Abstract
Purpose
Patients with myeloproliferative neoplasms (MPNs), including polycythemia vera, essential thrombocythemia, and primary myelofibrosis, have a propensity to develop acute myeloid leukemia (AML) and myelodysplastic syndromes (MDSs). Using population-based data from Sweden, we assessed the role of MPN treatment and subsequent AML/MDS risk with special focus on the leukemogenic potential of hydroxyurea (HU).
Methods
On the basis of a nationwide MPN cohort (N = 11,039), we conducted a nested case-control study, including 162 patients (153 and nine with subsequent AML and MDS diagnosis, respectively) and 242 matched controls. We obtained clinical and MPN treatment data for all patients. Using logistic regression, we calculated odds ratios (ORs) as measures of AML/MDS risk.
Results
Forty-one (25%) of 162 patients with MPNs with AML/MDS development were never exposed to alkylating agents, radioactive phosphorous (P32), or HU. Compared with patients with who were not exposed to HU, the ORs for 1 to 499 g, 500 to 999 g, more than 1,000 g of HU were 1.5 (95% CI, 0.6 to 2.4), 1.4 (95% CI, 0.6 to 3.4), and 1.3 (95% CI, 0.5 to 3.3), respectively, for AML/MDS development (not significant). Patients with MPNs who received P32 greater than 1,000 MBq and alkylators greater than 1 g had a 4.6-fold (95% CI, 2.1 to 9.8; P = .002) and 3.4-fold (95% CI, 1.1 to 10.6; P = .015) increased risk of AML/MDS, respectively. Patients receiving two or more cytoreductive treatments had a 2.9-fold (95% CI, 1.4 to 5.9) increased risk of transformation.
Conclusion
The risk of AML/MDS development after MPN diagnosis was significantly associated with high exposures of P32 and alkylators but not with HU treatment. Twenty-five percent of patients with MPNs who developed AML/MDS were not exposed to cytotoxic therapy, supporting a major role for nontreatment-related factors.
INTRODUCTION
Patients with myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) have a propensity to develop acute myeloid leukemia (AML) and myelodysplastic syndromes (MDSs).1,2 In PV, the reported incidence of AML/MDS ranges between 5% and 10% after 10 years of observation, with a time-dependent increase of risk.3–5 The risk seems higher (8% to 20%) in PMF6,7 and lower (2% to 5%) in ET.8–10 The underlying mechanisms of transformation remain an area of controversy.5 Use of alkylating agents and radioactive phosphorous (P32)3,11,12 is associated with an established increased risk for leukemic transformation. Hydroxyurea (HU) has been found to be less leukemogenic,9,10,13,14 although there are reports on increased transformation risk, especially when combined with other cytotoxic agents.15,16 Thus, its long-term effect on AML/MDS development remains controversial.5
The launching of large randomized trials addressing the risk of treatment-related AML/MDS has been hampered by the rarity of MPNs, late-appearing events in a long-term disease course, and reluctance to randomly assign patients to receive potentially leukemogenic therapies. To overcome this dilemma, we conducted the first large comprehensive study using population-based data from Sweden. The aim was to determine to what extent cytoreductive therapies add to the inherent risk of transformation, with a focus on the role of HU.
METHODS
Central Registries, Hospitals, and Patients
All residents of Sweden are assigned a national registration number, which is used in government-maintained nationwide health care and population registries, whereby record linkage is possible with high accuracy. For each individual, date of death is centrally registered in the cause of death registry.
Sweden has provided universal medical health care for the entire population, currently 9 million people, since the mid 1950s. Patients with hematologic malignancies are almost exclusively diagnosed, treated, and observed by physicians at hospital-based hematology/oncology centers.
All physicians and pathologists/cytologists in Sweden are obliged by law to report each incident case of cancer they diagnose to the centralized nationwide cancer registry established in 1958. The registry contains information on diagnosis, sex, date of birth, date of diagnosis, and region/hospital in which the diagnosis was made.17 A recent validation study focusing on lymphoproliferative tumors diagnosed from 1964 to 2003 found more than 90% to 95% completeness and diagnostic accuracy.18 Since 1993, patients with MDSs have been reported to the registry. In Sweden, from the mid 1970s to late 1990s, MPN diagnostics were based on Polycythemia Vera Study Group criteria.19,20 Since the late 1990s, WHO criteria have been used.19,21,22
As described previously,23 we identified 11,039 patients in a nationwide MPN cohort, including all patients with MPNs diagnosed from 1958 to 2005, from the Swedish Cancer Registry. In parallel, we retrieved information on patients with MPNs through our national MPN network to include patients who were not reported to the cancer registry.
In the present study, we conducted record linkage with the cancer registry to obtain information regarding subsequent AML and MDS diagnoses among patients in the MPN cohort. We identified 292 patients with MPNs who developed AML (n = 271) and MDSs (n = 21). Detailed information on treatment (type of therapy, cumulative dose, duration of treatment) and laboratory variables at diagnosis, including full blood count, bone marrow examination (at MPN diagnosis and at transformation), and any other tumor preceding AML/MDS, was collected from medical records. Patients were excluded if there was lack of relevant medical information (n = 51). Also, four and five patients were excluded because the original MPN or AML/MDS diagnosis, respectively, was found to be incorrect or unclear during review, and five patients were excluded because they had received prior chemotherapy or radiotherapy for a non-MPN malignancy.
