Abstract
The aim of this pilot study was to quantify the impact of sleep deprivation on psychophysiological reactivity to emotional stimuli. Following an adaptation night of sleep in the lab, healthy young adults were randomly assigned to either one night of total sleep deprivation or to a normal sleep control condition. The next afternoon, responses to positive, negative, and neutral picture stimuli were examined with pupillography, an indicator of cognitive and affective information processing. Only the sleep-deprived group displayed significantly larger pupil diameter while viewing negative pictures compared to positive or neutral pictures. The sleep-deprived group also showed anticipatory pupillary reactivity during blocks of negative pictures. These data suggest that sleep deprivation is associated with increased reactions to negative emotional information. Such responses may have important implications for psychiatric disorders, which may be triggered or characterized by sleep disturbances.
Keywords: Sleep deprivation, Affect, Emotional reactivity, Pupil dilation
1. Introduction
Effects of sleep deprivation on neurobehavioral function (e.g., vigilance and cognition) are well documented. The prefrontal cortex (PFC) is particularly sensitive to sleep loss (Drummond et al., 1999; Thomas et al., 2000), with corresponding impairments observed in PFC-associated executive functions (Harrison and Horne, 1998, 1999; Jones and Harrison, 2001; Muzur et al., 2002) and vigilance (Belenky et al., 2003; Van Dongen et al., 2003). The adverse impact of sleep deprivation on mood and affective reactivity and regulation has been less thoroughly explored in the experimental literature. This is surprising given the role of prefrontal cortical circuits in regulating mood, particularly by inhibiting brain structures important to the generation and recognition of affect such as the amygdala (Davidson et al., 2000; Phillips et al., 2003; Urry et al., 2006). Recent evidence suggests such processes may be impaired by sleep deprivation. In a recent neuroimaging study (Yoo et al., 2007), sleep deprived individuals demonstrated heightened amygdala reactivity to negative picture stimuli, which was also associated with reduced functional connectivity between the amygdala and the medial PFC.
Mood responses to sleep deprivation are variable and at times labile. Sleep deprivation can induce giddiness, child-like behaviors, and silliness (Bliss et al., 1959; Horne, 1993), as well as more widely recognized negative effects including dysphoria, increased irritability, and lowered frustration tolerance. The increased irritability that frequently accompanies sleep deprivation suggests that sleep-deprived individuals are highly reactive to emotional cues. These effects on mood can lead to negative consequences and impact functioning. For example, an inverse association between sleep duration and interpersonal difficulties and even violence has been observed in medical residents (Baldwin and Daugherty, 2004), and sleeping less than 8 h is associated with increased risk for adolescent suicidal behavior (Liu, 2004). Chronic sleep deprivation/restriction has also been associated with the development of psychopathology, including bipolar disorder (Kasper and Wehr, 1992; Wright, 1993; Frank et al., 1997), pre- and post-partum psychosis (Brockington et al., 1990; Sharma et al., 2004), and depression in both new mothers and fathers (Hiscock and Wake, 2001). However, the relationship between sleep deprivation and mood is not simple. Sleep deprivation transiently improves mood symptoms in 40–60% of individuals with clinical depression, although some patients’ symptoms worsen (Naylor et al., 1993; Wirz-Justice and Van den Hoofdakker, 1999; Giedke and Schwarzler, 2002; Giedke et al., 2003).
Previous experimental studies of chronic sleep restriction and acute sleep deprivation in healthy individuals have documented a deterioration of mood, with larger effect sizes than for either cognitive or motor responses (Pilcher and Huffcutt, 1996). However, previous studies almost exclusively used self-report outcomes. The reliability of such self-report data is uncertain due to contextual factors (e.g., reporting bias, demand characteristics, and scale interpretation). Objective measures of affective responding may help to characterize and quantify the affective consequences of sleep deprivation. Ultimately, such information may help to uncover pathways by which sleep is related to affective impairments and the development of mood disorders. For instance, sleep deprivation may be associated with disruptions in the dynamic time-course of responses to cognitive and emotional information as seen in depression (Challis and Krane, 1988; Deldin et al., 2001; Siegle et al., 2001, 2002, 2003a,b, 2007; Wagner et al., 2006). Self-report measures cannot capture this information, which occurs on the time-scale of milliseconds or seconds.
