Abstract
DSPG3, the human homolog to chick PG-Lb, is a member of the small leucine-rich repeat proteoglycan (SLRP) family, including decorin, biglycan, fibromodulin, and lumican. In contrast to the tissue distribution of the other SLRPs, DSPG3 is predominantly expressed in cartilage. In this study, we have determined that the human DSPG3 gene is composed of seven exons: Exon 2 of DSPG3 includes the start codon, exons 4–7 code for the leucine-rich repeats, exons 3 and 7 contain the potential glycosaminoglycan attachment sites, and exon 7 contains the potential N-glycosylation sites and the stop codon. We have identified two polymorphic variations, an insertion/deletion composed of 19 nucleotides in intron 1 and a tetranucleotide (TATT)n repeat in intron 5. Analysis of 1.6 kb of upstream promoter sequence of DSPG3 reveals three TATA boxes, one of which is 20 nucleotides before the transcription start site. The transcription start site precedes the translation start site by 98 nucleotides. There are 14 potential binding sites for SOX9, a transcription factor present in cartilage, in the promoter, and in the first intron of DSPG3. We have examined the evolution of the SLRP gene family and found that gene products clustered together in the evolutionary tree are encoded by genes with similarities in genomic structure. Hence, it appears that the majority of the introns in the SLRP genes were inserted after the differentiation of the SLRP genes from an ancestral gene that was most likely composed of 2–3 exons.
[The sequence data described in this paper have been submitted to GenBank under accession nos. AF031658 and U63814.]
DSPG3 is the human homolog to chick PG-Lb, an extracellular matrix proteoglycan originally isolated from epiphyseal cartilage (Shinomura et al. 1983; Deere et al. 1996). DSPG3 (PG-Lb) is a member of the small leucine-rich repeat proteoglycan (SLRP) family, including decorin, biglycan, fibromodulin, and lumican (Krusius and Ruoslahti 1986; Fisher et al. 1989; Oldberg et al. 1989; Shinomura and Kimata 1992; Deere et al. 1996). The core proteins of the SLRPs are composed of 6–10 tandem repeats of 24 amino acid residues that are rich in leucine (for review, see Kobe and Deisenhofer 1994). The leucine-rich repeats (LRR) are preceded by four cysteines and followed by two cysteines that are presumed to form disulfide bonds on either side of the LRRs. Related LRR glycoproteins include prolargin (PRELP), osteoglycin (formerly known as osteoinductive factor), and osteomodulin (Madisen et al. 1990; Bengtsson et al. 1995; Grover et al. 1996; Ohno et al. 1996).
The SLRPs are clustered in a few chromosomal regions. Decorin, lumican, and DSPG3 map to human chromosome 12q21–q22 (McBride et al. 1990; Danielson et al. 1993; Vetter et al. 1993; Chakravarti et al. 1995; Grover et al. 1995; Deere et al. 1996). Fibromodulin and PRELP are localized to human chromosome 1q32 (Sztrolovics et al. 1994; Grover et al. 1996). Currently, only one SLRP, biglycan, maps to human chromosome Xq28 (McBride et al. 1990; Fisher et al. 1991; Traupe et al. 1992).
The SLRPs have related genomic structures. The fibromodulin, lumican, and PRELP genes are composed of three exons, and the first intron, in each case, immediately precedes the start site of translation, whereas the second intron is in the last LRR (Antonsson et al. 1993; Grover et al. 1995, 1996). The decorin and biglycan genes are both composed of eight exons, with the positions of two introns corresponding with those identified in fibromodulin, lumican, and PRELP (Fisher et al. 1991; Danielson et al. 1993; Vetter et al. 1993). The other five introns are present in the LRRs at identical sites.
DSPG3, in contrast to the other SLRPs, is predominantly expressed in cartilage (Shinomura and Kimata 1992; Deere et al. 1996; Kurita et al. 1996). There are several important extracellular matrix proteins expressed primarily in cartilage: collagen types II, IX, X, and XI, aggrecan, and link protein (for reviews, see Heinegard and Oldberg 1989; Hall and Newman 1991). Promoter studies for these genes have identified regions that may be important for the cartilage-specific transcription of these genes, including a binding site for SOX9 in the first intron of type II collagen (Nishimura et al. 1989; Rhodes and Yamada 1995; Thomas et al. 1995; Krebsbach et al. 1996, Lefebvre et al. 1997). SOX9 is a HMG (high-mobility group) transcription factor. Mutations in SOX9 cause campomelic dysplasia, a skeletal dysplasia, which suggests that SOX9 is important for the regulation of genes in normal cartilage development (Foster et al. 1994; Wagner et al. 1994).
In this study we have delineated the genomic structure of human DSPG3 and compared the exon/intron boundaries with those of lumican and decorin. We have also sequenced the promoter region of human DSPG3 and have identified transcriptional elements that may be important for the cartilage-specific expression of DSPG3. In addition, using available protein sequence data, we have performed the most complete evolutionary analysis of the SLRP gene family.
