Abstract
Muscle paralysis after spinal cord injury is partly caused by a loss of brainstem-derived serotonin (5-HT), which normally maintains motoneuron excitability by regulating crucial persistent calcium currents. Here we examine how over time motoneurons compensate for lost 5-HT to regain excitability. We find that, months after a spinal transection in rats, changes in post-transcriptional editing of 5-HT2C receptor mRNA lead to increased expression of 5-HT2C receptor isoforms that are spontaneously active (constitutively active) without 5-HT. Such constitutive receptor activity restores large persistent calcium currents in motoneurons in the absence of 5-HT. We show that this helps motoneurons recover their ability to produce sustained muscle contractions and ultimately enables recovery of motor functions such as locomotion. However, without regulation from the brain, these sustained contractions can also cause debilitating muscle spasms. Accordingly, blocking constitutively active 5-HT2C receptors with SB206553 or cyproheptadine, in both rats and humans, largely eliminates these calcium currents and muscle spasms, providing a new rationale for antispastic drug therapy.
Severe spinal cord injury (SCI) causes an immediate paralysis of muscles innervated by motoneurons directly caudal to the injury site. This results not only from a loss of supraspinal tracts that subserve voluntary initiation of movement (for example, corticospinal and reticulospinal tracts that use fast glutamatergic synaptic transmission1,2) but also from a loss of descending brainstem tracts that provide spinal motoneurons with their major source of neuromodulators, such as 5-HT (refs. 1,3–5). Normally, brainstem-derived 5-HT sets spinal motoneurons and interneurons into an excitable state, ready to respond to fast glutamate synaptic inputs and cause appropriate muscle contractions2,4,6,7. 5-HT does this by activating 5-HT2 receptors that facilitate ionic currents intrinsic to the motoneurons, including voltage-gated persistent Ca2+ and Na+ currents (termed persistent inward currents: PICs)1,8–11. These PICs are easily activated by brief synaptic inputs because of their unusually low threshold and, thus, serve a crucial role in amplifying and prolonging the action of synaptic inputs, ultimately enabling sustained muscle contractions2,7,11–14. Consequently, when SCI eliminates brainstem-derived 5-HT, motoneurons are left in an unexcitable state with small PICs7,9,12,15, consistent with the paralysis, areflexia and spinal shock seen early after SCI16–18. The key role of brainstem-derived 5-HT is demonstrated by the repeated finding that motoneuron excitability (PICs) and associated motor functions (locomotion) can be regained shortly after SCI with exogenous application of 5-HT or selective agonists that activate 5-HT2 receptors7,9–11,19.
Remarkably, over the weeks after SCI (chronic injury), motoneurons spontaneously recover their excitability, with large PICs and associated sustained firing12,20, despite the continued absence of brainstem-derived 5-HT. However, unlike before injury, the powerful depolarizing actions of PICs are difficult to terminate, because after injury motoneurons have weaker inhibitory inputs21, especially from spinal interneurons that are normally regulated by descending tracts12,17,22–26. Thus, the PICs (especially Ca2+ PICs) can lead to excessive motoneuron activity that produces uncontrolled and debilitating muscle contractions (spasms, lasting many seconds), in both humans27 and rats12,17. To make matters worse, these PICs and spasms are readily triggered by synaptic inputs arising from normally innocuous cutaneous stimulation or muscle stretch, because these synaptic inputs are enhanced after SCI12,17,23,28–30.
A major question that remains is how motoneurons adapt so profoundly, recovering large PICs in the absence of brainstem-derived 5-HT. Here we consider the hypothesis that 5-HT2 receptors on spinal motoneurons become constitutively active to compensate for lost brainstem 5-HT, ultimately helping to produce recovery of motoneuron excitability (PICs) and related motor functions such as locomotion. Constitutively active receptors spontaneously couple to their Gq proteins and initiate intracellular signaling without being bound to 5-HT or any other ligand31–37, a process well understood in isolated cell culture systems but not previously considered for motoneurons. The 5-HT2C receptor is an ideal candidate for such constitutive activity because it has a number of native isoforms that have a high degree of constitutive activity in humans and rats (>50% active)32,35. Furthermore, expression of these constitutively active isoforms increases in the cortex after depletion of 5-HT38, suggesting that a similar change may be possible after SCI. We thus examined whether recovery of motoneuron function after SCI depends on constitutive 5-HT receptor activity. We initially focused on showing that constitutive receptor activity causes spasms, because the emergence of spasms after SCI is an indirect measure of recovery of motoneuron and general motor function (albeit maladaptive) that is readily studied in rats and humans (motoneuron PICs cause spasms). After this, we evaluated how constitutive activity contributes to locomotor recovery. For studying spasms, we used a complete spinal transection model (chronic spinal rat, Fig. 1), which eliminates brainstem-derived 5-HT, thus minimizing the chance that receptors remain activated by 5-HT. Nevertheless, we still had to consider the role of other 5-HT sources, because even with a complete transection, some residual spinal 5-HT remains caudal to the injury39, and motoneurons are extremely sensitive to small amounts of 5-HT after SCI8,10.
Figure 1.
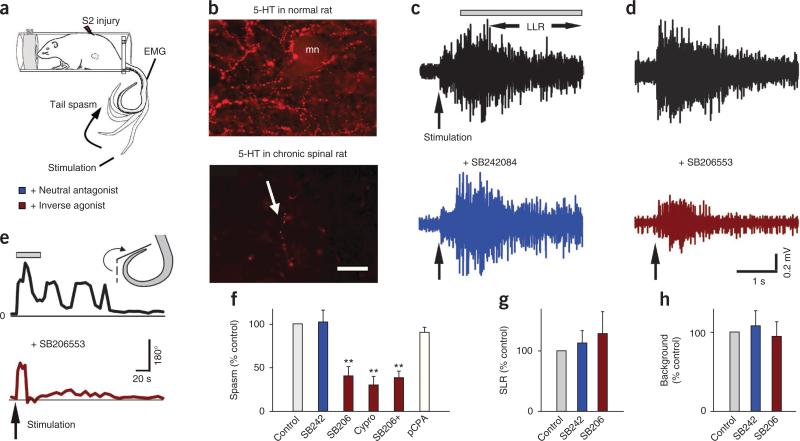
Constitutive 5-HT2 receptor activity, but not residual 5-HT, causes spasms. (a) Schematic of tail spasm in an awake chronic spinal rat with S2 sacral transection. (b) Representative immunofluorescence images of 5-HT fibers (beaded) in the S4 ventral horn of normal rats (top; mn, motoneuron, n = 5 rats) and chronic spinal rats (bottom; the arrow indicates a residual fiber, n = 5; scale bar, 50 μm). (c,d) Spasms in chronic spinal rat evoked by cutaneous electrical stimulation of the tail (pulse three times the threshold (3×T)) and recorded with EMG (quantified during the length of time indicated by the bar, LLR) before and after blocking effects of residual 5-HT with i.t. injection of the neutral antagonist SB242084 (3 mM in 30 μl saline). (d) Lack of spasm (LLR) after blocking constitutive receptor activity with the inverse agonist SB206553 (i.t., 3 mM in 30 μl saline). (e) Tail flexion angle during spasms before and after SB206553 injection, quantified during the length of time indicated by the bar. (f) Group means of spasms (normalized to predrug control) with SB242084 (abbreviated SB242; LLR), SB206553 (SB206 for LLR EMG recording; and SB206+ for tail-angle spasms) and cyproheptadine (cypro; LLR; 10 mg per kg body weight, orally), and after depletion of residual 5-HT with para-chlorophenylalanine-methylester (pCPA) (two 300 mg per kg body weight intraperitoneal injections over 48 h; tail-angle), with n = 5 rats per drug. (g,h) Normalized group means of SLR and background EMG with SB242084 and SB206553. **P < 0.01 relative to predrug control, 100%. Error bars indicate s.e.m.
RESULTS
Lack of contribution of residual 5-HT to spasms
Before injury, the spinal cord was densely innervated by 5-HT fibers along its whole length, particularly in the ventral horn (Fig. 1b). In contrast, after SCI in the chronic spinal rat, only a few short (43.3 ± 25.0 μm) fibers remained (Fig. 1b) with, on average, 18 ± 11 such fibers along the whole length of the spinal cord below the injury.
To examine whether the remaining 5-HT fibers in chronic spinal rats had any functional effect on spasms and associated 5-HT2 receptors, we blocked the action of 5-HT with an intrathecal (i.t.) injection of the highly selective 5-HT2C receptor antagonist SB242084 (Fig. 1c,f). This injection did not significantly change the tail muscle spasms recorded with EMG in vivo (Fig. 1c; evoked by cutaneous stimulation), indicating that the 5-HT2 receptors were not activated by residual 5-HT (or other endogenous ligands). Notably, SB242084 is a neutral antagonist that blocks only the action of 5-HT (or other agonists) on the 5-HT2 receptors, and does not inhibit constitutive receptor activity31,34. We also found that depleting residual 5-HT with pCPA38 did not significantly influence spasms (Fig. 1f).
Spasms depend on constitutive 5-HT2 receptor activity
We next examined whether the loss of 5-HT after injury was compensated for by constitutive activity in 5-HT2 receptors by intrathecally injecting SB206553, which selectively binds 5-HT2C receptors and potently inhibits their constitutive activity (termed an inverse agonist31,34,37). This injection reduced the magnitude of the spasms recorded with either electromyography (EMG) (Fig. 1d,f) or tail kinematics (Fig. 1e,f) by well over 50%, whereas control saline injections had no effect. Likewise, oral application of the non-selective 5-HT2 receptor inverse agonist cyproheptadine33 significantly reduced spasms (Fig. 1f).
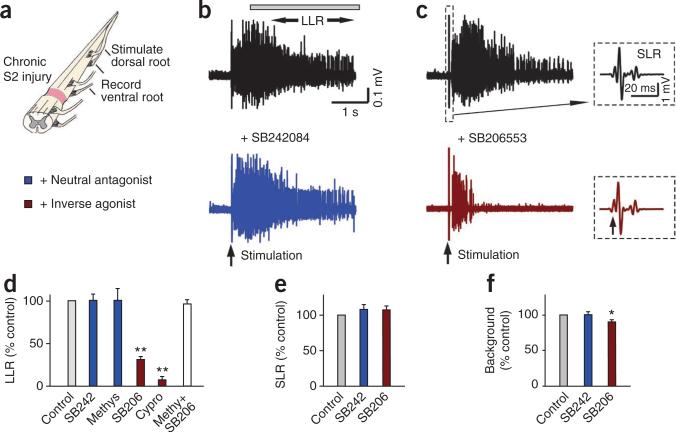
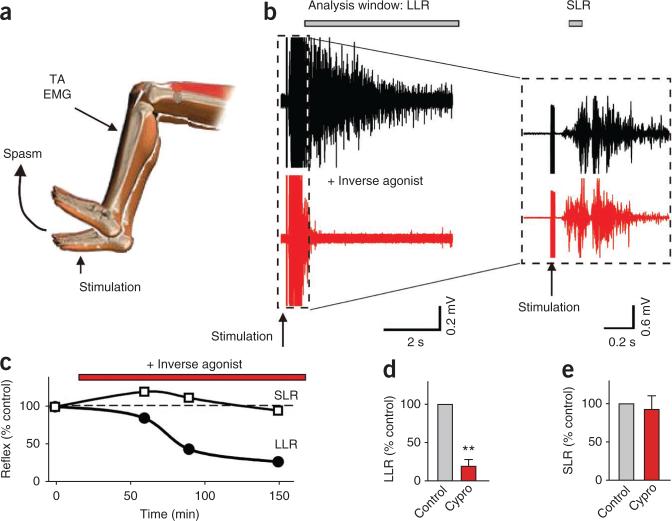
We next examined the whole spinal cord from chronic spinal rats (caudal to the injury) after it was removed and maintained in vitro, which eliminated possible peripheral or brain-derived 5-HT influences. We recorded long-lasting reflexes (LLRs) from the ventral roots in response to a brief stimulation of dorsal roots (Fig. 2a–c); these LLRs have previously been shown to underlie muscle spasms recorded in vivo12,17. The LLRs were not significantly affected by blocking the possible action of endogenous 5-HT with the 5-HT2C receptor neutral antagonists SB242084 or methysergide33 (Fig. 2b,d), even though these antagonists blocked the increase in LLRs induced by exogenous application of selective 5-HT2C agonists (Supplementary Fig. 1). Furthermore, enhancing available residual endogenous 5-HT with either the 5-HT transport-blocker citalopram or 5-HT releaser fenfluramine did not significantly affect LLRs (Supplementary Fig. 2). In contrast, the LLRs were markedly inhibited by blocking constitutive 5-HT2 receptor activity with inverse agonists (SB206553 and cyproheptadine; Fig. 2c,d). This inhibitory action of SB206553 was blocked by a prior application of methysergide (Fig. 2d), which competitively inhibits SB206553 binding to 5-HT2C receptors34.
Figure 2.
