Abstract
Purpose
The Study of Tamoxifen and Raloxifene (STAR) demonstrated that raloxifene was as effective as tamoxifen in reducing the risk of invasive breast cancer (IBC) in postmenopausal women and had lower risks of thromboembolic events, endometrial cancer, and cataracts but had a nonstatistically significant higher risk of noninvasive breast cancer. There is a need to summarize the risks and benefits of these agents.
Patients and Methods
Baseline incidence rates of IBC and other health outcomes, absent raloxifene and tamoxifen, were estimated from breast cancer chemoprevention trials; the Surveillance, Epidemiology and End Results Program; and the Women's Health Initiative. Effects of raloxifene and tamoxifen were estimated from STAR and the Breast Cancer Prevention Trial. We assigned weights to health outcomes to calculate the net benefit from raloxifene compared with placebo and tamoxifen compared with placebo.
Results
Risks and benefits of treatment with raloxifene or tamoxifen depend on age, race, breast cancer risk, and history of hysterectomy. Over a 5-year period, postmenopausal women with an intact uterus had a better benefit/risk index for raloxifene than for tamoxifen. For postmenopausal women without a uterus, the benefit/risk ratio was similar. The benefits and risks of raloxifene and tamoxifen are described in tables that can help identify groups of women for whom the benefits outweigh the risks.
Conclusion
We developed a benefit/risk index to quantify benefits from chemoprevention with tamoxifen or raloxifene. This index can complement clinical evaluation in deciding whether to initiate chemoprevention and in comparing the benefits and risks of raloxifene versus tamoxifen.
INTRODUCTION
Chemoprevention trials in the United States and Europe have evaluated selective estrogen receptor modulators to prevent breast cancer in high-risk women.1–4 The Breast Cancer Prevention Trial (BCPT) demonstrated that tamoxifen produced a 49% reduction in invasive breast cancer (IBC) in US women at increased risk.1 The US Food and Drug Administration (FDA) subsequently approved tamoxifen for breast cancer chemoprevention among women age 35 years or older with a 5-year breast cancer risk of 1.67% or higher. Because tamoxifen use is associated with adverse events such as endometrial cancer and stroke, a previous analysis5 developed a benefit/risk index to identify levels of breast cancer risk for which benefits outweighed risks.
Raloxifene, another selective estrogen receptor modulator, reduced IBC risk in studies for the prevention and treatment of other conditions in postmenopausal women.6 The Multiple Outcomes Raloxifene Evaluation trial for osteoporosis7 and the Raloxifene Use for the Heart trial8 both demonstrated a substantial reduced risk of IBC in postmenopausal women. These trials provided the scientific basis for the National Cancer Institute (NCI) to initiate the National Surgical Adjuvant Breast and Bowel Project (NSABP) Study of Tamoxifen and Raloxifene (STAR) in 1999.9 STAR did not include a placebo group but directly compared tamoxifen with raloxifene in a population of US postmenopausal women at increased risk of breast cancer. Over a mean follow-up of 3.9 years, STAR demonstrated that raloxifene was as effective as tamoxifen in reducing risk of IBC. Raloxifene also resulted in lower risk of endometrial cancer, thromboembolic events, and cataracts, but a nonstatistically significant higher risk of noninvasive breast cancer. The risk of fractures, ischemic heart disease, and stroke were similar for raloxifene and tamoxifen.
In 2007, FDA approved the use of raloxifene to reduce the risk of IBC in postmenopausal women with osteoporosis or at high risk of IBC.10 Several cancer networks and professional societies have issued guidelines on the use of these chemopreventive agents in breast cancer risk reduction.11–13 A recent systematic review compared the effectiveness and safety of several breast cancer chemopreventive agents but reached limited conclusions.14 Relying on data from the BCPT and STAR trials and on methods for weighing risks and benefits in Gail et al,5 we produced tables of benefit/risk indices to compare raloxifene with no treatment (placebo) and tamoxifen with no treatment. Our tables and results can be used for counseling postmenopausal women regarding the use of these agents.
PATIENTS AND METHODS
To compute net benefit/risk indices, we assigned weights to various health outcomes and used background incidence rates for relevant health outcomes in the absence of raloxifene and tamoxifen (Table 1) and relative risk (RR) estimates of the effects of raloxifene and tamoxifen on these incidence rates from BCPT and STAR (Table 2). Net benefit/risk indices were calculated for raloxifene as the difference in the sums of weighted expected events in the absence and presence of raloxifene. Analogous indices were computed for tamoxifen.
Table 1.