In this study, we used pupillary response as an indicator of cognitive and emotional information processing in order to examine the magnitude and time-course of responses to affective picture stimuli in healthy adults following sleep deprivation or normal sleep. We used pupillography for several reasons. First, as there is extensive overlap between cognitive and affective processes (e.g., Davidson, 2003) it is appropriate that pupillary responses reflect each of these phenomena. Many studies have demonstrated pupil dilation to be a reliable correlate of cognitive load. For example, the pupil dilates more under conditions of higher attentional allocation, memory use, or interpretation of more difficult material (see Beatty, 1982; Steinhauer and Hakerem, 1992 for reviews). The pupil has also been shown to dilate in response to emotional information (Janisse, 1974; Bradley et al., 2008). Second, sustained cognitive load leads to sustained pupil dilation (Beatty, 1982). Thus, pupillography is an appropriate measure to examine immediate and sustained processing of emotional information. Third, the pupil is innervated by brain structures involved in cognitive and emotional processing, such as the anterior cingulate cortex (Szabadi and Bradshaw, 1996). Stimulation of limbic regions such as the amygdala increases pupil dilation (Koikegami and Yoshida, 1953; Fernandez De Molina and Hunsperger, 1962), as does stimulation of the midbrain reticular formation (Beatty, 1986), which receives afferent projections from the frontal cortex and sends efferent projections to the ocular motor nuclei. Concurrent pupil dilation/functional magnetic resonance imaging assessment has confirmed that pupil dilation reflects the time-course of activity in brain areas associated with cognitive processing including the dorsolateral prefrontal cortex (Siegle et al., 2003a,b). Urry and colleagues (2006) recently used this method to demonstrate that pupil dilation reflects the time-course of neural responses to affective picture stimuli as well as explicit instructions to regulate emotions. The pupil therefore reflects initial reactivity as well as brain processes associated with subsequent affect regulation, and aspects of arousal (Critchley et al., 2005), even though it may be difficult to distinguish specific cognitive and emotional subprocesses in a given instant.
For this pilot study, we used passive viewing of visual stimuli to examine automatic reactivity to emotional stimuli (e.g., Cuthbert et al., 2000) in healthy adults following either one night of total sleep deprivation or following a night of normal sleep. A passive viewing task was employed to assess naturalistic reactivity outside the context of laboratory-induced explicit cognitive or emotional demands. We expected negative stimuli to induce larger pupillary responses than neutral or positive stimuli in the sleep deprivation condition compared to a non-sleep-deprived control group. Such data would reflect a fundamental tendency towards increased reactivity in the seconds following emotional stimuli in sleep deprivation. That said, our analysis path also allowed for the potential that sleep deprivation was associated more diffusely with increased arousal which would be evidenced by increased pupillary responses to both negative and positive stimuli compared to neutral stimuli.
2. General methods
Following an adaptation night of sleep in the lab, participants were randomly assigned to either one night of total sleep deprivation (SD group) or to a control condition (non-SD group). Emotional reactivity to affective picture stimuli was assessed the next afternoon, after approximately 31–33 h of wakefulness in the SD group.
2.1. Participants
Participants included 30 healthy adult volunteers ages 21–30: 15 female and 15 male (8 females and 7 males assigned to the sleep deprivation group); 20 Caucasian, 4 African–American, 4 Asian, 2 Hispanic; mean age ± standard deviation M = 24.4 ± 2.76 years. Potential participants were excluded if they had any of the following: current or past psychiatric or sleep disorders; presence of significant sleep disordered breathing or leg movements (10 or more events per hour of sleep); significant hearing or vision problems or major medical problems and any medications other than contraception; bedtimes earlier than 10:00 p.m. or later than 2:00 a.m., or an irregular sleep/wake schedule (i.e., 2 or more hours of variability in sleep/wake times based on a week of sleep diary data); tobacco use; or consumption of more than 2 caffeinated beverages daily.
2.2. Procedure
Following an initial telephone screening, interested participants were scheduled for an in-person assessment interview at the Clinical Neuroscience Research Center (CNRC), a satellite of the University of Pittsburgh’s General Clinical Research Center. At the screening, the experiment was explained to participants, who signed a consent form approved by the University of Pittsburgh Institutional Review Board. Structured clinical interview assessments for psychiatric (SCID I, First et al., 1995) and sleep disorders were conducted by trained and reliable research nurses. Eligible participants were scheduled for an adaptation night in the CNRC lab, including an overnight diagnostic polysomnogram to rule out clinically significant sleep disordered breathing or leg movements. Participants returned the next evening and stayed at the lab for 24 h. Participants assigned to the non-SD control group went to bed at their habitual bedtime and were allowed 8 h of time in bed from the time of sleep onset. Those assigned to the SD group remained awake all night under continual polysomnographic and frequent behavioral monitoring. During the SD night, participants had access to food, TV, and the internet. Participants were run in pairs when possible to help facilitate wakefulness for the sleep-deprived group. Participants completed emotional information processing and cognitive tasks (the latter to be presented in subsequent reports) in an afternoon test session that started at 2:30–4:30 p.m.; task order was randomized. Multiple self-report and physiological measures of sleepiness were also administered across the day. These measures have been described separately (Franzen et al., 2008). Participants refrained from caffeine and alcohol for 24 h prior to the adaptation night and throughout remainder of their time in the lab.
