Abstract
Purpose
Allogeneic hematopoietic cell transplantation (HCT) is curative but is associated with life-threatening complications. Most deaths occur within the first 2 years after transplantation. In this report, we examine long-term survival in 2-year survivors in the largest cohort ever studied.
Patients and Methods
Records of 10,632 patients worldwide reported to the Center for International Blood and Marrow Transplant Research who were alive and disease free 2 years after receiving a myeloablative allogeneic HCT before 2004 for acute myelogenous or lymphoblastic leukemia, myelodysplastic syndrome, lymphoma, or severe aplastic anemia were reviewed.
Results
Median follow-up was 9 years, and 3,788 patients had been observed for 10 or more years. The probability of being alive 10 years after HCT was 85%. The chief risk factors for late death included older age and chronic graft-versus-host disease (GVHD). For patients who underwent transplantation for malignancy, relapse was the most common cause of death. The greatest risk factor for late relapse was advanced disease at transplantation. Principal risk factors for nonrelapse deaths were older age and GVHD. When compared with age, sex, and nationality-matched general population, late deaths remained higher than expected for each disease, with the possible exception of lymphoma, although the relative risk generally receded over time.
Conclusion
The prospect for long-term survival is excellent for 2-year survivors of allogeneic HCT. However, life expectancy remains lower than expected. Performance of HCT earlier in the course of disease, control of GVHD, enhancement of immune reconstitution, less toxic regimens, and prevention and early treatment of late complications are needed.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is a common treatment for a variety of malignant and benign hematologic diseases. Advances in transplantation practices and supportive care have led to improved outcomes and an increasing number of long-term HCT survivors.
Most deaths after HCT occur within the first 2 years as a result of relapse, acute or chronic graft-versus-host disease (GVHD), infection, or other acute or subacute toxicities of HCT. Death beyond 2 years is infrequent. In 1999, the Late Effects Working Committee of the Center for International Blood and Marrow Transplant Research (CIBMTR) undertook a study to determine the long-term survival of 2-year allogeneic HCT survivors and relative mortality rates compared with the general population.1 In that analysis of 6,691 survivors who underwent transplantation for severe aplastic anemia (SAA), acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), or chronic myelogenous leukemia (CML), who were alive and free of recurrent disease at 2 years after HCT, survival at 5 years was 89% (95% CI, 88% to 90%). The risk of death of survivors who underwent transplantation for SAA had returned to that of the normal population by the sixth year but had remained higher than that of the normal population matched for age, sex, ethnicity, and nationality throughout the study (5 years later) for patients who underwent transplantation for AML, ALL, or CML. Recurrent leukemia was the chief cause of death for patients who underwent transplantation for leukemia, but chronic GVHD was the chief cause of death for patients who underwent transplantation for SAA. Advanced disease before transplantation and active chronic GVHD were risk factors for late death.
In the decade since that analysis, there have been many changes in transplantation practices. There are greater numbers of survivors. The duration of long-term follow-up has extended. Accordingly, we conducted another analysis at this time to investigate new insights into long-term survival after allogeneic HCT. Our objectives were to determine long-term survival, evaluate risk factors for late mortality, and compare survival with that of the general population.
PATIENTS AND METHODS
CIBMTR
Data reported to the CIBMTR by 318 centers worldwide formed the basis of this study. The CIBMTR is a group of nearly 500 HCT centers worldwide that contribute detailed data on all the allogeneic HCT performed at their centers. The quality of the data is monitored by computerized checks for errors, review of data by physicians, and periodic on-site audits of patient records of participating centers.
Patients
Survivors of first allogeneic HCT who received myeloablative regimens for AML, ALL, myelodysplastic syndrome (MDS), lymphoma, and SAA with related or unrelated donors of all ages who were alive and disease free ≥ 2 years post HCT with follow-up data reported to the CIBMTR were considered for study inclusion. Follow-up information regarding both survival and disease control was required. Recipients of identical twin transplantations and umbilical cord blood transplantations were excluded.
The records of 31,818 potentially eligible patients who underwent transplantation between January 1980 and December 2003 were reviewed. Patients who, within 2 years after transplantation, experienced relapse or died (n = 15,543), received a second transplantation (n = 584), or were lost to follow-up (n = 417) were excluded. In addition, patients who underwent transplantation at centers whose team follow-up completeness index, which is the ratio of total observed person-time to the potential person-time of follow-up,2 was less than 80% at 5 years were excluded (n = 4,615) to avoid bias of selective reporting. This left a final study population of 10,632 patients. The overall survival for 4,615 patients excluded from centers with incomplete follow-up was 91% at 5 years (compared with 92% for patients included in the analysis; P = .62) and was 80% at 10 years (compared with 85%; P = .02).
Definitions
Relapse was defined as disease recurrence. The event was summarized by the cumulative incidence estimate, and death in remission was the competing risk. Nonrelapse mortality (NRM) was defined as death in continuous remission. The event was summarized by the cumulative incidence estimate, for which relapse was the competing risk. Disease-free survival was defined as survival without death or disease relapse. Overall survival (OS) was defined as absence of death as a result of any cause. The event was summarized by a survival curve.3 Patients who were alive were censored at the time of last contact. All probabilities were calculated from the date of transplantation, on the condition that the patient was disease free for at least 2 years after transplantation. Relative mortality was defined as the relative or excess risk of death in the transplantation cohort compared with general populations matched on sex, age, country, and ethnicity (for US population only).4 The causes of late deaths were categorized hierarchically.5 Deaths in those who had experienced relapse of disease at any time were considered to be caused by relapse. Deaths in patients with active GVHD or during active treatment for GVHD were deemed a result of GVHD, even if other complications occurred. Deaths as a result of infection included only those infections that occurred without active GVHD or GVHD therapy. HLA-matching status for recipients of unrelated donor HCT was classified as well matched, partially matched, or mismatched, according to previously published criteria.6
Statistical Analysis
The primary objective of this study was to determine overall mortality in HCT survivors who were free of disease recurrence at 2 years. Patients with different diseases were analyzed separately because of differences in disease biology, the likelihood of relapse, pretransplantation treatment, and conditioning regimens. The probabilities of disease-free survival and OS were estimated by the Kaplan-Meier method. Estimates of the hazard rate for all the patients and of the hazard rates according to the stage of diseases were obtained with the use of the Nelson-Aalen estimator.3
Estimates of relative mortality were performed as described by Andersen and Vaeth,4 taking into account differences among patients with regard to age, sex, ethnicity (in the United States only), and nationality. Relative mortality with respect to transplantation recipient was the relative risk of dying at a given time after HCT compared with a person of similar age, sex, and nationality in the general population. Rates for which the 95% CI for relative mortality included 1.0 were not considered significantly different from the rates in a normal population.