For each patient with MPNs with a subsequent AML/MDS diagnosis (ie, cases), up to two patients with MPNs without AML/MDS matched for MPN subtype, year of birth (± 5 years), sex, and date of MPN diagnosis (± 1 year) were identified (ie, controls). A control patient could be used for more than one patient case but with adjusted follow-up time. For each control, similar to patient cases, we obtained detailed clinical and treatment information from medical records. Controls were excluded if MPN diagnosis was found to be incorrect or unclear during review (n = 2) or if they had received prior chemotherapy or radiotherapy for a non-MPN malignancy (n = 12). For the matched case-control analysis, 65 patient cases were excluded because there were no matched controls available in the database. Approval for this study was obtained from the Karolinska Institutet Review Board.
Statistical Analysis
The risk of AML was analyzed in the nationwide MPN cohort. All patients were observed from date of MPN diagnosis to date of death, emigration, diagnosis of AML, or end of follow-up, whichever occurred first. The absolute risks with respect to MPN subtype are presented by means of Kaplan-Meier curves. The risk in relation to the expected risk in the population is presented as standardized incidence ratio (SIR; ie, ratio of observed to expected numbers of AML cases). The expected number of cases was estimated by multiplying the age-, sex-, and calendar year–specific person-years of follow-up for patients with MPNs who developed AML, with the corresponding AML rates in the general population obtained from the Swedish Cancer Registry. CIs for the SIRs were determined based on the assumption of Poisson-distributed number of observed cases. Patients who developed MDSs were not included in these analyses because of the later-introduced MDS registration in the cancer registry.
The risk of transformation in relation to cumulative doses of HU, P32, and alkylating agents was analyzed by conditional logistic regression. The matching factors were adjusted for age, sex, calendar period, and MPN subtype. Crude associations for each treatment as well as associations adjusted for other treatments were estimated. Trend tests were performed by considering the categorized variables to be equidistantly numeric. Subset analyses of patients with PV/ET and AML were performed on the matched sets fulfilling these criteria. Results are presented as odds ratios (ORs) together with 95% CIs. Analysis of survival after AML in relation to MPN treatment was performed on all patients with relevant clinical information. All analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
The study population in the nested case-control study consisted of 162 patients with MPNs (59% men; median age, 64 years) who developed AML/MDS (ie, cases) and their 242 matched control patients with MPNs. Characteristics of patient cases and controls are listed in Table 1. Among patient cases, 153 experienced transformation to AML and nine to MDSs. A majority had a preceding PV diagnosis (68%). Thirty-four percent were diagnosed with MPNs before 1980.
Table 1.
Clinical Characteristics of Patients With MPNs Who Developed AML/MDS and Matched Controls
| Characteristic | Patient Cases |
Controls |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Total | 162 | 100 | 242 | 100 |
| Sex | ||||
| Female | 67 | 41 | 101 | 42 |
| Male | 95 | 59 | 141 | 58 |
| MPN subtype | ||||
| PV | 110 | 68 | 176 | 73 |
| ET | 26 | 16 | 39 | 16 |
| PMF | 15 | 9 | 23 | 9 |
| MPN NOS | 11 | 7 | 4 | 2 |
| Age at MPN diagnosis, years | ||||
| < 49 | 17 | 10 | 20 | 8 |
| 50-59 | 35 | 22 | 55 | 23 |
| 60-69 | 55 | 34 | 91 | 38 |
| > 70 | 55 | 34 | 76 | 31 |
| Median | 64 | 65 | ||
| Range | 17-85 | 35-86 | ||
| Type of transformation | ||||
| AML | 153 | 94 | — | |
| MDS | 9 | 6 | — | |
| Calendar period of MPN diagnosis | ||||
| 1958-1969 | 32 | 20 | 44 | 18 |
| 1970-1979 | 23 | 14 | 39 | 16 |
| 1980-1989 | 41 | 25 | 58 | 24 |
| 1990-1999 | 51 | 31 | 81 | 33 |
| 2000-2005 | 15 | 9 | 20 | 8 |
| Treatment | ||||
| None | 41 | 25 | 78 | 32 |
| Alk only | 12 | 7 | 29 | 12 |
| P32 only | 39 | 24 | 59 | 24 |
| HU only | 34 | 21 | 50 | 21 |
| Alk + P32 | 19 | 12 | 13 | 5 |
| Alk + HU | 5 | 3 | 4 | 2 |
| HU + P32 | 10 | 6 | 9 | 4 |
| Alk + P32 + HU | 2 | 1 | 0 | 0 |
Abbreviations: Alk, alkylating agent; AML, acute myeloid leukemia; ET, essential thrombocythemia; HU, hydroxyurea; MDS, myelodysplastic syndrome; MPN, chronic myeloproliferative neoplasm; NOS, not otherwise specified; P32, radioactive phosphorus; PMF, primary myelofibrosis; PV, polycythemia vera.