Incidence Rates per 1,000 Woman-Years by Race
| Type of Event | Incidence Rates for Women (by age groups, in years) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| White |
Black |
Hispanic |
|||||||
| 50-59 | 60-69 | 70-79 | 50-59 | 60-69 | 70-79 | 50-59 | 60-69 | 70-79 | |
| Hip fracture* | 0.43 | 1.41 | 4.84 | 0.22 | 0.3 | 1.9 | 0.25 | 0.61 | 1.32 |
| Endometrial cancer† | 0.92 | 1.80 | 1.70 | 0.53 | 1.48 | 1.11 | 0.5 | 0.71 | 0.86 |
| Stroke* | 0.83 | 2.22 | 5.49 | 2.03 | 3.69 | 6.19 | 0.75 | 2.56 | 5.14 |
| Pulmonary embolism* | 0.56 | 0.86 | 1.08 | 0.82 | 0.8 | 1.43 | 0.0 | 0.1 | 0.95 |
| Deep vein thrombosis* | 0.66 | 1.28 | 2.04 | 0.99 | 1.47 | 2.28 | 0.25 | 0.89 | 0.96 |
| Colles fracture* | 0.97 | 1.34 | 1.64 | 0.32 | 0.35 | 0.49 | 0.64 | 1.13 | 2.0 |
| Spine fracture* | 0.98 | 2.13 | 4.40 | 0.26 | 0.3 | 0.83 | 0.59 | 1.01 | 1.62 |
| Cataracts‡ | 15.91 | 52.18 | 98.49 | 15.91 | 52.18 | 98.49 | 15.91 | 52.18 | 98.49 |
Age-specific incidence rates for stroke, pulmonary embolism, deep vein thrombosis, and fractures of the proximal femur (hip), vertebra (spine), and distal forearm (Colles fractures) were obtained from the placebo arm of the Women's Health Initiative.15
We based estimates of endometrial cancer incidence rates on age- and race-specific incidence rates from the Surveillance, Epidemiology and End Results (SEER) Program for 1998 through 2002. To predict risk for women with a uterus, SEER rates were divided by the estimated age-specific prevalence of having a uterus by using data from the 2000 National Health Interview Survey.16
Baseline estimates of cataract incidence were calculated from data in the placebo arm of the Breast Cancer Prevention Trial because this cohort reflects current ophthalmologic practice and is the largest cohort with reports on cataracts in women.1
Table 2.
Computed RRs for Events for Raloxifene Versus Placebo on the Basis of BCPT and STAR
| Type of Event | No. of Events in BCPT |
No. of Events in STAR |
RR for BCPT ×RR for STAR(raloxifene v placebo) |
|||||
|---|---|---|---|---|---|---|---|---|
| Placebo/ Tamoxifen | RR | 95% CI | Tamoxifen/Raloxifene | RR | 95% CI | RR | 95% CI | |
| Invasive breast cancer | 175/89 | 0.51 | 0.39 to 0.66 | 181/212 | 1.16 | 0.95 to 1.42 | 0.59 | 0.43 to 0.82 |
| Hip fracture | 22/12 | 0.55 | 0.25 to 1.15 | 26/23 | 0.88 | 0.48 to 1.60 | 0.48 | 0.20 to 1.16 |
| Endometrial cancer | 1.14* | 0.65 to 1.98 | ||||||
| All women | 15/36 | 2.53 | 1.35 to 4.97 | 36/23 | 0.62 | 0.35 to 1.08 | ||
| Women age ≥ 50 years | 7/27 | 4.01 | 1.70 to 10.90 | 35/23 | 0.65 | 0.37 to 1.13 | ||
| Stroke | 24/38 | 1.59 | 0.93 to 2.77 | 53/51 | 0.96 | 0.64 to 1.43 | 1.53 | 0.81 to 2.85 |
| Pulmonary embolism | 6/18 | 3.01 | 1.15 to 9.27 | 54/35 | 0.64 | 0.41 to 1.00 | 1.93 | 0.74 to 5.07 |
| In situ breast cancer | 69/35 | 0.50 | 0.33 to 0.77 | 57/80 | 1.40 | 0.98 to 2.00 | 0.70 | 0.41 to 1.18 |
| Deep vein thrombosis | 22/35 | 1.60 | 0.91 to 2.86 | 87/65 | 0.74 | 0.53 to 1.03 | 1.18 | 0.64 to 2.19 |
| Colles fracture | 23/14 | 0.61 | 0.29 to 1.23 | 27/23 | 0.85 | 0.46 to 1.53 | 0.52 | 0.22 to 1.20 |
| Spine fracture | 31/23 | 0.74 | 0.41 to 1.32 | 53/52 | 0.98 | 0.65 to 1.46 | 0.73 | 0.38 to 1.38 |
| Cataracts | 507/574 | 1.14 | 1.01 to 1.29 | 394/313 | 0.79 | 0.68 to 0.92 | 0.90 | 0.75 to 1.09 |
Abbreviations: RR, relative risk; BCPT, Breast Cancer Prevention Trial; STAR, Study of Tamoxifen and Raloxifene.