Potential risk factors for late death were analyzed by Cox regression models. These included geographic region, age at HCT, sex, Karnofsky performance score at HCT, disease risk, time from diagnosis to HCT, donor source, HLA match, stem-cell source, conditioning regimen, dose of total-body irradiation (TBI), GVHD prophylaxis regimen, use of T-cell depletion, year of HCT, and acute and chronic GVHD within the first 2 years after HCT. Multivariate models also considered center effects. All potential risk factors were checked with time-dependent covariates to ensure that assumptions of proportionality were met. Factors were then tested for their association with late death by means of forward stepwise selection of variables.3
RESULTS
The characteristics of the final study population are in Table 1. The median follow-up intervals were 9 years (range, 2-27 years) for AML, 9 years (range, 2 to 26 years) for ALL, 8 years (range, 2-20 years) for MDS, 9 years (range, 2 to 27 years) for lymphoma, and 9 years (range, 2-31 years) for SAA. The percentages of patients observed for 10 years or longer after HCT were 37% for AML, 35% for ALL, 24% for MDS, 30% for lymphoma, and 40% for SAA.
Table 1.
Characteristics of Patients Receiving Myeloablative Allogeneic Transplantations Through 2003 Surviving for at Least 2 years in Remission Post Transplantation Reported to the CIBMTR
| Variable | AML |
ALL |
MDS |
Lymphoma |
SAA |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 4,017 | 2,895 | 930 | 619 | 2,171 | |||||
| No. of centers | 280 | 267 | 203 | 157 | 243 | |||||
| Age, years | ||||||||||
| Median | ||||||||||
| Range | 28 | < 1-66 | 16 | < 1-64 | 34 | < 1-66 | 34 | 2-61 | 18 | < 1-66 |
| < 10 | 466 | 12 | 918 | 32 | 160 | 17 | 46 | 7 | 453 | 21 |
| 10-19 | 742 | 18 | 932 | 32 | 125 | 13 | 84 | 14 | 805 | 37 |
| 20-29 | 937 | 23 | 562 | 19 | 107 | 12 | 122 | 20 | 563 | 26 |
| 30-39 | 925 | 23 | 294 | 10 | 198 | 21 | 160 | 26 | 245 | 11 |
| 40-49 | 711 | 18 | 150 | 5 | 199 | 21 | 163 | 26 | 84 | 4 |
| ≥ 50 | 236 | 6 | 39 | 1 | 141 | 15 | 44 | 7 | 21 | 1 |
| Male | 2,102 | 52 | 1,826 | 63 | 508 | 55 | 392 | 63 | 1,303 | 60 |
| Region of transplantation centers | ||||||||||
| United States | 1,390 | 35 | 1,025 | 35 | 470 | 51 | 300 | 48 | 654 | 30 |
| Canada | 208 | 5 | 115 | 4 | 59 | 6 | 62 | 10 | 71 | 3 |
| Europe | 1,742 | 43 | 1,289 | 45 | 259 | 28 | 178 | 29 | 729 | 34 |
| Asia | 177 | 4 | 109 | 4 | 32 | 3 | 31 | 5 | 122 | 6 |
| Australia/New Zealand | 233 | 6 | 208 | 7 | 46 | 5 | 25 | 4 | 110 | 5 |
| Mideast/Africa | 156 | 4 | 98 | 3 | 28 | 3 | 18 | 3 | 109 | 5 |
| Central/South America | 111 | 3 | 51 | 2 | 36 | 4 | 5 | 1 | 376 | 17 |
| Karnofsky score ≥ 90 | 3,170 | 81 | 2,361 | 83 | 619 | 69 | 442 | 72 | 1,153 | 56 |
| Missing | 115 | 63 | 29 | 9 | 122 | |||||
| Year of transplantation | ||||||||||
| ≤ 1985 | 457 | 11 | 372 | 13 | 20 | 2 | 31 | 5 | 459 | 21 |
| 1986-1990 | 908 | 23 | 649 | 22 | 121 | 13 | 108 | 17 | 373 | 17 |
| 1991-1995 | 1,142 | 28 | 788 | 27 | 244 | 26 | 181 | 29 | 555 | 26 |
| 1996-2000 | 1,008 | 25 | 767 | 26 | 360 | 39 | 202 | 33 | 519 | 24 |
| 2001-2003 | 502 | 12 | 319 | 11 | 185 | 20 | 97 | 16 | 265 | 12 |
| Disease risk prior to transplantation* | ||||||||||
| AML, ALL, early | 2,683 | 67 | 1,271 | 44 | — | — | — | |||
| AML, ALL, intermediate | 677 | 17 | 1,150 | 40 | — | — | — | |||
| AML, ALL, late | 640 | 16 | 469 | 16 | — | — | — | |||
| MDS, treated | — | — | 377 | 51 | — | — | ||||
| MDS, untreated | — | — | 361 | 49 | — | — | ||||
| Missing | 17 | 5 | 192 | |||||||
| Lymphoma histology | ||||||||||
| Hodgkin's lymphoma | — | — | — | 25 | 4 | — | ||||
| Follicular | — | — | — | 164 | 26 | — | ||||
| DLCL/immunoblastic | — | — | — | 63 | 10 | — | ||||
| Lymphoblastic/Burkitt's/Burkitt-like | — | — | — | 81 | 13 | — | ||||