RESULTS AND DISCUSSION
Genomic Structure of DSPG3
The genomic structure of human DSPG3 is composed of seven exons and spans more than 12 kb (Table 1). This structure is conserved with murine PG-Lb (Iwata et al. 1998). Exon 1 consists of 5′-untranslated region, and the start codon is present in the second exon. Exons 4–7 encode the LRRs. Exons 3 and 7 contain the potential glycosaminoglycan attachment sites (codons 64, 96, and 320), and exon 7 includes the consensus N-glycosylation sites (codons 283 and 302), the stop codon, and 3′-untranslated region. The majority of the intron sizes are ∼1 kb. Exceptions are intron 1 (2.2 kb) and intron 2, whose size as determined by Southern blot analysis is at least 5 kb (data not shown). All of the splice donor and acceptor sites follow the consensus GT–AG rule. Two intronic polymorphisms, with low heterozygosities, were identified: An insertion/deletion of 19 nucleotides (TTGAACATCTGGCAGCAAT, nucleotides 3747–3765) in intron 1 (heterozygosity = 0.21) and a tetranucleotide (TATT)n repeat in intron 5 (heterozygosity = 0.39) (Table 2) (GenBank accession nos. AF031658 and U63814, respectively).
Table 1.
Genomic Structure of Human DSPG3
| Exon no. | Exon size | Intron | Splice donor | Splice acceptor | AA site | Type | |
|---|---|---|---|---|---|---|---|
| location | size | ||||||
| 1 | 85 | 85/86 | 2368 | AAG gtaag | tag GAA | ||
| 2 | 178 | 263/264 | 5000+ | GAG gtaat | cag ATT | Glu/Ile | 0 |
| 3 | 175 | 438/439 | 830+ | AAG gtcag | tag ACT | Asp | I |
| 4 | 159 | 597/598 | 819 | TAA gtatg | tag GTG | Ser | I |
| 5 | 203 | 800/801 | 825+ | AAA gtaag | cag GAC | Lys/Asp | 0 |
| 6 | 96 | 896/897 | 800+ | CAG gtagg | tag AAT | Gln/Asn | 0 |
| 7 | 671 | ||||||
Table 2.
Allele Frequencies
| Allele | Size (bp) | Frequency |
|---|---|---|
| a. For the insertion/deletion in intron 1 of the DSPG3 gene | ||
| A1 | 449 | 0.8 |
| A2 | 430 | 0.2 |
| b. For the tetranucleotide repeat in intron 5 of the DSPG3 gene | ||
| A1 | 279 | 0.04 |
| A2 | 275 | 0.14 |
| A3 | 271 | 0.69 |
| A4 | 267 | 0.08 |
| A5 | 263 | 0.02 |
| A6 | 255 | 0.03 |
Promoter Sequence of DSPG3
The sequence of the promoter and first intron of DSPG3 has been deposited in GenBank (accession no. AF031658). The start site of transcription is 98 nucleotides upstream of the start codon (mRNA sequence) as demonstrated by a ribonuclease protection assay (data not shown). The transcription start site is 20 nucleotide downstream of a TATA box (Fig. 1). There are several potential transcription factor binding sites present in the promoter sequence. The most noteable site, (A/T)(A/T)CAA(A/T)G, is the consensus DNA-binding site for HMG domain transcription factors, including SOX9 (Grosschedl et al. 1994; Sudbeck et al. 1996; Lefebvre et al. 1997). This site is present 4 times in the promoter region and 10 times in the first intron of DSPG3. Several of these sites are conserved with murine PG-Lb (Iwata et al. 1998). Mutations in SOX9 cause campomelic dysplasia, a skeletal dysplasia associated with sex reversal and lethality (Foster et al. 1994; Wagner et al. 1994). These mutations suggest that SOX9 is an important transcription factor in cartilage, as well as testis development. DSPG3 is primarily expressed in cartilage, and SOX9 may play an important role in the tissue-specific expression of this gene. Support for this conclusion comes from the cartilage-specific, type II collagen gene, COL2A1. COL2A1 was recently shown to contain a SOX9-binding site in the first intron that was necessary for the correct tissue expression of the gene (Bell et al. 1997; Lefebvre et al. 1997). Further studies will be necessary to prove whether the potential SOX9-binding sites are necessary for DSPG3 expression in cartilage.
Figure 1.

Sequence of the promoter of DSPG3 with the potential SOX9-binding sites (underlined), TATA boxes (bold and underlined), and transcription start site (bold) noted. Exon 1 is in uppercase letters.
