Abstract
The pupose of this study was to evaluate the activity of ON 01910.Na, a mitotic inhibitor, in in vitro and in vivo models of pancreatic cancer and to discover biomarkers predictive of efficacy. Successive in vitro and in vivo models were used; these included cell line-derived and patient-derived tumors from our PancXenoBank, a live collection of freshly generated pancreatic cancer xenografts. ON 01910.Na showed equivalent activity to gemcitabine against pancreatic cancer cell lines in vitro. The activity of the agent correlated with suppression of phospho-CDC25C and cyclin B1. These markers were optimized for a fine-needle aspirate ex vivo rapid assay. Cyclin B1 mRNA evaluation yielded the most optimal combination of accuracy and reproducibility. Next, nine patient-derived tumors from the PancXenoBank were profiled using the assay developed in cell lines and treated with ON01910.Na for 28 days. Two cases were cataloged as potential responders and seven as resistants. There was a correlation between the ex vivo assay and sensitivity to the tested agent, as the two cases prospectively identified as sensitive met prespecified criteria for response. Of the seven tumors of predictive resistant, only one was found to be sensitive to ON 01910.Na. In addition, there was a good correlation between cyclin B1 downregulation ex vivo and changes in cyclin B1 protein post-treatment. The novel mitotic inhibitor, ON 01910.Na, showed activity in preclinical model of pancreatic cancer. A rapid assay was rationally developed that not only identified cases sensitive to ON 01910.Na, but also anticipated the pharmacodynamic events occurring after in vivo exposure.
Keywords: pancreatic cancer, xenograft model, biomarker, ex vivo, cyclin B1, mitotic modulator
Introduction
Pancreatic cancer remains a devastating disease, as shown by the equivalence of incidence and mortality rates (Jemal et al., 2007). One key mediator of the intrincate and overlapping control points into the mitotic phase is the polo-like kinase 1 (Plk1)-centered regulatory loop (Nigg, 1998; Smits et al., 2000) that modulates the transition through the G2/M checkpoint in the cell cycle by influencing the activation of the phosphatase CDC25C and cyclin B1 (Jackman et al., 2003). ON 01910.Na is a small molecule drug that disrupts G2/M cell cycle transition at least in part by modulating Plk1 activity and induces mitotic arrest of tumor cells leading to their apoptosis (Gumireddy et al., 2005). This compound is the subject of several active clinical trials, and a phase I study has been completed (Jimeno et al., 2008).
Often, there is no information on biomarkers that may predict the activity of drugs entering clinical development. Our group has developed a novel direct xenograft model (Rubio-Viqueira et al., 2006) to screen drugs for activity in pancreatic cancer and to discover predictive biomarkers. In this study, we aimed at characterizing the in vitro and in vivo efficacy of ON 01910.Na in pancreatic cancer, and discovering markers that may predict efficacy in this tumor type. In a work reported earlier (Hidalgo et al., 2006), we have optimized the use of fine-needle aspirate biopsies as a platform to conduct ex vivo predictive assays. Currently, there are limited basis to prioritize which agents should be administered to a given patient. Chemosensitivity and resistance assays have addressed this issue, but have largely failed as they are based on clonogenicity and/or proliferation indexes, and both require large amounts of tissue and maintaining cell viability for extended periods of time (Samson et al., 2004; Schrag et al., 2004). On the other hand, it is possible to elicit pharmacodynamic responses by briefly exposing small amounts of tumor cells to a drug, an approach termed as ex vivo testing (Jimeno et al., 2006). We have hypothesized that these dynamic responses may render more information in such a complex disease as pancreatic cancer (Jones et al., 2008), and are more likely to predict the patient's outcome than static features.
Results
In vitro growth inhibition analysis
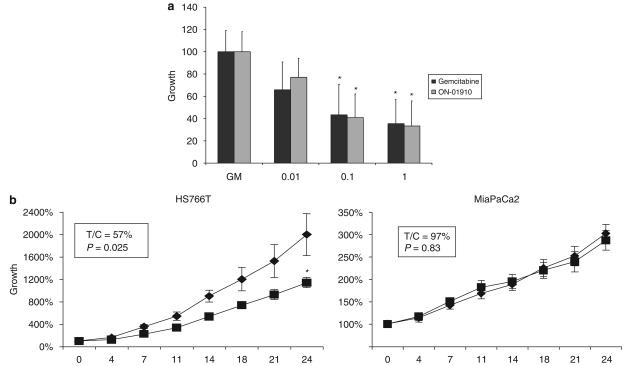
We first tested ON 01910. Na and gemcitabine in a panel of 12 pancreatic cancer cell lines in an attempt to identify sensitive and resistant cell lines. ON 01910.Na showed a high level of activity and equivalent efficacy with gemcitabine in this broad panel of pancreatic cancer cell lines (Figure 1a). Some cell lines were sensitive to both agents (HS766T and Panc1), others were resistant to both (MiaPaca2) and others had a mixed sensitivity profile (XPA3).