Based on analysis of raloxifene versus placebo studies by Nelson et al.14
Projecting Risks in the Absence of Raloxifene and Tamoxifen
To calculate the projected 5-year risk of IBC for white and Hispanic women with particular risk factors but with no history or current evidence of IBC, ductal carcinoma in situ, or lobular carcinoma in situ, we used the breast cancer risk assessment model developed by Gail et al,17 modified by Costantino18 and by Anderson et al,19 and recently modified for African American women.20 We estimated the risk for in situ breast cancer as 0.22 times the estimated IBC risk. This ratio was obtained from the three placebo arms of the Women's Health Initiative (WHI) trial.15,21
We based estimates of endometrial cancer incidence rates on age- and race-specific incidence rates from the Surveillance, Epidemiology and End Results (SEER) Program for 1998 through 2002. To predict risk for women with a uterus, SEER rates were divided by the estimated age-specific prevalence of having a uterus with data from the 2000 National Health Interview Survey.16 For white, black, and Hispanic women, the age-specific incidence rates for stroke, pulmonary embolism, deep vein thrombosis (DVT), and fractures of the proximal femur (hip), vertebra (spine), and distal forearm (Colles fractures) were obtained from the three placebo arms of WHI.15,21 Detailed eligibility criteria and recruitment methods for WHI have been published.22 Baseline estimates of cataract incidence were calculated from the placebo arm of BCPT1 (Table 1).
Projecting Risks With Raloxifene and Tamoxifen
Fisher et al1 described the RRs comparing tamoxifen to placebo in BCPT. Protective RRs were found for IBC, in situ breast cancer, and hip fracture (Table 2). Adverse RRs were found for endometrial cancer, stroke, pulmonary embolism, DVT, and cataracts. Vogel et al9 described RRs of raloxifene compared with those for tamoxifen in STAR. RRs favored raloxifene for endometrial cancer, pulmonary embolism, and DVT, but tamoxifen was more effective in preventing in situ breast cancer than raloxifene (RR, 1.40; 95% CI, 0.98 to 2.00; Table 2). For IBC, we used the estimated RR of 1.16 (95% CI, 0.95 to 1.42) from recent STAR data.23
There was no statistically significant evidence of heterogeneity of RRs for IBC or in situ breast cancer in either trial across groups defined by age, number of affected first-degree relatives, projected 5-year risk of IBC, or lobular carcinoma in situ status. Therefore, we assumed that the RRs for IBC and in situ breast cancer are uniform across all subgroups.
Because STAR did not include a placebo group, it did not provide an RR compared with no treatment. To estimate the RR of raloxifene compared with placebo, we multiplied the RR estimates from STAR by the RR estimates from BCPT (Table 2). We used log-normal approximations to construct 95% CIs on RRs. The variance of the log-RR estimate for raloxifene versus placebo was the sum of the corresponding variances from both trials. RRs for raloxifene versus placebo (Table 2) were 0.59 (95% CI, 0.43 to 0.82) for IBC, 0.48 (95% CI, 0.2 to 1.16) for hip fracture, 1.53 (95% CI, 0.81 to 2.85) for stroke, 1.93 (95% CI, 0.74 to 5.07) for pulmonary embolism, 0.70 (95% CI, 0.41 to 1.18) for in situ breast cancer, and 1.18 (95% CI, 0.64 to 2.19) for DVT. For endometrial cancer, we used an RR of 1.14 (95% CI, 0.65 to 1.98) that was based on several studies comparing raloxifene to placebo.14 This RR was used instead of 4.01 × 0.65 = 2.60 from Table 2 because intensive follow-up in the tamoxifen arm in STAR with hysterectomies for conditions such as endometrial bleeding and atypical hyperplasia biased the RR comparing raloxifene to tamoxifen (0.65) upward. Our raloxifene-to-placebo RRs (Table 2) are similar to those in trials of raloxifene for osteoporosis.6
Benefit/Risk Comparisons for Women Age 50 Years or Older
Although BCPT included pre- and postmenopausal women age 35 years and older, we confined our benefit/risk analyses to women age 50 years and older because only postmenopausal women were eligible for STAR. Few women in STAR were younger than age 50 years, and raloxifene is not approved for reduction of breast cancer risk in premenopausal women.
As in Gail et al,5 we considered eight non–breast cancer conditions whose rates were potentially influenced by raloxifene (Tables 1 and 2). We grouped these outcomes plus IBC and in situ breast cancer into the categories “life-threatening events,” “severe events,” and “other events.” Life-threatening events included invasive breast cancer, hip fracture, endometrial cancer, stroke, and pulmonary embolism. Severe events included in situ breast cancer and DVT. Other events included Colles and spine fracture and cataracts.