Evolutionary Analysis of the SLRP Gene Family
In this study we have compared the genomic structures of the human SLRP genes and related proteins to determine whether there were indications of shared placement of the introns (Fig. 2). Analysis of the newly determined genomic structure of DSPG3 demonstrates that the first intron is present in the 5′-untranslated region, whereas the last (sixth) intron is present in the last LRR, a pattern similar to the genomic structures of the other SLRPs. However, the placement of introns 2–5 in the LRR region of DSPG3 shows no correspondence with introns 2–6 in decorin/biglycan (which are identical with each other) that are also located in the region encoding the LRRs (Fig. 2). Also, the last (seventh) LRR of DSPG3 aligns with the seventh LRR of decorin and biglycan and not the last (tenth) LRR. Therefore, the genomic structures of DSPG3 and decorin/biglycan appear to have evolved independently but with a shared tendency for the introns to occur in the LRR-encoding region. The other major group of SLRP genes (fibromodulin, lumican, and PRELP) have three exons. As with DSPG3, the first intron occurs upstream of the start site, whereas the second occurs in the final LRR. It should be noted that the final LRR of DSPG3 is in repeat seven (LRRs 8–10 are absent or deleted) and the exact position of the final intron differs between biglycan/decorin and fibromodulin/lumican/PRELP. Also, the final intron in fibromodulin is shifted 1 bp relative to that of lumican and PRELP. Therefore, it seems that the ancestral SLRP had one intron upstream of the start codon, and that most, if not all, of the additional introns were introduced separately in at least three lineages: DSPG3; biglycan/decorin; fibromodulin/lumican/PRELP. This observation is in accord with the introns late hypothesis (Stolzfus et al. 1997).
Figure 2.
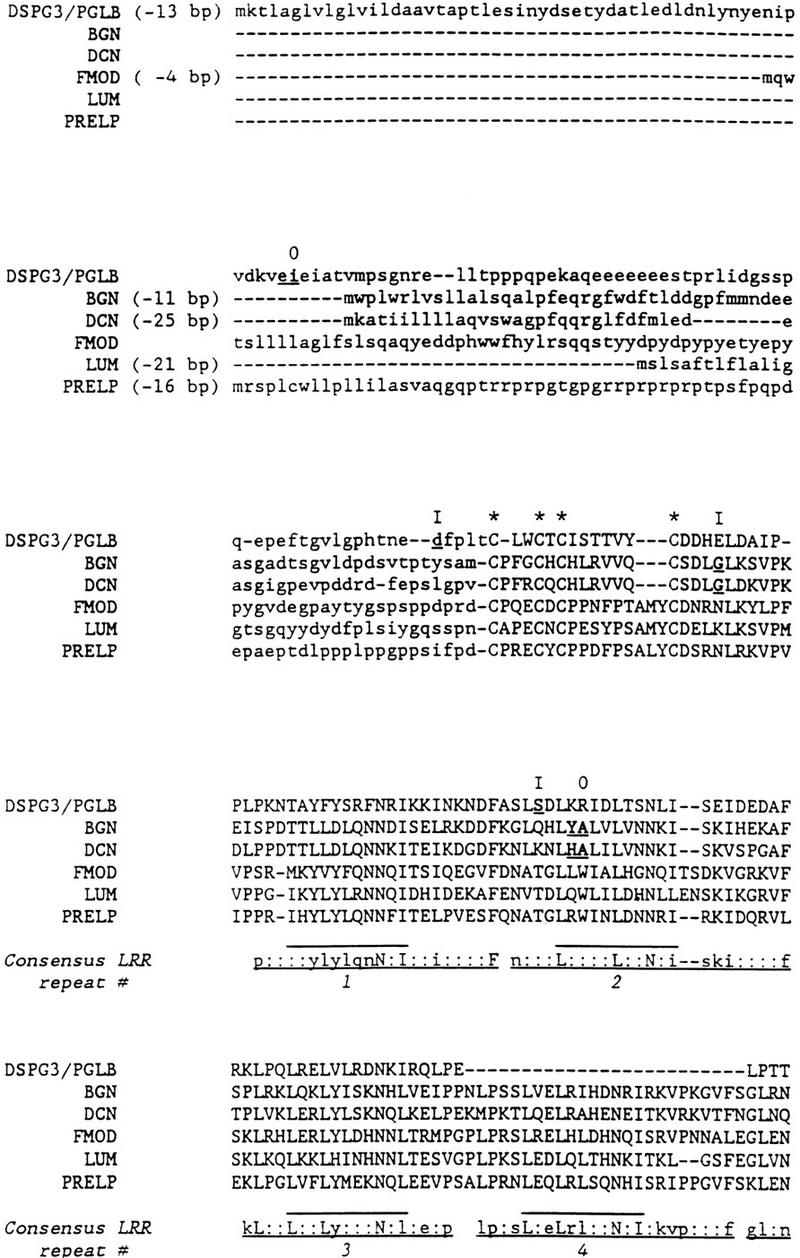

Protein sequence alignment of human DSPG3/PGLB, biglycan (BGN), decorin (DCN), lumican (LUM), fibromodulin (FMOD), and prolargin (PRELP). Amino acids whose codons are interrupted by an intron are in bold and underlined; where an intron occurs between two codons, both encoded amino acids are underlined. Intron phase is shown at top (0) Intron between codons; (I) intron after first base of codon; (II) intron after second base of codon. The first intron of each gene is upstream of the start codon; distance upstream is shown (e.g., −13 bp for DSPG3/PGLB). The LRRs are numbered and indicated by lines at bottom; consensus sequence of each LRR is also shown. (Uppercase letters) Amino acids completely conserved; (lowercase letters) >50% conserved in the six sequences; (*) the conserved cysteines flanking the LRRs. The central portion of the protein alignment that was used in the evolutionary analysis is in uppercase letters.