Figure 1.
(a) Comparison of the average growth inhibition of the 12 cell lines to ON 01910.Na and gemcitabine, where they showed equivalent potency. Error bars represent s.d. (experiments conducted in triplicate three times). (b) In vivo tumor growth inhibition of the sensitive (HS766T) and resistant (MiaPaCa2) cell lines after being xenografted on nude mice. HS766T tumors treated with ON 01910.Na intraperitoneally (■) decreased in size by 43% compared with controls (♦; P=0.025 by t-test means comparison). Mice lived 8.4 days more than untreated controls (P=0.05 by log-rank). In addition, ON 01910.Na-treated tumors tended to ulcerate less and later in time. In MiaPaCa2 tumors, no biologic activity was observed in terms of differences in tumor growth or survival. Error bars represent s.e.(n=10 tumors per group); asterisk indicates P<0.05 (Student's t-test) compared with control.
Initial in vivo growth inhibition analysis and pharmacokinetic analysis
Next, we confirmed the in vitro sensitivity data by xenografting on mice HS766T and MiaPaCa2, after which a course consisting of ON 01910.Na 250 mg/kg/day for 24 days was administered. In HS766T, the drug was active as shown by decreased local invasion, growth reduction, and also by increasing the survival of mice, whereas in MiaPaCa2 xenografts, there was no indication of antitumor effect (Figure 1b). We performed a comparative plasma, normal tissue (liver) and tumor tissue pharmacokinetic analysis to determine the peak concentrations that were achieved in vivo at steady state. The concentrations achieved after repeated dosing were (average±s.d.) 35.3±6.1 μM, 9.0±2.4 μg/g (equivalent to 11.2±3.0 μM) and 107.2±29.2 μg/g (equivalent to 134.1±36.4 μM) in plasma, tumor and liver, respectively.
Pharmacodynamic factors of activity: initial screen
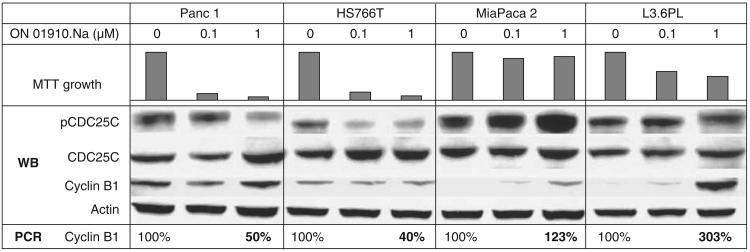
We then aimed at assessing factors that may indicate the degree of Plk1 and G2/M checkpoint inhibition, first in vitro and then in vivo/ex vivo. We correlated growth inhibition after exposure to ON 01910.Na with Plk1 levels (both by western blot and reverse transcriptase PCR (RT–PCR) analysis), CDC25C activity (measuring total and phospho-CDC25C), cyclin B1 levels (both by western blot and by RT–PCR analysis), phospho-H3, MPM-2, c-fos and c-myc. For this purpose, we used four cell lines with differential ON 01910.Na sensitivity (two sensitive, Panc1 and HS766T; two resistant, MiaPaca 2 and L3.6PL; Figure 2). In both sensitive cell lines, phospho-CDC25C decreased after treatment, whereas in the resistant, it increased. By protein assessment, cyclin B1 did not change significantly in the sensitive strains, whereas it increased in a dose-dependent manner upon exposure to ON 01910.Na in the resistant strains; using RT–PCR, cyclin B1 decreased to 50% or less in HS766T and Panc1 and increased in the resistant cell lines. The rest of the markers did not show an informative pattern (data not shown) and were not evaluated further.
Figure 2.
Pharmacodynamic marker evaluation in two sensitive (Panc 1 and HS766T) and two resistant (MiaPaca2 and L36PL) cell lines after ON-01910 treatment. In both sensitive strains, pCDC25C decreases after treatment, whereas in the resistant strains, it increases. Also, cyclin B1, both by western blot and RT–PCR, increases in a dose-dependent manner upon exposure to ON 01910.Na in the resistant strains.