Constitutive 5-HT2 receptor activity contributes to LLRs in the isolated spinal cord in vitro. (a) Whole sacrocaudal spinal cord below chronic S2 transection maintained in vitro. (b) Long-lasting reflex triggered by dorsal root stimulation (single pulse, 3×T) and recorded from the ventral roots (LLR, quantified during the length of time indicated by the horizontal bar; counterpart of spasms in Figure 1) before and after blocking effects of residual 5-HT with the neutral 5-HT2 receptor antagonist SB242084 (3–5 μM). (c) Elimination of LLR, but not SLR, after blocking constitutive 5-HT2 receptor activity with the inverse agonist SB206553 (3–5 μM). Inset, SLR (expanded time scale). (d) Group means of LLRs (normalized to predrug LLRs) with SB242084 (abbreviated SB242, n = 11), methysergide (Methys, 10 μM, neutral antagonist, n = 12), SB206553 (SB206, n = 24), cyproheptadine (Cypro, 20 μM; n = 6), and SB206553 after prior application of methysergide (30 μM; white bar; Methy+SB206; n = 8). (e,f) Normalized group means of the SLR and background ventral root activity with SB206553 and SB242084. *P < 0.05, **P < 0.01 relative to control, 100%. Error bars indicate s.e.m.
The transient short latency reflexes (SLRs) evoked immediately after stimulation (Fig. 2c) were not affected by SB206553, both in vitro (Fig. 2e) and in vivo (Fig. 1g), and did not correlate with the LLRs (spasms; r2 = 0.10)29, consistent with a negligible modulation of SLRs by Ca2+ PICs and associated 5-HT2C receptors. Also, the background activity before the LLRs had relatively little (Fig. 2f, in vitro) or no (Fig. 1h, in vivo) change with SB206553 treatment.
Constitutive 5-HT2 receptor activity in motoneurons
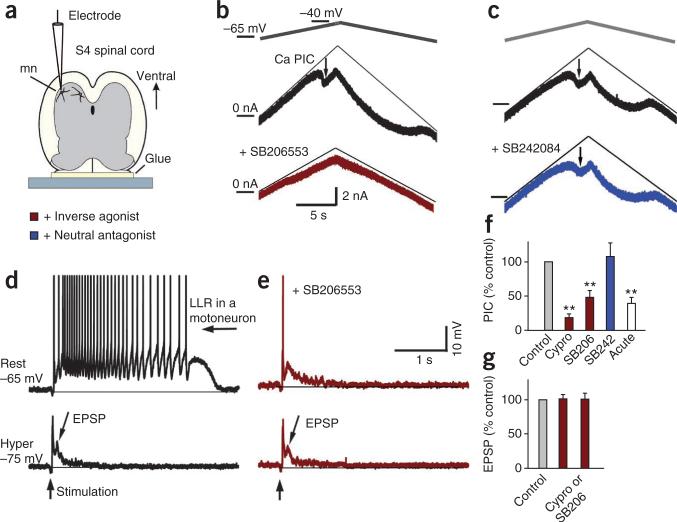
Given that spasms result from persistent calcium currents (Ca2+ PICs) in motoneurons12,27, we made intracellular recordings from motoneurons after SCI to investigate whether there were constitutively active 5-HT2C receptors on motoneurons that regulate Ca2+ PICs (Fig. 3). As previously described, the large voltage-dependent Ca2+ PICs in motoneurons were readily observed in isolation as a sharp downward deflection in the current response during an increasing voltage ramp (Fig. 3b) after sodium currents and synaptic inputs were eliminated with tetrodotoxin12. Blocking constitutively active 5-HT2 receptors with the inverse agonists SB206553 or cyproheptadine markedly decreased the magnitude of these Ca2+ PICs (Fig. 3b,f), whereas SB242084 had no effect on Ca2+ PICs (Fig. 3c,f). The portion of the Ca2+ PICs that resulted from constitutive 5-HT2 receptor activity (SB206553-sensitive decrease) was 1.99 ± 0.42 nA, which was 42.9 ± 8.9% of the maximum possible Ca2+ PICs produced by activating all 5-HT2 receptors (with 1 μM 5-HT). The small remaining Ca2+ PICs with inverse agonists in chronic spinal rats was similar to the small Ca2+ PICs observed acutely after spinal transection (Fig. 3f).
Figure 3.
Constitutively active 5-HT2 receptors on motoneurons contribute to Ca2+ PICs underlying spasms. (a,b) Intracellular recording from motoneuron (mn) in whole spinal cord, in vitro. (b) Top, Ca2+ PIC in motoneuron of chronic spinal rat, activated by slowly increasing the membrane potential under voltage-clamp in presence of 2 μM tetrodotoxin (TTX) and quantified at its initial peak, where it produced a downward deflection in the recorded current (thick black plot, at arrow, Ca2+ PIC) relative to the leak current (thin line). Bottom plot, small Ca2+ PIC after SB206553 application (5 μM). (c) Ca2+ PIC in another motoneuron (arrow), which is unaffected by SB242084 application (5 μM). (d) Top, PIC-mediated plateau and sustained firing (LLR) evoked by dorsal root stimulation (3×T; without TTX) in a motoneuron at rest (without injected current; top). Bottom, with a hyperpolarizing bias current to prevent PIC activation, the same stimulation only evoked a polysynaptic EPSP (lower plot). (e) Response of same motoneuron as in d to dorsal root stimulation after application of SB206553 (5 μM), at rest (top) and with a hyperpolarizing bias current (bottom). (f) Group means of Ca2+ PIC (normalized to predrug Ca2+ PIC in chronic spinal rats, control), with SB206553 (SB206; n = 7), cyproheptadine (cypro, 20 μM; n = 16) and SB242084 (SB242; n = 5) in chronic spinal rats and in acute spinal rats (white bar, no drugs, n = 7). (g) Normalized group means of EPSP amplitude (middle bar; control mean 4.4 mV) and duration (right bar, control 480 ms) with inverse agonists cyproheptadine or SB206553 (chronic). **P < 0.01 relative to control, 100%. Error bars represent s.e.m.
When we stimulated the dorsal roots during recording from a motoneuron at rest and in the absence of tetrodotoxin, the PIC produced a sustained depolarization (plateau)12 that caused many seconds of repetitive firing (LLR; Fig. 3d). As expected, the LLR and plateau were eliminated by the inverse agonist SB206553 (Fig. 3e). The LLR and plateau were also eliminated by simply hyperpolarizing the motoneuron to prevent activation of the underlying voltage-dependent PIC (Fig. 3d)12, although there remained a polysynaptic excitatory postsynaptic potential (EPSP) lasting about 0.5 s. The inverse agonists SB206553 and cyproheptadine had no effect on this EPSP (Fig. 3e,g).
Increase in constitutively active 5-HT2C receptor isoform
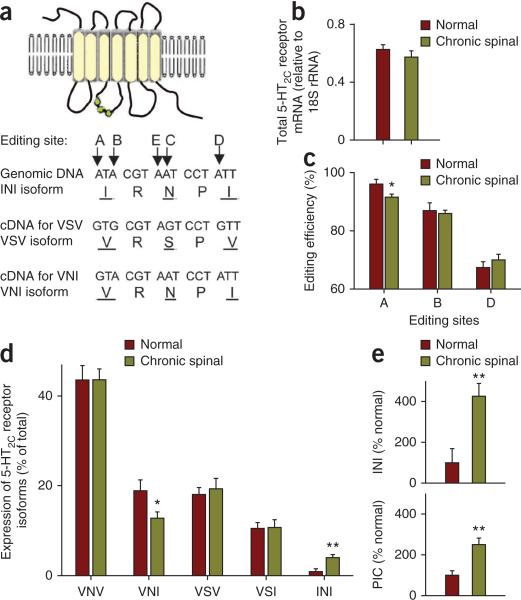
The 5-HT2C receptor RNA undergoes post-transcriptional editing at five sites (labeled A to E) that leads to numerous native receptor isoforms in rats and humans, by changing three amino acids on an intracellular loop of the receptor (isoforms are named after the amino acid sequences, such as INI, VSV and VNI, as depicted in Fig. 4a)32–35,40. Functionally, the unedited isoform (INI) shows a high degree of constitutive activity, whereas editing reduces this activity, producing isoforms with less constitutive activity, such as VNI (with 51% of INI activity) and VSV (32% of INI)36. We thus compared 5-HT2C receptor mRNA levels from spinal cords of normal (unlesioned) and chronic spinal rats (below S2 injury level). The total amount of 5-HT2C mRNA did not change with SCI (Fig. 4b). However, there was a decrease in the amount of RNA editing at the A site (Fig. 4c). Corresponding to this, there was also a decrease in the relative proportion of the VNI receptor isoform and an increase in the relative proportion of the highly constitutively active INI isoform (Fig. 4d). The increase in INI isoform expression (400%) was similar to the increase in PIC with chronic injury (Fig. 4e).
Figure 4.
A highly constitutively active 5-HT2C receptor isoform is upregulated with injury. (a) Schematic showing 5-HT2C receptor with various isoforms produced by changing three amino acids on its intracellular loop (green; isoforms named by amino acid triplet). These three amino acids (underlined) are changed by post-transcriptional editing of RNA at five sites (A–E; adenosine editing), leading to various native receptor isoforms, of which the unedited isoform (INI) is most highly constitutively active. (b) Total 5-HT2C receptor mRNA (normalized to an internal control, 18S rRNA) in chronic spinal rats (n = 6) and normal uninjured rats (n = 6). (c) Proportion of 5-HT2C receptor mRNA with editing at sites A, B and D (editing efficiency) in chronic spinal and normal rats (C and E site editing efficiency < 30% and not changed, data not shown). (d) Distribution of 5-HT2C receptor isoform mRNA in the spinal cord of normal and chronic spinal rats (15 isoforms detected; the five most prevalent are shown). (e) Comparison of change in INI isoform expression (top) and Ca2+ PIC (bottom, recorded in vitro) after chronic spinal injury. *P < 0.05, **P < 0.01, significant change with injury. Error bars indicate s.e.m.
We directly confirmed that the motoneurons of the sacral spinal cord had 5-HT2C receptors after SCI by immunolabeling (Supplementary Fig. 3). Furthermore, a large fraction of the 5-HT2C receptor labeling was inside the motoneurons (intracellular) in chronic spinal rats, and this receptor internalization was reduced by SB206553, consistent with the presence of constitutively active isoforms of the receptor on motoneurons, the hallmark of which is a high degree of activity-dependent internalization (INI isoform34,37; Supplementary Figs. 3 and 4).
Antispastic action of inverse agonists in humans with SCI
In humans with SCI, we evoked leg muscle spasms with cutaneous stimulation of the foot while recording tibialis anterior muscle EMG (Fig. 5)27. Blocking constitutive 5-HT2 receptor activity with oral administration of the inverse agonist cyproheptadine significantly decreased the muscle spasms (Fig. 5b,d). Furthermore, the effect was again selective to the long-lasting portion of the spasm (LLR, Fig. 5b–d), with no drug-induced change in the SLR (Fig. 5b,e). Spasms were equally reduced by cyproheptadine in subjects with varying impairment of motor function (B–D on the American Spinal Injury Association Impairment Scale, which ranges from A–E; Supplementary Table 1).
Figure 5.
5-HT2 receptor inverse agonist blocks spasms in spinal cord injured humans. (a) Leg spasm triggered by brief electrical stimulation of the medial arch of the foot (3–5×T). TA, tibialis anterior. (b) Spasm recorded with tibialis anterior muscle surface EMG and quantified over the time windows indicated (LLR and SLR), before and 2 h after blocking constitutively active 5-HT2 receptors with cyproheptadine (8 mg administered orally). The inset on a different scale shows SLR. (c) Gradual reduction in the spasms (LLRs), but not SLRs, over time after inverse agonist application. (d,e) Normalized group means for LLRs (d) and SLRs (e) with cyproheptadine (n = 7 subjects). **P < 0.01 relative to control, 100%. Error bars represent s.e.m.
Dependence of walking on constitutively active 5-HT2 receptors
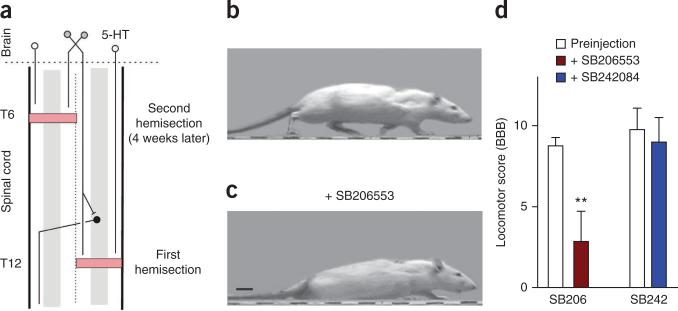
To evaluate whether constitutive 5-HT receptor activity contributes to recovery of locomotion after partial SCI, we used a staggered hemisection injury model (Fig. 6a) that transects all descending 5-HT axons but spares enough propriospinal neurons that traverse the injury site to allow the rat to voluntarily initiate functional hindlimb locomotion41. Three weeks after this injury, rats regained good hindlimb locomotor ability, voluntarily initiating walking with near normal weight support, although they retained a deficit in forelimb-hindlimb coordination (with a BBB score42 < 12; Fig. 6b). Blocking constitutively active 5-HT2 receptors with the inverse agonist SB206553 (i.t.) dramatically reduced weight support (hindlimbs dragged; Fig. 6c) and overall locomotor ability (BBB score, Fig. 6c,d). In contrast, blocking possible action of residual 5-HT with the neutral antagonist SB242084 had no significant effect (Fig. 6d).