To analyze further the evolutionary history of the SLRP gene family, protein sequences from SLRPs and related LRR genes were aligned. Because the alignment was uncertain in the amino- and carboxy-terminal regions, only the LRR-containing region bounded by the four amino-terminal and two carboxy-terminal cysteines was used in the analysis (see Fig. 2) (The full alignment used is available on request.). Table 3 summarizes data on the SLRPs and related genes used in this analysis. neighbor-joining trees (Saitou and Nei 1987) were built with both p-distances (no correction for multiple mutations) and poisson-corrected distance matrices, each calculated by pairwise or complete deletions (Kumar et al. 1993). Because all four trees were very similar, only that using the p-distance with complete deletion of sites in which one or more sequence has a deletion is shown in Figure 3. The tree shows that the SLRP genes with similar genomic structures group together. DSPG3 (PG-Lb) and osteoglycin appear to have evolved separately from biglycan/decorin and lumican/fibromodulin/PRELP/osteomodulin. Our data correlates with previous dendrograms that have been constructed (Bengtsson et al. 1995; Iozzo 1997, 1998; Sommarin et al. 1998). However, this is the first study to include sequence data from multiple species allowing us to analyze the conservation of these genes as a family.
Table 3.
SLRP and Related Gene Sequences Used in this Study
Figure 3.
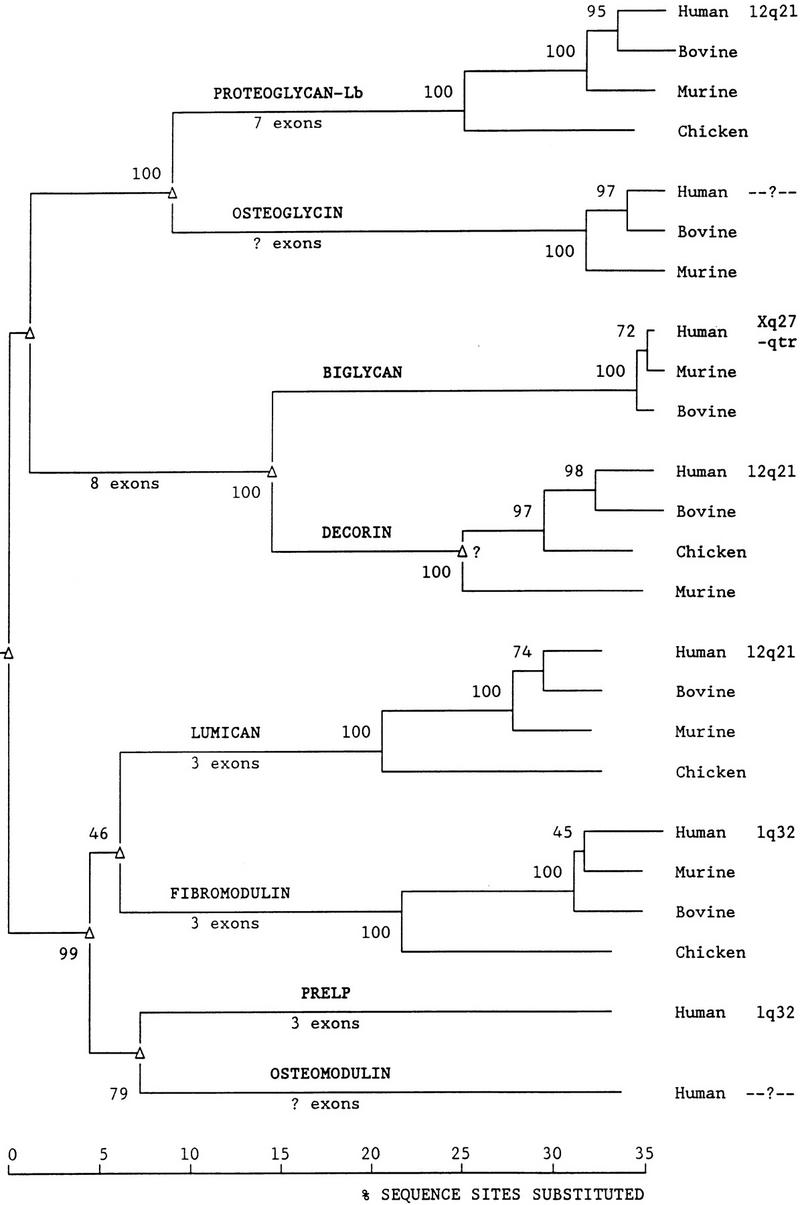
Phylogenetic tree of the SLRP gene family and related genes. (▵) Gene duplication. Numbers on branches (at left of node) represent the percent bootstrap replicates supporting that node. Chromosomal location of the human genes is shown. Branches are to scale and represent percent amino acid sequence sites substituted. No correction for undetected multiple replacements at a site was made in the tree shown. Root of the tree is arbitrarily placed.
Interestingly, in all four trees (Fig. 3; data not shown), the chicken decorin gene is more closely related to the human and bovine decorin genes than is the murine decorin gene. Because the bootstrap support for this unexpected finding is highly significant (97%, Fig. 3), it indicates that there was probably a duplication of an ancestral decorin gene prior to the bird/reptile/mammal divergence and that the cloned mouse gene represents one paralog, whereas the chicken, bovine, and human genes represent the other paralog. Somatic cell mapping of decorin demonstrated two signals on chromosome 12, also indicating that there may potentially be another member of the SLRPs present at that chromosomal region that is highly homologous to decorin (McBride et al. 1990). This may prove to be the human ortholog of the cloned mouse decorin gene.