Coupling phospho-CDC25C and cyclin B1 to an ex vivo assay
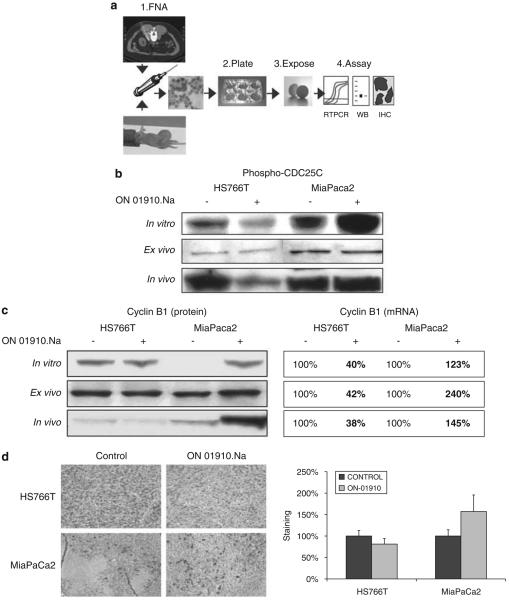
We next attempted to test these end points in fine-needle aspiration (FNA)-acquired baseline tumor tissue of HS766T and MiaPaCa2 xenografted tumors in an ex vivo assay (Figure 3a). In this process, a small amount of viable tumor cells are subjected to short-term in vitro conditions to elicit a pharmacodynamic response. We chose a concentration of 10 μM for the ex vivo assay based on the plasma and intratumor exposure, as discussed before. Tumor tissue obtained at the end of the treatment period was also interrogated for in vivo confirmation. We observed that there was a correlation of in vivo phospho-CDC25C with prior in vitro experiments (Figure 3b), where the activation level decreased in HS766T and increased in MiaPaCa2. However, repeated ex vivo blots showed a poor dynamic range and reproducibility. When cyclin B1 was analysed, the results faithfully followed prior in vitro data. As depicted in Figure 3c, the levels of cyclin B1 mRNA decreased in HS766T and increased in MiaPaca2, both in ex vivo and in vivo testing. The results of the protein analyses were less evident for HS766T, where no change or a minor decrease in cyclin B1 protein ex vivo were documented. We measured cyclin B1 levels by immunohistochemistry (IHC) in paraffin-embedded tumors at the end of the in vivo experiments (Figure 3d). In HS766T, there was a mild downregulation of cyclin B1, whereas in all ON 01910.Na-treated MiaPaCa2 tumors, there was an upregulation in cyclin B1. Thus, cyclin B1 was selected for further testing.
Figure 3.
(a) The ex vivo approach. A small sample of tissue is acquired either from a patient's tumor or from a tumor xenografted on mice, and is plated and exposed to the drug for a short period of time. The cells are then collected and analysed by the assay that is specifically coupled to that drug, and can be mRNA expression by reverse transcriptase PCR (RT–PCR), and/or protein assessment by western blot or immunohistochemistry (IHC). (b) Western blot analysis of pCDC25C in vitro, ex vivo and in vivo. Upon treatment with ON 01910.Na in the sensitive HS766t, there is a decrease, whereas in the resistant MiaPaCa2, there is a clear increase in the activation status of CDC25C, both in vitro and in vivo. However, the ex vivo analysis rendered largely inconclusive results, partly because of the high detection threshold for the tested end point. (c) Western blot and RT–PCR analysis of cyclin B1 in vitro, ex vivo and in vivo. Upon treatment with ON 01910.Na in the sensitive HS766T, there is a mild decrease, whereas in the resistant MiaPaCa2, there is a clear increase in cyclin B1 levels, both at the protein and mRNA levels. The ex vivo assay faithfully correlated with the pharmacodynamic events that occurred in the xenografted tumors (in vivo). (d) Four tumors per treatment group were examined by IHC for cyclin B1 expression, confirming the western blot analyses. In HS766T, there was a mild downregulation of cyclin B1, whereas in all ON 01910.Na-treated MiaPaCa2 tumors, there was an upregulation in cyclin B1.