| Mantle cell | — | — | — | 26 | 4 | — | ||||
| Other | — | — | — | 260 | 42 | — | ||||
| Conditioning regimen | ||||||||||
| Malignant disease | ||||||||||
| Cy + TBI ± other | 2,135 | 53 | 2,154 | 74 | 417 | 45 | 472 | 76 | — | |
| Bu + Cy ± other (no TBI) | 1,498 | 37 | 248 | 9 | 437 | 47 | 85 | 14 | — | |
| Other or unknown | 384 | 10 | 493 | 17 | 76 | 8 | 62 | 10 | — | |
| SAA | ||||||||||
| Cy + TBI ± other | — | — | — | — | 364 | 17 | ||||
| Bu + Cy ± other (no TBI) | — | — | — | — | 219 | 10 | ||||
| Cy ± other (No Bu, no TBI) | — | — | — | — | 1,496 | 69 | ||||
| Other or unknown | — | — | — | — | 92 | 4 | ||||
| Use of TBI | 2,411 | 60 | 2621 | 91 | 443 | 48 | 513 | 83 | 368 | 17 |
| TBI dose, Gy | ||||||||||
| No TBI | 1,606 | 40 | 274 | 10 | 487 | 52 | 106 | 17 | 1,803 | 83 |
| < 12 | 819 | 20 | 651 | 23 | 110 | 12 | 118 | 19 | 243 | 11 |
| ≥ 12 | 1,582 | 39 | 1,953 | 68 | 331 | 36 | 392 | 64 | 124 | 6 |
| Missing | 10 | 17 | 2 | 3 | 1 | |||||
| Interval from diagnosis to HCT, months | ||||||||||
| Median | 6 | 12 | 7 | 14 | 3 | |||||
| Range | < 1-133 | 1-238 | < 1-252 | 1-192 | < 1-316 | |||||
| < 6 | 2,040 | 51 | 833 | 29 | 373 | 40 | 98 | 16 | 1,471 | 68 |
| 6-12 | 1,082 | 27 | 610 | 21 | 265 | 29 | 175 | 28 | 279 | 13 |
| ≥ 12 | 889 | 22 | 1,447 | 50 | 288 | 31 | 344 | 56 | 413 | 19 |
| Missing | 6 | 5 | 4 | 2 | 8 | |||||
| Donor type | ||||||||||
| HLA-identical sibling | 3,029 | 75 | 1,881 | 65 | 481 | 52 | 494 | 80 | 1,758 | 81 |
| Other related | 210 | 5 | 217 | 7 | 44 | 5 | 21 | 3 | 117 | 5 |
| Unrelated | 778 | 19 | 797 | 28 | 405 | 44 | 104 | 17 | 296 | 14 |
| Degree of HLA match† | ||||||||||
| HLA-identical sibling | 3,029 | 75 | 1,881 | 65 | 481 | 52 | 494 | 80 | 1,758 | 81 |
| Well matched | 266 | 7 | 226 | 8 | 182 | 20 | 41 | 7 | 96 | 4 |
| Partially matched | 287 | 7 | 297 | 10 | 132 | 14 | 45 | 7 | 85 | 4 |
| Mismatched | 327 | 8 | 376 | 13 | 96 | 10 | 31 | 5 | 170 | 8 |
| Unknown | 108 | 3 | 115 | 4 | 39 | 4 | 8 | 1 | 62 | 3 |
| Graft type | ||||||||||
| BM | 3,414 | 85 | 2,647 | 91 | 747 | 80 | 458 | 74 | 2,056 | 95 |
| PBSC | 603 | 15 | 248 | 9 | 183 | 20 | 161 | 26 | 115 | 5 |
| GVHD prophylaxis | ||||||||||
| T-cell depletion (ex vivo) | 491 | 12 | 443 | 15 | 130 | 14 | 109 | 18 | 115 | 5 |
| Tac + MTX ± other | 190 | 5 | 105 | 4 | 74 | 8 | 48 | 8 | 46 | 2 |
| Tac ± other | 39 | 1 | 9 | < 1 | 17 | 2 | 18 | 3 | 7 | < 1 |
| Cyclosporine + MTX ± other | 2,262 | 56 | 1,520 | 53 | 584 | 63 | 326 | 53 | 1,211 | 56 |
| Cyclosporine ± other | 679 | 17 | 540 | 19 | 100 | 11 | 86 | 14 | 474 | 22 |
| Other/unknown | 356 | 9 | 278 | 10 | 25 | 3 | 32 | 5 | 318 | 15 |
| T-cell depletion (as part of conditioning or GVHD prophylaxis) | ||||||||||
| No T-cell depletion | 3,232 | 80 | 2,209 | 76 | 676 | 73 | 481 | 78 | 1,308 | 60 |
| Ex vivo | 491 | 12 | 443 | 15 | 130 | 14 | 109 | 18 | 115 | 5 |
| In vivo‡ | 294 | 7 | 243 | 8 | 124 | 13 | 29 | 5 | 748 | 34 |
| Grades II to IV acute GVHD§ | 1,565 | 39 | 1,328 | 46 | 419 | 45 | 260 | 42 | 545 | 25 |
| Chronic GVHD§ | 1,844 | 47 | 1,172 | 41 | 505 | 56 | 307 | 50 | 701 | 33 |
| Missing | 107 | 67 | 22 | 11 | 62 | |||||
| Follow-up of survivors, months | ||||||||||
| Median | 110 | 108 | 92 | 102 | 109 | |||||
| Range | 24-318 | 24-306 | 24-243 | 24-322 | 24-368 | |||||
| Duration of follow-up, years | ||||||||||
| ≥ 3 | 3,761 | 93 | 2,705 | 93 | 846 | 91 | 577 | 93 | 2,032 | 93 |
| ≥ 5 | 3,046 | 76 | 2,183 | 75 | 628 | 68 | 444 | 72 | 1,692 | 78 |
| ≥ 10 | 1,478 | 37 | 1,022 | 35 | 226 | 24 | 188 | 30 | 874 | 40 |
| ≥ 15 | 474 | 12 | 307 | 11 | 39 | 4 | 44 | 7 | 343 | 16 |
NOTE. The follow-up completeness indices at 5 years were 96% for AML, 96% for ALL, 94% for MDS, 95% for lymphoma, and 95% for SAA. The follow-up completeness indices at 10 years were 84% for AML, 83% for ALL, 80% for MDS, 82% for lymphoma, and 83% for SAA.