In this study we have identified and sequenced the intron/exon borders of human DSPG3 and determined that the gene is composed of seven exons. Two intronic polymorphisms were identified and characterized. We have also cloned and sequenced the promoter and first intron of DSPG3. Several putative transcription factor-binding sites, including the potential SOX9-binding sites, were identified. Further analysis of the transcriptional elements present in DSPG3 will be necessary to determine the mechanisms involved in the specific regulation and expression of DSPG3 in cartilage. We have compared the genomic structures of DSPG3 and other members of the SLRP gene family, and have shown that the introns within the LRRs must have arisen separately in DSPG3 and decorin/biglycan. Our evolutionary analysis of the SLRP gene family confirms this hypothesis. SLRP genes with similar gene structures were more closely related to each other than they were to the other SLRP genes. It appears that the ancestral SLRP gene was composed of two (or possibly three) exons and that additional introns were inserted in DSPG3, decorin/bigycan, and (probably) fibromodulin/lumican/PRELP. In addition, there appears to have been intron slippage in the intron upstream of the start codon and in the second fibromodulin intron. It will be interesting to see how additional data (genomic structure, chromosomal location) on the osteoglycin and osteomodulin genes help shape the evolutionary scheme proposed, and whether a second decorin gene is present.
METHODS
Identification of Cosmids Containing DSPG3
cDNA template was amplified with primers hepn3/hepn2 (Deere et al. 1996). This PCR product was random prime labeled and used to hybridize a dot blot of chromosome 12-specific cosmids following standard procedures. Cosmids 167H5, 24C10, 231B8, 133C5, 204F1 196B7, 61B9, and 207C11 were positive for the DSPG3 probe.
Identification and Sequencing of the Intron/Exon Borders of Human DSPG3
cDNA primers were used to amplify genomic DNA from cosmid 167H5 to identify the locations of introns in the gene. The primer sets used were hepn3/hepn15, hepn1/239861, bepn4/hepn6, and hepn5/hepn8 (Deere et al. 1996; Table 4). Intron/exon borders were sequenced by a series of primers (Table 4) by direct sequencing of cosmid 167H5 using an ABI automated sequencer. Sequencing of each region was performed at least twice in two separate laboratories. The resulting sequence was analyzed by the GCG database system (Genetics Computer Group 1994).
Table 4.
Sequencing Primers for the Introns and Promoter of DSPG3
| Primer | Intron/ promoter | Sequence (5′–3′) |
|---|---|---|
| hepn1 | intron 4 | CCGCTTATTTCTATTCCCGCTTTA |
| hepn2 | intron 3 | GCGGGAATAGAAATAAGCGGTGGT |
| hepn4 | intron 1 | GGTGGCATCATAGGTTTCTG |
| hepn6 | intron 6 | GCTTGTGGAGTTTTGCTGAG |
| hepn7 | intron 1 | TAGAGTTGGGGCAGTCACAG |
| hepn9 | intron 2 | CATACCTGTTGATAAAGTTG |
| hepn15 | intron 2 | GAGAAGAGCCATCAATCAGC |
| hepn16 | intron 2 | ATTCCTCCTCCTCCTCCTCT |
| hepn19 | intron 2 | CTCATGTGTTTTCAATATTA |
| hepn21 | intron 2 | ATTGAAAACACATGAGAAAT |
| hepn23 | introns 2 and 3 | AAGCAGGATGGTCAAACT |
| hepn25 | intron 6 | GTATGATCTCCATCATCTGT |
| hepn26 | intron 5 | TGAAGGGCTCGTAGATTTTC |
| hepn29 | intron 5 | TTTGCTGTCATTGACTACC |
| hepn30 | intron 5 | GCGAAACCATGTCTCTAC |
| hepn33 | intron 1 | GATGACGGTGATGATGACTG |
| hepn35 | intron 1 | ATTCCCTTGTATGCCTGTGG |
| hepn36 | intron 1 | CTCTATTTCAGTTGCCTTTG |
| hepn37 | intron 1 | TCACACAGGATAAACTAAGC |
| hepn40 | intron 1 | CCTTATCATCTTCAACTTCA |
| hepn42 | intron 1 | GCATTTTGCATCACTCCT |
| hepn43 | intron 1 | GTCAATAACAAAAATAAACCAA |
| hepn45 | promoter | CTGGTCTGTGGGAGGTGGAA |
| hepn47 | promoter | GCAAGTATAAAAACTTACCT |
| hepn48 | promoter | TTTTTCCTTAGAATAGAAGC |
| hepn49 | promoter | AACAGGGAAAAATGAACCTT |
| hepn50 | promoter | TTTGAAAGCGGGATTACT |
| bepn3 | intron 5 | AGGCAGCTCCCAGAA |
| bepn4 | intron 4 | GTCACGCAGGACAAGC |
Analysis of Polymorphic Repeats
The 19-bp insertion/deletion in intron one was amplified from 120 unrelated caucasian individuals by PCR primers (forward, 5′-TCTTCACCTATAAAATGGTATGACA-3′; and reverse, 5′-TCTTCATTTTTCAAGCTTTCC-3′) following standard conditions (Sambrook et al. 1989). The PCR products were analyzed on 6% acrylamide gels.