Risk of AML Transformation in Relation to MPN Subtype, Duration of Disease, WBC Count at Diagnosis, and Sex
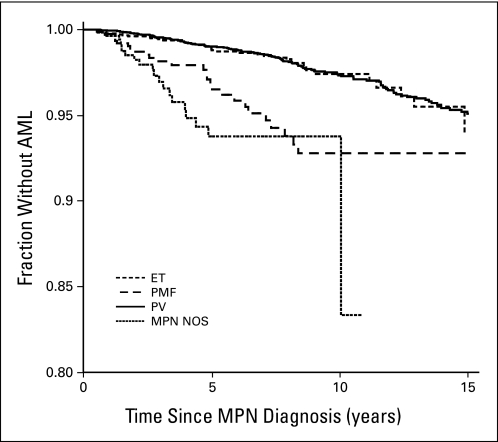
The overall risk of AML transformation was a SIR of 35.1 (95% CI, 30.6 to 39.9); the risk increased with time after a diagnosis of MPNs (approximate SIR after 15 years, 60). Among MPN subtypes, PMF carried the highest risk of AML development (SIR, 63.8; 95% CI, 42.7 to 91.6), followed by PV (SIR, 33.0; 95% CI, 27.8 to 38.9) and ET (SIR, 24.7; 95% CI, 17.3 to 34.2). Risk of AML transformation in relation to time after MPN diagnosis and according to subtype is graphically illustrated in Figure 1. WBC count greater than 9.0 × 109/L at MPN diagnosis was observed in 62.0% of patient cases and 66.4% of controls, respectively. The median WBC count of patient cases and controls with AML at diagnosis was 10.6 and 10.8 × 109/L, respectively. The risk of transformation was equal among men and women when all MPN diagnoses were analyzed together. Among patients with ET, women had a lower risk, whereas the risk among women with PV was higher than that among men. However, the differences were not statistically significant (data not shown).
Fig 1.
Risk of acute myeloid leukemia (AML) transformation in relation to time since myeloproliferative neoplasm (MPN) diagnosis according to subtype. Determined for all patients with MPNs with AML transformation who were identified from the Swedish Cancer Registry (n = 235). ET, essential thrombocythemia; NOS, not otherwise specified; PMF, primary myelofibrosis; PV, polycythemia vera.
Risk of AML/MDS Transformation in Relation to MPN Therapy
Twenty-five percent of patients with MPNs who developed AML/MDS were never exposed to alkylating agents, P32, or HU (v 32% of controls; Table 1). A total of 22% and 11% of patient cases and controls, respectively, received treatment with two or three types of cytoreductive treatments (Table 1). The proportion of patients administered HU alone was identical (21%) among patient cases and controls (Table 1).
Cumulative doses of alkylators, P32, and HU among patient cases and controls are listed in Table 2. Eight percent of patient cases and controls received 1,000 g or more of HU (Table 2). Previous exposure to HU was not significantly associated with an increased risk of AML/MDS transformation at any cumulative dose level, neither in a crude analysis nor after adjustment for other treatments (Table 2).
Table 2.
Risk of AML/MDS Transformation in Patients With MPNs in Relation to Cumulative Dose
| Cumulative Dose | All Patient Cases and Controls |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Cases |
Controls |
Risk of AML/MDS |
Risk of AML/MDS |
Risk of AML Only |
Patient Cases and Controls With PV/ET Risk of AML/MDS |
|||||||
| No. | % | No. | % | Crude OR | 95% CI | Adjusted OR* | 95% CI | Adjusted OR* | 95% CI | Adjusted OR* | 95% CI | |
| Total No. of patients | 162 | 100 | 242 | 100 | ||||||||
| HU, g | ||||||||||||
| 0 | 111 | 69 | 179 | 74 | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| 1-499 | 24 | 15 | 29 | 12 | 1.3 | 0.7 to 2.6 | 1.5 | 0.6 to 2.4 | 1.3 | 0.6 to 2.5 | 1.0 | 0.4 to 2.5 |
| 500-999 | 14 | 9 | 15 | 6 | 1.3 | 0.6 to 3.1 | 1.4 | 0.6 to 3.4 | 1.5 | 0.6 to 3.6 | 0.9 | 0.3 to 2.6 |
| ≥ 1,000 | 13 | 8 | 19 | 8 | 1.0 | 0.4 to 2.3 | 1.3 | 0.5 to 3.3 | 1.1 | 0.4 to 3.0 | 1.2 | 0.5 to 3.2 |
| Trend test P | .510 | .320 | .370 | .600 | ||||||||
| P32, MBq | ||||||||||||
| 0 | 92 | 57 | 161 | 67 | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| 1-499 | 14 | 9 | 21 | 9 | 1.3 | 0.6 to 2.8 | 1.5 | 0.6 to 3.3 | 1.3 | 0.6 to 3.1 | 1.4 | 0.6 to 3.2 |
| 500-999 | 16 | 10 | 32 | 13 | 0.9 | 0.5 to 1.8 | 1.1 | 0.5 to 2.2 | 0.9 | 0.4 to 1.9 | 1.2 | 0.6 to 2.5 |
| ≥ 1,000 | 40 | 25 | 28 | 12 | 4.0 | 1.9 to 8.3 | 4.6 | 2.1 to 9.8 | 4.8 | 2.0 to 9.9 | 4.4 | 2.0 to 9.6 |
| Trend test P | .006 | .002 | .006 | .003 | ||||||||
| Alkylating agents, g | ||||||||||||
| 0 | 124 | 77 | 196 | 81 | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 | Ref |
| 0.1-0.49 | 15 | 9 | 26 | 11 | 1.0 | 0.5 to 2.0 | 1.1 | 0.5 to 2.3 | 1.0 | 0.5 to 2.1 | 1.2 | 0.5 to 2.7 |
| 0.50-0.99 | 11 | 7 | 12 | 5 | 1.7 | 0.6 to 4.4 | 1.7 | 0.6 to 5.0 | 1.4 | 0.5 to 4.4 | 2.1 | 0.7 to 6.4 |
| ≥ 1.00 | 12 | 7 | 8 | 3 | 3.0 | 1.0 to 8.8 | 3.4 | 1.1 to 10.6 | 3.2 | 1.0 to 10.0 | 3.6 | 1.1 to 11.2 |
| Trend test P | .030 | .015 | .032 | .007 | ||||||||
Abbreviations: AML, acute myeloid leukemia; ET, essential thrombocythemia; HU, hydroxyurea; MDS, myelodysplastic syndrome; MPN, chronic myeloproliferative neoplasm; OR, odds ratio; P32, radioactive phosphorus; PV, polycythemia vera; Ref, reference.