2.3. Apparatus
Testing occurred with the participant alone in a moderately lit room. Stimuli were presented using E-Prime software (Psychology Software Tools, Pittsburgh, PA). Pupil diameter was recorded with a sampling frequency of 60 Hz using an ISCAN RK726 pupillometer (Burlington, MA), which consisted of a video camera and infrared light source pointed at the participant’s left eye to track pupil size. The pupillometer’s resolution is typically better than 0.05 mm pupil diameter.
2.4. Preparation of pupil data for analysis
Data were cleaned using our lab’s standard methodology, derived from Granholm et al. (1996), including blink and artifact rejection. Eye blinks were removed and then corrected for by linear interpolation. Trials which consisted of over 50% blinks were removed from consideration. Data cleaning procedures resulted in the elimination of median Md = 2, mean ± standard deviation M = 2.4 ± 2.1 trials per subject out of 45 trials presented. Data were smoothed by applying a 3-point flat filter twice. Linear trends in pupil dilation were calculated over blocks of 20 trials and removed from pupil dilation data to eliminate effects of slow drift in pupil diameter unrelated to trial characteristics. Prior to removing these trends, we examined the slopes over blocks of 20 trials (in three blocks). All three slopes were slightly negative. Only block 2 was significantly different from 0 (t(29) = −2.35, p = 0.026), whereas blocks 1 and 3 did not have a significant slope effect (t(29) = −1.21, p = 0.236 and t(29) = −1.40, p = 0.173, respectively); moreover, the three slopes did not differ from one another (F(2,58) = 1.05, p = 0.358). Thus it is unlikely that results observed in any block were affected by ceiling or floor effects of drift across the block. Data were corrected for baseline by subtracting tonic, baseline pupil diameter (100 ms prior to the warning cue) from waveforms representing pupillary responses. Baseline pupil diameters between the SD and non-SD groups did not significantly differ (t(28) = 0.46, p = 0.65). As a final data reduction step prior to statistical analysis, the original 60-Hz waveforms were binned into 0.5 s intervals.
Pupillary waveforms were analyzed using a mixed effects analysis of variance (ANOVA) with subject treated as a random factor, and group (SD, non-SD), valence (negative, positive, neutral), and time (in .5 s intervals over the baseline-corrected 60-Hz waveforms) as fixed factors. An autoregressive covariance structure (AR1) was used to account for autocorrelation in the time-series data. We used an alpha level of .05 for all statistical tests.
2.5. Emotional task and stimulus materials
The passive picture-viewing task consisted of viewing positive, neutral, and negative pictures (15 each) from the International Affective Picture System (IAPS; Center for the Study of Emotion and Attention, 1999). Two sets of stimuli matched for normative ratings of valence and arousal were presented in a counterbalanced order. Selected picture stimuli included arousing highly pleasant (positive; e.g., baby, happy people, cute animals, money, ice cream) and unpleasant (negative; e.g., car accident, crying child, eye disease) stimuli, along with neither pleasant nor unpleasant and nonarousing (neutral; e.g., chess board, building, truck) stimuli. As rated from 1 (very unpleasant) to 9 (very pleasant), mean ± standard deviation normative valence ratings for the positive, neutral, and negative stimuli were 7.63 ± .30, 4.99 ± .28, and 2.27 ± .34, respectively. As rated from 1 (very calm) to 9 (very aroused), normative arousal ratings for positive, neutral, and negative pictures were 5.34 ± 0.94, 3.34 ± 0.97, and 5.69 ± 0.69, respectively. Neutral stimuli were significantly less unpleasant than the negative stimuli and significantly less pleasant than the positive stimuli based on normative IAPS ratings (both p’s < 0.001). Compared to normative arousal ratings of neutral pictures, both negative and positive pictures were significantly more arousing (both p’s < .001); arousal ratings, however, did not differ for the positive and negative stimuli (p = .122).
Stimuli were presented via a computer monitor 65-cm in front of the participant. In order to control for varying light levels in the individual IAPS stimuli, they were converted to equiluminant black and white images (512 × 384 resolution). The stimuli were presented in a randomized mixed block/event-related design; a block consisted of five trials of the same valence. Each picture was preceded by a 2-s warning cue, consisting of a 6-mm black square in the center of an equiluminant mask screen. Following a 6-s picture presentation period, the mask was again presented during a 6–8 s inter-stimulus interval. Participants were instructed to push a button at picture onset as quickly as possible, and to keep their focus on the computer monitor at all times. Within several hours after completing this task, participants viewed the same picture stimuli while subjective valence and arousal ratings were collected using the 9-point Likert scale self-assessment manikin (Bradley and Lang, 1994).