PCR primers (forward, 5′-TTTGCTGTCATTGACTACC-3′; and reverse, 5′-GCGAAACCATGTCTCTAC-3′) were designed to amplify the tetranucleotide repeat (TATT)n in intron 5 of DSPG3 with a predicted PCR product size of 275 bp. Fifty-six unrelated individuals were amplified following standard procedures (Sambrook et al. 1989). The samples were analyzed on 6% denaturing polyacrylamide gels and silver-stained by the GelCode System (Pierce).
Sequencing Promoter Region of Human DSPG3
Cosmid 167H5 does not contain the promoter region of DSPG3. Therefore, cosmid 207C11 was used to sequence the promoter region. Cosmid 207C11 was subcloned into the EcoRI site in pBlueScript SK(+). Clones were then sequenced with a series of primers (Table 4) on an ABI automated sequencer. The promoter sequence was verified by amplification and sequencing of the promoter region from genomic DNA in a separate laboratory. The sequence was analyzed by the GAP program from the GCG database system (Genetics Computer Group 1994).
Ribonuclease Protection Assay
Primers, hepn46 (5′-GAATTTGTTACAGATGAGG-3′) and hepn47 (5′-GCAAGTATAAAAACTTACCT-3′), were used to amplify the first exon and 313 bp of the promoter region. The PCR product was cloned into the pGEM-T vector (Promega), and the clone was digested with NcoI to linearize the DNA for the probe. The probe was transcribed with SP6 polymerase and gel purified. The ribonuclease protection assays were performed with the RPA II kit from Ambion, Inc.
Evolutionary Analysis of the SLRP Gene Family
Published protein sequences of the human, bovine, murine, and chicken SLRP genes and related proteins were collected and an alignment made of the region between the first and last cysteines flanking the leucine-rich repeats by the LINEUP, PILEUP, and PRETTY programs from the GCG database system (Genetics Computer Group 1994). The proteins included DSPG3 (PG-Lb), osteoglycin, biglycan, decorin, lumican, fibromodulin, PRELP, and osteomodulin (Table 3). Alignments were verified with TBLASTN output of the DSPG3 protein sequence for the GenBank database (Genetics Computer Group 1994). Phylogenetic trees were built with the Molecular Evolutionary Genetics Analysis program, version 1.01 (MEGA) (Kumar et al. 1993). Four different neighbor-joining trees were built from p-distance and poisson-corrected distance matrices with both complete and pairwise deletions with 1000 bootstraps (Saitou and Nei 1987; Kumar et al. 1993).
Acknowledgments
We thank Dr. Raju Kucherlapati for providing the chromosome 12-specific cosmids. This research was supported by a Schissler Foundation Fellowship to M.D., Shriners Hospital for Children grants 15,955 and 15,957 to J.T.H., and grant support from The Academy of Finland and The Ulla Hjelt Fund to A.C. and J.L.D. The Department of Biomathematics at M.D. Anderson Cancer Center is supported by grant NCI CA-16672.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL jhecht@ped1.med.uth.tmc.edu; FAX (713) 500-5689.
REFERENCES
- Antonsson P, Heinegard D, Oldberg A. Structure and deduced amino acid sequence of the human fibromodulin gene. Biochim Biophys Acta. 1993;1174:204–206. doi: 10.1016/0167-4781(93)90117-v. [DOI] [PubMed] [Google Scholar]
- Bell DM, Leung KKH, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PPL, Cheah KSE. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- Bengtsson E, Neame PJ, Heinegard D, Sommarin Y. The primary structure of a leucine-rich repeat protein, PRELP, found in connective tissues. J Biol Chem. 1995;270:25639–25644. doi: 10.1074/jbc.270.43.25639. [DOI] [PubMed] [Google Scholar]
- Blochberger TC, Vergnes JP, Hempel J, Hassell JR. cDNA to chick lumican (corneal keratan sulfate proteoglycan) reveals homology to the small interstitial proteoglycan gene family and expression in muscle and intestine. J Biol Chem. 1992;267:347–352. [PubMed] [Google Scholar]
- Chakravarti S, Stallings RL, SundarRaj N, Cornuet PK, Hassell JR. Primary structure of human lumican (keratan sulfate proteoglycan) and localization of the gene (LUM) to chromosome 12q21.3–q22. Genomics. 1995;27:481–488. doi: 10.1006/geno.1995.1080. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Fazzio A, Cohen I, Cannizzaro LA, Eichstetter I, Iozzo RV. The human decorin gene: Intron-exon organization, discovery of two alternatively spliced exons in the 5′ untranslated region, and mapping of the gene to chromosome 12q23. Genomics. 1993;15:146–160. doi: 10.1006/geno.1993.1022. [DOI] [PubMed] [Google Scholar]
