Abstract
Background. Chronic kidney disease (CKD) is a growing health problem worldwide that leads to end-stage kidney failure and cardiovascular complications. We aimed to determine the prevalence of CKD in Turkey, and to evaluate relationships between CKD and cardiovascular risk factors in a population-based survey.
Methods. Medical data were collected through home visits and interviews. Serum creatinine, blood glucose, total cholesterol, triglycerides, HDL, LDL and uric acid were determined from 12-h fasting blood samples, and spot urine tests were performed for subjects who gave consent to laboratory evaluation.
Results. A total of 10 872 participants were included in the study. The final analysis was performed on 10 748 subjects (mean age 40.5 ± 16.3 years; 55.7% women) and excluded 124 pregnant women. A low glomerular filtration rate (GFR) (< 60 mL/min/1.73 m2) was present in 5.2% of the subjects who were evaluated for GFR, while microalbuminuria and macroalbuminuria were observed in 10.2% and 2% of the subjects, respectively. The presence of CKD was assessed in subjects who gave consent for urinary albumin excretion measurement (n = 8765). The overall prevalence of CKD was 15.7%; it was higher in women than men (18.4% vs. 12.8%, P < 0.001) and increased with increasing age of the subjects. The prevalence of hypertension (32.7% in the general population), diabetes (12.7%), dyslipidaemia (76.3%), obesity (20.1%) and metabolic syndrome (31.3%) was significantly higher in subjects with CKD than subjects without CKD (P < 0.001 for all).
Conclusions. The prevalence of CKD in Turkey is 15.7%. Cardiovascular risk factors were significantly more prevalent in CKD patients.
Keywords: chronic kidney disease, epidemiology and outcomes, risk factors
Introduction
Chronic kidney disease (CKD) is a growing health problem worldwide that leads to end-stage kidney failure and cardiovascular complications [1]. CKD is defined as kidney damage and/or decreased kidney function expressed as glomerular filtration rate (GFR) for at least 3 months, regardless of the cause [2,3].
CKD is classified into five stages based on the severity of the disease [2,3]. Annual reports from the United States Renal Data System (USRDS) and the European Renal Association–European Dialysis and Transplant Association (ERA–EDTA) have shown that incidence and prevalence rates of stage 5 CKD, also known as end-stage renal disease (ESRD), are on the rise [4,5]. International comparisons published in the 2009 USRDS atlas indicated that Taiwan [(415 per million people (pmp)], Jalisco-Mexico (372 pmp) and the USA (361 pmp) are the countries having the highest incidences of ESRD. In terms of prevalence, Taiwan (2288 pmp), Japan (2060 pmp) and the USA (1698 pmp) had the highest rates [5]. The data from the Turkish Society of Nephrology (TSN) 2008 annual registry indicated that the incidence of ESRD in Turkey has increased nearly 4-fold since 2000, while the prevalence of ESRD has doubled (incidence, 52 vs. 188 pmp; prevalence, 358 vs. 756 pmp, respectively) [6,7].
In the USA, the prevalence of stage 1–4 CKD was 13.1% according to results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004 data [8]. In a systematic review of population-based studies, the median CKD (defined as < 60 mL/min/1.73 m2) was 7.2% for persons aged 30 years or older but varied from 23.4% to 35.8% in the elderly population [9].
CKD patients at high risk for developing ESRD need to be identified and monitored for timely transition to renal replacement therapy. Moreover, angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARB) appear to have renoprotective effects and may be used in early-stage CKD to prevent progression of the disease [10–13].
In this large population-based study, we aimed to determine the prevalence of CKD in Turkey, and to assess the relationship between CKD and other cardiovascular risk factors such as diabetes, hypertension, dyslipidaemia, obesity and metabolic syndrome.
Materials and methods
Study design
We performed a population-based, national survey in Turkey on populations aged over 18 years. All subjects included in this survey gave informed consent to participate in the field study. Subjects with cognitive dysfunction that interfered with understanding and answering the study questionnaire were excluded from the study.
The study was approved by the Ethics Committee of the Akdeniz University Faculty of Medicine and the Turkish Ministry of Health.
Sampling method
A cluster sampling technique was used to select the study participants. A sampling frame was defined as the seven official geographical regions of Turkey, which included 81 cities. The study sample comprised 23 cities such that a minimum 50% of the total population in each geographical region formed a sampling outline by including both the city with the highest population and a randomly selected city with a low population in each geographical area.
The list of districts involved in the postal area codes of the selected cities was used to determine the cluster sampling outline for urban areas. The list of residences within 80 km of the centre of the selected cities, which are defined as rural areas according to the Turkish Statistical Institute [14] classification, was used to determine the cluster sampling outline for rural areas.
The sample size of the study included ~ 10 500 participants, and the prevalence of CKD was estimated with a 95% confidence interval according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiatives (NKF KDOQI) practice guidelines and the NHANES-III [2,15]. The number of subjects in each study subgroup was calculated on the basis of age distribution of women and men in each geographical area (since the sampling method was cluster sampling, the effect size was set at 1.2).
Field study
The data were collected through home visit and interviews of potential participants by specially trained field study teams. During interviews, the study questionnaire was given which included questions on subject demographics, socioeconomic status, diet, lifestyle, current diseases and drugs, family history, and other relevant medical history. In addition, height, weight, waist circumference and blood pressure were measured. Blood pressure was measured two times at 2-min intervals from both arms, and the average of the two measurements was recorded.