Figure 6.
Spontaneous recovery of locomotion in staggered-hemisected rats depends on constitutively active 5-HT2 receptors. (a) Schematic of staggered-hemisection SCI, which transects all descending axons from the brain, including 5-HT neurons (white circles), but leaves local propriospinal neurons (black) that transverse the injury and help relay descending signals for initiation of locomotion (gray)41. (b) Rat walking with good weight support and toe clearance three weeks after the staggered-hemisection (after second hemisection). (c) Same rat with little hindlimb weight support (just foot paddling motions), while the forelimbs dragged the hindquarters during walking after blocking constitutively active 5-HT2 receptors with SB206553 (3 mM in 30 μl saline, i.t.; same dose as in Fig. 1). Scale bar, 2 cm. (d) Group means of BBB locomotor scores before and after SB206553 injection (n = 8) and control SB242084 injection (3 mM in 30 μl saline, i.t.; n = 8 rats). **P < 0.01 relative to preinjection. Error bars represent s.e.m.
DISCUSSION
A loss of brainstem-derived 5-HT after SCI acutely reduces motoneuron excitability6–9,15 and accordingly depresses all motor functions. Our results demonstrate a previously undescribed mechanism for how spinal motoneurons compensate for this lost 5-HT over the months after injury (chronic injury). Decreased editing at a single site on the 5-HT2C receptor RNA (A site) leads to increased expression of the constitutively active INI isoform of this receptor. Constitutive 5-HT2 receptor activity in turn leads to large Ca2+ PICs in motoneurons, which ultimately enable motoneurons to recover their excitability, as evidenced by their sensitivity to inverse agonists. Because large PICs in motoneurons have been shown to have key roles in normal motor function in uninjured humans and animals11, these results suggest that constitutive 5-HT receptor activity (with its associated PICs) is essential in recovery of motor function after SCI. Indeed, we show that constitutive 5-HT2 receptor activity is crucial for spontaneous recovery of hindlimb locomotor function after partial SCI, because inverse agonists impair locomotion.
Given that inverse agonists inhibit conventional activation of 5-HT2 receptors by 5-HT, as well as constitutive activity, their action alone is not definitive proof of constitutive activity, without ruling out the influence of endogenous residual 5-HT31,34. We thus ruled out residual 5-HT by showing a complete lack of effect of neutral antagonists, 5-HT depletion, in vitro spinal cord isolation, SERT blockers and 5-HT releasers after SCI in rats.
Our results also show that, without normal descending supraspinal control, these constitutively active 5-HT2 receptors and associated PICs can, unfortunately, lead to uncontrolled motoneuron firing and associated muscle spasms (LLRs), which emerge over the weeks after injury17. However, blocking this constitutive receptor activity with inverse agonists decreases spasms in rats and humans with SCI, suggesting a new rationale for antispastic drug development, although care must be taken to use a dose that preserves some residual function. For example, the high dose of SB206553 used here to maximally block spasms in the transected rat also eliminates locomotion in the rat after partial SCI. In contrast, low doses of the broad-spectrum inverse agonist cyproheptadine have been shown to improve locomotion in humans43, presumably by reducing the amplitude and incidence44 of spasms that can interfere with stepping without completely eliminating PICs and muscle strength. The EPSPs that trigger spasms (and associated SLRs) are not affected by 5-HT2C receptor inverse agonists, whereas they are inhibited by traditional antispastic drugs such as baclofen, because they are regulated by other receptors presynaptically45,46. Thus, inverse agonists provide an independent and complementary approach to traditional spasticity management29,30,46.
Taken together, our pharmacological, mRNA and immunolabeling data suggest that the large PICs on motoneurons after SCI are facilitated by constitutive activity in 5-HT2C type receptors on motoneurons (perhaps with additional involvement of 5-HT2B receptors, because SB206553 blocks both 5-HT2B and 5-HT2C receptors47–49). 5-HT2C receptors activate the intracellular phospholipase C (PLC) pathway that leads to inositol phosphate synthesis and mobilization of intracellular Ca2+ stores50,51. Constitutive 5-HT2C receptor activity leads to a basal level of activity in this PLC pathway, which is inhibited by receptor blockade with inverse agonists such as SB206553 but not by neutral antagonists such as SB242084 (refs. 31,34,47,48). Our analogous results with SB206553 and SB242084 suggest that an intracellular PLC pathway in motoneurons may be tonically activated after SCI by constitutive activity, especially considering that motoneurons (PICs) are known to be regulated by PLC, inositol phosphate and intracellular Ca2+ concentrations52–54.
The INI 5-HT2C receptor isoform that we find upregulated in the spinal cord after chronic SCI shows substantial constitutive activity, with basal levels of inositol phosphate production approaching that achieved by 5-HT (fully active)32,35. Other isoforms show substantially less constitutive activity32,36 and do not increase in expression with injury. However, these isoforms probably contribute to a basal level of constitutive receptor activity in the normal rat, which should persist acutely after injury, contributing to the small PICs measured in vitro in the acutely isolated spinal cord of normal rats15. Also, the increase in total 5-HT2C receptor expression reported with severe chronic SCI55 should increase the constitutive activity contributed from all isoforms. This might explain why the PIC that is produced by constitutive activity (SB206553-sensitive) after chronic SCI is about 40% of the maximum PIC that can be induced by activating all 5-HT2 receptors, even though INI isoform represents only about 4% of all 5-HT2C receptors after injury. This discrepancy might also be explained by the especially effective intracellular signaling capacity of INI receptor isoforms, producing many times more inositol phosphate than other isoform56, and thus perhaps producing a disproportionately large PIC.
We do not know what initiates the remarkable adaptation in 5-HT2C receptors that we see after SCI. Perhaps it is the loss of 5-HT itself38. Alternatively, the lack of motoneuron activity and associated intracellular calcium signaling may trigger the adaptation, as in synaptically isolated single neurons57. That is, motoneurons may require an optimal amount of activity, regardless of where it arises or what form it takes (spasms or walking), and activity-dependent tuning of constitutive activity in 5-HT receptors may help achieve such optimal activity. Perhaps this explains why intense locomotor training activity after SCI in humans not only improves walking but also reduces spastic muscle activity58.
Our finding of constitutive 5-HT receptor activity opens up new possibilities for understanding spinal cord plasticity in disease and injury. Although the spinal cord is densely innervated by brainstem-derived 5-HT fibers, there are actually relatively few neurons in the brainstem that provide all of this innervation (<10,000; each neuron branches extensively)4, leaving motoneurons and spinal functions vulnerable to injury or disease that affects activity in these few 5-HT neurons. Constitutive 5-HT2 receptor activity provides a safeguard against such loss of 5-HT innervation of the spinal cord and probably even contributes to basal receptor activity in normal rats. With the loss of 5-HT after SCI, this constitutive activity increases dramatically, replacing the lost 5-HT–mediated activity.
In summary, we have demonstrated that substantial constitutive 5-HT2C receptor activity emerges after SCI and contributes to recovery of motoneuron function, with both positive (walking) and negative (spasms) outcomes. This constitutive activity must work in concert with the many other factors that contribute to locomotion and spasticity5,22,23,28–30,41,45,59.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturemedicine/.
Supplementary Material
SUPPLEMENTARY INFORMATION
Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors.
Katherine C. Murray, Aya Nakae, Marilee J. Stephens, Michelle Rank, Jessica D'Amico, Philip J. Harvey, Xiaole Li, R. Luke W. Harris, Edward W. Ballou, Roberta Anelli, Charles J. Heckman, Takashi Mashimo, Romana Vavrek, Leo Sanelli, Monica A. Gorassini, David J. Bennett, and Karim Fouad
Supplementary Figure 1. 5-HT2C receptor agonists augment spasms and antagonists reverse this. (A) Same chronic spinal rat preparation and format as in Fig. 2. The selective 5-HT2C agonist MK21230 (0.3 μM, n = 8) and the selective 5-HT2A/B/C receptor agonist α-methyl-5-HT30 (0.3 μM, n = 8) increased the LLR (spasm) recorded in vitro, showing that 5-HT2C receptors are involved in spasms. (B, C) Application of SB242084 (SB242; 3 μM, n = 8), methysergide (Methys; 10μM, n = 8) or SB206553 (SB206; 3–5 μM, n = 8) antagonized the action of the agonist α-methyl-5-HT (0.3 μM). This demonstrates that both SB242084 and methysergide are effective antagonists, even though they have no effect in the absence of exogenously applied agonists (neutral antagonists, Fig. 2b,d). Also, the finding that SB206553 blocked the action of this agonist, confirms its specificity to 5-HT2 receptors, and is consistent with the action of inverse agonists, which block both constitutive and ligand-bound receptor activity31. **P < 0.01. Error bars, s.e.m.
Supplementary Figure 2. Enhancing available residual 5-HT has no effect on spasms. (A) In the chronic spinal rat, dorsal root stimulation (3×T) triggered long-lasting reflex responses recorded on the ventral roots in vitro (LLRs, quantified as mean rectified activity during horizontal bar). Blocking re-uptake of residual 5-HT with the SERT blocker citalopram32 (10 μM) did not alter the LLR. (B) Group means of LLRs demonstrate no significant change with citalopram (citalo; n = 8). Additionally, forcing presynaptic release of 5-HT with the potent 5-HT releaser fenfluramine33 (fenflu; 10 μM; n = 12) had no significant effect on the LLRs. As a positive control, we showed that the LLR was significantly enhanced by citalopram (10 μM; n = 16) in sacral spinal cords from normal rats maintained in vitro (normal cords have abundant stores of endogenous 5-HT for citalopram to act on34; Fig. 1b). These normal spinal cords do not have LLRs in control pre-drug conditions35. Thus, to match control conditions in the chronic spinal rat, we treated normal cords with a low dose of the glycine receptor blocker strychnine (3 μM) to induce LLRs36 prior to citalopram application. Citalopram was then tested in the continued presence of strychnine in normal cords. **P < 0.01. Error bars, s.e.m.
Supplementary Figure 3. 5-HT2C receptor distribution on motoneurons after SCI. (A) Immunofluorescence labeling of 5-HT2C receptors (green, SC-15081 antibody) in the S4 sacral ventral horn of a normal rat, showing extensive receptor distribution, including labeling of the soma of motoneurons (m; nucleus, n), as visualized with 0.5 μm thin optical sections with confocal microscopy (n = 5). (B) Double-labeling of the same tissue with SMI32 (red), a selective marker of motoneurons in the ventral horn (m, SMI32-positive motoneuron soma), which specifically labels neurofilament in the intracellular space29. (C) An overlay of 5-HT2C and SMI32-labeling revealed extensive co-localization (yellow and orange), indicating 5-HT2C receptors inside motoneuron soma and dendrites (note perinuclear staining; yellow near nucleus, n). Additionally, there was 5-HT2C receptor labeling (green) in isolation, including a halo of green labeling that surrounded SMI-labeled motoneurons (arrows, putative membrane), suggesting 5-HT2C receptors in (or adjacent to) the motoneuron membrane in normal rats (all rats tested showed a similar receptor distribution, n = 5 normal rats). The halo of 5-HT2C receptor labeling not co-localized with SMI32 was quantified for individual motoneurons by computing the receptor density in a 0.7 μm wide band around the perimeter of the SMI32-labeled soma. The receptor density in this perimeter band around motoneurons (not co-localized with SMI32) was 59.6 ± 4.4% of the mean receptor density inside the motoneuron (co-localized with SMI32, n = 5). Mean ± s.e.m.
(D–F) Immunolabeling in the sacral ventral horn (below injury) of a chronic spinal rat also showed 5-HT2C receptors inside motoneurons (yellow/orange co-localization with SMI32), but in this case there was less 5-HT2C labeling in isolation (less green in F), suggesting fewer receptors in the membrane (all rats tested showed a similar receptor distribution, n = 5 chronic spinal rats). On average, the receptor density not co-localized with SMI32 around the perimeter of motoneurons (in the 0.7 μm band) was 27.3 ± 2.7% of the receptor density inside motoneurons (co-localized with SMI32) in chronic spinal rats, significantly lower than that in normal rats (50% lower, n = 5, P < 0.01). Thus, after SCI the 5-HT2C receptors appear to be highly internalized in motoneurons, consistent with the presence of constitutively active isoforms of the receptors (like INI isoform), which are characteristically found internalized. That is, the INI isoform has previously been shown to be so constitutively active that as soon as it enters the membrane it couples its G protein and causes intracellular signaling that culminates in receptor phophorylation and internalization37,38. This is followed by recycling of the receptor back into the membrane and further constitutive activity, but the internalization rate exceeds the recycling rate, and so the receptor isoform accumulates mostly inside the cell (even though there is a steady G protein activation), unlike in other isoforms37,38.