Mutually adjusted for other treatments.
A total of 25% of patient cases were exposed to 1,000 MBq or more of P32 in comparison with 12% of controls. Similarly, cumulative doses of alkylators (mainly busulphan) exceeding 1.0 g were recorded in 7% of patient cases and 3% of controls (Table 2). The risk for AML/MDS transformation was strongly associated with high exposure of P32 and alkylating agents in both crude and adjusted analyses. Lower exposure to P32 and alkylating agents was not associated with a significantly increased transformation risk (Table 2). Patients receiving two or three types of cytoreductive treatment had a higher risk of transformation than patients receiving single-agent treatment at any dose level (Table 3). When analysis was restricted to patients with PV and ET, results were essentially unchanged (Table 2). The same was true when patients with transformation to MDSs were excluded (Table 2).
Table 3.
Risk of AML/MDS Transformation According to No. of Cytoreductive Treatment Types
| Treatment | OR | 95% CI |
|---|---|---|
| None | 1.0 | Ref |
| P32 only | 1.5 | 0.8 to 2.8 |
| Alkylating agent only | 0.9 | 0.4 to 2.1 |
| HU only | 1.2 | 0.6 to 2.4 |
| Mixed treatment (two or three) | 2.9 | 1.4 to 5.9 |
Abbreviations: AML, acute myeloid leukemia; HU, hydroxyurea; MDS, myelodysplastic syndrome; OR, odds ratio; P32, radioactive phosphorus; Ref, reference.
Time to AML/MDS transformation differed between the treatment groups. Among patients who received no treatment or HU only, 40% and 42% experienced transformation 5 years or more after MPN diagnosis, respectively. In contrast, a majority of patients treated with alkylating agents (76%), P32 (77%), or combinations of the two (91%) experienced transformation after more than 5 years.
Survival in Patients With AML/MDS After MPNs
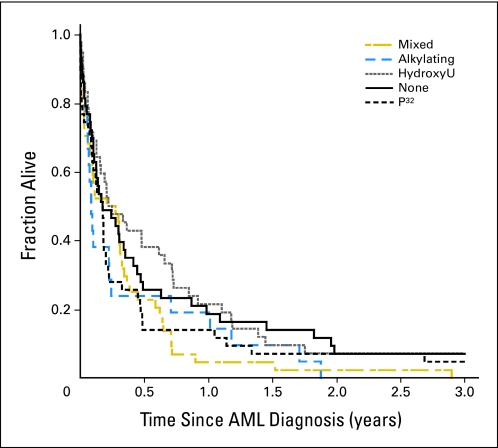
Patients with MPNs with AML transformation had a median survival of 3 months from time of AML diagnosis. Patients with preceding ET tended to have better survival compared with patients with PV/PMF; however, the difference was not significant (data not shown). Outcome after AML transformation was not influenced by type of previous MPN treatment (Fig 2).
Fig 2.
Acute myeloid leukemia (AML) survival in relation to previous therapy. Mixed combinations of two to three cytoreductive treatments (n = 46), alkylators only (n = 21), none (n = 47), hydroxyurea (HydroxyU) only (n = 45), and radioactive phosphorous (P32) only (n = 47).
DISCUSSION
To assess the role of treatment-related risk factors for AML/MDS development in patients with MPNs, we took advantage of a large (N = 11,039) national MPN cohort.23 By identifying patients who experienced transformation to AML/MDS and their matched controls from this cohort, a nested case-control study was performed. The SIR of AML transformation was 24.7 to 63.8, depending on MPN subtype. This is probably a conservative estimate because reporting of transformation in certain patient categories, such as the elderly, occasionally may have been neglected. The increased transformation risks, calculated as ORs, associated with the highest exposure to HU, P32, and alkylators were only 1.3, 4.6, and 3.4, respectively (Table 2). Importantly, 25% of patients with transformation to AML/MDS were never exposed to HU, P32, or alkylating agents. In addition, only 32% of patients with transformed disease were exposed to cumulative doses of P32 and/or alkylating agents shown here to be leukemogenic. We therefore conclude that the risk of transformation is mainly associated with the disease itself, whereas exposure to cytoreductive agents is of less importance.