Laboratory assessment
Laboratory assessment was performed for subjects who gave written consent for the laboratory evaluation during interviews. These subjects were visited the next day to collect 12-h fasting blood samples. At the same time, morning spot urine samples were obtained from the participants. Additionally, spot urine analysis (by dipsticks) was performed during home visits, and results were recorded on the study questionnaire. Albuminuria measurements were excluded in pregnant or menstruating women and in all patients suffering from febrile illness [16].
Urine albumin (immunoturbidimetric method) and creatinine (alkaline picrate method), serum creatinine (alkaline picrate method), blood glucose (hexokinase method), total cholesterol (cholesterol oxidase method), triglycerides (glycerol phosphate oxidase method), HDL cholesterol (accelerator selective detergent method), LDL cholesterol, and uric acid (uricase method) concentrations were determined.
Definitions
Serum creatinine levels, estimated GFR and spot urine microalbuminuria were studied as markers for kidney function.
CKD was defined as kidney damage with or without a decrease in GFR, which was calculated using a simplified version of the Modification of Diet in Renal Disease (MDRD) formula [186 × (Scr)− 1.154 × (Age)− 0.203 × (0.742 if woman) × (1.212 if African American)] [17]. Since there were no African American subjects in our study population, the last variable of the formula was not used.
CKD was categorized into five stages based on the classification system established by the NKF KDOQI [2]: stage 1, signs of kidney damage as evidenced by microalbuminuria or macroalbuminuria and normal GFR (≥ 90 mL/min/1.73 m2); stage 2, microalbuminuria or macroalbuminuria and with mild decrease in GFR (60–89 mL/min/1.73 m2); stage 3, moderate decrease in GFR (30–59 mL/min/1.73 m2); stage 4, severe decrease in GFR (15–29 mL/min/1.73 m2); and stage 5, GFR < 15 mL/min/1.73 m2 or dialysis.
The urinary albumin-to-creatinine ratio was determined as milligrams per gram. Albuminuria is defined as an albumin-to-creatinine ratio of 30 mg/g or higher, with microalbuminuria defined as an albumin-to-creatinine ratio of 30–299 mg/g, and macroalbuminuria defined as an albumin-to-creatinine ratio of 300 mg/g or higher [18].
For the purpose of this study, ‘hypertension’ was assumed to be present if there was a recorded diagnosis of hypertension, systolic blood pressure > 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg. ‘Diabetes mellitus’ was defined as diagnosis of diabetes mellitus or fasting blood glucose ≥ 126 mg/dL. The term ‘dyslipidaemia’ indicated patients having anti-lipid treatment or dyslipidaemia shown in the lipid profile (total cholesterol > 240 mg/dL or LDL cholesterol > 130 mg/dL or HDL cholesterol < 40 mg/dL for men, < 46 mg/dL for women, or serum triglyceride > 150 mg/dL). The term ‘obesity’ indicated a body mass index ≥ 30 kg/m2. The American Heart Association (AHA) criteria were used for the diagnosis of metabolic syndrome, which included the presence of three or more of the following components: elevated waist circumference (for men ≥ 102 cm and for women ≥ 88 cm), elevated triglycerides (≥ 150 mg/dL), reduced HDL (for men < 40 mg/dL and for women < 50 mg/dL), elevated blood pressure (≥ 130/85 mmHg) or elevated fasting glucose (≥ 100 mg/dL) [19].
Statistical analysis
Analyses were performed on populations weighted according to age groups, gender, geographical area and residence. Student's t-tests and Mann–Whitney U-tests were used for comparisons of continuous variables, which showed normal and non-normal distribution, respectively. Chi-square tests were used to compare proportions between groups. The Mantel–Haenszel test for linear association was used to test the significance of linear relationships between ordinal variables. The relative risk of risk factors for CKD prevalence was expressed as odds ratios with 95% confidence intervals (CI). Statistical significance was defined as P < 0.05.
Results
Study population
A total of 10 872 participants from throughout the country were included in the study. The data from 124 pregnant women were excluded, giving a final total of 10 748 subjects. The majority of the study population (71.6%) came from urban areas, while the remainder lived in rural areas. The distribution of subjects according to the geographical regions of Turkey was parallel to the population density of the regions, which is highest in the Marmara, Central Anatolia and the Aegean Regions. The mean age of subjects was 40.5 ± 16.3 years. The distribution of subject age groups showed that the majority of subjects (> 70%) were aged below 50 years. Of all the subjects, 55.7% were women (Table 1).
Table 1.
Characteristics of the study population (n = 10 748)
| n (%) or mean ± SD | ||
|---|---|---|
| Gender | Female | 5983 (55.7%) |
| Male | 4765 (44.3%) | |
| Mean age (years) | Total | 40.54 ± 16.30 |
| Female | 41.16 ± 16.71 | |
| Male | 39.91 ± 15.86 | |
| Age groups | < 30 years | 1902 (17.7%) |
| 30–39 years | 2540 (23.6%) | |
| 40–49 years | 2196 (20.4%) | |
| 50–59 years | 1816 (16.9%) | |
| 60–69 years | 1594 (14.8%) | |
| 70–79 years | 573 (5.3%) | |
| ≥ 80 years | 127 (1.2%) | |
| Residence | Urban | 7697 (71.6%) |
| Rural | 3051 (28.4%) | |
| Geographical region | Central Anatolia | 2073 (19.3%) |
| Mediterranean | 975 (9.1%) | |
| Marmara | 4502 (41.9%) | |
| Aegean | 910 (8.5%) | |
| East Anatolia | 646 (6%) | |
| Southeastern Anatolia | 676 (6.3%) | |
| Black Sea | 966 (9%) |
The number of subjects is crude (unadjusted) figures.
Kidney function
Creatinine levels and GFR were determined in 10 056 subjects, and microalbuminuria as well as urinalysis was performed in 9064 subjects.