(G–I) Blocking the INI receptor isoform activity with the inverse agonist SB206553 stops the process of receptor internalization and allows the receptor to accumulate in the membrane37,38. Thus, we examined the 5-HT2C receptor distribution in chronic spinal rats treated for 2 hrs with SB206553 (with two IT injections of 10 mM in 30 μL saline, separated by 1 hour, n = 4). In these treated rats there was considerable 5-HT2C receptor labeling in isolation (green in I), including labeling that surrounded SMI-labeled motoneurons (green halo, shown at arrows), suggesting substantial 5-HT2C receptors in the motoneuron membrane, unlike in untreated chronic spinal rats. On average, the receptor density not co-localized with SMI32 around the perimeter of motoneurons (in the 0.7 μm band) was 58.7 ± 3.6 % of the receptor density inside motoneurons (co-localized with SMI32) in treated chronic spinal rats, significantly higher than that in untreated chronic spinal rats (double, P < 0.01). This is consistent with the concept that inactive receptors accumulate in the membrane. It also verifies that the present immunolabeling can adequately distinguish membrane receptors from internalized receptors, similar to how internalized μ opiod receptors can be visualized with immunolabeling39. Scale bar 50 μm.
Supplementary Figure 4. 5-HT2C receptor internalization is reduced by SB206553. Similar format to Supplementary Fig. 3, but labeling done with a different 5-HT2C receptor antibody (ab32172) and DAB immunohistochemistry. (A) Typical 5-HT2C receptor labeling in ventral horn of a chronic spinal rat (black/brown, 10 μm transverse section). Black arrows indicate weakly labeled membrane of a motoneuron and its primary dendrite. Most of the receptor labeling was internalized, in diffuse clumps (e.g., at white arrow near the nucleus). (B) Typical 5-HT2C receptor labeling in a chronic spinal rat pre-treated with inverse agonist SB206553 (4 hours incubation in 30 μM SB206553, in vitro). In contrast to untreated chronic spinal rats, membranes of motoneuron soma and dendrites were more intensely labeled after treatment (at black arrows). Also, there were punctate, intensely labeled receptor clusters (black dots) that were mostly on or near the cell surface (non-confocal images), as demonstrated by punctate labeling on the surface of another motoneuron that happened to be deeper, and thus mainly the cell surface was in focus (at red arrow). These observations are consistent with the concept that SB206553 enables receptors to accumulate in the membrane by blocking their constitutive activity, whereas in untreated chronic spinal rats the receptors are largely internalized. Scale bar 50 μm.
Supplementary Table 1. Human spinal cord injured subjects. The table shows subject number plus sex (M, male; F, female), muscle studied (TA, tibialis anterior), age of subject at the time of experiment, injury level, years post-injury, the AIS – International Standards for Neurological and Functional Classification of Spinal Cord Injury40, and the MRC manual muscle test score. The latter is a qualitative assessment of the voluntary muscle strength of the examined muscle with 0 = no muscle contraction visible or palpable; 3– = greater than 50% range of motion against gravity; 3 = complete range of motion against gravity; 3+ = complete range of motion against gravity with minimal resistance; 4+ = can contract against moderate to strong pressure.
Supplementary Methods
Sacral spinal injury model in rat and relation to human spasticity. The S2 sacral spinal cord was transected in rats as described previously1-3. Briefly, under general anesthetic (sodium pentobarbital, 58.5 mg·kg–1) and sterile conditions, a laminectomy was performed on the L2 vertebrae to expose the S2 spinal cord. The dura was slit transversely, and 0.1–0.3 ml Xylocaine (1%) was applied topically. Under a surgical microscope, the spinal cord was transected by holding the pia with forceps and sucking under the pia with a fine suction tip. Caution was needed to avoid damaging the anterior artery or posterior/dorsal vein, since the sacrocaudal spinal cord dies without this midline vasculature. The dura was closed with two 8-0 silk sutures, and the muscle layers and skin were tightly sutured over the cord, and the rat allowed to recover. We evaluated spasticity and motoneuron properties 6–12 weeks post-injury (chronic spinal rats). This injury only affects the tail (not bladder or hindlimbs).
Previously, we have shown that the spasticity that emerges in the tail of chronic spinal rats closely mimics the human spasticity syndrome seen in the legs after SCI, with clonus, hypertonus, hyperreflexia, spasms, and general delayed onset of symptoms3. Flexor spasms emerge first, followed by extensor spasm (months), as also seen in humans3. Finally, we have recently shown that the same ionic mechanism that mediates spasms in the tail of rats also mediates spasms in humans (PICs in motoneuron)4, thus justifying the use of the sacral spinal rat in the current paper.
Staggered hemisection model of locomotor recovery after SCI and rationale for use. To examine recovery of locomotion after SCI, rats underwent a staggered hemisection5. Rats were first hemisected on the right at the T12 spinal vertebrae (L1–L2 spinal level)6. Then, four weeks later, they were hemisected on the left at the spinal T6 vertebrae (T6–T7 level). Locomotion was evaluated after another 3 weeks using the BBB score7. Bladders were expressed 3 times daily for 3 days, until bladder function recovered. In this staggered hemisection model all direct descending inputs, including 5-HT, are cut (Fig. 6a, white and gray neurons), whereas spared local propriospinal neurons (Fig. 6a, black neurons) relay descending signals (originating from supraspinal neurons; Fig 6a, gray neurons) around the lesion site. This allows robust spontaneous recovery of voluntary locomotion (unlike in transected animals) in the absence of most 5-HT5,8.
Spinal neurons involved in locomotion require neuromodulators like 5-HT to function, setting them into a state of readiness for movement generation9,10. The critical importance of these neuromodulators has been demonstrated by the finding in animal models that locomotor-activity can be regained soon after spinal transection with the exogenous application of drugs that activate the neuromodulatory 5-HT, norepinephrine and dopamine receptors (in vivo and in vitro)11,12-15, including 5-HT2 and 5-HT7 receptor agonists16-18. Notably, over the weeks after injury, locomotor-like movements spontaneously emerge and improve with use19-21, as if the neuromodulatory receptor systems (e.g. 5-HT2 receptors) are somehow re-activated in the absence of 5-HT. We propose that this spontaneous recovery of locomotion is in part due to constitutive 5-HT receptor activity, and we investigated this with the staggered hemisection model.
Spasms in awake spastic rat. Tail muscle spasms were measured with percutaneous EMG (electromyogram) wires inserted in segmental tail muscles at the midpoint of tail and with kinematic recordings of tail flexion (with video), while the rat was in a Plexiglas tube, as detailed previously (Fig. 1a,e)2. During EMG recording, muscle spasms were evoked with electrical stimulation of the skin at the distal tip of the tail (cutaneous stimulation; 0.2 ms, 10 mA pulse; 2– 3x afferent threshold [T]; 6 spasms evoked at 10 s intervals for a trial; trials repeated at 12 min intervals) and the tail was restrained from moving. During kinematic recording, spasms were evoked with mechanical stimulation of the tail skin2,22, and the tail was free to move, which caused sensory input that extended spasms over many minutes (Fig. 1e). EMG was sampled at 5 kHz, rectified and averaged over a 10–40 ms interval post-stimulus to quantify the short latency reflex (SLR), and a 500–4000 ms interval to quantify spasms (long lasting reflex, LLR; using Axoscope, Axon Instr., and Matlab, Mathworks). EMG over 300 ms prior to stimulation was also averaged (background). Tail flexion angle was computed as described previously22 and averaged over 3 mins post-stimulation.
Human subjects with spinal cord injury and leg spasms. The human subjects studied had varied severity of SCI, as summarized in Supplementary Table 1. The subjects were seated in their wheelchair with limbs free to move, since immobilization interfered with generating muscle spasms. Two disposable surface electrodes (Kendall Soft-E), separated by 1 cm were placed over the tibialis anterior (TA) muscle to record EMG. Spasms were evoked at rest by a brief electrical stimulation of the medial arch of the foot (with a 24 ms train of 7 pulses at 300 Hz, 0.2 ms and 50 mA each pulse; 3–5×T; using a Digitimer DS7A). Stimulation was repeated 8 times at 15 s intervals for each trial, and this was repeated every 30 mins. Spasms were quantified by rectifying and averaging the EMG over the windows in time indicated in Fig. 5.
Ventral root recordings of long-lasting reflexes in rats, in vitro. Under urethane anesthesia (1.8 g·kg–1) the whole spinal cord caudal to the S2 injury level was removed from chronic spinal rats (and age-matched normal rats) and immersed in oxygenated artificial cerebrospinal fluid (ACSF; flowing 8 ml·min–1); recordings were made starting 2.5 hr later, as detailed previously1,23. Ventral (S4 and Co1) and dorsal (Co1, coccygeal) roots were mounted on silver wires above the ACSF and covered with Vaseline. The dorsal root was stimulated with a single pulse (0.1 ms, 0.02 mA, 3×T; repeated 5 times at 10 s intervals for one trial, trials repeated every 12 mins), and the long-lasting reflex (LLR) response was recorded on the ventral roots, and then analyzed as for the EMG. We quantified the LLR by averaging the rectified ventral root activity over a time-window 500–4000 ms post stimulus, a period previously shown to reflect the motoneuron Ca PIC activity in isolation. We also measured the transient short latency reflex (SLR) by averaging ventral root responses over a window 10–40 ms post stimulus (polysynaptic reflex with about 10 ms central delay). Because of slow diffusion in whole spinal cord preparations24,25, drugs required 10x higher concentrations than in thin slice preparations, and peak effects of even TTX (2 μM; sodium channel blocker) required 10–15 mins. To assure selectivity of drugs, they were titrated to a minimal dose that produced peak effect, and results were reported after 25–45 min of drug application.
Intracellular recordings, in vitro. Sharp intracellular electrodes (30 MΩ; filled with 1M K-acetate and 1M KCl) were advanced into the spinal cord with a stepper motor (666, Kopf, Instr.) to penetrate motoneurons (identified by antidromic ventral root stimulation), as detailed before1,26. Intracellular recordings were made with an Axoclamp2B amplifier (Axon) running in discontinuous-single-electrode voltage-clamp (gain 0.8–2.5 nA·mV–1; for Ca PICs) or discontinuous-current-clamp (switching rate 5 kHz; for EPSPs) mode, filtered at 3 kHz, and sampled at 6.7 kHz (Clampex and Clampfit used; Axon Instr.). In the presence of TTX (2 μM) to block synaptic transmission and sodium currents (Na PIC), slow triangular voltage ramps (3.5 mV s–1 voltage-clamp) were applied to the motoneurons to measure the Ca PIC (Fig. 3b)1,26. During the upward portion of this ramp, the current response initially increased linearly with voltage in response to the passive leak conductance. A linear relation was fit to the current in the region just below the Ca PIC onset (5 mV below) and extrapolated to the whole range of the ramp (leak current, thin line in Fig. 3b). At depolarized potentials above the Ca PIC onset threshold, the Ca PIC induced a downward deviation from the extrapolated leak current, and this Ca PIC was estimated as the difference between the leak current and the total current (leak-subtracted current). The amplitude of the peak Ca PIC was quantified as the initial peak amplitude of this downward deviation below the leak line (downward arrow Fig. 3b). The Ca PIC is thought to be mediated by Cav1.3 L-type calcium channels1, because it is activated at an unusually low threshold (about 10 mV above rest), blocked by high doses of nimodipine and highly persistent (in contrast, transient currents are inactivated during the slow ramp). There is also a small contribution to the Ca PIC from calcium-activated channels27.
The excitatory postsynaptic potential (EPSP) and associated reflexes were also measured in motoneurons by stimulating the Co1 dorsal roots (at 2–3×T, as in ventral root reflex recording), while applying hyperpolarizing bias currents to block the PICs (Fig. 3d).
mRNA measurements. Following the methods of Nakae et al.28, RNA was extracted from spinal cords of chronic spinal rats and age-matched normal rats with RNAeasy-Lipid-tissue Mini-Kits (Qiagen, Japan) and used for first-strand cDNA synthesis by Thermoscript (Invitrogen). Quantitative RT-PCR was then performed on the cDNA with ABI-PRISM-7900HT using TaqMan probes (Applied Biosystems) to estimate total 5-HT2C receptor mRNA (and internal control 18SrRNA). To determine the 5-HT2C receptor isoforms in the tissue, rat 5-HT2C receptor fragments were amplified from the cDNA using RT-PCR with the primers ATCATGGCAGTAAGCATGGAGAAGA and ATTGATATTGCCCAAACGATGGCA. The PCR product DNA was cloned into the pCR2.1 vector (Invitrogen), transfected into single E. coli bacterial cells, and > 300 recombinant colonies grown (each corresponding to a single 5-HT2C receptor isoform). DNA extracted from 80 randomly picked colonies underwent nucleotide sequencing with an ABI3700 genetic analyzer (Applied Biosystems) to determine the isoform DNA adopted by each colony.