Abbreviations: CIBMTR, Center for International Blood and Marrow Transplant Research; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; SAA, severe aplastic anemia; DLCL, diffuse large-cell lymphoma; Cy, cyclophosphamide; TBI, total-body irradiation; Bu, bufsulfan; HCT, hematopoietic cell transplantation; BM, bone marrow; PBSC, peripheral-blood stem cell; GVHD, graft-versus-host disease; Tac, tacrolimus; MTX, methotrexate.
Early-stage acute leukemia is AML or ALL in first complete remission; intermediate-stage leukemia is AML or ALL in second or subsequent complete remission; advanced-stage leukemia is AML or ALL not in remission.
Unrelated donors and other related donors were classified as HLA well matched, partially matched, or mismatched.6
In vivo T-cell depletion included use of anti-thymocyte globulin, alemtuzumab, or anti-CD3 monoclonal antibody.
Within the first 2 years post transplantation.
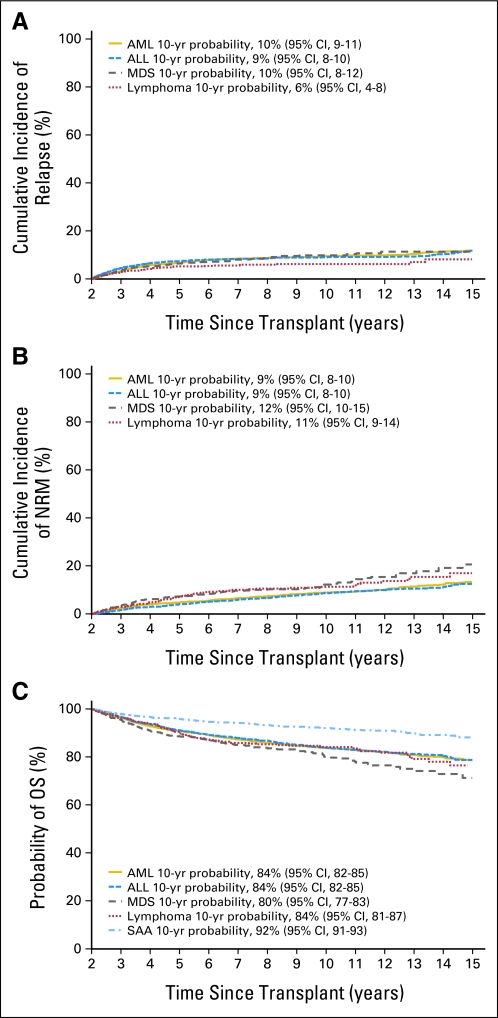
The probabilities for survival at 10 years after HCT were 84% for AML, 84% for ALL, 80% for MDS, 84% for lymphoma, and 92% for SAA (Fig 1; Data Supplement). For patients who underwent transplantation for malignancy, cumulative incidences of relapse at 10 years after HCT were 10% for AML, 9% for ALL, 10% for MDS, and 6% for lymphoma (Fig 1). Similarly, for patients who underwent transplantation for malignancy, cumulative incidences of NRM 10 years after HCT were 9% for AML, 9% for ALL, 12% for MDS, and 11% for lymphoma (Fig 1). Probabilities of disease-free survival at 10 years were 82% for AML, 82% for ALL, 78% for MDS, and 82% for lymphoma.
Fig 1.
(A) Cumulative incidence of relapse for patients undergoing myeloablative allogeneic transplantations surviving at least 2 years in remission post transplantation, by disease group. (B) Cumulative incidence of nonrelapse mortality (NRM) for patients undergoing myeloablative allogeneic transplantations surviving at least 2 years in remission post transplantation, by disease group. (C) Probability of overall survival (OS) for patients undergoing myeloablative allogeneic transplantations surviving at least 2 years in remission post transplantation, by disease group. AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; SAA, severe aplastic anemia; yr, year.
Primary causes of death are listed in Table 2. Recurrent disease was the most common cause of death for patients who underwent transplantation for malignancy. Chronic GVHD, infection, organ toxicity, and second cancers were the next most frequent causes of death for patients who underwent transplantation for malignancy. The most common causes of death for patients who underwent transplantation for SAA were GVHD, infection, organ toxicity, and second cancers.
Table 2.
Causes of Death of Patients Receiving Myeloablative Allogeneic Transplantations and Surviving at Least 2 Years in Remission Post Transplantation After Undergoing Transplantation Through 2003 As Reported to the CIBMTR
| Cause of Death | Years After Transplantation by Disease |
|||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AML |
ALL |
MDS |
Lymphoma |
SAA |
||||||||||||||||||||||||||
| 2-4 |
5-9 |
≥ 10 |
2-4 |
5-9 |
≥ 10 |
2-4 |
5-9 |
≥ 10 |
2-4 |
5-9 |
≥ 10 |
2-4 |
5-9 |
≥ 10 |
||||||||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Recurrent or persistent disease | 162 | 47 | 81 | 43 | 11 | 15 | 132 | 55 | 48 | 36 | 11 | 23 | 33 | 34 | 16 | 43 | 3 | 17 | 15 | 27 | 7 | 28 | 2 | 20 | 0 | 0 | 0 | |||
| GVHD | 63 | 18 | 12 | 6 | 7 | 9 | 27 | 11 | 16 | 12 | 4 | 9 | 14 | 14 | 1 | 3 | 0 | 12 | 22 | 1 | 4 | 1 | 10 | 21 | 25 | 12 | 23 | 2 | 6 | |
| Infection | 45 | 13 | 17 | 9 | 4 | 5 | 27 | 11 | 11 | 8 | 2 | 4 | 15 | 15 | 4 | 11 | 3 | 17 | 8 | 15 | 4 | 16 | 0 | 17 | 20 | 6 | 11 | 8 | 24 | |
| Organ failure | 19 | 5 | 21 | 11 | 8 | 11 | 23 | 10 | 11 | 8 | 5 | 11 | 13 | 13 | 3 | 8 | 4 | 22 | 10 | 18 | 6 | 24 | 1 | 10 | 13 | 16 | 10 | 19 | 4 | 12 |
| Interstitial pneumonitis | 5 | 1 | 3 | 2 | 0 | 3 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 2 | 4 | 1 | 4 | 0 | 2 | 2 | 0 | 0 | ||||||
| Secondary malignancy | 14 | 4 | 19 | 10 | 7 | 9 | 11 | 5 | 18 | 14 | 5 | 11 | 3 | 3 | 2 | 5 | 2 | 11 | 1 | 2 | 0 | 1 | 10 | 6 | 7 | 7 | 13 | 4 | 12 | |
| Hemorrhage | 6 | 2 | 0 | 1 | 1 | 1 | < 1 | 0 | 2 | 4 | 2 | 2 | 1 | 3 | 0 | 0 | 0 | 1 | 10 | 4 | 5 | 5 | 9 | 0 | ||||||
| Graft rejection | 1 | < 1 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 6 | 3 | 6 | 1 | 3 | |||||||||
| Other causes* | 7 | 2 | 6 | 3 | 1 | 1 | 3 | 1 | 6 | 5 | 0 | 5 | 5 | 2 | 5 | 0 | 1 | 2 | 1 | 4 | 0 | 5 | 6 | 0 | 1 | 3 | ||||
| Unknown | 25 | 7 | 28 | 15 | 35 | 47 | 12 | 5 | 20 | 15 | 17 | 36 | 11 | 11 | 8 | 22 | 6 | 33 | 6 | 11 | 5 | 20 | 4 | 40 | 10 | 12 | 10 | 19 | 14 | 41 |
Abbreviations: CIBMTR, Center for International Blood and Marrow Transplant Research; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; SAA, severe aplastic anemia; GVHD, graft-versus-host disease.