Our results support the hypothesis that nontreatment-related factors play a major role in AML/MDS development after MPNs. In a recent study based on 18 patients with MPNs who experienced transformation to leukemia, AML1/RUNX1 mutations were detected in five patients at transformation; one patient was never exposed to cytoreductive therapy.24 Furthermore, when AML1/RUNX1 mutants were transduced into stem cells of patients with MPNs in chronic phase, it resulted in proliferation of immature myeloid cells, enhanced self-renewal capacity, and proliferation of primitive progenitors.24 In another study involving 24,577 first-degree relatives of the 11,039 patients with MPNs described in the present study, we found a 1.8-fold (95% CI, 0.9 to 3.8; P = .09) excess risk of AML development in relatives of patients with ET.23
Another main finding is the lack of a significant association between HU exposure and AML/MDS risk. The potential leukemogenic effect of HU has remained a controversial issue for many years.5,25,26 AML transformation has been reported in up to 22% of patients after therapy with HU alone16,27 and 30% of patients after therapy with HU in combination with busulphan.16 Other studies, including the large prospective ECLAP (European Collaboration on Low-dose Aspirin in Polycythemia Vera) study, have found no association between HU treatment and leukemic transformation in patients with ET and PV.12,25,28–31 In the randomized trial comparing HU and pipobroman (piperazine derivative that acts via alkylating mechanism) in patients with PV, leukemic transformation was significantly more common after pipobroman therapy compared with HU therapy, with cumulative incidences of 22% versus 56% at 20 years.27 Importantly, in this study, half of the patients who developed AML/MDS in the HU group received more than one cytoreductive drug, whereas in the pipobroman group, 85% of AML/MDS cases received pipobroman only.14,32 The discrepant results published regarding HU exposure and risk of AML/MDS are probably related to differences in patient characteristics (including MPN subtypes, MPN therapy, follow-up, and study size). On the basis of our findings, we cannot totally exclude a leukemogenic effect of HU. However, this potential risk is limited compared with the risk associated with the disease itself. Although sickle-cell anemia is not a myeloproliferative disorder, we believe the fact that AML/MDS development is a rare event even after many years of HU treatment in patients with sickle-cell anemia provides additional support for this notion.33,34 In vitro studies have also suggested that the mutagenic and carcinogenic potential of in vivo HU therapy is low.35
As expected, patients exposed to P32 or alkylating agents carried an increased risk of AML/MDS transformation, though only at cumulative doses greater than 1,000 MBq or 1 g, respectively. No dose dependency was observed in the lower dose intervals. Although based on small patient numbers, this may indicate the existence of a threshold exposure of P32 and alkylators for AML/MDS transformation in MPNs.36 However, patients may be at higher risk of transformation not because of treatment-related factors but rather because of longer disease span and more aggressive disease biology, causing exposure to higher doses and/or multiple drugs. Interestingly, most of the patients who developed AML/MDS after no treatment or HU treatment only did so within 5 years of MPN diagnosis. This may corroborate the notion that HU is nonleukomogenic because the majority of patients administered P32 and/or alkylators experienced transformation at a later time point.
SIRs for AML transformation among men and women did not differ when all MPN diagnoses were included in the analysis. We observed a lower SIR for women with ET, whereas the SIR for women with PV was slightly higher than that for men. None of these differences were statistically significant; thus we could not verify the previously reported higher incidence of transformation in women.1,25 In addition, we did not observe any association between leukocytosis and risk of AML transformation, which has been suggested by some investigators37 but not others.38 As reported previously, the risk of transformation was highest among patients with PMF.1,2 The median survival of patients after AML transformation was 3 months, confirming the dismal prognosis associated with this event.1,6
Our study has several strengths, including its large size and the application of high-quality data from Sweden in a stable population with access to standardized universal medical health care. The use of the nationwide register-based case-control design ruled out recall bias, ensured a population-based setting, and provided generalizability of our findings. Patient cases and controls were diagnosed with MPNs over a period of 47 years, with almost 60% diagnosed before 1990. A long observation time is clearly advantageous when studying diseases with long and indolent courses and late-appearing events of interest.
Although we used the largest population-based MPN database (N = 11,039) to date, we were limited by numbers for the nested case-control study. More specifically, when we matched patient cases and controls, we applied conservative matching criteria (MPN subtype, year of birth [± 5 years], sex, date of MPN diagnosis [± 1 year]). Consequently, 65 patient cases were excluded because to lack of matched controls. We obtained clinical data on 44 of these patient cases and found clinical and treatment features for the excluded patient cases to be similar to patients included in the study (Appendix Table A1, online only). In a sensitivity analysis in which we relaxed the matching criteria to include only duration of disease and MPN subtype, these 44 patients could be included in an analysis of a larger number of patients (206 patient cases and 366 controls). The results were similar to those obtained in the main analysis (Appendix Table A2, online only). Thus, we feel confident in the correctness and robustness of our results.
Only 2.6% of the 11,039 patients with MPNs in the cohort experienced transformation to AML/MDS. Given the long observation time, this proportion is lower than that previously reported,1–3,7–9,27 although AML transformation was observed in 1.4% and 2% of patients receiving busulphan and P32, respectively, at a median follow-up time of 8 years in a randomized trial by the European Organisation for Research and Treatment of Cancer.36 We speculate that this may be the result of selection bias in prior clinical studies, under-reporting in our population-based study, or most probably a combination of both factors. Such a discrepancy would, however, not likely affect the results of this nested case-control study because of its design and the large number of patients included.