To summarize risks and benefits in an index, we assigned weight 1.0 for life-threatening events, 0.5 for severe events, and 0.0 for other events. We defined the expected number of life-threatening equivalent events in a population of 10,000 women followed for 5 years as the life-threatening events plus half the severe events. On the basis of a woman's risk factors, one can calculate her probability of having each of the health outcomes in 5 years in the absence of chemoprevention and in the presence of chemoprevention. The net benefit index is the expected number of life-threatening equivalent events without chemoprevention in 10,000 such women minus the expected number of events with chemoprevention. To assess variability, we used a Bayesian bootstrap. The posterior distribution of RR for each event from each trial is a constant times an F distribution.5 In each bootstrap replication, we resampled the RRs in both trials,5 and the RR of raloxifene versus placebo was calculated as the product of the RR estimates from both trials. The expected number of adverse events in each treatment group and the net benefit were recalculated in 100,000 independent bootstrap replications. We defined “strong evidence” of a positive net benefit of a chemoprevention group versus placebo if the net benefit was positive in 90% or more of the replications (coded blue in Figs 1 through 4), and “moderate evidence” if the net benefit was positive in 60% to 89.99% (coded gold). If the probability was less than 60%, the cell was coded gray in Figures 1 through 4. The net benefit index is also shown in each cell. Some gray cells have positive net benefit indices. The 95% CIs on the difference in net benefits comparing raloxifene to tamoxifen were based on the 2.5th and 97.5th percentiles of the bootstrap distribution. In sensitivity analyses, we examined other choices of weights.
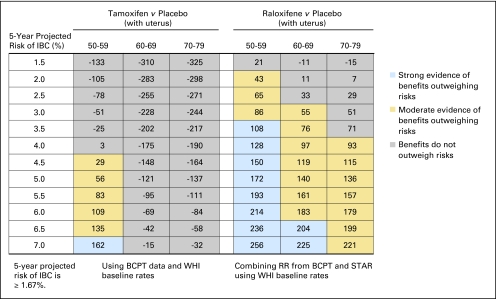
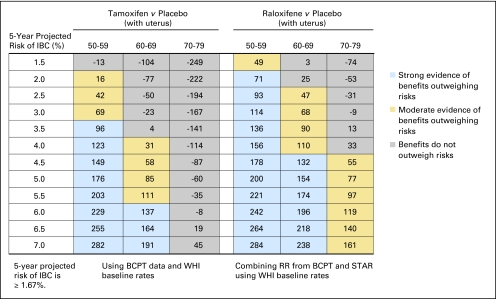
Fig 1.
Benefit/risk indices for tamoxifen and raloxifene chemoprevention by level of 5-year projected risk for invasive breast cancer (IBC) for white non-Hispanic women with a uterus, by age group. On the basis of a woman's risk factors (age, ethnicity, breast cancer risk, and whether she has a uterus), one can calculate her probability of having a health event in 5 years in the absence or presence of chemoprevention. To summarize risks and benefits in a single index, we assigned weights of 1.0 for life-threatening events (IBC, hip fracture, endometrial cancer, stroke, and pulmonary embolism) and 0.5 for severe events (in situ breast cancer and deep vein thrombosis). The net benefit index is the expected number of life-threatening equivalent events in 5 years without chemoprevention in 10,000 such women minus the expected number of life-threatening equivalent events if chemoprevention is used. (A severe event is regarded as equivalent to half a life-threatening event). For example, in this table, among 10,000 non-Hispanic white women with a uterus, age 50 to 59 years, and with a 5-year IBC risk of 3.5%, one expects that 108 life-threatening equivalent events would be prevented in 5 years by taking raloxifene instead of placebo, and there is strong evidence (P > .9; blue) that the benefits of taking raloxifene outweigh the risks. If tamoxifen were used instead, we estimate chemoprevention would result in 25 excess life-threatening events (P < .6, gray). BCPT, Breast Cancer Prevention Trial; WHI, Women's Health Initiative; RR, relative risk; STAR, Study of Tamoxifen and Raloxifene.
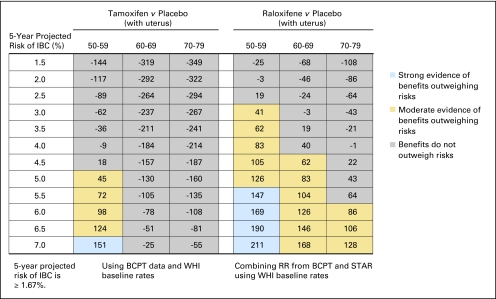
Fig 4.
Benefit/risk indices for tamoxifen and raloxifene chemoprevention by level of 5-year projected risk of invasive breast cancer (IBC) for black women without uterus, by age group. On the basis of a woman's risk factors (age, ethnicity, breast cancer risk, and whether she has a uterus), one can calculate her probability of having a health event in 5 years in the absence of chemoprevention and in the presence of chemoprevention. To summarize risks and benefits in a single index, we assigned weights of 1.0 for life-threatening events (IBC, hip fracture, endometrial cancer, stroke, and pulmonary embolism) and 0.5 for severe events (in situ breast cancer and deep vein thrombosis). The net benefit index is the expected number of life-threatening equivalent events in 5 years without chemoprevention in 10,000 such women minus the expected number of life-threatening equivalent events if chemoprevention is used. (A severe event is regarded as equivalent to half a life-threatening event.) For example, in this table, among 10,000 black women without a uterus, age 50 to 59 years, and with a 5-year IBC risk of 3.5%, one expects that 66 life-threatening equivalent events would be prevented in 5 years by taking raloxifene instead of placebo, and there is moderate evidence (P > 6 but < 0.9; gold) that the benefits of taking raloxifene outweigh the risk. If tamoxifen were used instead, we estimate chemoprevention would result in 42 life-threatening equivalent events being prevented, with moderate evidence of the benefits outweighing the risks (P > 0.6 but < 0.9; gold). BCPT, Breast Cancer Prevention Trial; WHI, Women's Health Initiative; RR, relative risk; STAR, Study of Tamoxifen and Raloxifene.