Other causes include accidental death (suicide, n = 3); vascular, not otherwise specified; thromboembolism; thrombotic thrombocytopenic purpura; and other transplantation-related causes.
Risk factors for late deaths obtained from multivariate analyses are listed in Table 3 for the various diseases. For all diseases, older age and the development of chronic GVHD before 2 years were associated with late deaths. Additional factors were also associated with late death for certain diseases. For AML and ALL, more advanced disease before transplantation; for AML, transplantations performed in the United States compared with other certain geographic regions (ie, Canada, Europe, Asia, and Australia/New Zealand); for ALL, use of a peripheral-blood graft; for MDS, acute GVHD and transplantation before 1985; for lymphoma, use of an unrelated donor graft; and for SAA, the occurrence of acute GVHD and a greater interval between diagnosis and transplantation were associated with a greater risk for late death.
Table 3.
Multivariate Analysis of Overall Survival in Patients Receiving Myeloablative Allogeneic Transplantations and Surviving for at Least 2 Years in Remission Post Transplantation After Undergoing Transplantation Through 2003 As Reported to the CIBMTR
| Variable by disease | No. of Patients | Relative Risk | 95% CI | P |
|---|---|---|---|---|
| AML | ||||
| Age, years | < .001* | |||
| 0-14 | 776 | 1.00 | ||
| 15-29 | 1,354 | 1.53 | 1.14 to 2.05 | .004 |
| 30-44 | 1,325 | 2.24 | 1.69 to 2.97 | < .001 |
| ≥ 45 | 531 | 3.21 | 2.35 to 4.37 | < .001 |
| Disease risk | < .001* | |||
| Early | 2,676 | 1.00 | ||
| Intermediate | 673 | 1.53 | 1.23 to 1.89 | < .001 |
| Late | 637 | 1.56 | 1.27 to 1.91 | < .001 |
| History of cGvHD† | < .001* | |||
| No | 2,053 | 1.00 | ||
| Yes | 1,831 | 1.60 | 1.35 to 1.90 | < .001 |
| Unknown | 102 | 1.92 | 1.23 to 2.98 | .004 |
| Region | .001* | |||
| United States | 1,373 | 1.00 | ||
| Canada | 205 | 0.65 | 0.45 to 0.93 | .020 |
| Europe | 1,733 | 0.63 | 0.52 to 0.76 | < .001 |
| Asia | 176 | 0.48 | 0.28 to 0.84 | .011 |
| Australia/New Zealand | 232 | 0.78 | 0.56 to 1.09 | .145 |
| Mideast/Africa | 156 | 0.92 | 0.58 to 1.45 | .714 |
| Central/South America | 111 | 0.85 | 0.51 to 1.42 | .541 |
| ALL | ||||
| Age, years | < .001* | |||
| 0-14 | 1,347 | 1.00 | ||
| 15-44 | 1,436 | 1.72 | 1.38 to 2.15 | < .001 |
| ≥ 45 | 87 | 3.76 | 2.43 to 5.82 | < .001 |
| Disease risk | < .001* | |||
| Early | 1,259 | 1.00 | ||
| Intermediate | 1,144 | 1.25 | 0.99 to 1.58 | .062 |
| Late | 467 | 1.77 | 1.37 to 2.28 | < .001 |
| Graft type | .045* | |||
| BM | 2,625 | 1.00 | ||
| PB | 245 | 1.44 | 1.01 to 2.07 | |
| History of cGvHD† | .003* | |||
| No | 1,642 | 1.00 | ||
| Yes | 1,162 | 1.34 | 1.10 to 1.64 | .004 |
| Unknown | 66 | 1.83 | 1.13 to 2.97 | .015 |
| MDS | ||||
| Age, years | < .001* | |||
| 0-29 | 390 | 1.00 | ||
| 30-54 | 478 | 1.61 | 1.11 to 2.33 | .012 |
| ≥ 55 | 56 | 3.45 | 1.93 to 6.18 | < .001 |
| Year of transplantation | .002* | |||
| ≤ 1985 | 20 | 1.00 | ||
| 1986-1990 | 120 | 0.26 | 0.13 to 0.56 | .001 |
| 1991-1995 | 243 | 0.24 | 0.12 to 0.49 | < .001 |
| 1996-2000 | 357 | 0.28 | 0.14 to 0.56 | < .001 |
| 2001-2003 | 184 | 0.23 | 0.10 to 0.52 | < .001 |
| History of aGvHD II-IV | .020* | |||
| No | 511 | 1.00 | ||
| Yes | 413 | 1.49 | 1.07 to 2.08 | |
| History of cGvHD† | .012* | |||
| No | 402 | 1.00 | ||
| Yes | 500 | 1.65 | 1.13 to 2.40 | .009 |
| Unknown | 22 | 0.59 | 0.18 to 1.97 | .391 |
| Lymphoma | ||||
| Age, years | .002* | |||
| 0-25 | 184 | 1.00 | ||
| ≥ 25 | 430 | 2.47 | 1.39 to 4.40 | |
| Degree of HLA match | .003* | |||
| HLA-identical siblings | 491 | 1.00 | ||
| Unrelated, well matched | 40 | 3.72 | 1.92 to 7.24 | < .001 |
| Unrelated, partially matched | 45 | 1.58 | 0.74 to 3.36 | .238 |
| Unrelated, mismatched | 30 | 0.99 | 0.35 to 2.76 | .980 |
| Unknown | 8 | 2.21 | 0.53 to 9.19 | .274 |
| Negative history of aGvHD II-VI‡ | ||||
| History of cGvHD† | ||||
| No | 219 | 1.00 | ||
| Yes | 129 | 0.71 | 0.37 to 1.38 | .317 |
| Unknown | 6 | 3.40 | 0.98 to 11.9 | .055 |
| Positive history of aGvHD II-IV | ||||
| History of cGvHD† | ||||
| No | 80 | 1.00 | ||
| Yes | 175 | 6.35 | 1.96 to 20.6 | .002 |
| Unknown | 5 | 11.1 | 1.81 to 68.0 | .009 |
| SAA | ||||
| Age, years | < .001* | |||
| 0-19 | 1,252 | 1.00 | ||