Serum creatinine
Overall, the mean creatinine level of the subjects was 0.90 ± 0.28 mg/dL. The creatinine level was significantly higher in men than women (1.00 ± 0.29 mg/dL and 0.80 ± 0.23 mg/dL, respectively, P < 0.001). There was a slight increase in creatinine level with increasing age (Table 2).
Table 2.
Mean serum creatinine, glomerular filtration rate and microalbuminuria with respect to gender and age groups of the study population
| Serum creatinine (mg/dL) |
GFR (mL/min/1.73 m2) |
Albuminuria (mg/g creatinine) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | P-value | n | Mean ± SD | P-value | n | Mean ± SD (median; IQR) | P-value | |
| Gender | |||||||||
| Female | 5110 | 0.80 ± 0.23 | < 0.001 | 5110 | 89.6 ± 19.9 | < 0.001 | 4615 | 35.1 ± 165.6 (7.55; 10.95) | < 0.001 |
| Male | 4947 | 1.00 ± 0.29 | 4947 | 94.2 ± 21.6 | 4449 | 28.9 ± 145.2 (5.71; 8.75) | |||
| Age groupsa | |||||||||
| < 30 | 3137 | 0.86 ± 0.19 | 3137 | 105.6 ± 20.4 | 2696 | 24.4 ± 91.5 (6.18; 8.36) | |||
| 30–39 | 2241 | 0.88 ± 0.25 | 0.064 | 2241 | 94.4 ± 16.8 | < 0.001 | 2030 | 21.4 ± 100.4 (5.76; 7.63) | 0.021 |
| 40–49 | 1860 | 0.90 ± 0.22 | 0.43 | 1860 | 87.8 ± 15.4 | < 0.001 | 1700 | 33.3 ± 228.6 (5.90; 8.75) | 0.68 |
| 50–59 | 1333 | 0.95 ± 0.48 | < 0.001 | 1333 | 81 ± 15.8 | < 0.001 | 1259 | 43.9 ± 189 (7.27; 13.21) | < 0.001 |
| 60–69 | 790 | 0.95 ± 0.33 | 0.99 | 790 | 77 ± 16 | < 0.001 | 728 | 53.5 ± 201.3 (8.76; 18.95) | 0.051 |
| 70–79 | 543 | 0.99 ± 0.27 | 0.12 | 543 | 71 ± 17.4 | < 0.001 | 502 | 46 ± 153.3 (11.65; 21.34) | 0.001 |
| ≥ 80 | 153 | 0.99 ± 0.22 | 0.99 | 153 | 67.6 ± 14.6 | 0.32 | 150 | 50.3 ± 99.2 (14.93; 29.96) | 0.006 |
| Total | 10 056 | 0.90 ± 0.28 | 10 056 | 91.8 ± 20.9 | 9064 | 32.1 ± 156 (6.59; 10.05) | |||
The numbers of subjects are adjusted (to age, gender, geographic region and residence) figures.
GFR, glomerular filtration rate; SD, standard deviation; IQR, interquartile range.
P-values correspond to the comparisons with previous age group.
Glomerular filtration rate
The estimated mean GFR was 91.8 ± 20.9 mL/min/1.73 m2, and it was significantly higher in men and in younger groups of subjects (P < 0.001, for both analyses) (Table 2).
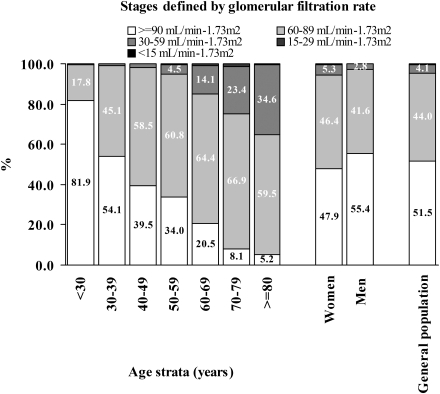
Decreased kidney function, defined as a moderate or severe decrease in GFR, was detected in 4.4% of the subjects who were evaluated for GFR. A moderate decrease (GFR, 30–59 mL/min/1.73 m2) was observed in 4.1%, while a GFR of 15–29 mL/min/1.73 m2 was noted in 0.24%, and a GFR < 15 mL/min/1.73 m2 GFR in 0.13%. Increasing subject age produced a consistent increase in the prevalence of mild and moderate decreases in GFR (P < 0.001) (Figure 1).
Fig. 1.
Percentage of subjects according to glomerular filtration rate with respect to gender and age groups. There was a significant relation between the stage of glomerular filtration rate and age group (P < 0.001).
Microalbuminuria
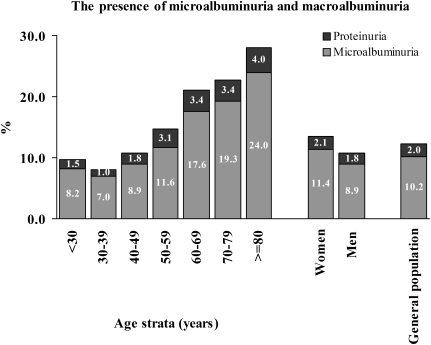
Of all the subjects, 10.2% had microalbuminuria, and 2% had macroalbuminuria. Both of these, particularly microalbuminuria, were higher in females and in older age groups (Figure 2). Spot urine tests revealed that the median (IQR) albuminuria level was 6.59 (10.05) mg/g creatinine. The median albuminuria was significantly higher for women than men (7.55 vs. 5.71 mg/g creatinine, respectively, P < 0.001). It was also significantly higher for patients aged over 50 years (Table 2).
Fig. 2.