RESULTS
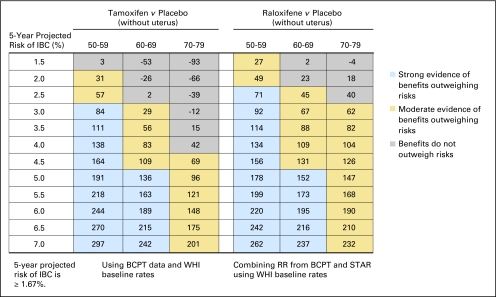
In Figure 1, we show benefit/risk indices for tamoxifen versus placebo and for raloxifene versus placebo by age group and level of 5-year IBC risk for non-Hispanic white women with a uterus. Figure 2 gives such results for white women without a uterus, and Figures 3 and 4 give results for black women. Appendix Figures A1 and A2 (online only) give these results for Hispanic women. From Figure 1, among 10,000 non-Hispanic white women with a uterus, age 50 to 59 years, with a 5-year IBC risk of 3.5%, one expects that 108 life-threatening equivalent events would be prevented in 5 years by taking raloxifene; there is strong evidence (P ≥ .9; blue) that benefits outweigh risk. If tamoxifen were used instead, we estimate chemoprevention would result in 25 excess life-threatening events (negative index). The direct comparison between raloxifene and tamoxifen indicates 108 + 25 = 133 (95% CI, −28 to 352) fewer life-threatening equivalent events on raloxifene. A similar pattern is seen for black women with an intact uterus and 5-year projected IBC risk of 3.5% (Fig 3).
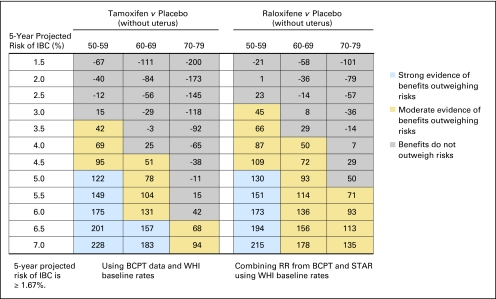
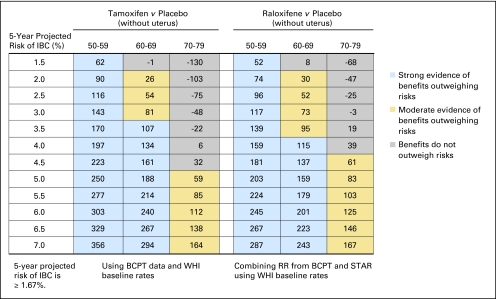
Fig 2.
Benefit/risk indices for tamoxifen and raloxifene chemoprevention by level of 5-year projected risk of invasive breast cancer (IBC) for white non-Hispanic women without uterus, by age group. On the basis of a woman's risk factors (age, ethnicity, breast cancer risk, and whether she has a uterus), one can calculate her probability of having a health event in 5 years in the absence of chemoprevention and in the presence of chemoprevention. To summarize risks and benefits in a single index, we assigned weights of 1.0 for life-threatening events (IBC, hip fracture, endometrial cancer, stroke, and pulmonary embolism) and 0.5 for severe events (in situ breast cancer and deep vein thrombosis). The net benefit index is the expected number of life-threatening equivalent events in 5 years without chemoprevention in 10,000 such women minus the expected number of life-threatening equivalent events if chemoprevention is used. (A severe event is regarded as equivalent to half a life-threatening event). For example, in this table, among 10,000 non-Hispanic white women without a uterus, age 50 to 59 years, and with a 5-year IBC risk of 3.5%, one expects that 114 life-threatening equivalent events would be prevented in 5 years by taking raloxifene instead of placebo, and there is strong evidence (P > 0.9; blue) that the benefits of taking raloxifene outweigh the risks. If tamoxifen were used instead, we estimate chemoprevention would also result in the prevention of 111 life-threatening events (P < 0.9; blue). Among 10,000 non-Hispanic white women without a uterus, age 70 to 79 years, and with a 5-year IBC risk of 3.0%, one expects that 62 life-threatening equivalent events would be prevented in 5 years by taking raloxifene instead of placebo, and there is moderate evidence (P ≥ 0.6 but < 0.9; gold) that the benefits of taking raloxifene outweigh the risks. If tamoxifen were used instead, we estimate chemoprevention would result in 12 excess life-threatening events (P < 0.6; gray). BCPT, Breast Cancer Prevention Trial; WHI, Women's Health Initiative; RR, relative risk; STAR, Study of Tamoxifen and Raloxifene.
Fig 3.