Immunolabeling. Rats were euthanized with Euthanyl (Bimeda-MTC; 700 mg·kg–1) and perfused intracardially with 100ml saline followed by 400 ml paraformaldehyde (PFA; in phosphate buffer at room temperature; over about 15 mins), with 4% PFA used for 5-HT immunolabeling and 2% PFA for 5-HT2C and SMI32 immunolabeling. Spinal cords were postfixed in PFA (overnight for 5-HT-labeling and 90 mins for others) at 4 °C, cryoprotected in 20% sucrose and 2% ethylene-glycol, frozen and cut on a cryostat in horizontal or transverse 25–40 μm sections. Spinal cord sections were incubated overnight at 4 °C with primary antibodies to 5-HT (1:1000 S5545, Sigma, raised in rabbit), 5-HT2C receptors (1:900, SC-15081, epitope near N-terminus, Santa Cruz, goat) or SMI32 (1:000, Sternberger, mouse) in phosphate or Tris buffered saline (PBS or TBS) and 1% normal serum. Antibody labeling was visualized with fluorescent secondary antibodies (5-HT: Texas-Red[TI1000], 1:200, Vector, USA, overnight at 4 °C; 5-HT2C: Alexa488[A11055 or A11078], 1:1000, Invitrogen, 90 min at room-temperature; SMI32: Alexa568[A11008], 1:1000, Invitrogen, 90 min RT) and using a Zeiss-CLSM510 microscope and laser-scanning confocal microscopy.
Controls in which the primary antibody was pre-absorbed with the antigen that it was raised against were used to verify the selectivity of the antibody labeling. For example, the 5-HT2C receptor antigen (1:180; 5.5 μl·ml–1, SC-15081P, Santa Cruz) was incubated with the 5-HT2C receptor antibody (SC-15081) for 80 mins at room temperature prior to dilution and application to tissue. Also, controls in which the primary antibody was omitted were used to confirm that the secondary antibody produced no labeling by itself.
SMI32 labels non-phosphorylated neurofilament H, and does not change with spinal cord injury29. We have previously demonstrated that SMI32 labels motoneurons, and not interneurons in the ventral horn, though it also labels large cells elsewhere in the brain. Thus, SMI32 provided a fairly selective marker of motoneurons in the ventral horn29, and in particular labels the intracellular space (neurofilament). Using image analysis software (ImageJ), we defined two regions for 5-HT2C receptor quantification in motoneurons: 1) the internal region inside motoneuron soma, co-localized with SMI32 (region with SMI32 above standardized threshold, as we described in Anelli et al.29), and 2) the membrane region, immediately adjacent to the internal SMI32 region. The later was produced by detecting the edge of the SMI32-positive region inside motoneuron soma and expanding this edge to a 0.7 μm wide band that surrounded the perimeter of the soma (not co-localized with SMI32). The mean 5-HT2C immunofluorescence signal density was then computed in these two regions, from which we computed the ratio of the receptor density in the membrane relative to the density inside the cell (x100%) for comparison across normal and injured rats.
We also labeled 10 μm transverse spinal cord sections from chronic spinal rats with a different 5-HT2C antibody (ab32172, epitope on C-terminus, Abcam), and then visualized the antibody with ABC amplification (Vector-Labs) followed by DAB labeling. For this, spinal cords were initially placed in vitro for a 4 hr incubation with and without SB206553 (30 μM) and then fixed in 4% PFA. After cryoprotection, frozen sections were cut and then incubated 15 min in 0.5% triton-x100 in PBS, 5 mins in 1.5% hydrogen peroxide in PBS, 1 hour in 10% normal goat serum in PBS, and then overnight in the primary 5-HT2C antibody in PBS (1:200; ab32172), all at RT. Then, sections were incubated in a biotinylated secondary antibody (1:200; BA9400; Vector-Labs) overnight at 4 °C, followed by incubation in the Vector ABC elite complex, overnight at 4 °C, and then 5-HT2C receptors were visualized with the Vector DAB kit and viewed with conventional light microscopy.
Supplementary References
1. Li, Y., Gorassini, M.A. & Bennett, D.J. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91, 767-783 (2004).
2. Bennett, D.J., Sanelli, L., Cooke, C., Harvey, P.J. & Gorassini, M.A. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J. Neurophysiol. 91, 2247-58 (2004).
3. Bennett, D.J. et al. Spasticity in rats with sacral spinal cord injury. J Neurotrauma 16, 69-84 (1999).
4. Gorassini, M.A., Knash, M.E., Harvey, P.J., Bennett, D.J. & Yang, J.F. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127, 2247-58 (2004).
5. Courtine, G. et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14, 69-74 (2008).
6. Ballermann, M. & Fouad, K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci 23, 1988-96 (2006).
7. Basso, D.M., Beattie, M.S. & Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12, 1-21 (1995).
8. Cowley, K.C., Zaporozhets, E. & Schmidt, B.J. Propriospinal neurons are sufficient for bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol 586, 1623-35 (2008).
9. Jordan, L.M., Liu, J., Hedlund, P.B., Akay, T. & Pearson, K.G. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57, 183-91 (2008).
10. Jacobs, B.L., Martin-Cora, F.J. & Fornal, C.A. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40, 45-52 (2002).
11. Kiehn, O. & Kjaerulff, O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol 75, 1472-82 (1996).
12. Cowley, K.C. & Schmidt, B.J. A comparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett 171, 147-50 (1994).
13. Viala, D. & Buser, P. [Methods of obtaining locomotor rhythms in the spinal rabbit by pharmacological treatments (DOPA, 5-HTP, D-amphetamine)]. Brain Res 35, 151-65 (1971).
14. Chau, C., Barbeau, H. & Rossignol, S. Effects of intrathecal alpha1- and alpha2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. J Neurophysiol 79, 2941-63 (1998).
15. Courtine, G. et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12, 1333-42 (2009).
16. Madriaga, M.A., McPhee, L.C., Chersa, T., Christie, K.J. & Whelan, P.J. Modulation of locomotor activity by multiple 5-HT and dopaminergic receptor subtypes in the neonatal mouse spinal cord. J Neurophysiol 92, 1566-76 (2004).
17. Landry, E.S. & Guertin, P.A. Differential effects of 5-HT1 and 5-HT2 receptor agonists on hindlimb movements in paraplegic mice. Prog Neuropsychopharmacol Biol Psychiatry 28, 1053-60 (2004).
18. McEwen, M.L., Van Hartesveldt, C. & Stehouwer, D.J. L-DOPA and quipazine elicit air-stepping in neonatal rats with spinal cord transections. Behav Neurosci 111, 825-33 (1997).
19. Kuhn, R.A. & Macht, M.B. Some manifestations of reflex activity in spinal man with particular reference to the occurrence of extensor spasm. Bull. Johns Hopkins Hosp. 84, 43-75 (1948).
20. de Leon, R.D., Hodgson, J.A., Roy, R.R. & Edgerton, V.R. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol 79, 1329-40 (1998).
21. Barbeau, H. & Rossignol, S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412, 84-95 (1987).
22. Rank, M.M., Li, X., Bennett, D.J. & Gorassini, M.A. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol 97, 3166-80 (2007).
23. Li, Y., Li, X., Harvey, P.J. & Bennett, D.J. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol 92, 2694-2703 (2004).
24. Harvey, P.J., Li, X., Li, Y. & Bennett, D.J. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96, 1171-86 (2006).
25. Perrier, J.F. & Hounsgaard, J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol 89, 954-9 (2003).
26. Harvey, P.J., Li, Y., Li, X. & Bennett, D.J. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96, 1141-57 (2006).
27. Li, X. & Bennett, D.J. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97, 3314-30 (2007).
28. Nakae, A. et al. Serotonin2C receptor mRNA editing in neuropathic pain model. Neurosci Res 60, 228-31 (2008).
29. Anelli, R., Sanelli, L., Bennett, D.J. & Heckman, C.J. Expression of L-type calcium channel alpha(1)-1.2 and alpha(1)-1.3 subunits on rat sacral motoneurons following chronic spinal cord injury. Neuroscience 145, 751-63 (2007).
30. Knight, A.R. et al. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol 370, 114-23 (2004).
31. Herrick-Davis, K., Grinde, E. & Niswender, C.M. Serotonin 5-HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J Neurochem 73, 1711-7 (1999).
32. Henry, L.K. et al. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem 281, 2012-23 (2006).
33. Rothman, R.B. & Baumann, M.H. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther 95, 73-88 (2002).
34. Wallis, D.I., Wu, J. & Wang, X. Descending inhibition in the neonate rat spinal cord is mediated by 5-hydroxytryptamine. Neuropharmacology 32, 73-83 (1993).
35. Li, Y., Harvey, P.J., Li, X. & Bennett, D.J. Spastic long-lasting reflexes in the chronic spinal rat, studied in vitro. J Neurophysiol 91, 2236-46 (2004).
36. Jiang, M.C. & Heckman, C.J. In vitro sacral cord preparation and motoneuron recording from adult mice. J Neurosci Methods 156, 31-6 (2006).
37. Chanrion, B. et al. Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-hydroxytryptamine2C receptors: differential modulation of cell surface expression and signal transduction. Mol Pharmacol 73, 748-57 (2008).
38. Marion, S., Weiner, D.M. & Caron, M.G. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J Biol Chem 279, 2945-54 (2004).
39. Sternini, C. et al. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci U S A 93, 9241-6 (1996).
40. Maynard, F.M., Jr. et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 35, 266-74 (1997).
ACKNoWLEDGMENTS
Thanks to F. Geddes, T. Tanaka, K. Miyake, G. Van Patten, J. Nevett-Duchcherer, G. Funk, M. Finlay and L. Hahn for assistance. This research was supported by the Alberta Heritage Foundation, Canadian Institutes of Health Research and the US National Institutes of Health (NS47567 and NS48170).
ONLINE METHODS
Spinal lesions
All rat use was approved by the University of Alberta Animal Care and Use Committee: Health Sciences. We completely transected spinal cords of adult female Sprague-Dawley rats (locally bred) at the S2 sacral spinal level and evaluated spasticity and motoneuron properties 6–12 weeks post-injury (chronic spinal state, see Supplementary Methods)12,17. Also, a separate group of female rats underwent a staggered hemisection41, which, like a transection, removes most descending supraspinal axons below the injury (including 5-HT axons), but leaves intact some propriospinal neuron connections that enable the rat to voluntarily initiate walking, as detailed in the Supplementary Methods.
All human experiments were carried out with signed, informed consent of subjects and approved by the University of Alberta Health Research Ethics Board. Human subjects had chronic SCI with varied severity (Supplementary Table 1) and did not take their antispastic medications on the experiment day.
Spasms in awake chronic spinal rats
We evoked tail muscle spasms with brief electrical (3× afferent threshold (T)) or manual stimulation of the skin of the tail and recorded these spasms with tail muscle EMG and video kinematic analysis, as detailed in the Supplementary Methods. Briefly, EMG was rectified and averaged over 10–40 ms after stimulus (SLR) and 500–4,000 ms after stimulus (LLR), and tail flexion angle measured.
Spasms in humans with SCI
We evoked leg spasms with a brief electrical stimulation of the medial arch of the foot (3–5×T) and recorded surface EMG responses over the tibialis anterior (TA) muscle (Fig. 5)27. We computed the SLR and LLR by averaging EMG over the intervals 50–100 and 500–5,000 ms after stimulation, respectively, and then subtracting background EMG (see Supplementary Methods).
Ventral root and intracellular motoneuron recording in rats, in vitro
The whole spinal cord caudal to the S2 injury level was removed from chronic spinal rats and maintained in vitro for ventral root and intracellular motoneuron recordings12,45, as described in the Supplementary Methods. Briefly, we stimulated a coccygeal dorsal root (Co1) with a single pulse (0.1 ms, 0.02 mA, 3×T), recorded the reflex response on the S4 and Co1 sacrocaudal ventral roots, and computed the mean SLR (over 10–40 ms after stimulation), LLR (500–4,000 ms after stimulation) and background activity (over 300 ms before stimulation). For intracellular recordings, sharp intracellular electrodes were advanced into motoneurons, and the Ca2+ PIC was measured under voltage-clamp. The Ca2+ PIC was quantified as the downward current deflection (Fig. 3b, thick black line, at arrow) recorded during a slow upward voltage ramp (Fig. 3b, top, gray), relative to the leak current (thin line), in tetrodotoxin. Characteristically, this Ca2+ PIC was activated at low voltages (–56.7 ± 6.0 mV), deactivated at even lower voltages (on downward ramp) and mediated by L-type calcium channels (nimodipine-sensitive), as previously reported12,15.
Locomotor assessment after spinal cord injury in rats
Locomotion was evaluated 3 weeks after the staggered hemisection using the BBB score42, as detailed in the Supplementary Methods.