Percentage of subjects with microalbuminuria (30–299 mg/g creatinine) or macroalbuminuria (> 300 mg/g creatinine) with respect to gender and age group.
Prevalence of CKD
The presence of CKD was assessed in all subjects who volunteered for urinary albumin excretion measurements. The prevalence of CKD was 15.7% (n = 1373) among 8765 subjects evaluated. CKD was significantly more common among women than men [18.4% vs. 12.8%, P < 0.001, odds ratio 1.54 (95% CI: 1.37–1.74)] (Table 3). The prevalence of CKD also increased with increasing age of the subjects. The odds ratios of CKD ranged from 1.45 (95% CI: 1.14–1.84) to 2.18 (95% CI: 1.76–2.70) for every 10-year increase in age for subjects over 30 years (Table 3). The prevalence of CKD was found to be 11.5% in the population aged < 60 years, while it was as high as 38.3% in subjects aged 60 years or over [P < 0.001, odds ratio 1.43 (95% CI: 1.37–1.49)]. Stage 3 CKD was especially more common among subjects aged over 60 years (1.6% vs. 21.5%). CKD prevalence was slightly higher among subjects living in rural areas than in those living in urban areas (16.8% vs. 15.2%, P = 0.049). CKD prevalence was highest among subjects from the Marmara region (19.7%) followed by Southeastern Anatolia (18.6%), the Black Sea (16.1%), East Anatolia (14.2%), the Aegean (13.8%), Central Anatolia (12.6%) and the Mediterranean (11.7%) regions.
Table 3.
The prevalence of chronic renal disease with respect to gender and age groups
| Total number of subjects | Subjects with CKD, n (%) | P-value | Odds ratio (95% CI) | |
|---|---|---|---|---|
| Gender | ||||
| Female | 4490 | 827 (18.4%) | < 0.001 | 1.54 (1.37–1.74) |
| Male | 4273 | 546 (12.8%) | ||
| Age groupsa | ||||
| < 30 | 2595 | 259 (10%) | ||
| 30–39 | 1947 | 165 (8.5%) | 0.084 | 0.84 (0.68–1.02) |
| 40–49 | 1645 | 207 (12.6%) | < 0.001 | 1.56 (1.25–1.93) |
| 50–59 | 1218 | 222 (18.2%) | < 0.001 | 1.55 (1.26–1.90) |
| 60–69 | 719 | 235 (32.7%) | < 0.001 | 2.18 (1.76–2.70) |
| 70–79 | 491 | 203 (41.3%) | 0.002 | 1.45 (1.14–1.84) |
| ≥ 80 | 150 | 82 (54.7%) | 0.004 | 1.71 (1.18–2.47) |
| Total | 8765 | 1373 (15.7%) | ||
The numbers of subjects are adjusted (to age, gender, geographic region and residence) figures.
CKD, chronic renal disease; CI, confidence interval.
P-values correspond to the comparisons with previous age group.
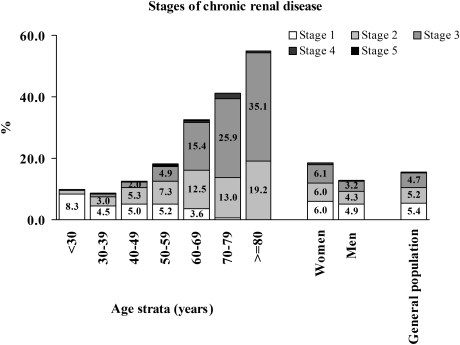
The majority of subjects with CKD were in stage 1–3. The prevalence rates for CKD stage 1, 2, 3, 4 and 5 were 5.4%, 5.2%, 4.7%, 0.3% and 0.2%, respectively. The percentage of subjects in stage 2 and 3 increased and those with stage 1 decreased with increasing age (Figure 3).
Fig. 3.
Percentage of subjects with chronic renal disease according to the stages of the disease with respect to gender and age group.
Concomitant cardiovascular risk factors
In the general population, the prevalence rates for hypertension, diabetes, dyslipidaemia, obesity and metabolic syndrome were 32.7%, 12.7%, 76.3%, 20.1% and 31.3%, respectively. The prevalence of hypertension was higher in subjects with CKD than in those without CKD (56.3% and 31%, P < 0.001). Similarly, the prevalence rates of diabetes (26.6% vs. 10.1%), dyslipidaemia (83.4% vs. 75.8%), obesity (29.2% vs. 20%) and metabolic syndrome (46% vs. 29.8%) were significantly higher in subjects with CKD compared with subjects without CKD (P < 0.001 for all analyses). Furthermore, the prevalence of these cardiovascular risk factors gradually increased in subjects having advanced stages of the disease. Thus, CKD was found to be strongly associated with these cardiovascular risk factors (Table 4).
Table 4.