| 20-39 | 805 | 1.43 | 1.02 to 1.99 | .036 |
| ≥ 40 | 105 | 3.28 | 2.01 to 5.34 | <.001 |
| Interval from diagnosis to transplantation, months | < .001* | |||
| < 6 | 1,470 | 1.00 | ||
| 6-12 | 279 | 1.57 | 1.02 to 2.41 | .039 |
| ≥ 12 | 413 | 2.04 | 1.40 to 2.96 | < .001 |
| History of aGvHD II-IV | .009* | |||
| No | 1,619 | 1.00 | ||
| Yes | 543 | 1.55 | 1.11 to 2.15 | |
| History of cGvHD† | < .001* | |||
| No | 1,404 | 1.00 | ||
| Yes | 698 | 2.15 | 1.54 to 3.02 | < .001 |
| Unknown | 60 | 3.29 | 1.75 to 6.19 | < .001 |
| Conditioning regimen | .002* | |||
| TBI + Cy | 359 | 1.00 | ||
| Bu + Cy | 219 | 1.28 | 0.68 to 2.42 | .452 |
| Cy + other (no TBI or Bu) | 1,494 | 1.07 | 0.69 to 1.66 | .764 |
| Other/unknown | 90 | 3.23 | 1.67 to 6.24 | < .001 |
Abbreviations: CIBMTR, Center for International Blood and Marrow Transplant Research; AML, acute myelogenous leukemia; cGVHD, chronic graft-versus-host disease; ALL, acute lymphoblastic leukemia; BM, bone marrow; PB, peripheral blood; MDS, myelodysplastic syndrome; aCGVD, acute graft-versus-host disease; SAA, severe aplastic anemia; TBI, total-body irradiation; Cy, cyclophosphamide; Bu, busulfan.
Test of overall equality among categories P value.
Within first 2 years after HCT.
P for aGvHD II-IV and cGvHD interaction = .005.
Risk factors from multivariate analyses for late relapse are listed in the Data Supplement. For AML, later stages of disease before transplantation, absence of acute GVHD, lower performance scores at time of transplantation, and T-cell depletion of the hematopoietic graft were associated with higher likelihood for relapse. For ALL, later stages of disease before transplantation, older age, absence of acute GVHD, and use of busulfan plus cyclophosphamide for the conditioning regimen were associated with greater risk of relapse. For MDS, there were no risk factors identified. For lymphoma, the occurrence of chronic GVHD and use of TBI were associated with a lower risk for relapse.
Risk factors from multivariate analyses for late NRM are listed in the Data Supplement. For AML, older age, occurrence of chronic GVHD, later stages of disease at the time of transplantation, use of both bone marrow and peripheral blood for the graft, performance of the transplantation in the United States, and being a man were factors associated with greater risk for NRM. For ALL, older age, development of acute or chronic GVHD, later stages of disease at the time of transplantation, use of both bone marrow and peripheral blood for the graft, performance of the transplantation in the United States, and being a man were factors associated with greater risk for NRM. For MDS, older age, development of acute or chronic GVHD, and a more remote date of transplantation were factors associated with greater risk for NRM. For lymphoma, older age and chronic GVHD were associated with a greater risk for NRM.
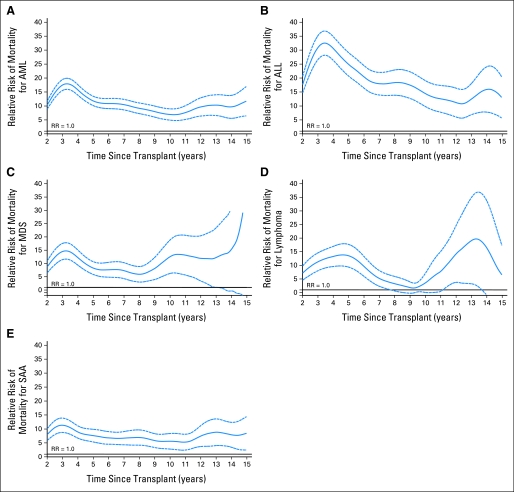
Relative rates of mortality were calculated for each disease category and compared with general population matched for sex, age, nationality, and ethnicity (for US population only). As shown in Figure 2, the relative mortality rates for all diseases were higher compared with the general population at 2 years. Although the relative risks declined for all diseases over time, they did not return to expected general population rates, except for in patients with lymphoma.
Fig 2.
(A) Relative mortality curves for patients with acute myelogenous leukemia (AML) receiving myeloablative allogeneic transplants surviving at least 2 years in remission post transplantation. (B) Relative mortality curves for patients with acute lymphoblastic leukemia (ALL) receiving myeloablative allogeneic transplants surviving at least 2 years in remission post transplantation. (C) Relative mortality curves for patients with myelodysplastic syndrome (MDS) receiving myeloablative allogeneic transplants surviving at least 2 years in remission post transplantation. (D) Relative mortality curves for patients with lymphoma receiving myeloablative allogeneic transplants surviving at least 2 years in remission post transplantation. (E) Relative mortality curves for patients with severe aplastic anemia (SAA) receiving myeloablative allogeneic transplants surviving at least 2 years in remission post transplantation.