2.6. Data analysis
For the picture-viewing task, we examined waveforms aligned to the time of cue presentation. Because the stimuli were presented in blocks consisting of 5 trials of the same valence, this additionally permitted an assessment of potential anticipatory reactivity during the 2-s warning cue. Because our primary interest was in group differences in reactivity over time which were specific to valence, effects associated with a light-reflex that were independent of stimulus type were removed by subtracting the mean waveform across all three valences from the average waveform for each valence. This technique had the effect of removing aspects of individual differences which were not of interest to the study. It also allowed simple-effects tests of valence to be interpreted as tests of the deviation of pupillary responses to one valence from the others.
For post hoc analyses of interactions involving time (i.e., variation of the pupillary response within a trial), we examined detailed time-courses of the 60-Hz waveforms. Differences in the time-course of reactivity between the SD versus non-SD groups were examined with independent samples t-tests, and within-group valence-related time-course comparisons were examined with an ANOVA in which valence was a repeated measure, at each sample along the pupillary response waveforms. Regions showing significant differences were detected with an empirical rather than an a priori approach. We adopted Guthrie and Buchwald’s (1991) technique for controlling type-I error in testing the significance of potential differences. This procedure uses adjacent tests as replications, requiring a temporal region of consecutive tests to be significant for any one test to be judged as significant at p < .05 after accounting for the temporal autocorrelation of the waveform. Consistent with our previous uses of this procedure to examine pupil dilation data (Siegle et al., 2003a,b, 2004), we used this method to determine the number of contiguous tests significant at p < 0.1 that were needed to judge a given window significant at p < 0.05. Given that we assumed fundamentally different brain processes associated with reactivity and regulation would operate during the warning cue (the 2-s interval before the stimulus was presented), the period while the stimulus was present (6 s), and the recovery period following the removal of the stimulus (6 s), these intervals were considered to be different families of tests. Monte Carlo simulations accounting for the temporal autocorrelation within each region suggested that to control type-I error at p < 0.05 for the cue period, 16 consecutive samples (0.27 s) had to be significant at p < 0.1. Similarly, for the stimulus presentation period, 58 samples (0.97 s) were required, and for the recovery period, 45 samples (0.75 s) were required. When results are reported for an entire window of significance, they represent tests of the mean pupillary response in the window. Importantly, though the mean level of significance in these windows may be between p < 0.1 and p < 0.05, the region of tests significant at p < 0.1 was longer than would be expected by chance, p < 0.05.
Behavioral data, which included reaction times and post-task subjective valence and arousal ratings, were examined with mixed effects ANOVAs with subject treated as a random factor, and group (SD, non-SD) and valence (negative, positive, neutral) as fixed factors. An unstructured covariance structure provided the best fit. Due to technical problems, subjective rating data were missing for 7 participants (2 in the SD group, and 5 in the non-SD group).
3. Results
3.1. Behavioral performance and post-task subjective ratings
There was a significant main effect of valence in subjective ratings of both pleasantness (valence) and arousal, F(2,21) = 244.1, p < 0.001 and F(2,21) = 73.4, p < 0.001, but no main effect of group, and no group by valence interaction (F’s < 0.2, p’s > 0.6). As expected, positive pictures were rated as more pleasant (t(22) = 13.1, p < .001) and arousing (t(22) = 8.7, p < 0.001) than neutral pictures, while negative pictures were rated as less pleasant (t(22) = 22.4, p < 0.001) and more arousing (t(22) = 10.4, p < 0.001) than neutral pictures. Negative pictures were slightly but significantly rated more arousing than positive pictures (t(22) = 2.8, p = 0.010). Subjective valence was rated from 1 (very pleasant) to 9 (very unpleasant), and subjective arousal was rated from 1 (very aroused) to 9 (very calm). Mean ± standard deviation valence ratings for positive, neutral, and negative pictures were 2.8 ± 0.8, 4.8 ± 0.2, and 7.6 ± 0.2, respectively; arousal ratings for positive, neutral, and negative pictures were 5.2 ± 1.7, 7.4 ± 1.3, and 4.3 ± 1.3, respectively. These results suggest that sleep deprivation did not impact subjective ratings. There were no significant findings in the reaction time data (F’s < 2.1, p’s > 0.15), suggesting that the sleep-deprived individuals were as engaged in the task as the non-deprived controls.