Benefit/risk indices for tamoxifen and raloxifene chemoprevention by level of 5-year projected risk of invasive breast cancer (IBC) for black women with uterus, by age group. On the basis of a woman's risk factors (age, ethnicity, breast cancer risk, and whether she has a uterus), one can calculate her probability of having a health event in 5 years in the absence of chemoprevention and in the presence of chemoprevention. To summarize risks and benefits in a single index, we assigned weights of 1.0 for life-threatening events (IBC, hip fracture, endometrial cancer, stroke, and pulmonary embolism) and 0.5 for severe events (in situ breast cancer and deep vein thrombosis). The net benefit index is the expected number of life-threatening equivalent events in 5 years without chemoprevention in 10,000 such women minus the expected number of life-threatening equivalent events if chemoprevention is used. (A severe event is regarded as equivalent to half a life-threatening event). For example, in this table, among 10,000 black women with a uterus, age 50 to 59 years, and with a 5-year IBC risk of 3.5%, one expects that 62 life-threatening equivalent events would be prevented in 5 years by taking raloxifene instead of placebo, and there is moderate evidence (P ≥ 6 but < 0.9; gold) that the benefits of taking raloxifene outweigh the risks. If tamoxifen were used instead, we estimate chemoprevention would result in 36 excess life-threatening equivalent events (P < .6; gray). BCPT, Breast Cancer Prevention Trial; WHI, Women's Health Initiative; RR, relative risk; STAR, Study of Tamoxifen and Raloxifene.
For non-Hispanic white women age 50 years or older with a uterus, raloxifene displayed a better benefit/risk profile than tamoxifen overall (Fig 1). For tamoxifen, women age 50 to 59 years with a 5-year IBC risk of 4.5% to 6.5% showed moderate evidence (gold) of net positive benefit, and women with IBC risk of 7.0% or higher showed strong evidence (blue). For women age 50 to 59 years with a 5-year IBC risk less than 4.0%, the risks outweighed the benefits (gray cells with negative net benefit indices). The risks outweighed the benefits for women age 60 years or older, regardless of IBC risk. In contrast, for raloxifene, there was strong evidence (blue) that benefits outweighed risks, compared with placebo, for women age 50 to 59 years with a 5-year IBC risk of 3.5% or higher and for women age 60 to 69 years with an IBC risk of 6.5% risk or higher. There was moderate evidence (gold) of a net benefit for women age 50 to 59 years with a 5-year IBC risk of 2.0% to 3.0%, women age 60 to 69 years with a 5-year IBC risk of 3.0% to 6.0%, and women age 70 to 79 years with a 5-year IBC risk of 4.0% or higher. For postmenopausal black and Hispanic women with a uterus, raloxifene also displayed a better benefit/risk profile than tamoxifen and in a similar pattern to that for whites (Fig 3 and Appendix Table A1). Net benefit indices tended to be larger in Hispanic women and smaller in black women than in white women, however.
For non-Hispanic white women age 50 years or older without a uterus, the benefit/risk ratios were similar for raloxifene and tamoxifen (Fig 2). For tamoxifen, there was moderate or strong evidence for benefits outweighing risks among women age 50 to 59 years with an IBC risk of 2.0% or more, women age 60 to 69 years with a risk of 3.0% or more, and women age 70 to 79 years with a risk of 4.5% or more. For raloxifene, there was moderate or strong evidence for benefits outweighing risks among women age 50 to 59 years with a projected 5-year IBC risk of 1.5% or more, women age 60 to 69 years with a risk of 2.5% or more, and women age 70 to 79 years with a risk of 3.0% or more. Direct comparison of raloxifene with tamoxifen showed that the 95% CI on the difference in benefit indices usually included zero (data not shown). For postmenopausal black women without a uterus, both tamoxifen and raloxifene also displayed a benefit/risk profile pattern similar to that of white women (Fig 4). Similar results were found for postmenopausal Hispanic women without a uterus, (Appendix Table A2), except among women age 50 to 59 years in whom tamoxifen had a better benefit/risk profile. Net benefit indices were smaller for black women than for white women, but Hispanic and white women without a uterus had similar indices.
In sensitivity analyses with weights ranging from 0.5 to 1.0 for severe events and from 1.0 to 0.25 for other events, the patterns of evidence for benefit were similar to those in Figures 1 through 4 and Appendix Tables A1 and A2 (data not shown). However, putting more weight on other events favored raloxifene, primarily because tamoxifen is associated with increased risk of cataracts (Table 2), which are common (Table 1), whereas raloxifene is not associated with risk of cataracts.
DISCUSSION
The benefit/risk indices in this article indicated that raloxifene is better than tamoxifen for women age 50 years or older with a uterus. For women without a uterus, the benefit/risk profile for raloxifene is similar to that for tamoxifen. Our tables can help physicians and patients summarize the benefits and risks of tamoxifen and raloxifene for chemoprevention. By using NCI's Breast Cancer Risk Assessment Tool (BCRAT) to estimate the projected 5-year risk of IBC,24 a health care provider can obtain a benefit/risk index from the corresponding table entries. By combining this information with information on clinical features and personal preferences, the health care provider and patient can make an informed decision.