Cyclin B1 specificity as a marker in the mitotic pathway
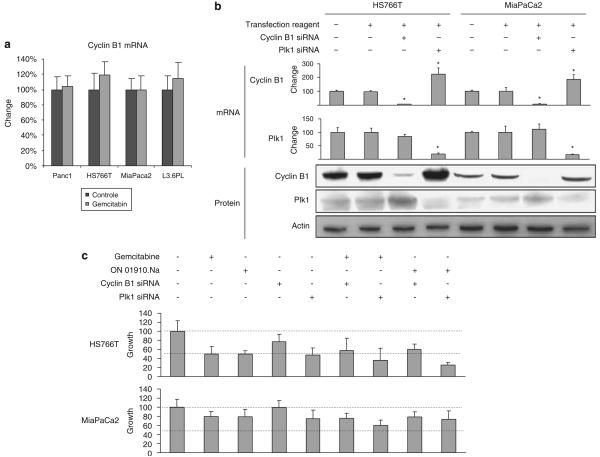
To assess the specificity of cyclin B1, a set of the same four cell lines were treated in parallel with gemcitabine. Neither in the gemcitabine-sensitive cell lines (Panc1 and HS766T; IC50 20 and 45 nM, respectively) nor in those resistant (MiaPaCa2 and L36PL; both IC50>10 μM) there was any modification in cyclin B1 levels after exposure to the gemcitabine (Figure 4a).
Figure 4.
(a) Cyclin B1 evaluation after gemcitabine in highly sensitive (Panc1 and HS766T) and resistant (MiaPaca2 and L36PL) cell lines after ON 01910.Na treatment. No change in cyclin B1 is observed after gemcitabine treatment in sensitive or resistant cells. (b and c) Small interference RNA (siRNA) of cyclin B1 and Plk1 decreased both mRNA and protein levels in Hs766T and MiaPaca2. Downregulation of polo-like kinase 1 (Plk1) induced increases in cyclin B1 mRNA also in both cell lines, but the effects at the protein level were more evident in HS766T. After assessing the effects of siRNA, cyclin B1 and Plk1 in conjunction with treatment with gemcitabine and ON 01910.Na, it can be concluded that cyclin B1 had no role in determining sensitivity status, nor did have an effect of its own. However, Plk1 downregulation had an effect in HS766T, but not in MiaPaca2, confirming that in the former cell line, cell growth is Plk1-dependent. There was also some additive effect when Plk1 siRNA and ON 01910.Na were given together. Error bars represent s.d.; asterisk indicates Po0.05 (Student's t-test) compared with control.
Knockdown of both cyclin B1 and Plk1 mRNA induced decreases in protein content. Interestingly, there was a release effect with both markers, and the downregulation of one induced an increment of the other, especially at the protein level (Figure 4b). Knockdown of cyclin B1 by specific small interfering RNA (siRNA) before exposure to either of the drugs had no meaningful effect in the growth of the sensitive HS766T or the resistant MiaPaca2, or in their response to ON 01910.Na or gemcitabine cotreatment (Figures 4b and c), suggesting that cyclin B1 is indeed a marker of activity and not a mediator of it. Plk1 siRNA had a growth inhibitory effect on HS766T, and Plk1 siRNA plus ON 01910.Na had a superior effect than either modality alone.
Prospective assessment in the pancreatic cancer direct xenograft bank
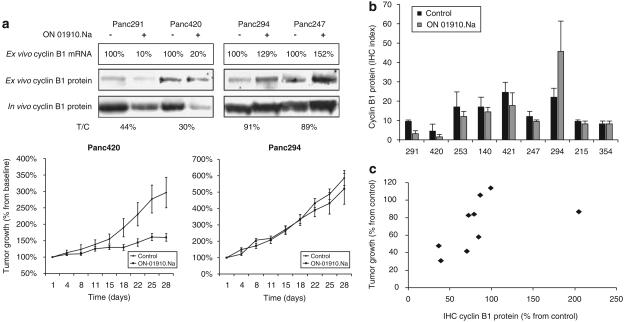
Next, we aimed at testing the ex vivo assay in a prospective manner. Nine unselected cases from the PancXenoBank were assessed by the ex vivo assay, mimicking the scenario, when a patient undergoes an FNA before therapy. All procedures rendered enough material to conduct RT–PCR analyses, but only in five cases, there was sufficient protein to run a western blot assay. In a second repeat, again in only four cases, the western blot analyses were reproducible. Thus, cyclin B1 mRNA expression analysis was chosen as the assay's end point.
Table 1 summarizes the results of the predictive assay and the efficacy observed. Of the cases tested, two showed lower than 25% cyclin B1 mRNA levels compared with the control, four had either a minor decrement or no change and three presented an increment (Figure 5a). Groups of 20 tumors per each case were then randomized and treated with vehicle or ON 01910.Na during 28 days. The two cases predicted to be sensitive met criteria for efficacy, whereas of the seven cases predictive to be resistant, only one met the criteria of susceptibility. Of the second group, only one was sensitive. Of the cases, where cyclin B1 increased ex vivo, none showed evidence of antitumor effect.
Table 1.