3.2. Pupillary responses to picture stimuli
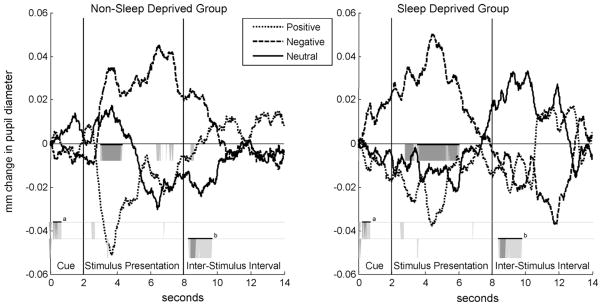
Light-reflex corrected pupillary waveform averages for the non-SD and SD groups are shown in Fig. 1. Mixed effects analysis revealed a significant group × valence × time interaction, F(54,1695.8) = 1.47, p = .015. In other words, the SD and non-SD groups had different pupillary responses to specific valenced stimuli. To clarify the nature of this interaction, separate sets of ANOVA analyses were performed for the SD and non-SD groups, as well as comparing each valence between the two groups.
Fig. 1.
Light-reflex corrected pupillary waveform averages during trials of positive, negative, and neutral pictures for the non-SD (left) and SD (right) groups. The regions immediately below the x-axis highlighted in gray (dark gray, p < .05; light gray, p < .10) show where the effect of valence was significant within-groups. Between-group differences are highlighted in gray further below the x-axis, first for negative stimuli during the cue (a), and below that for neutral stimuli during the inter-stimulus interval (b). Highlighted sections that are depicted with bars above them are the regions that were long enough for the entire window to be considered statistically significant at p < .05 using the Guthrie and Buchwald (1991) method of type-I error control.
For the SD group, a contiguous window during the stimulus presentation was significant from 3.55 to 6.07 s (relative to cue onset), F(2,13) = 11.82, p = 0.001. Responses in this window were larger for negative pictures (mean ± standard deviation, M = 0.037 mm ± 0.029) compared to both positive (M = −0.023 mm ± 0.052) and neutral (M = −0.015 ± 0.055) pictures, t(14) = 3.66, p = 0.003, d = 0.94 and t(14) = 2.77, p = 0.015, d = 0.72, respectively. For the non-SD group, differential responses to the valences were apparent from 3.02 to 4.33 s, F(2,13) = 5.73, p = 0.016. Pupillary responses in this window were smaller for positive pictures (M = −0.042 mm ± 0.045) compared to both negative (M = 0.029 mm ± 0.054) and neutral (M = 0.013 mm ± 0.044), t(14) = 3.07, p = 0.008, d = 0.79 and t(14) = 2.91, p = 0.011, d = 0.75, respectively. Although the non-SD group had larger responses to negative stimuli than neutral and positive stimuli during the second half of the stimulus presentation period, there were no regions long enough to be considered statistically significant.
We also examined differences between the SD and non-SD groups for each valence separately. Compared to the non-SD group, the SD group had significantly larger responses from 0.25 to 0.77 s of the warning cue during blocks of negative stimuli, indicating anticipatory reactivity (t(28) = 2.04, p = 0.05, d = 0.75, SD group M = 0.011 mm ± 0.022, non-SD group M = −0.006 ± 0.023). This difference is illustrated in Fig. 1 by an elevated waveform for negative stimuli in the “cue” region in the SD group compared to the non-SD group. To examine how the anticipatory response developed over time, we compared the average response within this window for each of the three blocks of negative trials (five stimuli per block) with a 2 (group) by 3 (blocks) repeated measures ANOVA. As expected, the ANOVA revealed a significant main effect of group, where the SD group had larger responses overall (F(1,28) = 4.76, p = 0.038), and a significant group by block interaction, F(2,56) = 4.04, p = 0.023. Post hoc independent samples t-tests revealed that the SD group had significantly larger responses than the non-SD group only during block 2 (t(28) = 2.87, p = 0.008), but not for blocks 1 or 3 (p’s > 0.36).
The SD group also had larger responses during the inter-stimulus interval following neutral trials, from 8.90 to 9.58 (t(28) = 2.03, p = 0.05, d = 0.74, SD group M = 0.029 mm ± 0.084, non-SD group M = −0.021 ± 0.043) and from 9.62 to 10.33 s (t(28) = 1.90, p = 0.07, d = 0.70, SD group M = 0.029 mm ± 0.065, non-SD group M = −0.013 ± 0.057). This is illustrated in Fig. 1 by an elevated waveform to neutral stimuli during the inter-stimulus interval for the SD group compared to the non-SD group. There were no significant group differences during trials of positive stimuli.