- Day AA, McQuillan CI, Termine JD, Young MR. Molecular cloning and sequence analysis of the cDNA for small proteoglycan II of bovine bone. J Biochem. 1987;248:801–805. doi: 10.1042/bj2480801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deere M, Johnson J, Garza S, Harrison WR, Yoon SJ, Elder FFB, Kucherlapati R, Hook M, Hecht JT. Characterization of human DSPG3, a small dermatan sulfate proteoglycan. Genomics. 1996;38:399–404. doi: 10.1006/geno.1996.0643. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Termine JD, Young MF. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989;264:4571–4576. [PubMed] [Google Scholar]
- Fisher LW, Heegaard AM, Vetter U, Vogel W, Just W, Termine JD, Young MF. Human biglycan gene: Putative promoter, intron-exon junctions, and chromosomal localization. J Biol Chem. 1991;266:14371–14377. [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Brown SJ, Vergnes J-P, Hassell JR, Mann MM, Conrad GW. Sequence and structural implications of a bovine corneal keratan sulfate proteoglycan core protein: Protein 37B represents bovine lumican and proteins 37A and 25 are unique. J Biol Chem. 1993;268:11874–11880. [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Hevelone ND, Stech ME, Justice MJ, Liu C-Y, Kao WW-Y, Conrad GW. Sequence, molecular properties, and chromosomal mapping of mouse lumican. Invest Opthalmol Vis Sci. 1995;36:2296–2303. [PubMed] [Google Scholar]
- Genetics Computer Group. Program manual for the Wisconsin package, version 8. Madison, WI 53711: University of Wisconsin; 1994. [Google Scholar]
- Grosschedl R, Giese K, Pagel J. HMG domain proteins: Architechtural elements in the assembly of nucleoprotein structures. Trends Genet. 1994;10:94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Grover J, Chen X-N, Korenberg JR, Roughley PJ. The human lumican gene: Organization, chromosomal location, and expression in articular cartilage. J Biol Chem. 1995;270:21942–21949. doi: 10.1074/jbc.270.37.21942. [DOI] [PubMed] [Google Scholar]
- Grover J, Chen X-N, Korenberg JR, Recklies AD, Roughley PJ. The gene organization, chromosomal location, and expression of a 55-kD matrix protein (PRELP) of human articular cartilage. Genomics. 1996;38:109–117. doi: 10.1006/geno.1996.0605. [DOI] [PubMed] [Google Scholar]
- Hall B, Newman S. Cartilage: Molecular aspects. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Heinegard D, Oldberg O. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989;3:2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The family of small leucine-rich proteoglycans: Key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- ————— Matrix proteoglycans: From molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Shinomura T, Kurita K, Zako M, Kimata K. The gene structure and organization of mouse PG-Lb, a small chondroitin/dermatan sulfate proteoglycan. Biochem J. 1998;331:959–964. doi: 10.1042/bj3310959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HJ, Rosenberg L, Choi HU, Garza S, Hook M, Neame PJ. Characterization of epiphycan, a small proteoglycan with a leucine-rich repeat core protein. J Biol Chem. 1997;272:18709–18717. doi: 10.1074/jbc.272.30.18709. [DOI] [PubMed] [Google Scholar]
- Kobe B, Deisenhofer J. The leucine-rich repeat: A versatile binding motif. Trends Biochem Sci. 1994;19:415–421. doi: 10.1016/0968-0004(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Nakata K, Bernier SM, Hatano O, Miyashita T, Rhodes CS, Yamada Y. Identification of a minimum enhancer sequence for the Type II collagen gene reveals several core sequence motifs in common with the link protein gene. J Biol Chem. 1996;271:4298–4303. doi: 10.1074/jbc.271.8.4298. [DOI] [PubMed] [Google Scholar]
- Krusius T, Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci. 1986;83:7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA: Molecular evolutionary genetics analysis, version 1.01. University Park, PA: Pennsylvania State University; 1993. [Google Scholar]
- Kurita K, Shinomura T, Ujita M, Zako M, Kida D, Iwata H, Kimata K. Occurence of PG-Lb, a leucine-rich small chondroitin/dermatan sulfate proteoglycan in mammalian epiphyseal cartilage-molecular cloning and sequence analysis of the mouse cDNA. Biochem J. 1996;318:909–914. doi: 10.1042/bj3180909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Zhou G, Mukhopadhyay K, Smith CN, Zhang A, Eberspaecher H, Zhou X, Sinha S, Maity SN, de Crombrugghe B. An 18-bp sequence in the mouse proα1(II) collagen gene is sufficient for expression in cartilage and binds nuclear proteins that are selectively expressed in chondrocytes. Mol Cell Biol. 1996;16:4512–4523. doi: 10.1128/mcb.16.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the proα1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Vergnes JP, Cornuet PK, Hassell JR. cDNA clone to chick corneal chondroitin/dermatan sulfate proteoglycan reveals identity to decorin. Arch Biochem Biophys. 1992;296:190–197. doi: 10.1016/0003-9861(92)90562-b. [DOI] [PubMed] [Google Scholar]