The diagnostic criteria for MPNs changed during the study period; many patients with ET in this study would today be diagnosed with PMF. In a Swedish follow-up study of 60 patients treated with anagrelide between 1998 and 2002, the diagnostic bone marrow samples were reevaluated blindly according to WHO criteria. Twenty-one of 42 patients with ET were identified as truly having ET.39 Thus, the AML/MDS SIRs reported here among patients with ET may be overestimations. However, this is not likely to affect the results regarding treatment-related risk.
In summary, we conclude that the inherent propensity of MPNs to AML/MDS transformation is substantial, as judged from the fact that a quarter of the patients who developed AML/MDS were never exposed to cytoreductive therapy. In addition, HU exposure, even at high doses, is not associated with a significantly increased risk of transformation to AML/MDS. These findings have important implications regarding treatment strategies in MPNs, especially in younger patients requiring decades of active treatment.
Appendix
Table A1.
Clinical Characteristics of Included and Excluded Patients With MPNs Who Developed AML/MDS
| Characteristic | Included |
Excluded |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Total No. of patients | 162 | 100 | 44 | 100 |
| Sex | ||||
| Female | 67 | 41 | 21 | 48 |
| Male | 95 | 59 | 23 | 52 |
| MPN subtype | ||||
| PV | 110 | 68 | 30 | 68 |
| ET | 26 | 16 | 7 | 16 |
| PMF | 15 | 9 | 4 | 9 |
| MPN NOS | 11 | 7 | 3 | 7 |
| Age at MPN diagnosis, years | ||||
| < 49 | 17 | 10 | 10 | 22 |
| 50-59 | 35 | 22 | 9 | 20 |
| 60-69 | 55 | 34 | 12 | 27 |
| > 70 | 55 | 34 | 13 | 30 |
| Calendar period of MPN diagnosis | ||||
| 1958-1969 | 32 | 20 | 10 | 23 |
| 1970-1979 | 23 | 14 | 10 | 23 |
| 1980-1989 | 41 | 25 | 10 | 23 |
| 1990-1999 | 51 | 31 | 11 | 25 |
| 2000-2005 | 15 | 9 | 3 | 7 |
| Treatment | ||||
| None | 41 | 25 | 7 | 16 |
| Alk only | 12 | 7 | 9 | 20 |
| P32 only | 39 | 24 | 8 | 18 |
| HU only | 34 | 21 | 9 | 20 |
| Alk + P32 | 19 | 12 | 4 | 9 |
| Alk + HU | 5 | 3 | 4 | 9 |
| HU + P32 | 10 | 6 | 2 | 5 |
| Alk + P32 + HU | 2 | 1 | 1 | 2 |
NOTE. Included patients had available matched controls; patients were excluded because of lack of available matched controls.
Abbreviations: Alk, alkylating agent; AML, acute myeloid leukemia; ET, essential thrombocythemia; HU, hydroxyurea; MDS, myelodysplastic syndrome; MPN, chronic myeloproliferative neoplasm; NOS, not otherwise specified; P32, radioactive phosphorus; PMF, primary myelofibrosis; PV, polycythemia vera.
Table A2.
Risk of AML/MDS Transformation in Patients With MPNs in Relation to Cumulative Doses When Matching Criteria Includes Only Duration of Disease and Type of MPN Diagnosis
| Cumulative Dose | Patient Cases |
Controls |
Risk of AML/MDS |
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | Crude OR | 95% CI | Adjusted OR* | 95% CI | |
| Total No. of patients | 206 | 100 | 366 | 100 | ||||
| HU, g | ||||||||
| 0 | 139 | 67 | 265 | 72 | 1.0 | Ref | 1.0 | Ref |
| 1-499 | 35 | 17 | 46 | 13 | 1.4 | 0.8 to 2.5 | 1.4 | 0.8 to 2.5 |
| 500-999 | 17 | 8 | 28 | 8 | 1.0 | 0.5 to 2.2 | 1.2 | 0.5 to 2.9 |
| ≥ 1,000 | 15 | 7 | 27 | 7 | 0.9 | 0.4 to 2.2 | 1.2 | 0.5 to 2.9 |
| Trend test P | .45 | .26 | ||||||
| P32, MBq | ||||||||
| 0 | 121 | 59 | 246 | 67 | 1.0 | Ref | 1.0 | Ref |
| 1-499 | 16 | 8 | 28 | 8 | 1.4 | 0.7 to 2.9 | 1.6 | 0.7 to 3.5 |
| 500-999 | 20 | 10 | 45 | 12 | 1.3 | 0.7 to 2.4 | 1.5 | 0.8 to 2.9 |
| ≥ 1,000 | 49 | 24 | 47 | 13 | 2.1 | 1.2 to 3.8 | 2.6 | 1.4 to 4.8 |
| Trend test P | .019 | .0027 | ||||||
| Alkylating agents, g | ||||||||
| 0 | 150 | 73 | 299 | 82 | 1.0 | Ref | 1.0 | Ref |
| 0.1-0.49 | 21 | 10 | 35 | 10 | 1.2 | 0.6 to 2.2 | 1.3 | 0.7 to 2.6 |
| 0.50-0.99 | 15 | 7 | 19 | 5 | 1.5 | 0.7 to 3.2 | 1.7 | 0.7 to 4.0 |
| ≥ 1.00 | 20 | 10 | 13 | 3 | 2.7 | 1.2 to 5.9 | 3.4 | 1.5 to 7.8 |
| Trend test P | .015 | .0025 | ||||||
Abbreviations: AML, acute myeloid leukemia; HU, hydroxyurea; MDS, myelodysplastic syndrome; MPN, chronic myeloproliferative neoplasm; OR, odds ratio; P32, radioactive phosphorus; Ref, reference.