4. Discussion
We administered positive, negative, and neutral affective stimuli to sleep-deprived and non-deprived individuals and measured pupil diameter responses during a passive picture-viewing task. Both groups demonstrated larger pupillary responses while viewing blocks of negative pictures compared to positive and neutral blocks, although this difference was only statistically reliable in the SD group. This reactivity started after stimulus onset in the non-SD group, whereas the SD group began reacting during the warning cue during blocks of negative trials. Together, these data suggest that sleep deprivation altered emotional reactivity responses while anticipating and viewing unpleasant picture stimuli. These findings suggest that pupil diameter changes may represent a sensitive measure of sleep deprivation-related emotional reactivity. Although many previous studies have reported changes in subjective mood with sleep deprivation (e.g., Lingenfelser et al., 1994; Pilcher and Huffcutt, 1996; Dinges et al., 1997; Van Dongen et al., 2004; Haack and Mullington, 2005), we are aware of only one other recent study (Yoo et al., 2007) that employed objective, physiological measures in sleep-deprived individuals, finding greater amygdala reactivity to negative picture stimuli. We also observed greater pupillary reactivity to negative pictures. Such effects could lead to functional deficits specifically in responding to negative emotional information in sleep-deprived individuals.
These results are analogous to findings in depressed individuals, who have sustained pupil dilation in response to emotional stimuli (Siegle et al., 2001, 2003a,b). We interpreted this phenomenon as resulting from sustained attention and elaboration. Like depressed individuals, sleep-deprived individuals may display sustained engagement to emotional information but less sustained engagement in non-emotional tasks. This hypothesis is consistent with attentional deficits in sleep-deprived people that are most easily revealed during boring and monotonous situations. We suggest that sleep-deprived individuals do not effectively engage attention mechanisms unless the material has a sufficiently affective load. This explanation is consistent not only with data suggesting that sleep-deprived individuals perform poorly on cognitive tasks requiring prefrontal engagement, but also with neuroimaging data suggesting that sleep-deprived individuals may show either decreased or increased activity in prefrontal regions depending on task characteristics (Drummond et al., 1999, 2001). Studies in healthy young adult samples are an important first step for examining the effects of SD on objective measures of affect reactivity/regulation. These data will establish benchmarks for subsequent comparisons with at-risk and clinical populations such as people with depression.
This study has several limitations. Pupillography is an imperfect measure of underlying brain activity. Many factors other than sleep deprivation and affective/cognitive processing affect pupil dilation, including the luminance of stimuli. We controlled for this factor across conditions by using equiluminant stimuli and masks. Slow pupillary oscillations resulting from sleepiness (e.g., Wilhelm et al., 1998) could theoretically affect these findings. Such pupillary instability was unlikely to confound the assessment of pupil dilation in this study, as participants were presumably as sleepy for the entirety of the task (i.e., for all stimuli, regardless of valence). Another important limitation is that the exact nature and functional significance of enhanced pupillary reactivity to negative emotional information under sleep-deprived conditions is unclear. Enhanced pupillary responses to affective stimuli in sleep-deprived individuals may reflect compensatory effort to regulate emotional responses. For example, greater PFC activation may be required in the sleep-deprived brain during more automatic aspects of emotional responses. Conversely, such responses may reflect an exaggerated, hyper-reactivity response pattern to emotional cues (possibly reflecting reduced PFC-related limbic inhibition). Concurrent neuroimaging and pupillographic assessments may clarify the neural pathways underlying sleep deprivation-related alterations in reactivity to emotional stimuli observed in this study. Either way, it is tempting to speculate that increased affective processing during sleep deprivation would leave fewer resources available for other cognitive processes. In this way, sleep deprivation-related alterations in emotional information processing could plausibly contribute to neurobehavioral deficits (e.g., Van Dongen et al., 2004) observed in sleep-deprived individuals.
Although group differences were detected in the pupillary response and self-report ratings of mood (i.e., increased negative mood ratings on visual analog scales, and reduced positive mood ratings on the Positive and Negative Affect Schedule, as reported in Franzen et al., 2008), differences for subjective valence and arousal ratings of the affective stimuli were not apparent. One possibility is that pupillary responses within a passive picture-viewing task paradigm may capture a more implicit reaction, as compared to an overt cognitive interpretation that is required when rating subjective arousal and valence. Neuroimaging studies (Hariri et al., 2000, 2003; Lieberman et al., 2007) have identified two different neural substrates which are involved when labeling affective stimuli versus just viewing or matching stimuli, and that the former was associated with lesser amygdala reactivity. Thus, viewing and rating the picture stimuli may involve two different processes, and the process of rating the stimuli may dampen emotional reactions. It is also possible that the small number of stimuli rated per valence, or the missing data in participants’ ratings, or that the stimuli were rated upon viewing the stimuli a second time after the initial task was completed may have contributed to the observed lack of group differences in the subjective ratings.