DISCUSSION
This report of 10,632 HCT survivors represents the largest group of survivors with long-term follow-up ever reported, to our knowledge; 3,788 patients were observed for more than 10 years, which is more than three times the number of survivors observed for 10 years in the previous CIBMTR report1 and which is a substantially larger number of patients than in other reports of allogeneic HCT survivorship.7–14 Strengths of this study include the efforts made for rigor of data collection, inclusion of all allogeneic HCT patients undergoing transplantation at participating centers, high levels of completeness of follow-up, and exclusion of all data from centers with more than 20% incomplete current follow-up to exclude the potential bias of selective reporting. We limited the analysis to diseases commonly treated by allogeneic HCT and excluded less common diseases and diseases for which allogeneic HCT are less commonly performed.
These data indicate that long-term prospects for survival are excellent, with 80% to 92% of 2-year survivors surviving at 10 years after HCT. There appeared to be a return to normal death rates in patients who underwent transplantation for SAA in the earlier CIBMTR analysis1; with more patients observed for longer time, this was not evident in this analysis, which included not only the survivors in the earlier analysis observed for a longer interval but also additional patients who more recently underwent transplantation. Although the 95% CIs of the mortality rates of patients who underwent transplantation for lymphoma crossed the expected mortality rate of a matched normal population at 8 years after HCT, there were only 287 patients in that cohort, which makes this estimate imprecise.
The issue of relative death rates after allogeneic HCT compared with expected population norms has been addressed by several studies.1,7–12 In those earlier studies, the mortality rates mostly remained higher than the mortality rate in the matched general population, and the relative risks declined over time, similar to the findings in this study. A recent study from the CIBMTR that included allogeneic HCT recipients with CML who had survived in remission for at least 5 years after transplantation showed that the relative mortality of these long-term survivors was similar to that of matched general population.11 Differences in mortality risk between the present and previously reported study could be attributed to cohort selection criteria (2-year v 5-year survivors) and diagnosis (less intense pre-HCT therapy for CML). However, both studies indicate that, as patients survive longer post transplantation, the risks of mortality continue to decrease over time and may eventually reach that of the general population. However, studies with longer follow-up are still needed to confirm this observation.
The finding of more late deaths in patients from the United States who underwent transplantation for AML compared with patients in other regions who underwent transplantation prompted exploratory analyses of transplantations performed in the United States, Canada, and Europe (the largest comparison groups). There was a higher proportion of patients in Europe and Canada who underwent transplantation in early stages of disease, the interval from diagnosis to transplantation was shorter, and a greater proportion of patients had sibling donors (rather than unrelated donors; data not shown). These differences in patient selection indicate that the patients in the United States were at higher risk of failure. Thus, patient selection appears to account for much of the geographic differences.
Late relapse was the leading cause of death among patients who underwent transplantation for malignancy (27% to 42% of all deaths), a finding similar in other HCT series.1,7–14 Relapse has also been shown to be a major long-term problem for survivors of childhood and adolescent cancer not receiving HCT,15–17 and there are only limited data in adult leukemia survivors who did not receive HCT.18 Of note, relapse tended to occur more during years 2 to 5 after HCT, and it tended to diminish after year 5. Thus, intense surveillance for late relapse after 5 years can be reduced. Multiple studies have shown advanced or recurrent disease before transplantation to be the most common factor associated with relapse after HCT, as was the case in this study. Identification of patients who are unlikely to be cured by nontransplantation therapy and who are suitable candidates for HCT would benefit by early referral for transplantation to address this challenge.
The prominence of GVHD as a cause of death was no surprise and has been noted earlier.1,7–12 Clearly, reduction of GVHD remains a high priority for HCT. Infection in the absence of GVHD as a cause of death suggests prolonged immunodeficiency may persist after HCT.19 An emphasis on avoidance of exposures, vaccination, and infection control are all important to address this challenge.20
Second cancers accounted for 2% to 10% of deaths in late survivors. There is an increasing recognition of second cancers in HCT survivors. Post-transplantation lymphomas typically occur within the first year,21,22 secondary myelodysplasia and AML typically occur several years after transplantation (most frequently after autologous rather than allogeneic HCT),23,24 and epithelial cancers often do not occur until 10 or more years later.25–28 As more survivors live longer, this problem may become more apparent. Alternately, changes in treatment approaches may reduce the risk for second cancers. More and more patients are undergoing transplantation with less intensive, so-called nonablative conditioning regimens. Clinicians are also increasingly avoiding various pretransplantation modalities subsequently found associated with cancers, such as head or craniospinal radiation for CNS leukemia prophylaxis. Thus, the patterns and frequency of secondary cancers may change in the years ahead. Efforts to develop transplantation regimens that minimize the risk for second cancers are needed.26,27 However, chemoradiotherapy is not the only factor that is contributory to second cancers after HCT. GVHD and immunodeficiency also play roles,27 and genetic susceptibility may also be contributory. Prevention behaviors and screening for second cancers are important for follow-up care of allogeneic HCT survivors.29 A recent report indicates there is considerable room for improvement for health promotion by HCT survivors and practitioners caring for HCT survivors.30
Cautions about these findings are necessary. Protocols for HCT and its complications were not uniform. Transplantation practices have changed tremendously over the decades spanning the collection of these data, and risks of current transplantation practices may differ considerably from those used decades ago. For some deaths, information about cause was lacking.
In conclusion, durable survival is likely in most 2-year survivors. Nonetheless, late life-threatening complications occur, and these observations emphasize the need for prolonged follow-up to prevent, identify, and treat late complications29,31 to optimize long-term outcomes.
Supplementary Material
Acknowledgment
We thank the participating CIBMTR centers and the members of the Late Effects Working Committee of the CIBMTR, without whom this trial would not have been completed.