Mutually adjusted for other treatments.
Footnotes
Supported by the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet; an unrestricted grant from Shire Pharmaceuticals; the Adolf H. Lundin Charitable Foundation; the Swedish Cancer Society; and the Intramural Program of the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Magnus Björkholm, Shire Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Magnus Björkholm, Åsa R. Derolf, Malin Hultcrantz, Sigurdur Y. Kristinsson, Ola Landgren
Provision of study materials or patients: Magnus Björkholm, Åsa R. Derolf, Malin Hultcrantz, Sigurdur Y. Kristinsson, Björn Andreasson, Gunnar Birgegård, Olle Linder, Claes Malm, Berit Markevärn, Lars Nilsson, Jan Samuelsson
Collection and assembly of data: Charlotta Ekstrand, Björn Andreasson, Gunnar Birgegård, Olle Linder, Claes Malm, Berit Markevärn, Lars Nilsson, Jan Samuelsson
Data analysis and interpretation: Magnus Björkholm, Åsa R. Derolf, Malin Hultcrantz, Sigurdur Y. Kristinsson, Lynn R. Goldin, Fredrik Granath, Ola Landgren
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Abdulkarim K, Girodon F, Johansson P, et al. AML transformation in 56 patients with Ph- MPD in two well defined populations. Eur J Haematol. 2009;82:106–111. doi: 10.1111/j.1600-0609.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 2.Cervantes F, Tassies D, Salgado C, et al. Acute transformation in nonleukemic chronic myeloproliferative disorders: Actuarial probability and main characteristics in a series of 218 patients. Acta Haematol. 1991;85:124–127. doi: 10.1159/000204873. [DOI] [PubMed] [Google Scholar]
- 3.Berk PD, Goldberg JD, Silverstein MN, et al. Increased incidence of acute leukemia in polycythemia vera associated with chlorambucil therapy. N Engl J Med. 1981;304:441–447. doi: 10.1056/NEJM198102193040801. [DOI] [PubMed] [Google Scholar]
- 4.Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: A Polycythemia Vera Study Group report on hydroxyurea in patients with polycythemia vera. Semin Hematol. 1997;34:17–23. [PubMed] [Google Scholar]
- 5.Barbui T. The leukemia controversy in myeloproliferative disorders: Is it a natural progression of disease, a secondary sequela of therapy, or a combination of both? Semin Hematol. 2004;41:15–17. doi: 10.1053/j.seminhematol.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Mesa RA, Li CY, Ketterling RP, et al. Leukemic transformation in myelofibrosis with myeloid metaplasia: A single-institution experience with 91 cases. Blood. 2005;105:973–977. doi: 10.1182/blood-2004-07-2864. [DOI] [PubMed] [Google Scholar]
- 7.Okamura T, Kinukawa N, Niho Y, et al. Primary chronic myelofibrosis: Clinical and prognostic evaluation in 336 Japanese patients. Int J Hematol. 2001;73:194–198. doi: 10.1007/BF02981937. [DOI] [PubMed] [Google Scholar]
- 8.Chim CS, Kwong YL, Lie AK, et al. Long-term outcome of 231 patients with essential thrombocythemia: Prognostic factors for thrombosis, bleeding, myelofibrosis, and leukemia. Arch Intern Med. 2005;165:2651–2658. doi: 10.1001/archinte.165.22.2651. [DOI] [PubMed] [Google Scholar]
- 9.Passamonti F, Rumi E, Arcaini L, et al. Prognostic factors for thrombosis, myelofibrosis, and leukemia in essential thrombocythemia: A study of 605 patients. Haematologica. 2008;93:1645–1651. doi: 10.3324/haematol.13346. [DOI] [PubMed] [Google Scholar]
- 10.Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: Safety of cytoreductive therapy. Am J Hematol. 2009;84:215–220. doi: 10.1002/ajh.21360. [DOI] [PubMed] [Google Scholar]
- 11.Modan B, Lilienfeld AM. Polycythemia vera and leukemia: The role of radiation treatment—A study of 1,222 patients. Medicine (Baltimore) 1965;44:305–344. doi: 10.1097/00005792-196507000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Radaelli F, Onida F, Rossi FG, et al. Second malignancies in essential thrombocythemia (ET): A retrospective analysis of 331 patients with long-term follow-up from a single institution. Hematology. 2008;13:195–202. doi: 10.1179/102453308X316022. [DOI] [PubMed] [Google Scholar]
- 13.Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Kiladjian JJ, Rain JD, Bernard JF, et al. Long-term incidence of hematological evolution in three French prospective studies of hydroxyurea and pipobroman in polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:417–421. doi: 10.1055/s-2006-942762. [DOI] [PubMed] [Google Scholar]
- 15.Najean Y, Rain JD. Treatment of polycythemia vera: Use of 32P alone or in combination with maintenance therapy using hydroxyurea in 461 patients greater than 65 years of age—The French Polycythemia Study Group. Blood. 1997;89:2319–2327. [PubMed] [Google Scholar]
- 16.Nielsen I, Hasselbalch HC. Acute leukemia and myelodysplasia in patients with a Philadelphia chromosome negative chronic myeloproliferative disorder treated with hydroxyurea alone or with hydroxyurea after busulphan. Am J Hematol. 2003;74:26–31. doi: 10.1002/ajh.10375. [DOI] [PubMed] [Google Scholar]
- 17.National Board of Health and Welfare: Cancer Incidence in Sweden 2008. http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/17841/2009-12-1.pdf.