Summary of the results of the predictive assay and the efficacy observed
| Case | Ex vivo cyclin B1 protein by WB |
Ex vivo cyclin B1 mRNA (% ±s.d.) |
Growth compared with control T/C (%) |
In vivo cyclin B1 protein by IHC (% ±s.d) |
Predicted efficacy |
Observed efficacy |
|---|---|---|---|---|---|---|
| 291 | Decrease | 10 ±9 | 44 | 38 ± 25 | Sensitive | Sensitive |
| 420 | Decrease | 20 ± 8 | 30 | 40 ± 30 | Sensitive | Sensitive |
| 140 | NA | 50 ± 10 | 57 | 86 ± 23 | Resistant | Resistant |
| 354 | NA | 65 ± 13 | 103 | 100 ± 29 | Resistant | Resistant |
| 421 | No change | 70 ± 1 | 82 | 73 ± 50 | Resistant | Resistant |
| 253 | NA | 100 ± 17 | 41 | 71 ± 29 | Resistant | Sensitive |
| 294 | Increase | 129 ± 11 | 86 | 206 ± 110 | Resistant | Resistant |
| 247 | Increase | 152 ± 6 | 83 | 80 ± 5 | Resistant | Resistant |
| 215 | NA | 373 ± 23 | 105 | 88 ± 25 | Resistant | Resistant |
Abbreviations: IHC, immunohistochemistry; NA, not available; WB; western blot.
All assays were repeated twice. The protein-based assay failed to yield sufficient material for analysis four of nine and five of nine times, respectively; the mRNA-based assay rendered adequate material nine of nine times in both repeats. Of the cases tested, two showed lower than 25% cyclin B1 mRNA levels compared with the control (average ± s.d., 10±9% and 20±8%, respectively), four had either a minor decrement or no change (50 ± 10%, 65 ± 13%, 70 ± 1%, and 100 ± 17%, respectively), and three presented an increment (129 ± 11%, 152 ± 6% and 373 ± 23%, respectively).
Figure 5.
Evaluation of the ex vivo assay in the pancreatic cancer xenograft bank. (a) Western blot and reverse transcriptase PCR (RT–PCR) analysis of cyclin B1 ex vivo in four of the nine selected cases that were prospectively assessed. The ex vivo assay faithfully correlated with the efficacy of the drug after it was administered to the xenografted tumors. Below, growth curves of two selected examples. (b) Plot of the nuclear cyclin B1-staining index (calculated as intensity of staining (0–3) multiplied by the percentage cells staining positive). (c) Correlation between the relative tumor growth (in T/C) and the cyclin B1 protein expression by immunohistochemistry (IHC; in % normalized to control). There was a significant direct correlation between tumor growth inhibition and changes in cyclin B1; cases with higher tumor inhibition after 28 days of ON 01910.Na showed decreases in nuclear cyclin B1. Error bars represent s.e.; asterisk indicates P<0.05 (Student's t-test) compared with control.
Correlation between cyclin B1 ex vivo with IHC and activity
Eight tumors per case (four controls and four treated) were blindly assessed by IHC. Baseline cyclin B1 levels did not correlate with activity, as the four cases with the lowest level corresponded with the two more sensitive and two more resistant cases. However, the dynamics of cyclin B1 did correlate with activity, as the only two cases where a meaningful (>50%) decrease was observed after 28 days of treatment were those from the two cases predicted sensitive (291 and 420; Figure 5b); in the third case that was sensitive (253), cyclin B1 was 70% of baseline. Globally, there was a significant correlation between tumor growth inhibition and changes in cyclin B1; cases with higher tumor inhibition after 28 days of ON 01910.Na showed higher decrease in nuclear cyclin B1.
Discussion
In this study, we evaluated a novel mitotic inhibitor against in vitro and in vivo models of pancreatic cancer. This agent had relevant antitumor activity compared to other agents tested in this model, including gemcitabine and erlotinib. We tested a panel of related candidate markers and selected one (cyclin B1) that could be incorporated to ex vivo testing to predict the treatment efficacy before a patient is exposed to the drug. The correlation between cyclin B1 in all three in vitro, ex vivo and in vivo analyses was remarkable, and the assay permitted the correct identification of two sensitive cases. The assay was imperfect, although one case that was ultimately sensitive was coded as resistant.
ON 01910.Na is a small molecule that inhibits mitotic progression by causing disruption of the centrosomal and spindle architecture in cancer cells and has shown preclinical antitumor activity (Gumireddy et al., 2005). Given the complexities of cancer, a given agent is usually only active in a fraction of the patients with a given disease; therefore, identifying the factors determining this benefit will not only prevent unnecessary treatments but, if discovered in early stages of drug development, also prove instrumental in its ultimate success (Slamon et al., 2001). In this case, we explored the potential value of a panel of related markers, of which cyclin B1 proved to be informative and feasible in small samples, similar to those obtained in a clinical scenario. ON 01910.Na is completing the first phase of clinical development (Jimeno et al., 2008).