Drugs and solutions
The drugs used were 5-HT, fenfluramine, SB242084, strychnine, para-chlorophenylalanine-methyl-ester (all Sigma-Aldrich), α-methyl-5-HT, citalopram, cyproheptadine, methysergide, MK212, nimodipine, SB206553 (all Tocris) and tetrodotoxin citrate (Alomone). In vitro, the artificial cerebrospinal fluid consisted of (in mM) 122 NaCl, 24 NaHCO3, 2.5 CaCl2, 3 KCl, 1 MgCl2 and 12 d-glucose, saturated with 95% O2 and 5% CO2 (pH 7.4) and maintained at 22–24 °C. Drugs were dissolved in the artificial cerebrospinal fluid. In vivo, drugs were administered via transcutaneous i.t. injection60, intraperitoneal injection or oral gavage, and peak effects were reported (at 5–20 min after i.t. and 60 min after oral gavage). SB206553 was used at a dose that produced maximal effects (on spasms) both in vivo and in vitro (determined by titration), and SB242084 was used at the same dose, because SB206553 and SB242084 have similar binding affinity at 5-HT2C receptors49.
mRNA measurements
We extracted RNA from the whole spinal cord below the S2 injury level and from this synthesized and amplified cDNA (with RT-PCR) to quantify the mRNA. We quantified RNA editing and 5-HT2C isoforms by sequencing the DNA of bacterial colonies grown from single bacteria cells transfected with DNA fragments synthesized and amplified from spinal cord cDNA (using 5-HT2C receptor–related PCR primers; each colony adopts a single 5-HT2C receptor isoform). We computed editing efficiency at each of five sites (A–E in Fig. 4a) as the proportion of colonies with editing at that site in their sequence. We computed the proportion of each 5-HT2C receptor isoform in the spinal cord, from the number colonies with that isoform, relative to the total number of colonies. See further details in the Supplementary Methods.
Histology
Immunofluorescence labeling for 5-HT and 5-HT2C receptors was performed as described in the Supplementary Methods.
Statistical analyses
Statistical comparisons were performed by a paired t test after verifying normality. Data are reported as means ± s.e.m.
Additional methods
Detailed methodology is described in the Supplementary Methods.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol. Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J. Physiol. (Lond.) 2003;552:945–952. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlsson A, Magnusson T, Rosengren E. 5-hydroxytryptamine of the spinal cord normally and after transection. Experientia. 1963;19:359. doi: 10.1007/BF02152316. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res. Brain Res. Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 5.Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res. Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Perrier JF, Delgado-Lezama R. Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J. Neurosci. 2005;25:7993–7999. doi: 10.1523/JNEUROSCI.1957-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J. Physiol. (Lond.) 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J. Neurophysiol. 2007;97:1236–1246. doi: 10.1152/jn.00995.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrier JF, Hounsgaard J. 5–HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J. Neurophysiol. 2003;89:954–959. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- 10.Harvey PJ, Li X, Li Y, Bennett DJ. 5–HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J. Neurophysiol. 2006;96:1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J. Neurophysiol. 2004;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- 13.Carlin KP, Jiang Z, Brownstone RM. Characterization of calcium currents in functionally mature mouse spinal motoneurons. Eur. J. Neurosci. 2000;12:1624–1634. doi: 10.1046/j.1460-9568.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 14.Brownstone RM, Gossard JP, Hultborn H. Voltage-dependent excitation of motoneurones from spinal locomotor centres in the cat. Exp. Brain Res. 1994;102:34–44. doi: 10.1007/BF00232436. [DOI] [PubMed] [Google Scholar]
- 15.Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J. Neurophysiol. 2006;96:1141–1157. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DJ, et al. Spasticity in rats with sacral spinal cord injury. J. Neurotrauma. 1999;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- 17.Bennett DJ, Sanelli L, Cooke C, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J. Neurophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn RA, Macht MB. Some manifestations of reflex activity in spinal man with particular reference to the occurrence of extensor spasm. Bull. Johns Hopkins Hosp. 1949;84:43–75. [PubMed] [Google Scholar]
- 19.Ung RV, et al. Role of spinal 5–HT2 receptor subtypes in quipazine-induced hindlimb movements after a low-thoracic spinal cord transection. Eur. J. Neurosci. 2008;28:2231–2242. doi: 10.1111/j.1460-9568.2008.06508.x. [DOI] [PubMed] [Google Scholar]
- 20.Button DC, et al. Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? J. Physiol. (Lond.) 2008;586:529–544. doi: 10.1113/jphysiol.2007.141499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boulenguez P, et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 22.Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- 23.Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain. 2008;131:1478–1491. doi: 10.1093/brain/awn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology. The Nervous System. Motor Control. American Physiological Society; Bethesda, Maryland: 1981. pp. 509–595. [Google Scholar]
- 25.Shefchyk SJ, Jordan LM. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J. Neurophysiol. 1985;53:1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- 26.Wallis DI, Wu J, Wang X. Descending inhibition in the neonate rat spinal cord is mediated by 5-hydroxytryptamine. Neuropharmacology. 1993;32:73–83. doi: 10.1016/0028-3908(93)90132-m. [DOI] [PubMed] [Google Scholar]
- 27.Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- 28.Baker LL, Chandler SH. Characterization of postsynaptic potentials evoked by sural nerve stimulation in hindlimb motoneurons from acute and chronic spinal cats. Brain Res. 1987;420:340–350. doi: 10.1016/0006-8993(87)91255-8. [DOI] [PubMed] [Google Scholar]
- 29.Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007;6:725–733. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- 30.Ashby P, McCrea DA. Neurphysiology of spinal spasticity. In: Davidoff RA, editor. Handbook of the Spinal Cord. Dekker; New York: 1987. pp. 119–143. [Google Scholar]
- 31.Seifert R, Wenzel-Seifert K. Constitutive activity of G protein–coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 32.Herrick-Davis K, Grinde E, Niswender CM. Serotonin 5–HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J. Neurochem. 1999;73:1711–1717. doi: 10.1046/j.1471-4159.1999.731711.x. [DOI] [PubMed] [Google Scholar]
- 33.Westphal RS, Sanders-Bush E. Reciprocal binding properties of 5-hydroxytryptamine type 2C receptor agonists and inverse agonists. Mol. Pharmacol. 1994;46:937–942. [PubMed] [Google Scholar]
- 34.Chanrion B, et al. Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-hydroxytryptamine2C receptors: differential modulation of cell surface expression and signal transduction. Mol. Pharmacol. 2008;73:748–757. doi: 10.1124/mol.107.041574. [DOI] [PubMed] [Google Scholar]
- 35.Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J. Biol. Chem. 1999;274:9472–9478. doi: 10.1074/jbc.274.14.9472. [DOI] [PubMed] [Google Scholar]
- 36.Berg KA, Dunlop J, Sanchez T, Silva M, Clarke WP. A conservative, single-amino acid substitution in the second cytoplasmic domain of the human Serotonin2C receptor alters both ligand-dependent and -independent receptor signaling. J. Pharmacol. Exp. Ther. 2008;324:1084–1092. doi: 10.1124/jpet.107.131524. [DOI] [PubMed] [Google Scholar]
- 37.Marion S, Weiner DM, Caron MG. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J. Biol. Chem. 2004;279:2945–2954. doi: 10.1074/jbc.M308742200. [DOI] [PubMed] [Google Scholar]
- 38.Gurevich I, Englander MT, Adlersberg M, Siegal NB, Schmauss C. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J. Neurosci. 2002;22:10529–10532. doi: 10.1523/JNEUROSCI.22-24-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newton BW, Hamill RW. The morphology and distribution of rat serotoninergic intraspinal neurons: an immunohistochemical study. Brain Res. Bull. 1988;20:349–360. doi: 10.1016/0361-9230(88)90064-0. [DOI] [PubMed] [Google Scholar]
- 40.Nakae A, et al. Serotonin2C receptor mRNA editing in neuropathic pain model. Neurosci. Res. 2008;60:228–231. doi: 10.1016/j.neures.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Courtine G, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 43.Wainberg M, Barbeau H, Gauthier S. The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J. Neurol. Neurosurg. Psychiatry. 1990;53:754–763. doi: 10.1136/jnnp.53.9.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbeau H, Richards CL, Bedard PJ. Action of cyproheptadine in spastic paraparetic patients. J. Neurol. Neurosurg. Psychiatry. 1982;45:923–926. doi: 10.1136/jnnp.45.10.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J. Neurophysiol. 2004;92:2694–2703. doi: 10.1152/jn.00164.2004. [DOI] [PubMed] [Google Scholar]
- 46.Davidoff RA. Antispasticity drugs: mechanisms of action. Ann. Neurol. 1985;17:107–116. doi: 10.1002/ana.410170202. [DOI] [PubMed] [Google Scholar]
- 47.Kennett GA, et al. In vitro and in vivo profile of SB 206553, a potent 5–HT2C/5–HT2B receptor antagonist with anxiolytic-like properties. Br. J. Pharmacol. 1996;117:427–434. doi: 10.1111/j.1476-5381.1996.tb15208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennett GA, et al. SB 242084, a selective and brain penetrant 5–HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- 49.Knight AR, et al. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2004;370:114–123. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- 50.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 51.Mizuno N, Itoh H. Functions and regulatory mechanisms of Gq-signaling pathways. Neurosignals. 2009;17:42–54. doi: 10.1159/000186689. [DOI] [PubMed] [Google Scholar]
- 52.Mejia-Gervacio S, Hounsgaard J, Diaz-Munoz M. Roles of ryanodine and inositol triphosphate receptors in regulation of plateau potentials in turtle spinal motoneurons. Neuroscience. 2004;123:123–130. doi: 10.1016/j.neuroscience.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 53.Perrier JF, Mejia-Gervacio S, Hounsgaard J. Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J. Physiol. (Lond.) 2000;528:107–113. doi: 10.1111/j.1469-7793.2000.t01-1-00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holohean AM, Hackman JC. Mechanisms intrinsic to 5–HT2B receptor-induced potentiation of NMDA receptor responses in frog motoneurones. Br. J. Pharmacol. 2004;143:351–360. doi: 10.1038/sj.bjp.0705935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayashi Y, et al. 5-HT precursor loading, but not 5-HT receptor agonists, increases motor function after spinal cord contusion in adult rats. Exp. Neurol. 2010;221:68–78. doi: 10.1016/j.expneurol.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGrew L, Price RD, Hackler E, Chang MS, Sanders-Bush E. RNA editing of the human serotonin 5–HT2C receptor disrupts transactivation of the small G-protein RhoA. Mol. Pharmacol. 2004;65:252–256. doi: 10.1124/mol.65.1.252. [DOI] [PubMed] [Google Scholar]
- 57.Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- 58.Gorassini MA, Norton JA, Nevett-Duchcherer J, Roy FD, Yang JF. Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J. Neurophysiol. 2009;101:969–979. doi: 10.1152/jn.91131.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rank MM, Li X, Bennett DJ, Gorassini MA. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J. Neurophysiol. 2007;97:3166–3180. doi: 10.1152/jn.01168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J. Pharmacol. Toxicol. Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY INFORMATION
Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors.
Katherine C. Murray, Aya Nakae, Marilee J. Stephens, Michelle Rank, Jessica D'Amico, Philip J. Harvey, Xiaole Li, R. Luke W. Harris, Edward W. Ballou, Roberta Anelli, Charles J. Heckman, Takashi Mashimo, Romana Vavrek, Leo Sanelli, Monica A. Gorassini, David J. Bennett, and Karim Fouad
Supplementary Figure 1. 5-HT2C receptor agonists augment spasms and antagonists reverse this. (A) Same chronic spinal rat preparation and format as in Fig. 2. The selective 5-HT2C agonist MK21230 (0.3 μM, n = 8) and the selective 5-HT2A/B/C receptor agonist α-methyl-5-HT30 (0.3 μM, n = 8) increased the LLR (spasm) recorded in vitro, showing that 5-HT2C receptors are involved in spasms. (B, C) Application of SB242084 (SB242; 3 μM, n = 8), methysergide (Methys; 10μM, n = 8) or SB206553 (SB206; 3–5 μM, n = 8) antagonized the action of the agonist α-methyl-5-HT (0.3 μM). This demonstrates that both SB242084 and methysergide are effective antagonists, even though they have no effect in the absence of exogenously applied agonists (neutral antagonists, Fig. 2b,d). Also, the finding that SB206553 blocked the action of this agonist, confirms its specificity to 5-HT2 receptors, and is consistent with the action of inverse agonists, which block both constitutive and ligand-bound receptor activity31. **P < 0.01. Error bars, s.e.m.