- 18.Turesson I, Linet MS, Björkholm M, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964-2003. Int J Cancer. 2007;121:2260–2266. doi: 10.1002/ijc.22912. [DOI] [PubMed] [Google Scholar]
- 19.Berlin NI. Diagnosis and classification of the polycythemias. Semin Hematol. 1975;12:339–351. [PubMed] [Google Scholar]
- 20.Kutti J, Wadenvik H. Diagnostic and differential criteria of essential thrombocythemia and reactive thrombocytosis. Leuk Lymphoma. 1996;22(suppl 1):41–45. doi: 10.3109/10428199609074359. [DOI] [PubMed] [Google Scholar]
- 21.Andreasson B, Löfvenberg E, Westin J. Management of patients with polycythaemia vera: Results of a survey among Swedish haematologists. Eur J Haematol. 2005;74:489–495. doi: 10.1111/j.1600-0609.2005.00424.x. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe E, Harris NL, Stein H, et al., editors. Lyon, France: IARC Press; 2001. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 23.Landgren O, Goldin LR, Kristinsson SY, et al. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008;112:2199–2204. doi: 10.1182/blood-2008-03-143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Y, Harada Y, Imagawa J, et al. AML1/RUNX1 point mutation possibly promotes leukemic transformation in myeloproliferative neoplasms. Blood. 2009;114:5201–5205. doi: 10.1182/blood-2009-06-223982. [DOI] [PubMed] [Google Scholar]
- 25.Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: An analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005;105:2664–2670. doi: 10.1182/blood-2004-09-3426. [DOI] [PubMed] [Google Scholar]
- 26.McMullin MF. A review of the therapeutic agents used in the management of polycythaemia vera. Hematol Oncol. 2007;25:58–65. doi: 10.1002/hon.809. [DOI] [PubMed] [Google Scholar]
- 27.Kiladjian J-J, Chevret S, Dosquet C, et al. Long-term outcome in polycythemia vera (PV): Final analysis of a randomised trial comparing hydroxyurea (HU) to pipobroman (Pi) Blood. 2008:112. abstr 1746. [Google Scholar]
- 28.Finazzi G, Ruggeri M, Rodeghiero F, et al. Second malignancies in patients with essential thrombocythaemia treated with busulphan and hydroxyurea: Long-term follow-up of a randomized clinical trial. Br J Haematol. 2000;110:577–583. doi: 10.1046/j.1365-2141.2000.02188.x. [DOI] [PubMed] [Google Scholar]
- 29.Randi ML, Fabris F, Girolami A. Leukemia and myelodysplasia effect of multiple cytotoxic therapy in essential thrombocythemia. Leuk Lymphoma. 2000;37:379–385. doi: 10.3109/10428190009089438. [DOI] [PubMed] [Google Scholar]
- 30.West WO. Hydroxyurea in the treatment of polycythemia vera: A prospective study of 100 patients over a 20-year period. South Med J. 1987;80:323–327. doi: 10.1097/00007611-198703000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Mavrogianni D, Viniou N, Michali E, et al. Leukemogenic risk of hydroxyurea therapy as a single agent in polycythemia vera and essential thrombocythemia: N- and K-ras mutations and microsatellite instability in chromosomes 5 and 7 in 69 patients. Int J Hematol. 2002;75:394–400. doi: 10.1007/BF02982131. [DOI] [PubMed] [Google Scholar]
- 32.Najean Y, Rain JD. Treatment of polycythemia vera: The use of hydroxyurea and pipobroman in 292 patients under the age of 65 years. Blood. 1997;90:3370–3377. [PubMed] [Google Scholar]
- 33.Maier-Redelsperger M, Labie D, Elion J. Long-term hydroxyurea treatment in young sickle cell patients. Curr Opin Hematol. 1999;6:115–120. doi: 10.1097/00062752-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanft VN, Fruchtman SR, Pickens CV, et al. Acquired DNA mutations associated with in vivo hydroxyurea exposure. Blood. 2000;95:3589–3593. [PubMed] [Google Scholar]
- 36.Leukemia and Hematosarcoma Cooperative Group. European Organisation for Research and Treatment of Cancer (EORTC): Treatment of polycythaemia vera by radiophosphorus or busulphan: A randomized trial. Br J Cancer. 1981;44:75–80. doi: 10.1038/bjc.1981.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tefferi A, Gangat N, Wolanskyj AP, et al. 20+ yr without leukemic or fibrotic transformation in essential thrombocythemia or polycythemia vera: Predictors at diagnosis. Eur J Haematol. 2008;80:386–390. doi: 10.1111/j.1600-0609.2008.01038.x. [DOI] [PubMed] [Google Scholar]
- 38.Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: The impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24:1574–1579. doi: 10.1038/leu.2010.148. [DOI] [PubMed] [Google Scholar]
- 39.Ejerblad E, Kvasnicka H, Thiele J, et al. Seven year follow-up shows that WHO bone marrow criteria effectively identifies true ET with no fibrosis. Presented at the 15th Congress of the European Hematology Association; June 10-13; Barcelona, Spain. 2010. abstr 0986. [Google Scholar]