Cyclin B1 has a key role in the regulation of cell cycle progression, but its relevance in cancer is not completely understood. Decrease in cyclin B1 has been linked to G2/M arrest (Dvory-Sobol et al., 2006), and stable gene silencing of cyclin B1 in HeLa cells increased susceptibility to paclitaxel and lead to growth arrest in vivo (Yuan et al., 2006). From a different perspective, elevated levels of cyclin B1 have been associated with resistance to radiation and increased risk of locoregional recurrence and metastasis in head-and-neck squamous (Hassan et al., 2002) and colorectal cancers (Korenaga et al., 2002). In our model, given that (1) the decrement in cyclin B1 did not induce cell kill, (2) the change in cyclin B1 was selectively elicited by ON 01910.Na, but not by gemcitabine and (3) its downregulation did not increase the sensitivity to mitotic inhibition, we conclude that in these pancreatic models, cyclin B1 is a marker of ON-01910.Na-induced G2/M arrest, and not a per se sustainer of neither tumor growth nor drug resistance. However, it has been reported that cyclin B1 increases may override the G2/M checkpoint (Park et al., 2000), so it could be hypothesized that certain cells could become resistant to ON 01910.Na because they can bypass its principal effect. In addition, the data suggest that cyclin B1 is a dynamic, rather than a static marker, as baseline levels were not informative. Indeed, proportional change, rather than absolute baseline levels, is correlated with activity, as has been suggested with other pharmacodynamic markers (Jimeno et al., 2005). The two tumors where a greater downregulation of ex vivo cyclin B1 was documented had a similar decrease in cyclin B1 protein in the post-treatment tumors. Thus, the ex vivo assay not only identified cases sensitive to ON 01910.Na, but also replicated the pharmacodynamic events occurring after in vivo exposure.
Cyclin B1 and Plk1 were found to be closely interrelated, but with differences between the sensitive and the resistant strains. Whereas siRNA-mediated downregulation of Plk1 induced the upregulation of cyclin B1 mRNA in both cell lines, this was only followed by the increased protein translation in HS766T, but not in MiaPaca2. After assessing the effects of siRNA cyclin B1 and Plk1 in conjunction with treatment with gemcitabine and ON 01910.Na, it can be concluded that cyclin B1 had no role in determining sensitivity status, nor did have an effect on its own. However, Plk1 downregulation had an effect in HS766T, but not in MiaPaca2, confirming that in the former cell line, cell growth is Plk1-dependent. There was also some degree of additive effect when Plk1 siRNA and ON 01910.Na were given together. This suggests that si-Plk1- and ON 01910.Na-mediated effects may impinge on both parallel and connected pathways in mitotic progression. The Plk1 pathway has been implicated in asymmetric cell division (Budirahardja and Gonczy, 2008) used by neuroblasts and progenitor cells (Gonczy, 2008) that are linked to chemotherapy resistance.
From a pharmacological standpoint, it is relevant to note that concentrations that achieved 90% in vitro inhibition only induced 50% growth inhibition in vivo, which could be a consequence of overprediction from the in vitro tests of pharmacokinetic (PK) issues such as drug protein binding. Interestingly, the in vivo intratumor drug concentrations are in range with clinical PK data (Jimeno et al., 2008).
This study highlights the potential value of an FNA-based ex vivo assay for the prediction of efficacy to targeted therapies (Hidalgo et al., 2006). If validated clinically, this approach may have implications in drug development, as it permits identifying which patients may obtain a benefit before receiving the drug. A key to successfully develop a clinical assay is choosing an end point that can be tested in a reasonably large proportion of the potential patients. Although different markers have been linked to Plk1 inhibition (phospho-CDC25C (Gumireddy et al., 2005), phospho-H3 (Schmidt et al., 2006) or mitotic protein monoclonal 2 (MPM-2) (Schmidt et al., 2006)), these are dependent on western blotting, require relatively large amounts of material and are not fully quantitative. mRNA expression analysis by RT–PCR, which is reproducible and fully quantitative, requires less material, and thus has a higher potential for miniaturization. It is important to stress that only profound variations in cyclin B1 levels indicated potential sensitivity to ON 01910.Na. The suggested fourfold reduction in cyclin B1 threshold selected only one of five prospective cases, but enrichment strategies require specific rather than sensitive tests (Slamon et al., 2001).