The frequency of concomitant hypertension, diabetes mellitus, dyslipidaemia, obesity or metabolic syndrome in the study population with respect to presence and stage of chronic renal disease
| Chronic renal disease status | Hypertension |
Diabetes mellitus |
Dyslipidaemia |
Obesity |
Metabolic syndrome |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | OR (95% CI) | % | OR (95% CI) | % | OR (95% CI) | % | OR (95% CI) | % | OR (95% CI) | |
| General population | 32.7 | 12.7 | 76.3 | 20.1 | 31.3 | |||||
| No CKD | 31 | reference | 10.1 | reference | 75.8 | reference | 20 | reference | 29.8 | reference |
| CKD (any stage)a | 56.3 | 2.86 (2.54–3.22) | 26.6 | 3.22 (2.77–3.74) | 83.4 | 1.60 (1.37–1.86) | 29.2 | 1.65 (1.45–1.88) | 46 | 2.01 (1.77–2.27) |
| Stage 1 CKDb | 34.8 | 1.19 (0.98–1.45) | 16.4 | 1.73 (1.33–2.26) | 74.3 | 0.92 (0.74–1.14) | 23.7 | 1.25 (1–1.55) | 31.1 | 1.06 (0.86–1.31) |
| Stage 2 CKDc | 55.1 | 2.30 (1.76–3) | 32.1 | 2.41 (1.74–3.35) | 84.2 | 1.85 (1.33–2.57) | 29.7 | 1.36 (1.01–1.82) | 51.4 | 2.34 (1.77–3.11) |
| Stage 3 CKDc | 79.8 | 3.21 (2.36–4.36) | 32.3 | 1.01 (0.74–1.38) | 92.6 | 2.36 (1.50–3.70) | 34.7 | 1.26 (0.95–1.68) | 57 | 1.26 (0.94–1.68) |
| Stage 4 CKDc | 82.6 | 1.21 (0.40–3.64) | 40.9 | 1.45 (0.60–3.49) | 87.5 | 0.56 (0.16–1.97) | 29.2 | 0.82 (0.33–2.04) | 68.2 | 1.62 (0.64–4.06) |
| Stage 5 CKDc | 92.3 | 2.53 (0.25–25.38) | 45.5 | 1.20 (0.28–5.18) | 91.7 | 1 57 (0.15–16.94) | 33.3 | 1.14 (0.26–5.09) | 66.7 | 0.93 (0.21–4.18) |
Significant ORs are denoted in italic font type.
Hypertension (HT), diagnosis of HT or blood pressure ≥ 140/90 mmHg; diabetes mellitus (DM), diagnosis of DM or fasting blood glucose ≥ 126 mg/dL; dyslipidaemia, anti-lipid use or dyslipidaemia in lipid profile; obesity, body mass index ≥ 30 kg/m2; metabolic syndrome, as defined according to the AHA criteria; OR, odds ratio.
CKD of any stage vs. no CKD.
Stage 1 vs. no CKD.
Versus previous stage.
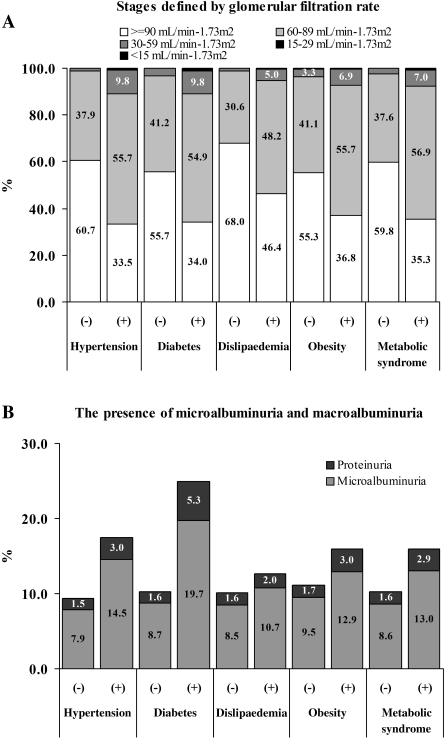
Mean values of kidney function and/or damage indicators, such as serum creatinine, GFR and microalbuminuria, in subjects with and without hypertension, diabetes, dyslipidaemia, obesity and metabolic syndrome also demonstrated that renal function in subjects with cardiovascular risk factors was significantly impaired compared with healthy individuals (Table 5). The prevalence of decreased GFR and microalbuminuria was higher in subjects having these risk factors, particularly in those having hypertension and diabetes (Figure 4). The prevalence of CKD among subjects with and without hypertension was 25.3% and 10.6%, respectively [P < 0.001, odds ratio 1.58 (95% CI: 1.48–1.68)]. Similarly, CKD was significantly more common in subjects with diabetes than in subjects without diabetes [32.4% vs. 13%; P < 0.001, odds ratio 1.22 (95% CI: 1.18–1.27)].
Table 5.
Mean serum creatinine, glomerular filtration rate and microalbuminuria in subjects with and without concomitant hypertension, diabetes mellitus, dyslipidaemia, obesity or metabolic syndrome
| Serum creatinine (mg/dL) |
GFR (mL/min/1.73 m2) |
Microalbuminuria (mg/g creatinine) |
|||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | P-value | Mean ± SD | P-value | Mean ± SD (median; IQR) | P-value | ||
| Hypertension | No | 0.88 ± 0.18 | < 0.001 | 96.5 ± 20.3 | < 0.001 | 25.9 ± 146.7 (5.98; 8.15) | < 0.001 |
| Yes | 0.94 ± 0.42 | 82.6 ± 19.2 | 45 ± 176.6 (8.10; 14.77) | ||||
| Diabetes mellitus | No | 0.88 ± 0.26 | < 0.001 | 94 ± 20.8 | < 0.001 | 27.1 ± 144.5 (6.23; 8.83) | < 0.001 |
| Yes | 0.94 ± 0.44 | 82 ± 19.4 | 74.1 ± 260.5 (10.76; 25.22) | ||||
| Dyslipidaemia | No | 0.85 ± 0.20 | < 0.001 | 100.5 ± 21.8 | < 0.001 | 32.1 ± 211.5 (6.31; 8.74) | 0.006 |
| Yes | 0.91 ± 0.30 | 89.2 ± 19.9 | 31.8 ± 137.3 (6.65; 10.45) | ||||
| Obesity | No | 0.90 ± 0.28 | 0.17 | 93.7 ± 21.1 | < 0.001 | 29.7 ± 143.7 (6.42; 9.33) | < 0.001 |
| Yes | 0.90 ± 0.30 | 84.7 ± 18.5 | 41.3 ± 196.2 (7.42; 12.35) | ||||
| Metabolic syndrome | No | 0.88 ± 0.25 | < 0.001 | 95.9 ± 21 | < 0.001 | 27.9 ± 156 (6.18; 8.73) | < 0.001 |
| Yes | 0.92 ± 0.35 | 83.9 ± 18.4 | 42.7 ± 178.5 (7.57; 13.42) | ||||
Hypertension (HT), diagnosis of HT or blood pressure ≥ 140/90 mmHg; diabetes mellitus (DM), diagnosis of DM or fasting blood glucose ≥ 126 mg/dL; dyslipidaemia, anti-lipid use or dyslipidaemia in lipid profile; obesity, body mass index ≥ 30 kg/m2; metabolic syndrome, as defined according to the AHA criteria; GFR, glomerular filtration rate; SD, standard deviation; IQR, interquartile range.