Supplementary Figure 2. Enhancing available residual 5-HT has no effect on spasms. (A) In the chronic spinal rat, dorsal root stimulation (3×T) triggered long-lasting reflex responses recorded on the ventral roots in vitro (LLRs, quantified as mean rectified activity during horizontal bar). Blocking re-uptake of residual 5-HT with the SERT blocker citalopram32 (10 μM) did not alter the LLR. (B) Group means of LLRs demonstrate no significant change with citalopram (citalo; n = 8). Additionally, forcing presynaptic release of 5-HT with the potent 5-HT releaser fenfluramine33 (fenflu; 10 μM; n = 12) had no significant effect on the LLRs. As a positive control, we showed that the LLR was significantly enhanced by citalopram (10 μM; n = 16) in sacral spinal cords from normal rats maintained in vitro (normal cords have abundant stores of endogenous 5-HT for citalopram to act on34; Fig. 1b). These normal spinal cords do not have LLRs in control pre-drug conditions35. Thus, to match control conditions in the chronic spinal rat, we treated normal cords with a low dose of the glycine receptor blocker strychnine (3 μM) to induce LLRs36 prior to citalopram application. Citalopram was then tested in the continued presence of strychnine in normal cords. **P < 0.01. Error bars, s.e.m.
Supplementary Figure 3. 5-HT2C receptor distribution on motoneurons after SCI. (A) Immunofluorescence labeling of 5-HT2C receptors (green, SC-15081 antibody) in the S4 sacral ventral horn of a normal rat, showing extensive receptor distribution, including labeling of the soma of motoneurons (m; nucleus, n), as visualized with 0.5 μm thin optical sections with confocal microscopy (n = 5). (B) Double-labeling of the same tissue with SMI32 (red), a selective marker of motoneurons in the ventral horn (m, SMI32-positive motoneuron soma), which specifically labels neurofilament in the intracellular space29. (C) An overlay of 5-HT2C and SMI32-labeling revealed extensive co-localization (yellow and orange), indicating 5-HT2C receptors inside motoneuron soma and dendrites (note perinuclear staining; yellow near nucleus, n). Additionally, there was 5-HT2C receptor labeling (green) in isolation, including a halo of green labeling that surrounded SMI-labeled motoneurons (arrows, putative membrane), suggesting 5-HT2C receptors in (or adjacent to) the motoneuron membrane in normal rats (all rats tested showed a similar receptor distribution, n = 5 normal rats). The halo of 5-HT2C receptor labeling not co-localized with SMI32 was quantified for individual motoneurons by computing the receptor density in a 0.7 μm wide band around the perimeter of the SMI32-labeled soma. The receptor density in this perimeter band around motoneurons (not co-localized with SMI32) was 59.6 ± 4.4% of the mean receptor density inside the motoneuron (co-localized with SMI32, n = 5). Mean ± s.e.m.
(D–F) Immunolabeling in the sacral ventral horn (below injury) of a chronic spinal rat also showed 5-HT2C receptors inside motoneurons (yellow/orange co-localization with SMI32), but in this case there was less 5-HT2C labeling in isolation (less green in F), suggesting fewer receptors in the membrane (all rats tested showed a similar receptor distribution, n = 5 chronic spinal rats). On average, the receptor density not co-localized with SMI32 around the perimeter of motoneurons (in the 0.7 μm band) was 27.3 ± 2.7% of the receptor density inside motoneurons (co-localized with SMI32) in chronic spinal rats, significantly lower than that in normal rats (50% lower, n = 5, P < 0.01). Thus, after SCI the 5-HT2C receptors appear to be highly internalized in motoneurons, consistent with the presence of constitutively active isoforms of the receptors (like INI isoform), which are characteristically found internalized. That is, the INI isoform has previously been shown to be so constitutively active that as soon as it enters the membrane it couples its G protein and causes intracellular signaling that culminates in receptor phophorylation and internalization37,38. This is followed by recycling of the receptor back into the membrane and further constitutive activity, but the internalization rate exceeds the recycling rate, and so the receptor isoform accumulates mostly inside the cell (even though there is a steady G protein activation), unlike in other isoforms37,38.
(G–I) Blocking the INI receptor isoform activity with the inverse agonist SB206553 stops the process of receptor internalization and allows the receptor to accumulate in the membrane37,38. Thus, we examined the 5-HT2C receptor distribution in chronic spinal rats treated for 2 hrs with SB206553 (with two IT injections of 10 mM in 30 μL saline, separated by 1 hour, n = 4). In these treated rats there was considerable 5-HT2C receptor labeling in isolation (green in I), including labeling that surrounded SMI-labeled motoneurons (green halo, shown at arrows), suggesting substantial 5-HT2C receptors in the motoneuron membrane, unlike in untreated chronic spinal rats. On average, the receptor density not co-localized with SMI32 around the perimeter of motoneurons (in the 0.7 μm band) was 58.7 ± 3.6 % of the receptor density inside motoneurons (co-localized with SMI32) in treated chronic spinal rats, significantly higher than that in untreated chronic spinal rats (double, P < 0.01). This is consistent with the concept that inactive receptors accumulate in the membrane. It also verifies that the present immunolabeling can adequately distinguish membrane receptors from internalized receptors, similar to how internalized μ opiod receptors can be visualized with immunolabeling39. Scale bar 50 μm.
Supplementary Figure 4. 5-HT2C receptor internalization is reduced by SB206553. Similar format to Supplementary Fig. 3, but labeling done with a different 5-HT2C receptor antibody (ab32172) and DAB immunohistochemistry. (A) Typical 5-HT2C receptor labeling in ventral horn of a chronic spinal rat (black/brown, 10 μm transverse section). Black arrows indicate weakly labeled membrane of a motoneuron and its primary dendrite. Most of the receptor labeling was internalized, in diffuse clumps (e.g., at white arrow near the nucleus). (B) Typical 5-HT2C receptor labeling in a chronic spinal rat pre-treated with inverse agonist SB206553 (4 hours incubation in 30 μM SB206553, in vitro). In contrast to untreated chronic spinal rats, membranes of motoneuron soma and dendrites were more intensely labeled after treatment (at black arrows). Also, there were punctate, intensely labeled receptor clusters (black dots) that were mostly on or near the cell surface (non-confocal images), as demonstrated by punctate labeling on the surface of another motoneuron that happened to be deeper, and thus mainly the cell surface was in focus (at red arrow). These observations are consistent with the concept that SB206553 enables receptors to accumulate in the membrane by blocking their constitutive activity, whereas in untreated chronic spinal rats the receptors are largely internalized. Scale bar 50 μm.
Supplementary Table 1. Human spinal cord injured subjects. The table shows subject number plus sex (M, male; F, female), muscle studied (TA, tibialis anterior), age of subject at the time of experiment, injury level, years post-injury, the AIS – International Standards for Neurological and Functional Classification of Spinal Cord Injury40, and the MRC manual muscle test score. The latter is a qualitative assessment of the voluntary muscle strength of the examined muscle with 0 = no muscle contraction visible or palpable; 3– = greater than 50% range of motion against gravity; 3 = complete range of motion against gravity; 3+ = complete range of motion against gravity with minimal resistance; 4+ = can contract against moderate to strong pressure.
Supplementary Methods
Sacral spinal injury model in rat and relation to human spasticity. The S2 sacral spinal cord was transected in rats as described previously1-3. Briefly, under general anesthetic (sodium pentobarbital, 58.5 mg·kg–1) and sterile conditions, a laminectomy was performed on the L2 vertebrae to expose the S2 spinal cord. The dura was slit transversely, and 0.1–0.3 ml Xylocaine (1%) was applied topically. Under a surgical microscope, the spinal cord was transected by holding the pia with forceps and sucking under the pia with a fine suction tip. Caution was needed to avoid damaging the anterior artery or posterior/dorsal vein, since the sacrocaudal spinal cord dies without this midline vasculature. The dura was closed with two 8-0 silk sutures, and the muscle layers and skin were tightly sutured over the cord, and the rat allowed to recover. We evaluated spasticity and motoneuron properties 6–12 weeks post-injury (chronic spinal rats). This injury only affects the tail (not bladder or hindlimbs).
Previously, we have shown that the spasticity that emerges in the tail of chronic spinal rats closely mimics the human spasticity syndrome seen in the legs after SCI, with clonus, hypertonus, hyperreflexia, spasms, and general delayed onset of symptoms3. Flexor spasms emerge first, followed by extensor spasm (months), as also seen in humans3. Finally, we have recently shown that the same ionic mechanism that mediates spasms in the tail of rats also mediates spasms in humans (PICs in motoneuron)4, thus justifying the use of the sacral spinal rat in the current paper.
Staggered hemisection model of locomotor recovery after SCI and rationale for use. To examine recovery of locomotion after SCI, rats underwent a staggered hemisection5. Rats were first hemisected on the right at the T12 spinal vertebrae (L1–L2 spinal level)6. Then, four weeks later, they were hemisected on the left at the spinal T6 vertebrae (T6–T7 level). Locomotion was evaluated after another 3 weeks using the BBB score7. Bladders were expressed 3 times daily for 3 days, until bladder function recovered. In this staggered hemisection model all direct descending inputs, including 5-HT, are cut (Fig. 6a, white and gray neurons), whereas spared local propriospinal neurons (Fig. 6a, black neurons) relay descending signals (originating from supraspinal neurons; Fig 6a, gray neurons) around the lesion site. This allows robust spontaneous recovery of voluntary locomotion (unlike in transected animals) in the absence of most 5-HT5,8.
Spinal neurons involved in locomotion require neuromodulators like 5-HT to function, setting them into a state of readiness for movement generation9,10. The critical importance of these neuromodulators has been demonstrated by the finding in animal models that locomotor-activity can be regained soon after spinal transection with the exogenous application of drugs that activate the neuromodulatory 5-HT, norepinephrine and dopamine receptors (in vivo and in vitro)11,12-15, including 5-HT2 and 5-HT7 receptor agonists16-18. Notably, over the weeks after injury, locomotor-like movements spontaneously emerge and improve with use19-21, as if the neuromodulatory receptor systems (e.g. 5-HT2 receptors) are somehow re-activated in the absence of 5-HT. We propose that this spontaneous recovery of locomotion is in part due to constitutive 5-HT receptor activity, and we investigated this with the staggered hemisection model.
Spasms in awake spastic rat. Tail muscle spasms were measured with percutaneous EMG (electromyogram) wires inserted in segmental tail muscles at the midpoint of tail and with kinematic recordings of tail flexion (with video), while the rat was in a Plexiglas tube, as detailed previously (Fig. 1a,e)2. During EMG recording, muscle spasms were evoked with electrical stimulation of the skin at the distal tip of the tail (cutaneous stimulation; 0.2 ms, 10 mA pulse; 2– 3x afferent threshold [T]; 6 spasms evoked at 10 s intervals for a trial; trials repeated at 12 min intervals) and the tail was restrained from moving. During kinematic recording, spasms were evoked with mechanical stimulation of the tail skin2,22, and the tail was free to move, which caused sensory input that extended spasms over many minutes (Fig. 1e). EMG was sampled at 5 kHz, rectified and averaged over a 10–40 ms interval post-stimulus to quantify the short latency reflex (SLR), and a 500–4000 ms interval to quantify spasms (long lasting reflex, LLR; using Axoscope, Axon Instr., and Matlab, Mathworks). EMG over 300 ms prior to stimulation was also averaged (background). Tail flexion angle was computed as described previously22 and averaged over 3 mins post-stimulation.
Human subjects with spinal cord injury and leg spasms. The human subjects studied had varied severity of SCI, as summarized in Supplementary Table 1. The subjects were seated in their wheelchair with limbs free to move, since immobilization interfered with generating muscle spasms. Two disposable surface electrodes (Kendall Soft-E), separated by 1 cm were placed over the tibialis anterior (TA) muscle to record EMG. Spasms were evoked at rest by a brief electrical stimulation of the medial arch of the foot (with a 24 ms train of 7 pulses at 300 Hz, 0.2 ms and 50 mA each pulse; 3–5×T; using a Digitimer DS7A). Stimulation was repeated 8 times at 15 s intervals for each trial, and this was repeated every 30 mins. Spasms were quantified by rectifying and averaging the EMG over the windows in time indicated in Fig. 5.
Ventral root recordings of long-lasting reflexes in rats, in vitro. Under urethane anesthesia (1.8 g·kg–1) the whole spinal cord caudal to the S2 injury level was removed from chronic spinal rats (and age-matched normal rats) and immersed in oxygenated artificial cerebrospinal fluid (ACSF; flowing 8 ml·min–1); recordings were made starting 2.5 hr later, as detailed previously1,23. Ventral (S4 and Co1) and dorsal (Co1, coccygeal) roots were mounted on silver wires above the ACSF and covered with Vaseline. The dorsal root was stimulated with a single pulse (0.1 ms, 0.02 mA, 3×T; repeated 5 times at 10 s intervals for one trial, trials repeated every 12 mins), and the long-lasting reflex (LLR) response was recorded on the ventral roots, and then analyzed as for the EMG. We quantified the LLR by averaging the rectified ventral root activity over a time-window 500–4000 ms post stimulus, a period previously shown to reflect the motoneuron Ca PIC activity in isolation. We also measured the transient short latency reflex (SLR) by averaging ventral root responses over a window 10–40 ms post stimulus (polysynaptic reflex with about 10 ms central delay). Because of slow diffusion in whole spinal cord preparations24,25, drugs required 10x higher concentrations than in thin slice preparations, and peak effects of even TTX (2 μM; sodium channel blocker) required 10–15 mins. To assure selectivity of drugs, they were titrated to a minimal dose that produced peak effect, and results were reported after 25–45 min of drug application.