We revised benefit/risk tables for tamoxifen compared with no treatment (placebo) previously developed by Gail et al5 by using baseline incidence rates from WHI. We confined our analysis to women age 50 years and older because raloxifene is approved only for postmenopausal women. However, net benefits of tamoxifen are greater in younger women with high risk of breast cancer for whom the earlier tables5 are still recommended.
We produced tables of indices to weigh the benefits and risks of raloxifene compared with no treatment by combining data from STAR and BCPT. This innovation improves the usefulness of STAR data in deciding whether to use raloxifene for chemoprevention because the STAR trial did not have a placebo arm.
Our tables are appropriate for the general population of women age 50 years or older without previous breast cancer. However, women with high risks for certain conditions in Tables 1 and 2 are at additional risk from chemoprevention. For example, a woman with a history of a thromboembolic event would be at greater risk of having a DVT than the average woman. Data are insufficient on the effect of chemoprevention in women with mutations in BRCA1 or BRCA2 genes.25
Principal strengths of this article include use of data from randomized chemoprevention trials and use of WHI data on incidence rates of health outcomes in the absence of chemoprevention.
Our study has some weaknesses or points of criticism. (1) The weights chosen for life-threatening, severe, and other events affect the benefit/risk values in our tables. If more weight is assigned to a particular severe event, such as in situ breast cancer, the relative benefit of tamoxifen increases. Some women may be concerned about a particular health outcome. For such women, different weighting of the outcomes might be more appropriate. (2) To estimate the effects of raloxifene versus placebo, we multiplied RRs from BCPT and STAR. This method increased the variability of our net benefit indices for raloxifene and required the assumption that the treatment RR comparing tamoxifen with placebo in BCPT would also have been observed in the STAR population. (3) Women in WHI may have lower baseline disease rates than the general population, which could affect the indices. (4) Much of the improved performance of raloxifene compared with tamoxifen is from reduced risk of endometrial cancer. Although we used the best available data,14 our findings in favor of raloxifene are sensitive to these estimates. (5) BRCAT does not predict the incidence of estrogen receptor–positive IBC, which is affected by tamoxifen and raloxifene, and the net index for black women may be less than that tabulated because a smaller proportion of black women have estrogen receptor–positive disease. (6) Some women who stand to gain the most from chemoprevention are younger than age 50 years,5 and are not covered by our tables. (7) Although tamoxifen shows continued chemopreventive efficacy and reduced adverse effects for up to 20 years,26,27 and updated data from STAR show continued efficacy in reducing breast cancer risk at 81 months of follow-up,23 we restricted our net benefit index tables to 5 years. An analysis over extended time periods may be worthwhile and health care providers may wish to consider this continued efficacy.
In summary, we have updated and extended a benefit/risk index for raloxifene and tamoxifen, and for Hispanic and non-Hispanic white and black women age 50 years or older. By giving quantitative indices that are color-coded for strength of evidence, we hope to help health care providers and their postmenopausal patients make better informed decisions about chemoprevention.
Acknowledgment
We thank women participating in the Breast Cancer Prevention Trial (BCPT), Study of Tamoxifen and Raloxifene (STAR), and Women's Health Initiative (WHI) studies for their valuable contribution to this research. We also thank Amy Monaco for research assistance and Linda Andersen for editorial assistance.
Appendix
Fig A1.
Benefit/risk indices for tamoxifen and raloxifene chemoprevention by level of 5-year projected risk of invasive breast cancer (IBC) for Hispanic women with uterus, by age group.
On the basis of a woman's risk factors (age, ethnicity, breast cancer risk, and whether she has a uterus), one can calculate her probability of having a health event in 5 years in the absence or presence of chemoprevention. To summarize risks and benefits in a single index, we assigned weights of 1.0 for life-threatening equivalent events (invasive breast cancer, hip fracture, endometrial cancer, stroke, and pulmonary embolism) and 0.5 for severe events (in situ breast cancer and deep vein thrombosis). The net benefit index is the expected number of life-threatening equivalent events in 5 years without chemoprevention in 10,000 such women minus the expected number of life-threatening equivalent events if chemoprevention is used. (A severe event is regarded as equivalent to half a life-threatening event.) For example, among 10,000 Hispanic women with a uterus, age 50 to 59 years, and with a 5-year IBC risk of 3.0%, one expects that 114 life-threatening equivalent events would be prevented in 5 years by taking raloxifene instead of a placebo, and there is strong evidence (P > .9, blue) that the benefits of taking raloxifene outweigh the risk. If tamoxifen were used instead, we estimate chemoprevention would also result in the prevention of 69 life-threatening equivalent events with moderate evidence that the benefits of taking tamoxifen outweigh the risk (P < .6, gold). BCPT, Breast Cancer Prevention Trial; WHI, Women's Health Initiative; RR, relative risk; STAR, Study of Tamoxifen and Raloxifene.