Fig. 4.
Glomerular filtration rate (A), and microalbuminuria [as microalbuminuria (30–299 mg/g creatinine) or macroalbuminuria (> 300 mg/g creatinine)] (B) in subjects with and without concomitant hypertension, diabetes mellitus, dyslipidaemia, obesity or metabolic syndrome.
Discussion
This was the first epidemiological study to assess the prevalence of CKD in Turkey. Using the criteria of CKD established by the KDOQI guidelines, the overall prevalence of CKD in Turkey was 15.7%. By employing similar KDOQI criteria, the prevalence of CKD was 13.1% in the USA based on the NHANES-III (1999–2004) data [8] and 10.2% in Norway based on the HUNT-II (1995–97) data [20]. Although the US and Norwegian studies showed the highest prevalence for stage 3 CKD (7.7% in the USA and 4.2% in Norway), our Turkish stage 1–3 cohort were more evenly distributed, and each comprised nearly one-third of the CKD population. This suggests that Turkey shows a higher proportion of albuminuria in the absence of kidney dysfunction.
A number of studies have focused on the prevalence of stage 3–5 CKD based on GFR < 60 mL/min/1.73 m2 and calculated GFRs using the MDRD equation. The prevalence of low estimated GFR in Turkey (5.2%) is lower compared with Italy (6.4%) [21], Switzerland (8.1%) [22] and Iceland (7.2%) [23], but is higher than India (4.2%) [24] and China (2.5%) [25].
The MDRD equation has mostly been replaced with the Cockcroft–Gault equation since the latter gives a more accurate estimate of GFR in CKD patients [17]. In epidemiological studies that reported CKD rates using both equations, the Cockcroft–Gault-calculated rate was always higher than the MDRD-calculated rate. As an example, the NHANES-III 1988–94 survey showed that the US prevalence of low GFR was 4.5% by the MDRD and 7% by the Cockcroft–Gault equation [15].
An earlier diagnosis of stage 4 CKD patients would allow for future planning, such as early access (peritoneal dialysis catheter or arteriovenous fistula) for dialysis or transplantation from a donor in the family [26,27]. Furthermore, knowledge on the prospective incidence of ESRD from a well-established population-based cohort will help in identifying those patients at the highest risk for developing this condition.
Predictors of ESRD have been studied in long-term prospective studies such as the Okinawa screen, the Multiple Risk Factor Intervention Trial (MRFIT) and the Norwegian HUNT study. In a 10-year follow-up of the Okinawa population, proteinuria, haematuria and hypertension were identified as predictors of ESRD [28]. In contrast, proteinuria and estimated GFR were ESRD predictors in the MRFIT study having a 25-year follow-up [29]. However, their predictive value was limited since the combined GFR and proteinuria could detect only 27% of the eventual ESRD cases [29,30]. A 10-year follow-up of HUNT-II showed that the combination of the albumin/creatinine ratio (not just proteinuria) and estimated GFR narrowed the population at risk for developing ESRD to 1.4% and correctly predicted 66% of the patients eventually progressing to ESRD [31].
The Kidney Early Evaluation Program (KEEP) study conducted by the National Kidney Foundation revealed an independent relationship between CVD and several measures of CKD, such as estimated GFR, microalbuminuria and anaemia [32]. The early detection and management of cardiovascular risk factors will be important for Turkey since the mortality pattern is very similar to that of developed nations even though industrialization is ongoing, the population is young, and overall cholesterol levels are comparatively low [33]. Turkey was one of the 22 European countries participating in the EUROASPIRE III study, which aimed to describe lifestyles, risk factors and therapeutic management of coronary heart disease patients. In this study, 20% of the Turkish patients hospitalized for coronary heart disease were under 50 years of age, constituting the youngest patients in all of Europe [34,35]. Moreover, the prospective Turkish Adult Risk Factors study, a large population-based survey that accumulated data on coronary heart disease and related risk factors since 1990, has shown that coronary mortality is highest in Turkey among 30 European countries at 7.6 per 1000 person-years for men and 3.84 for women in the age group of 45–74 years [36].
In the present cohort, we found that the overall prevalence of diabetes was 12.7%. In contrast, the TURDEP study in 2002 had predicted a prevalence of 7.2% in the overall Turkish population [37]. This increased prevalence may be related to increased life expectancy as well as lifestyle changes in the Turkish population, such as changes in dietary habits, more sedentary lifestyle and increased urbanization. In a more recent study in an older population cohort, the prevalence of diabetes was 11% of individuals aged over 35 years [38]. The prevalence of diabetes was more than doubled in subjects diagnosed with CKD (26.6%). Diabetes was clearly associated with CKD progression since the prevalence of diabetic patients increased with advanced stages of kidney disease. Kidney damage is very common in diabetes. For example, the NHANES-III showed that close to one-third of the diabetic population (28.8%) in the USA had microalbuminuria [39].