Intracellular recordings, in vitro. Sharp intracellular electrodes (30 MΩ; filled with 1M K-acetate and 1M KCl) were advanced into the spinal cord with a stepper motor (666, Kopf, Instr.) to penetrate motoneurons (identified by antidromic ventral root stimulation), as detailed before1,26. Intracellular recordings were made with an Axoclamp2B amplifier (Axon) running in discontinuous-single-electrode voltage-clamp (gain 0.8–2.5 nA·mV–1; for Ca PICs) or discontinuous-current-clamp (switching rate 5 kHz; for EPSPs) mode, filtered at 3 kHz, and sampled at 6.7 kHz (Clampex and Clampfit used; Axon Instr.). In the presence of TTX (2 μM) to block synaptic transmission and sodium currents (Na PIC), slow triangular voltage ramps (3.5 mV s–1 voltage-clamp) were applied to the motoneurons to measure the Ca PIC (Fig. 3b)1,26. During the upward portion of this ramp, the current response initially increased linearly with voltage in response to the passive leak conductance. A linear relation was fit to the current in the region just below the Ca PIC onset (5 mV below) and extrapolated to the whole range of the ramp (leak current, thin line in Fig. 3b). At depolarized potentials above the Ca PIC onset threshold, the Ca PIC induced a downward deviation from the extrapolated leak current, and this Ca PIC was estimated as the difference between the leak current and the total current (leak-subtracted current). The amplitude of the peak Ca PIC was quantified as the initial peak amplitude of this downward deviation below the leak line (downward arrow Fig. 3b). The Ca PIC is thought to be mediated by Cav1.3 L-type calcium channels1, because it is activated at an unusually low threshold (about 10 mV above rest), blocked by high doses of nimodipine and highly persistent (in contrast, transient currents are inactivated during the slow ramp). There is also a small contribution to the Ca PIC from calcium-activated channels27.
The excitatory postsynaptic potential (EPSP) and associated reflexes were also measured in motoneurons by stimulating the Co1 dorsal roots (at 2–3×T, as in ventral root reflex recording), while applying hyperpolarizing bias currents to block the PICs (Fig. 3d).
mRNA measurements. Following the methods of Nakae et al.28, RNA was extracted from spinal cords of chronic spinal rats and age-matched normal rats with RNAeasy-Lipid-tissue Mini-Kits (Qiagen, Japan) and used for first-strand cDNA synthesis by Thermoscript (Invitrogen). Quantitative RT-PCR was then performed on the cDNA with ABI-PRISM-7900HT using TaqMan probes (Applied Biosystems) to estimate total 5-HT2C receptor mRNA (and internal control 18SrRNA). To determine the 5-HT2C receptor isoforms in the tissue, rat 5-HT2C receptor fragments were amplified from the cDNA using RT-PCR with the primers ATCATGGCAGTAAGCATGGAGAAGA and ATTGATATTGCCCAAACGATGGCA. The PCR product DNA was cloned into the pCR2.1 vector (Invitrogen), transfected into single E. coli bacterial cells, and > 300 recombinant colonies grown (each corresponding to a single 5-HT2C receptor isoform). DNA extracted from 80 randomly picked colonies underwent nucleotide sequencing with an ABI3700 genetic analyzer (Applied Biosystems) to determine the isoform DNA adopted by each colony.
Immunolabeling. Rats were euthanized with Euthanyl (Bimeda-MTC; 700 mg·kg–1) and perfused intracardially with 100ml saline followed by 400 ml paraformaldehyde (PFA; in phosphate buffer at room temperature; over about 15 mins), with 4% PFA used for 5-HT immunolabeling and 2% PFA for 5-HT2C and SMI32 immunolabeling. Spinal cords were postfixed in PFA (overnight for 5-HT-labeling and 90 mins for others) at 4 °C, cryoprotected in 20% sucrose and 2% ethylene-glycol, frozen and cut on a cryostat in horizontal or transverse 25–40 μm sections. Spinal cord sections were incubated overnight at 4 °C with primary antibodies to 5-HT (1:1000 S5545, Sigma, raised in rabbit), 5-HT2C receptors (1:900, SC-15081, epitope near N-terminus, Santa Cruz, goat) or SMI32 (1:000, Sternberger, mouse) in phosphate or Tris buffered saline (PBS or TBS) and 1% normal serum. Antibody labeling was visualized with fluorescent secondary antibodies (5-HT: Texas-Red[TI1000], 1:200, Vector, USA, overnight at 4 °C; 5-HT2C: Alexa488[A11055 or A11078], 1:1000, Invitrogen, 90 min at room-temperature; SMI32: Alexa568[A11008], 1:1000, Invitrogen, 90 min RT) and using a Zeiss-CLSM510 microscope and laser-scanning confocal microscopy.
Controls in which the primary antibody was pre-absorbed with the antigen that it was raised against were used to verify the selectivity of the antibody labeling. For example, the 5-HT2C receptor antigen (1:180; 5.5 μl·ml–1, SC-15081P, Santa Cruz) was incubated with the 5-HT2C receptor antibody (SC-15081) for 80 mins at room temperature prior to dilution and application to tissue. Also, controls in which the primary antibody was omitted were used to confirm that the secondary antibody produced no labeling by itself.
SMI32 labels non-phosphorylated neurofilament H, and does not change with spinal cord injury29. We have previously demonstrated that SMI32 labels motoneurons, and not interneurons in the ventral horn, though it also labels large cells elsewhere in the brain. Thus, SMI32 provided a fairly selective marker of motoneurons in the ventral horn29, and in particular labels the intracellular space (neurofilament). Using image analysis software (ImageJ), we defined two regions for 5-HT2C receptor quantification in motoneurons: 1) the internal region inside motoneuron soma, co-localized with SMI32 (region with SMI32 above standardized threshold, as we described in Anelli et al.29), and 2) the membrane region, immediately adjacent to the internal SMI32 region. The later was produced by detecting the edge of the SMI32-positive region inside motoneuron soma and expanding this edge to a 0.7 μm wide band that surrounded the perimeter of the soma (not co-localized with SMI32). The mean 5-HT2C immunofluorescence signal density was then computed in these two regions, from which we computed the ratio of the receptor density in the membrane relative to the density inside the cell (x100%) for comparison across normal and injured rats.
We also labeled 10 μm transverse spinal cord sections from chronic spinal rats with a different 5-HT2C antibody (ab32172, epitope on C-terminus, Abcam), and then visualized the antibody with ABC amplification (Vector-Labs) followed by DAB labeling. For this, spinal cords were initially placed in vitro for a 4 hr incubation with and without SB206553 (30 μM) and then fixed in 4% PFA. After cryoprotection, frozen sections were cut and then incubated 15 min in 0.5% triton-x100 in PBS, 5 mins in 1.5% hydrogen peroxide in PBS, 1 hour in 10% normal goat serum in PBS, and then overnight in the primary 5-HT2C antibody in PBS (1:200; ab32172), all at RT. Then, sections were incubated in a biotinylated secondary antibody (1:200; BA9400; Vector-Labs) overnight at 4 °C, followed by incubation in the Vector ABC elite complex, overnight at 4 °C, and then 5-HT2C receptors were visualized with the Vector DAB kit and viewed with conventional light microscopy.
Supplementary References
1. Li, Y., Gorassini, M.A. & Bennett, D.J. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91, 767-783 (2004).
2. Bennett, D.J., Sanelli, L., Cooke, C., Harvey, P.J. & Gorassini, M.A. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J. Neurophysiol. 91, 2247-58 (2004).
3. Bennett, D.J. et al. Spasticity in rats with sacral spinal cord injury. J Neurotrauma 16, 69-84 (1999).
4. Gorassini, M.A., Knash, M.E., Harvey, P.J., Bennett, D.J. & Yang, J.F. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127, 2247-58 (2004).
5. Courtine, G. et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14, 69-74 (2008).
6. Ballermann, M. & Fouad, K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci 23, 1988-96 (2006).
7. Basso, D.M., Beattie, M.S. & Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12, 1-21 (1995).
8. Cowley, K.C., Zaporozhets, E. & Schmidt, B.J. Propriospinal neurons are sufficient for bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol 586, 1623-35 (2008).
9. Jordan, L.M., Liu, J., Hedlund, P.B., Akay, T. & Pearson, K.G. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57, 183-91 (2008).
10. Jacobs, B.L., Martin-Cora, F.J. & Fornal, C.A. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40, 45-52 (2002).
11. Kiehn, O. & Kjaerulff, O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol 75, 1472-82 (1996).
12. Cowley, K.C. & Schmidt, B.J. A comparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett 171, 147-50 (1994).
13. Viala, D. & Buser, P. [Methods of obtaining locomotor rhythms in the spinal rabbit by pharmacological treatments (DOPA, 5-HTP, D-amphetamine)]. Brain Res 35, 151-65 (1971).
14. Chau, C., Barbeau, H. & Rossignol, S. Effects of intrathecal alpha1- and alpha2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. J Neurophysiol 79, 2941-63 (1998).
15. Courtine, G. et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12, 1333-42 (2009).
16. Madriaga, M.A., McPhee, L.C., Chersa, T., Christie, K.J. & Whelan, P.J. Modulation of locomotor activity by multiple 5-HT and dopaminergic receptor subtypes in the neonatal mouse spinal cord. J Neurophysiol 92, 1566-76 (2004).
17. Landry, E.S. & Guertin, P.A. Differential effects of 5-HT1 and 5-HT2 receptor agonists on hindlimb movements in paraplegic mice. Prog Neuropsychopharmacol Biol Psychiatry 28, 1053-60 (2004).
18. McEwen, M.L., Van Hartesveldt, C. & Stehouwer, D.J. L-DOPA and quipazine elicit air-stepping in neonatal rats with spinal cord transections. Behav Neurosci 111, 825-33 (1997).
19. Kuhn, R.A. & Macht, M.B. Some manifestations of reflex activity in spinal man with particular reference to the occurrence of extensor spasm. Bull. Johns Hopkins Hosp. 84, 43-75 (1948).
20. de Leon, R.D., Hodgson, J.A., Roy, R.R. & Edgerton, V.R. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol 79, 1329-40 (1998).
21. Barbeau, H. & Rossignol, S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412, 84-95 (1987).
22. Rank, M.M., Li, X., Bennett, D.J. & Gorassini, M.A. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol 97, 3166-80 (2007).
23. Li, Y., Li, X., Harvey, P.J. & Bennett, D.J. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol 92, 2694-2703 (2004).
24. Harvey, P.J., Li, X., Li, Y. & Bennett, D.J. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96, 1171-86 (2006).
25. Perrier, J.F. & Hounsgaard, J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol 89, 954-9 (2003).
26. Harvey, P.J., Li, Y., Li, X. & Bennett, D.J. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96, 1141-57 (2006).
27. Li, X. & Bennett, D.J. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97, 3314-30 (2007).
28. Nakae, A. et al. Serotonin2C receptor mRNA editing in neuropathic pain model. Neurosci Res 60, 228-31 (2008).
29. Anelli, R., Sanelli, L., Bennett, D.J. & Heckman, C.J. Expression of L-type calcium channel alpha(1)-1.2 and alpha(1)-1.3 subunits on rat sacral motoneurons following chronic spinal cord injury. Neuroscience 145, 751-63 (2007).
30. Knight, A.R. et al. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol 370, 114-23 (2004).
31. Herrick-Davis, K., Grinde, E. & Niswender, C.M. Serotonin 5-HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J Neurochem 73, 1711-7 (1999).
32. Henry, L.K. et al. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem 281, 2012-23 (2006).
33. Rothman, R.B. & Baumann, M.H. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther 95, 73-88 (2002).
34. Wallis, D.I., Wu, J. & Wang, X. Descending inhibition in the neonate rat spinal cord is mediated by 5-hydroxytryptamine. Neuropharmacology 32, 73-83 (1993).
35. Li, Y., Harvey, P.J., Li, X. & Bennett, D.J. Spastic long-lasting reflexes in the chronic spinal rat, studied in vitro. J Neurophysiol 91, 2236-46 (2004).
36. Jiang, M.C. & Heckman, C.J. In vitro sacral cord preparation and motoneuron recording from adult mice. J Neurosci Methods 156, 31-6 (2006).
37. Chanrion, B. et al. Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-hydroxytryptamine2C receptors: differential modulation of cell surface expression and signal transduction. Mol Pharmacol 73, 748-57 (2008).
38. Marion, S., Weiner, D.M. & Caron, M.G. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J Biol Chem 279, 2945-54 (2004).
39. Sternini, C. et al. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci U S A 93, 9241-6 (1996).
40. Maynard, F.M., Jr. et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 35, 266-74 (1997).