To test this drug and its putative biomarkers, we used a patient-direct pancreas cancer xenograft model as a platform (Rubio-Viqueira et al., 2006). Before entering clinical development, agents are usually tested against high-passage commercially obtained cell lines and xenografts established from these lines. It is unclear how representative those models are of the biology of pancreas cancer. It has shown to be feasible, with a high engraftment rate, but more importantly, for new drug development, it was stable over time and passages, both genetically and from the perspective of drug sensitivity (Jimeno et al., 2008). As opposed to cell line-derived in vivo models, in these xenografts (as in the clinic), regressions occur infrequently. Therefore, growth retardation is the primary end point to determine the efficacy of tested agents.
We conclude that ON 01910.Na is a promising anticancer agent, shown by its equipotency with gemcitabine against pancreas cancer cell lines. The in vitro and in vivo activities correlated well, and ON 01910.Na had meaningful single-agent activity. The data also indicate that a cyclin B1-based ex vivo assay is able to identify tumors more likely to benefit from ON 01910.Na. Thus, ON 01910.Na will be tested in patients with pancreatic tumors using the developed cyclin B1 ex vivo assay as a potential marker of activity.
Materials and methods
Drugs
ON 01910.Na (Onconova Therapeutics Inc., Lawrenceville, NJ, USA) was dissolved in phosphate-buffered solution. Gemcitabine (Elli Lilly, Indianapolis, IN, USA) was dissolved in phosphate-buffered solution .
In vivo growth inhibition studies
Six-week-old female athymic nude mice (Harlan, IN, USA) were used. The research protocol was approved by the Johns Hopkins University Animal Care and Use Committee, and animals were maintained in accordance with the guidelines of the American Association of Laboratory Animal Care. The xenografts were generated and treated with control (vehicle) and ON 01910.Na (250 mg/kg 5 week intraperitonealy for 28 days) as described (Rubio-Viqueira et al., 2006).
Pharmacokinetic analyses
Mice bearing HS766T tumors were divided into two groups—two mice served as controls, four received five doses daily. Plasma, liver and tumor tissues were harvested 1 h after the last treatment. Mouse plasma samples containing ON 01910.Na were diluted 1:10 in human plasma before protein precipitation using acetonitrile. Liver or tumor tissue homogenates were prepared at a concentration of 200 mg/ml in phosphate-buffered solution and diluted 1:10 in human plasma. The analytes of interest were monitored by Micromass Quattro LC triple-quadrupole mass spectrometric detector (Beverly, MA, USA) with electrospray positive ionization (Li et al., 2007).
FNA and ex vivo rapid molecular assay
Fine-needle aspiration biopsies were performed according to standard cytopathologic practice under inhaled general anesthesia (isofluorane) using 10cc syringes and 25-gauge needles. During each FNA procedure, the first pass was smeared onto glass slides and used for morphologic analysis and 3–4 subsequent passes were used for viable cell harvesting. FNA material from untreated tumors of HS766T, MiaPaca2 and nine direct pancreatic cancer xenografts (140, 215, 247, 253, 291, 294, 354, 420 and 421) was aliquoted in growth media, seeded in 6-well plates and treated in duplicates for 6 h with growth media (GM) and GM plus ON 01910.Na at a concentration of 10 μM. The experiment was repeated twice in all the cases.
Immunohistochemical analysis
In all, 5 μm sections were used for cyclin B1 staining. After rehydrating, antigen retrieval was performed using a target retrieval solution (S1700; Dako, Carpinteria, CA, USA) heated for 40 min. Endogenous peroxidase activity was quenched by incubation in 3% hydrogen peroxide. Next, the cyclin B1 primary antibody (14-6712; eBioscience, San Diego, CA, USA) was diluted 1:100 and incubated at room temperature for 1 h, followed by an antirabbit secondary antibody (Envision+: Dako) for 30 min and DAB (K3468; Dako) for 5 min. Between steps, slides were washed in phosphate-buffered solution with 1% Tween 20. Four tumors per treatment group were blindly analysed.
Additional conventional/standard techniques as Supplementary Methods.