Hypertension is the second major cause of ESRD, and is also very common in Turkey. The prevalence, awareness, treatment and control of hypertension in Turkey (PatenT) study reported that the age- and sex-adjusted rate of hypertension was 31.8% in a population of ~ 15 million people [40]. A 4-year follow-up of this study showed that the incidence rate of hypertension in Turkey was 21.4%, and this reached as high as 43.4% for individuals aged over 65 years [41]. Our current study showed that the percentage of patients with hypertension increased steadily with progressing CKD, such that nearly all patients with ESRD (92.3%) had elevated blood pressure.
Obesity and metabolic syndrome are also risk factors for CKD. In a Swedish study, obesity (in men) and morbid obesity (in women) at anytime during the lifespan were associated with a 3–4-fold increase in the risk of CKD [42]. In a prospective 9-year study, the odds ratio of incident CKD was 1.24 among subjects with metabolic syndrome after adjustments for diabetes and hypertension [43]. Obesity and metabolic syndrome were highly prevalent in this cohort, and the prevalence of both risk factors was significantly greater in subjects with CKD. Even higher rates of obesity (one-third of the adult population) have been reported in some Turkish studies, while metabolic syndrome ranged from 18% to 41% depending on the definition [44,45].
The major limitation of this study was the use of single measurements to detect serum creatinine and albuminuria. Many of those screened may have had transient albuminuria or a transient decline in GFR. Ideally, a repeat test should be performed after a few months to eliminate cases with transient kidney injury, which is relevant at the individual level. Nevertheless, it is difficult to perform more than single testing in large-scale cross-sectional surveys at the population level [24,46]. Another limitation was the difficulty of diagnosing elderly subjects having reduced GFR with CKD. The prevalence of CKD, particularly stage 3 CKD, was found to be significantly higher in subjects aged 60 years and older. However, it is very likely that most of the elderly participants had age-related renal impairments rather than CKD [47], making it necessary to evaluate age-related renal functional changes during evaluation of high-prevalence CKD among subjects aged over 60 years. In addition, the MDRD equation has not been validated for the Turkish population, and its accuracy in predicting actual GFR in Turkish men and women is not known. A validation study for the MDRD equation in the Turkish population is currently being conducted by the Turkish Society of Nephrology.
In conclusion, similar to many other countries, CKD is a major health problem in Turkey. Associations between CKD and several cardiovascular risk factors emphasize that this is a major public health problem and a major predictor of overall morbidity and mortality. Longitudinal studies will be needed to establish relationships between GFR, microalbuminuria, cardiovascular outcomes and the risk for ESRD development. The population examined in the present study is currently being followed up as a longitudinal study, and results will be evaluated at the fifth year.
Acknowledgments
This study was designed, conducted and analyzed by Turkish Society of Nephrology. This study was financially supported by the Scientific and Technological Research Council of Turkey (TUBITAK) with the project code of SBAG-3184. We thank Fresenius Medical Care Company for laboratory services and additional financial support, Omega-CRO for conducting field study, and Dr Oktay Özdemir from Yorum Ltd. for statistical analysis.
Conflict of interest statement. None declared.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 2.K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 3.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 4.ERA-EDTA Registry . ERA-EDTA Registry Annual Report 2007. Amsterdam, the Netherlands: Academic Medical Center, Department of Medical Informatics; 2009. http://www.era-edta-reg.org/index.jsp?p=annrep. [Google Scholar]
- 5.U.S. Renal Data System . USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. http://www.usrds.org/atlas.htm. [Google Scholar]
- 6.Erek E, Suleymanlar G, Serdengecti K. Nephrology, dialysis and transplantation in Turkey. Nephrol Dial Transplant. 2002;17:2087–2093. doi: 10.1093/ndt/17.12.2087. [DOI] [PubMed] [Google Scholar]
- 7.Serdengeçti K, Süleymanlar G, Altiparmak M, et al. Registry of the Nephrology Dialysis and Transplantation in Turkey (Registry-2008) Istanbul: Published by the Turkish Society of Nephrology; 2009. [Google Scholar]
- 8.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 9.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 11.Asselbergs FW, Diercks GF, Hillege HL, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 12.Atthobari J, Asselbergs FW, Boersma C, et al. Cost-effectiveness of screening for albuminuria with subsequent fosinopril treatment to prevent cardiovascular events: a pharmacoeconomic analysis linked to the prevention of renal and vascular endstage disease (PREVEND) study and the prevention of renal and vascular endstage disease intervention trial (PREVEND IT) Clin Ther. 2006;28:432–444. doi: 10.1016/j.clinthera.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 14. Prime Minister Republic of Turkey, Turkish Statistical Institute, www.turkstat.gov.tr.
