared no conflicts of interest.
References
- 1.American Cancer Society. Cancer facts & figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 2.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–07. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 3.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–91. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the surveillance, epidemiology, and end results (SEER) program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 6.Al-Eid HS, Arteh SO. Cancer incidence report Saudi Arabia 2003. Riyadh: Kingdom of Saudi Arabia Ministry of Health National Cancer Registry; 2003. [Google Scholar]
- 7.Baudin E, Schlumberger M. New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol. 2007;8:148–56. doi: 10.1016/S1470-2045(07)70034-7. [DOI] [PubMed] [Google Scholar]
- 8.Sherman SI. Advances in chemotherapy of differentiated epithelial and medullary thyroid cancers. J Clin Endocrinol Metab. 2009;94:1493–99. doi: 10.1210/jc.2008-0923. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6:2012–21. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz HI, Dowlati A, Saini S, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15:4220–27. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 11.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–13. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iten F, Muller B, Schindler C, et al. [(90)Yttrium-DOTA]-TOC response is associated with survival benefit in iodine-refractory thyroid cancer: long-term results of a phase 2 clinical trial. Cancer. 2009;115:2052–62. doi: 10.1002/cncr.24272. [DOI] [PubMed] [Google Scholar]
- 13.Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–19. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–84. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ain KB, Lee C, Williams KD. Phase II trial of thalidomide for therapy of radioiodine-unresponsive and rapidly progressive thyroid carcinomas. Thyroid. 2007;17:663–70. doi: 10.1089/thy.2006.0289. [DOI] [PubMed] [Google Scholar]
- 16.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib Thyroid Cancer Study Group. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 17.Argiris A, Agarwala SS, Karamouzis MV, Burmeister LA, Carty SE. A phase II trial of doxorubicin and interferon alpha 2b in advanced, non-medullary thyroid cancer. Invest New Drugs. 2008;26:183–88. doi: 10.1007/s10637-007-9091-2. [DOI] [PubMed] [Google Scholar]
- 18.Mrozek E, Kloos RT, Ringel MD, et al. Phase II study of celecoxib in metastatic differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2006;91:2201–04. doi: 10.1210/jc.2005-2498. [DOI] [PubMed] [Google Scholar]
- 19.Woyach JA, Kloos RT, Ringel MD, et al. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009;94:164–70. doi: 10.1210/jc.2008-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pennell NA, Daniels GH, Haddad RI, et al. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid. 2008;18:317–23. doi: 10.1089/thy.2007.0120. [DOI] [PubMed] [Google Scholar]
- 21.Cabanillas ME, Waguespack SG, Bronstein Y, et al. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J Clin Endocrinol Metab. 2010;95:2588–95. doi: 10.1210/jc.2009-1923. [DOI] [PubMed] [Google Scholar]
- 22.Tsimberidou AM, Vaklavas C, Wen S, et al. Phase I clinical trials in 56 patients with thyroid cancer: the M. D. Anderson Cancer Center experience. J Clin Endocrinol Metab. 2009;94:4423–32. doi: 10.1210/jc.2009-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ain KB, Lee C, Holbrook KM, Dziba JM, Williams KD. Phase II study of lenalidomide in distantly metastatic, rapidly progressive, and radioiodine-unresponsive thyroid carcinomas: preliminary results. Proc Am Soc Clin Oncol. 2008;26(suppl):6027. (abstr) [Google Scholar]
- 24.Yu XM, Lo CY, Chan WF, Lam KY, Leung P, Luk JM. Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin Cancer Res. 2005;11:8063–69. doi: 10.1158/1078-0432.CCR-05-0646. [DOI] [PubMed] [Google Scholar]
- 25.Lennard CM, Patel A, Wilson J, et al. Intensity of vascular endothelial growth factor expression is associated with increased risk of recurrence and decreased disease-free survival in papillary thyroid cancer. Surgery. 2001;129:552–58. doi: 10.1067/msy.2001.112592. [DOI] [PubMed] [Google Scholar]
- 26.Klein M, Vignaud JM, Hennequin V, et al. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:656–58. doi: 10.1210/jcem.86.2.7226. [DOI] [PubMed] [Google Scholar]
- 27.Soh EY, Eigelberger MS, Kim KJ, et al. Neutralizing vascular endothelial growth factor activity inhibits thyroid cancer growth in vivo. Surgery. 2000;128:1059–65. doi: 10.1067/msy.2000.110430. [DOI] [PubMed] [Google Scholar]
- 28.Jo YS, Li S, Song JH, et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab. 2006;91:3667–70. doi: 10.1210/jc.2005-2836. [DOI] [PubMed] [Google Scholar]
- 29.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]