Supplementary Material
Acknowledgements
This study was supported in part by the Viragh Foundation and Onconova Inc.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Budirahardja Y, Gonczy P. PLK-1 asymmetry contributes to asynchronous cell division of C. elegans embryos. Development. 2008;135:1303–1313. doi: 10.1242/dev.019075. [DOI] [PubMed] [Google Scholar]
- Dvory-Sobol H, Cohen-Noyman E, Kazanov D, Figer A, Birkenfeld S, Madar-Shapiro L, et al. Celecoxib leads to G2/M arrest by induction of p21 and downregulation of cyclin B1 expression in a p53-independent manner. Eur J Cancer. 2006;42:422–426. doi: 10.1016/j.ejca.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- Gumireddy K, Reddy MV, Cosenza SC, Boominathan R, Baker SJ, Papathi N, et al. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell. 2005;7:275–286. doi: 10.1016/j.ccr.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Hassan KA, Ang KK, El-Naggar AK, Story MD, Lee JI, Liu D, et al. Cyclin B1 overexpression and resistance to radiotherapy in head and neck squamous cell carcinoma. Cancer Res. 2002;62:6414–6417. [PubMed] [Google Scholar]
- Hidalgo M, Amador ML, Jimeno A, Mezzadra H, Patel P, Chan A, et al. Assessment of gefitinib- and CI-1040-mediated changes in epidermal growth factor receptor signaling in HuCCT-1 human cholangiocarcinoma by serial fine needle aspiration. Mol Cancer Ther. 2006;5:1895–1903. doi: 10.1158/1535-7163.MCT-05-0525. [DOI] [PubMed] [Google Scholar]
- Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Jimeno A, Kulesza P, Kincaid E, Bouaroud N, Chan A, Forastiere A, et al. C-fos assessment as a marker of anti-epidermal growth factor receptor effect. Cancer Res. 2006;66:2385–2390. doi: 10.1158/0008-5472.CAN-05-2882. [DOI] [PubMed] [Google Scholar]
- Jimeno A, Li J, Messersmith WA, Laheru D, Rudek MA, Maniar M, et al. Phase I Study of ON 01910.Na, a novel modulator of the polo-like kinase 1 pathway, in adult patients with solid tumours. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.17.9788. e-pub ahead of print, 27 October 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, Rubio-Viqueira B, Amador ML, Oppenheimer D, Bouraoud N, Kulesza P, et al. Epidermal growth factor receptor dynamics influences response to epidermal growth factor receptor targeted agents. Cancer Res. 2005;65:3003–3010. doi: 10.1158/0008-5472.CAN-04-3586. [DOI] [PubMed] [Google Scholar]
- Jimeno A, Tan A, Garrido-Laguna I, Solomo A, Hruban R, Schulick R, et al. A prospective clinical validation of a direct xenograft pancreatic cancer approach; 2008 ASCO Annual Meeting, oral presentation; abstract 4500. [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenaga D, Takesue F, Yasuda M, Honda M, Nozoe T, Inutsuka S. The relationship between cyclin B1 overexpression and lymph node metastasis in human colorectal cancer. Surgery. 2002;131:S114–S120. doi: 10.1067/msy.2002.119362. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao M, Jimeno A, He P, Ramana Reddy MV, Hidalgo M, et al. Validation and implementation of a liquid chromatography/tandem mass spectrometry assay to quantitate ON 01910.Na, a mitotic progression modulator, in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:198–204. doi: 10.1016/j.jchromb.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 1998;10:776–783. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- Park M, Chae HD, Yun J, Jung M, Kim YS, Kim SH, et al. Constitutive activation of cyclin B1-associated cdc2 kinase overrides p53-mediated G2-M arrest. Cancer Res. 2000;60:542–545. [PubMed] [Google Scholar]
- Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- Samson DJ, Seidenfeld J, Ziegler K, Aronson N. Chemotherapy sensitivity and resistance assays: a systematic review. J Clin Oncol. 2004;22:3618–3630. doi: 10.1200/JCO.2004.04.077. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Hofmann HP, Sanders K, Sczakiel G, Beckers TL, Gekeler V. Molecular alterations after Polo-like kinase 1 mRNA suppression versus pharmacologic inhibition in cancer cells. Mol Cancer Ther. 2006;5:809–817. doi: 10.1158/1535-7163.MCT-05-0455. [DOI] [PubMed] [Google Scholar]
- Schrag D, Garewal HS, Burstein HJ, Samson DJ, Von Hoff DD, Somerfield MR. American Society of Clinical Oncology Technology Assessment: chemotherapy sensitivity and resistance assays. J Clin Oncol. 2004;22:3631–3638. doi: 10.1200/JCO.2004.05.065. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol. 2000;2:672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- Yuan J, Kramer A, Matthess Y, Yan R, Spankuch B, Gatje R, et al. Stable gene silencing of cyclin B1 in tumor cells increases susceptibility to taxol and leads to growth arrest in vivo. Oncogene. 2006;25:1753–1762. doi: 10.1038/sj.onc.1209202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.