Fig A2.
Benefit/risk indices for tamoxifen and raloxifene chemoprevention by level of 5-year projected risk of invasive breast cancer (IBC) for Hispanic women without uterus, by age group.
On the basis of a woman's risk factors (age, ethnicity, breast cancer risk, and whether she has a uterus), one can calculate her probability of having a health event in 5 years in the absence or presence of chemoprevention. To summarize risks and benefits in a single index, we assigned weights of 1.0 for life-threatening equivalent events (invasive breast cancer, hip fracture, endometrial cancer, stroke, and pulmonary embolism) and 0.5 for severe events (in situ breast cancer and deep vein thrombosis). The net benefit index is the expected number of life-threatening equivalent events in 5 years without chemoprevention in 10,000 such women minus the expected number of life-threatening equivalent events if chemoprevention is used. (A severe event is regarded as equivalent to half a life-threatening event.) For example, among 10,000 Hispanic women without a uterus, aged 50 to 59 years, and with a 5-year IBC risk of 3.0%, one expects that 117 life-threatening equivalent events would be prevented in 5 years by taking raloxifene instead of placebo, and there is strong evidence (P > .9, blue) that the benefits of taking raloxifene outweigh the risk. If tamoxifen were used instead, we estimate chemoprevention would also result in the prevention of 143 life-threatening equivalent events, also with strong evidence that the benefits of taking tamoxifen outweigh the risk (P < .9, blue). BCPT, Breast Cancer Prevention Trial; WHI, Women's Health Initiative; RR, relative risk; STAR, Study of Tamoxifen and Raloxifene.
Footnotes
See accompanying editorial on page 2296
Supported by Contracts No. N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221 from the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services; by the Intramural Research Program of the National Institute on Aging (B.Y.), by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (M.H.G. and B.I.G.), and by Grant No. U10-CA069974 from the Public Health Service (J.P.C.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Victor G. Vogel, Eli Lilly (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Andrew N. Freedman, Mitchell H. Gail, Joseph P. Costantino, Worta McCaskill-Stevens
Collection and assembly of data: Andrew N. Freedman, Mitchell H. Gail, Joseph P. Costantino, Barry I. Graubard, Garnet L. Anderson, Worta McCaskill-Stevens
Data analysis and interpretation: Andrew N. Freedman, Binbing Yu, Mitchell H. Gail, Joseph P. Costantino, Barry I. Graubard, Victor G. Vogel, Worta McCaskill-Stevens
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): A randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 3.Powles T, Eeles R, Ashley S, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352:98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: Preliminary findings from the Italian randomised trial among hysterectomised women—Italian Tamoxifen Prevention Study. Lancet. 1998;352:93–97. doi: 10.1016/s0140-6736(98)85011-3. [DOI] [PubMed] [Google Scholar]
- 5.Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–1846. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 6.Cranney A, Guyatt G, Griffith L, et al. Meta-analyses of therapies for postmenopausal osteoporosis: IX. Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002;23:570–578. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial—Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 9.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. Raloxifene hydrochloride, approved September 13, 2007. http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm129243.htm.
- 11.National Comprehensive Cancer Network (NCCN) NCCN Updates Breast Cancer and Breast Cancer Risk Reduction Guidelines, February 3, 2009. http://www.nccn.org/about/news/newsinfo.asp?NewsID=200.
- 12.US Preventive Services Task Force (USPSTF) Chemoprevention of Breast Cancer: Summary of recommendations/supporting documents, July 2002. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrpv.htm.
- 13.Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–3258. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson HD, Fu R, Griffin JC, et al. Systematic review: Comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151:703–715. doi: 10.7326/0003-4819-151-10-200911170-00147. [DOI] [PubMed] [Google Scholar]
- 15.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention: National Center for Health Statistics. National Health Interview Survey. http://www.cdc.gov/nchs/nhis.htm.
- 17.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 18.Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91:1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 19.Anderson SJ, Ahnn S, Duff K. University of Pittsburgh, Department of Biostatistics: Pittsburgh, PA; 1992. Aug 14, NSABP Breast Cancer Prevention Trial risk assessment program, Version 2. [Google Scholar]
- 20.Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782–1792. doi: 10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- 21.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 22.Design of the Women's Health Initiative clinical trial and observational study: The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 23.Vogel VG, Costantino JP, Wickerham LD, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Breast Cancer Risk Assessment Tool (BCRAT) http://www.cancer.gov/gov/bcrisktool/
- 25.Bermejo-Pérez MJ, Márquez-Calderón S, Llanos-Méndez A. Effectiveness of preventive interventions in BRCA1/2 gene mutation carriers: A systematic review. Int J Cancer. 2007;15:225–231. doi: 10.1002/ijc.22817. 121(2) [DOI] [PubMed] [Google Scholar]
- 26.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer–96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;21:272–282. doi: 10.1093/jnci/djk049. 99(4) [DOI] [PubMed] [Google Scholar]
- 27.Powles TJ, Ashley S, Tidy A, et al. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]