Further studies in larger samples are needed to confirm the findings from this preliminary study, and to examine inter-individual differences that are likely to be critically important in understanding differential vulnerability to the affective consequences of sleep deprivation. Recent evidence strongly suggests these individual differences are important in understanding other sleep deprivation-related consequences such as neurobehavioral impairments (Van Dongen et al., 2004).
These limitations notwithstanding, the present results provide a starting point for understanding important links between sleep and affect, and the potential effects of sleep loss on affective functioning. Little information is available regarding how sleep deprivation impacts the processing, generation, and regulation of affect, but understanding the impact of sleep deprivation on affective reactivity and regulation has important implications for public health. Sleep deprivation is increasingly common in industrialized societies (Bliwise, 1996), and its influence on affect regulation could lead to a host of adverse behavioral (e.g., impaired decision-making, risk-taking, and interpersonal transactions) and mental health outcomes by increasing vulnerability to mood, anxiety, and substance use disorders. Carefully examining the effects of sleep deprivation on affective function under controlled conditions may reveal predictors of sleep disturbance-related trait vulnerability for psychopathology, and serve a first step toward developing mechanistic models of the role of sleep in mental health. Such models could inform the development of effective prevention and intervention strategies for disorders in which sleep disturbances are a typical feature.
Acknowledgments
This research was supported by funds received from grants awarded by the National Institute of Health MH30915, RR000056, RR024153, MH16804, AG00972, MH24652, MH064159, and MH077106, and the National Sleep Foundation. We wish to thank Denise N. Duryea, B.A. and CNRC staff for assistance with data collection.
References
- Baldwin DC, Jr, Daugherty SR. Sleep deprivation and fatigue in residency training: results of a national survey of first- and second-year residents. Sleep. 2004;27:217–223. doi: 10.1093/sleep/27.2.217. [DOI] [PubMed] [Google Scholar]
- Beatty J. Phasic not tonic pupillary responses vary with auditory vigilance performance. Psychophysiology. 1982;19:167–172. doi: 10.1111/j.1469-8986.1982.tb02540.x. [DOI] [PubMed] [Google Scholar]
- Beatty J. The pupil system. In: Coles MGH, Donchin E, Porges SW, editors. Psychophysiology: Systems, Processes and Application. Guilford; New York: 1986. pp. 43–50. [Google Scholar]
- Belenky G, Wesensten NJ, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. Journal of Sleep Research. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Bliss EL, Clark LD, et al. Studies of sleep deprivation-relationship to schizophrenia. AMA Archives of Neurology and Psychiatry. 1959;81:348–359. doi: 10.1001/archneurpsyc.1959.02340150080009. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Historical change in the report of daytime fatigue. Sleep. 1996;19:462–464. doi: 10.1093/sleep/19.6.462. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, et al. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington IF, Oates M, et al. Prepartum psychosis. Journal of Affective Disorders. 1990;19:31–35. doi: 10.1016/0165-0327(90)90006-t. [DOI] [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention, C. The International affective picture system: digitized photographs. Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1999. [Google Scholar]
- Challis BH, Krane RV. Mood induction and the priming of semantic memory in a lexical decision task: asymmetric effects of elation and depression. Bulletin of the Psychonomic Society. 1988;26:309–312. [Google Scholar]
- Critchley HD, Tang J, et al. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, et al. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, et al. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychological Bulletin. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Deldin P, Deveney C, et al. A slow wave investigation of working memory biases in mood disorders. Journal of Abnormal Psychology. 2001;110:267–281. doi: 10.1037//0021-843x.110.2.267. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 h per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Drummond SP, Brown GG, et al. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10:3745–3748. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, et al. Increased cerebral response during a divided attention task following sleep deprivation. Journal of Sleep Research. 2001;10:85–92. doi: 10.1046/j.1365-2869.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- Fernandez De Molina A, Hunsperger RW. Organization of the subcortical system governing defence and flight reactions in the cat. Journal of Physiology. 1962;160:200–213. doi: 10.1113/jphysiol.1962.sp006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis I Disorders - Non-Patient Edition. New York State Psychiatric Institute; NY: 1995. [Google Scholar]
- Frank E, Hlastala S, et al. Inducing lifestyle regularity in recovering bipolar disorder patients: results from the maintenance therapies in bipolar disorder protocol. Biological Psychiatry. 1997;41:1165–1173. doi: 10.1016/s0006-3223(96)00241-7. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Siegle GJ, et al. Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedke H, Klingberg S, et al. Direct comparison of total sleep deprivation and late partial sleep deprivation in the treatment of major depression. Journal of Affective Disorders. 2003;76:1–3. 85–93. doi: 10.1016/s0165-0327(02)00071-x. [DOI] [PubMed] [Google Scholar]
- Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Medicine Reviews. 2002;6:361–377. [PubMed] [Google Scholar]
- Granholm E, Asarnow RF, et al. Pupillary responses index cognitive resource limitations. Psychophysiology. 1996;33:457–461. doi: 10.1111/j.1469-8986.1996.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:1–3. 56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, et al. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, et al. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. Sleep loss impairs short and novel language tasks having a prefrontal focus. Journal of Sleep Research. 1998;7:95–100. doi: 10.1046/j.1365-2869.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. One night of sleep loss impairs innovative thinking and flexible decision making. Organizational Behavior and Human Decision Processes. 1999;78:128–145. doi: 10.1006/obhd.1999.2827. [DOI] [PubMed] [Google Scholar]
- Hiscock H, Wake M. Infant sleep problems and postnatal depression: a community-based study. Pediatrics. 2001;107:1317–1322. doi: 10.1542/peds.107.6.1317. [DOI] [PubMed] [Google Scholar]
- Horne JA. Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. British Journal of Psychiatry. 1993;162:413–419. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- Janisse MP. Pupil size, affect, and exposure frequency. Social Behavior and Personality. 1974;2:125–146. [Google Scholar]
- Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Medicine Reviews. 2001;5:463–475. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- Kasper S, Wehr TA. The role of sleep and wakefulness in the genesis of depression and mania. Encephale. 1992;18(Spec No 1):45–50. [PubMed] [Google Scholar]
- Koikegami H, Yoshida K. Pupillary dilation induced by stimulation of amygdaloid nuclei. Folia Pychiatrica Neurologica Japonica. 1953;7:109–125. doi: 10.1111/j.1440-1819.1953.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, et al. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lingenfelser T, Kaschel R, et al. Young hospital doctors after night duty: their task-specific cognitive status and emotional condition. Medical Education. 1994;28:566–572. doi: 10.1111/j.1365-2923.1994.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Liu X. Sleep and adolescent suicidal behavior. Sleep. 2004;27:1351–1358. doi: 10.1093/sleep/27.7.1351. [DOI] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, et al. The prefrontal cortex in sleep. Trends in Cognitive Science. 2002;6:475–481. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- Naylor MW, King CA, et al. Sleep deprivation in depressed adolescents and psychiatric controls. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32:753–759. doi: 10.1097/00004583-199307000-00008. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, et al. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19:318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Sharma V, Smith A, et al. The relationship between duration of labour, time of delivery, and puerperal psychosis. Journal of Affective Disorders. 2004;83:2–3. 215–220. doi: 10.1016/j.jad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Granholm E, et al. Pupillary and reaction time measures of sustained processing of negative information in depression. Biological Psychiatry. 2001;49:624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, et al. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003a;27:365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, et al. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage. 2003b;20:114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, et al. Pupillary assessment and computational modeling of the Stroop task in depression. International Journal of Psychophysiology. 2004;52:63–76. doi: 10.1016/j.ijpsycho.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, et al. Can’t shake that feeling: fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, et al. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hakerem G. The pupillary response in cognitive psychophysiology and schizophrenia. Annals of the New York Academy of Sciences. 1992;658:182–204. doi: 10.1111/j.1749-6632.1992.tb22845.x. [DOI] [PubMed] [Google Scholar]
- Szabadi E, Bradshaw CM. Autonomic pharmacology of 2-adrenoceptors. Journal of Psychopharmacology. 1996;10:6–18. [Google Scholar]
- Thomas M, Sing H, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. Journal of Sleep Research. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen HP, Baynard MD, et al. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, et al. Cortical inefficiency in patients with unipolar depression: an event-related fMRI Study with the Stroop Task. Biological Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Wilhelm B, Wilhelm H, et al. Pupillographic assessment of sleepiness in sleep-deprived healthy subjects. Sleep. 1998;21:258–265. [PubMed] [Google Scholar]
- Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go. Biological Psychiatry. 1999;46:445–453. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- Wright JB. Mania following sleep deprivation. British Journal of Psychiatry. 1993;163:679–680. doi: 10.1192/bjp.163.5.679. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, et al. The human emotional brain without sleep—a prefrontal amygdala disconnect. Current Biology. 2007;17:R877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]