- 15.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 16.Carter JL, Tomson CR, Stevens PE, et al. Does urinary tract infection cause proteinuria or microalbuminuria? A systematic review. Nephrol Dial Transplant. 2006;21:3031–3037. doi: 10.1093/ndt/gfl373. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Gansevoort RT, Verhave JC, Hillege HL, et al. The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int Suppl. 2005:S28–S35. doi: 10.1111/j.1523-1755.2005.09408.x. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 20.Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17:2275–2284. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 21.Cirillo M, Laurenzi M, Mancini M, et al. Low glomerular filtration in the population: prevalence, associated disorders, and awareness. Kidney Int. 2006;70:800–806. doi: 10.1038/sj.ki.5001641. [DOI] [PubMed] [Google Scholar]
- 22.Nitsch D, Felber Dietrich D, von Eckardstein A, et al. Prevalence of renal impairment and its association with cardiovascular risk factors in a general population: results of the Swiss SAPALDIA study. Nephrol Dial Transplant. 2006;21:935–944. doi: 10.1093/ndt/gfk021. [DOI] [PubMed] [Google Scholar]
- 23.Viktorsdottir O, Palsson R, Andresdottir MB, et al. Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrol Dial Transplant. 2005;20:1799–1807. doi: 10.1093/ndt/gfh914. [DOI] [PubMed] [Google Scholar]
- 24.Singh NP, Ingle GK, Saini VK, et al. Prevalence of low glomerular filtration rate, proteinuria and associated risk factors in North India using Cockcroft-Gault and Modification of Diet in Renal Disease equation: an observational, cross-sectional study. BMC Nephrol. 2009;10:4. doi: 10.1186/1471-2369-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Wildman RP, Gu D, et al. Prevalence of decreased kidney function in Chinese adults aged 35 to 74 years. Kidney Int. 2005;68:2837–2845. doi: 10.1111/j.1523-1755.2005.00757.x. [DOI] [PubMed] [Google Scholar]
- 26.Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK) Am J Kidney Dis. 2003;42:617–622. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 27.Lameire N, van Biesen W. The pattern of referral of patients with end-stage renal disease to the nephrologist—a European survey. Nephrol Dial Transplant. 1999;14:16–23. doi: 10.1093/ndt/14.suppl_6.16. [DOI] [PubMed] [Google Scholar]
- 28.Iseki K. The Okinawa screening program. J Am Soc Nephrol. 2003;14:S127–S130. doi: 10.1097/01.asn.0000070153.91733.09. [DOI] [PubMed] [Google Scholar]
- 29.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 30.Gansevoort RT, Bakker SJ, de Jong PE. Early detection of progressive chronic kidney disease: is it feasible? J Am Soc Nephrol. 2006;17:1218–1220. doi: 10.1681/ASN.2006030247. [DOI] [PubMed] [Google Scholar]
- 31.Hallan SI, Ritz E, Lydersen S, et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough PA, Jurkovitz CT, Pergola PE, et al. Independent components of chronic kidney disease as a cardiovascular risk state: results from the Kidney Early Evaluation Program (KEEP) Arch Intern Med. 2007;167:1122–1129. doi: 10.1001/archinte.167.11.1122. [DOI] [PubMed] [Google Scholar]
- 33.Onat A. Risk factors and cardiovascular disease in Turkey. Atherosclerosis. 2001;156:1–10. doi: 10.1016/s0021-9150(01)00500-7. [DOI] [PubMed] [Google Scholar]
- 34.Kotseva K, Wood D, De Backer G, et al. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil. 2009;16:121–137. doi: 10.1097/HJR.0b013e3283294b1d. [DOI] [PubMed] [Google Scholar]
- 35.Tokgozoglu L, Baris Kaya E. Atherosclerotic vascular disease and risk factors in Turkey: from past to present. J Atheroscler Thromb. 2008;15:286–291. doi: 10.5551/jat.e614. [DOI] [PubMed] [Google Scholar]
- 36.Onat A, Ugur M, Tuncer M, et al. Age at death in the Turkish Adult Risk Factor Study: temporal trend and regional distribution at 56,700 person-years’ follow-up. Türk Kardiyol Dern Ars. 2009;37:155–160. [PubMed] [Google Scholar]
- 37.Satman I, Yilmaz T, Sengul A, et al. Population-based study of diabetes and risk characteristics in Turkey: results of the Turkish diabetes epidemiology study (TURDEP) Diab Care. 2002;25:1551–1556. doi: 10.2337/diacare.25.9.1551. [DOI] [PubMed] [Google Scholar]
- 38.Onat A, Hergenc G, Uyarel H, et al. Prevalence, incidence, predictors and outcome of type 2 diabetes in Turkey. Anadolu Kardiyol Derg. 2006;6:314–321. [PubMed] [Google Scholar]
- 39.Jones CA, Francis ME, Eberhardt MS, et al. Microalbuminuria in the US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39:445–459. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- 40.Altun B, Arici M, Nergizoglu G, et al. Prevalence, awareness, treatment and control of hypertension in Turkey (the PatenT study) in 2003. J Hypertens. 2005;23:1817–1823. doi: 10.1097/01.hjh.0000176789.89505.59. [DOI] [PubMed] [Google Scholar]
- 41.Arici M, Turgan C, Altun B, et al. Hypertension incidence in Turkey (HinT): a population-based study. J Hypertens. 2010;28:240–244. doi: 10.1097/HJH.0b013e328332c36b. [DOI] [PubMed] [Google Scholar]
- 42.Ejerblad E, Fored CM, Lindblad P, et al. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 43.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 44.Oguz A, Temizhan A, Abaci A, et al. Obesity and abdominal obesity; an alarming challenge for cardio-metabolic risk in Turkish adults. Anadolu Kardiyol Derg. 2008;8:401–406. [PubMed] [Google Scholar]
- 45.Sanisoglu SY, Oktenli C, Hasimi A, et al. Prevalence of metabolic syndrome-related disorders in a large adult population in Turkey. BMC Public Health. 2006;6:92. doi: 10.1186/1471-2458-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S, Lim CS, Han DC, et al. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: a population-based cross-sectional epidemiologic study. J Korean Med Sci. 2009;24:S11–S21. doi: 10.3346/jkms.2009.24.S1.S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nygaard H, Naik M, Ruths S, et al. Clinically important renal impairment in various groups of old persons. Scand J Prim Health Care. 2004;22:152–156. doi: 10.1080/02813430410006468. [DOI] [PubMed] [Google Scholar]