Supported by the following: Public Health Service Cooperative Agreement/grant No. U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); Cooperative Agreement/grant No. 5U01HL069294 from NHLBI and NCI; contract No. HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); grants No. N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; grants from AABB; Allos; and Amgen; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; Caridian BCT; Celgene; CellGenix, GmbH; Children's Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai; Genentech; Genzyme Corporation; Histogenetics; HKS Medical Information Systems; Hospira; Kirin Brewery; The Leukemia and Lymphoma Society; Merck; Medical College of Wisconsin; Millennium Pharmaceuticals; Miller Pharmacal Group; Milliman USA; Miltenyi Biotec; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics; Otsuka America Pharmaceutical; Pall Life Sciences; Pfizer; Schering; Sigma-Tau Pharmaceuticals; Soligenix; StemCyte; StemSoft Software; Sysmex America; THERAKOS; Vidacare; ViraCor Laboratories; ViroPharma; and Wellpoint.
Appendix
Writing contributors.
Other members of the writing committee are Saro Armenian, K. Scott Baker, Paolo Bartolomeo, Smita Bhatia, Linda Burns, Jean-Yves Cahn, Jing Chen, Mary Eapen, James Gajewski, Vikas Gupta, Gregory Hale, Joerg Halter, Ann Jakubowski, Hans-Jochem Kolb, Brandon Hayes-Lattin, Hillard Lazarus, Alison Loren, David Marks, Vijay Reddy, Harry Schouten, Andre Tichelli, Anne Warwick, and William Wood.
Footnotes
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: John R. Wingard, Pfizer (C), Merck (C), Astellas (C) Stock Ownership: None Honoraria: John R. Wingard, Pfizer, Merck, Astellas Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: John R. Wingard, Navneet S. Majhail, Kathleen A. Sobocinski, Mary M. Horowitz, Brian Bolwell, J. Douglas Rizzo, Gérard Socié
Administrative support: Mary M. Horowitz
Collection and assembly of data: Navneet S. Majhail, Zhiwei Wang, Kathleen A. Sobocinski, Mary M. Horowitz, J. Douglas Rizzo
Data analysis and interpretation: John R. Wingard, Navneet S. Majhail, Ruta Brazauskas, Zhiwei Wang, Kathleen A. Sobocinski, David Jacobsohn, Mohamed L. Sorror, Mary M. Horowitz, Brian Bolwell, J. Douglas Rizzo, Gérard Socié
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Socié G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation: Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 2.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 3.Klein J, Moeschberger M. Survival Analysis: Techniques of censored and truncated data. (ed 2) New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 4.Andersen PK, Vaeth M. Simple parametric and nonparametric models for excess and relative mortality. Biometrics. 1989;45:523–535. [PubMed] [Google Scholar]
- 5.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13:1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 6.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: Revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin PJ, Counts GW, Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28:1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: Report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeg HJ, Leisenring W, Storb R, et al. Long-term outcome after marrow transplantation for severe aplastic anemia. Blood. 1998;91:3637–3645. [PubMed] [Google Scholar]
- 10.Duell T, van Lint MT, Ljungman P, et al. Health and functional status of long-term survivors of bone marrow transplantation: EBMT Working Party on Late Effects and EULEP Study Group on Late Effects—European Group for Blood and Marrow Transplantation. Ann Intern Med. 1997;126:184–192. doi: 10.7326/0003-4819-126-3-199702010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Goldman JM, Majhail NS, Klein JP, et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. 2010;28:1888–1895. doi: 10.1200/JCO.2009.26.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pond GR, Lipton JH, Messner HA. Long-term survival after blood and marrow transplantation: Comparison with an age- and gender-matched normative population. Biol Blood Marrow Transplant. 2006;12:422–429. doi: 10.1016/j.bbmt.2005.11.518. [DOI] [PubMed] [Google Scholar]
- 13.Majhail NS, Bajorunaite R, Lazarus HM, et al. Long-term survival and late relapse in 2-year survivors of autologous haematopoietic cell transplantation for Hodgkin and non-Hodgkin lymphoma. Br J Haematol. 2009;147:129–139. doi: 10.1111/j.1365-2141.2009.07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majhail NS, Bajorunaite R, Lazarus HM, et al. High probability of long-term survival in 2-year survivors of autologous hematopoietic cell transplantation for AML in first or second CR. Bone Marrow Transplant. 2011;46:385–392. doi: 10.1038/bmt.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong GT, Pan Z, Ness KK, et al. Temporal trends in cause-specific late mortality among 5-year survivors of childhood cancer. J Clin Oncol. 2010;28:1224–1231. doi: 10.1200/JCO.2009.24.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacArthur AC, Spinelli JJ, Rogers PC, et al. Mortality among 5-year survivors of cancer diagnosed during childhood or adolescence in British Columbia, Canada. Pediatr Blood Cancer. 2007;48:460–467. doi: 10.1002/pbc.20922. [DOI] [PubMed] [Google Scholar]
- 18.Schiffer CA, Dodge R, Larson RA. Long-term follow-up of Cancer and Leukemia Group B studies in acute myeloid leukemia. Cancer. 1997;80:2210–2214. doi: 10.1002/(sici)1097-0142(19971201)80:11+<2210::aid-cncr8>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Antin JH. Immune reconstitution: The major barrier to successful stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:43–45. doi: 10.1016/j.bbmt.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: A global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis RE, Travis LB, Rowlings PA, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: A multi-institutional study. Blood. 1999;94:2208–2216. [PubMed] [Google Scholar]
- 22.Landgren O, Gilbert ES, Rizzo JD, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan A, Bhatia S, Slovak ML, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: An assessment of risk factors. Blood. 2000;95:1588–1593. [PubMed] [Google Scholar]
- 24.Metayer C, Curtis RE, Vose J, et al. Myelodysplastic syndrome and acute myeloid leukemia after autotransplantation for lymphoma: A multicenter case-control study. Blood. 2003;101:2015–2023. doi: 10.1182/blood-2002-04-1261. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 26.Rizzo JD, Curtis RE, Socié G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: An international case-control study. Blood. 2005;105:3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 29.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: Joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12:138–151. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207–214. doi: 10.1016/j.bbmt.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antin JH. Clinical practice: Long-term care after hematopoietic-cell transplantation in adults. N Engl J Med. 2002;347:36–42. doi: 10.1056/NEJMcp010518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.