- Madisen L, Neubauer M, Plowman G, Rosen D, Segarini P, Dasch J, Thompson A, Ziman J, Bentz H, Purchio AF. Molecular cloning of a novel bone-forming compound: Osteoinductive factor. DNA Cell Biol. 1990;9:303–309. doi: 10.1089/dna.1990.9.303. [DOI] [PubMed] [Google Scholar]
- McBride OW, Fisher LW, Young MF. Localization of PGI (biglycan, BGN) and PGII (decorin, DCN, PG-40) genes on human chromosomes Xq13-qter and 12q, respectively. Genomics. 1990;6:219–225. doi: 10.1016/0888-7543(90)90560-h. [DOI] [PubMed] [Google Scholar]
- Nishimura I, Muragaki Y, Olsen BR. Tissue-specific forms of type IX collagen-proteoglycan arise from the use of two widely separated promoters. J Biol Chem. 1989;264:20033–20041. [PubMed] [Google Scholar]
- Nurminskaya MV, Birk DE. Differential expression of fibromodulin mRNA associated with tendon fibril growth: Isolation and characterization of a chicken fibromodulin cDNA. Biochem J. 1996;317:785–789. doi: 10.1042/bj3170785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno I, Hashimoto J, Takaoka K, Ochi T, Okubo K, Matsubara K. The cloning of a cDNA for novel gene expressed in human osteoblast. 1996. DDBJ/EMBL/GenBank, accession no. AB000114. [DOI] [PubMed] [Google Scholar]
- Oldberg A, Antonnson P, Lindblom K, Heinegard D. A collagen binding 59 kD protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG S1 and PG S2 (decorin) EMBO J. 1989;8:2601–2604. doi: 10.1002/j.1460-2075.1989.tb08399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau W, Just W, Vetter U, Vogel W. A dinucleotide repeat in the mouse biglycan gene (EST) on the X chromosome. Mamm Genome. 1994;5:395–396. doi: 10.1007/BF00356564. [DOI] [PubMed] [Google Scholar]
- Rhodes C, Yamada Y. Characterization of a glucocorticoid responsive element and identification of an AT-rich element that regulate the link protein gene. Nucleic Acids Res. 1995;23:2305–2313. doi: 10.1093/nar/23.12.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saamanen AMK. M. musculus mRNA for fibromodulin. 1996. DDBJ/EMBL/GenBank accession no. X94998. [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch F, Maniatis T. Molecular cloning: A laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Scholzen T, Solursh M, Suzuki S, Reiter R, Morgan JL, Buchberg AM, Siracusa LD, Iozzo RV. The murine decorin. Complete cDNA cloning, genomic organization, chromosomal assignment, and expression during organogenesis and tissue differentiation. J Biol Chem. 1994;269:28270–28281. [PubMed] [Google Scholar]
- Shinomura T, Kimata K. Proteoglycan-Lb, a small dermatan sulfate proteoglycan expressed in embryonic chick epiphyseal cartilage, is structurally related to osteoinductive factor. J Biol Chem. 1992;267:1265–1270. [PubMed] [Google Scholar]
- Shinomura T, Kimata K, Oike Y, Noro A, Hirose N, Tanabe K, Suzuki S. The occurrence of three different proteoglycan species in chick embryo cartilage: Isolation and characterization of a second proteoglycan (PG-Lb) and its precursor form. J Biol Chem. 1983;258:9314–9322. [PubMed] [Google Scholar]
- Sommarin Y, Wendel M, Shen Z, Hellman U, Heinegard D. Osteoadherin, a cell-binding keratan sulfate proteoglycan in bone, belongs to the family of leucine-rich repeat proteins of the extracellular matrix. J Biol Chem. 1998;273:16723–16729. doi: 10.1074/jbc.273.27.16723. [DOI] [PubMed] [Google Scholar]
- Stolzfus A, Logsden JM, Jr, Palmer JD, Doolittle WF. Intron “sliding” and the diversity of intron positions. Proc Natl Acad Sci. 1997;94:10739–10744. doi: 10.1073/pnas.94.20.10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbeck P, Schmitz ML, Baeuerle PA, Sherer G. Sex reversal by loss of the C-terminal transactivation domain of human SOX9. Nat Genet. 1996;13:230–232. doi: 10.1038/ng0696-230. [DOI] [PubMed] [Google Scholar]
- Sztrolovics R, Chen X-N, Grover J, Roughley PJ, Korenberg JR. Localization of the human fibromodulin gene (FMOD) to chromosome 1q32 and completion of the cDNA sequence. Genomics. 1994;23:715–717. doi: 10.1006/geno.1994.1567. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Sweetman WA, Cresswell CJ, Wallis GA, Grant ME, Boot-Handford RP. Sequence comparison of three mammalian type-X collagen promoters and preliminary functional analysis of the human promoter. Gene. 1995;160:291–296. doi: 10.1016/0378-1119(95)00189-d. [DOI] [PubMed] [Google Scholar]
- Traupe H, van den Ouweland AMW, van Oost BA, Vogel W, Vetter U, Warren ST, Rocchi M, Darlison MG, Ropers HH. Fine mapping of the human biglycan (BGN) gene within the Xq28 region employing a hybrid cell panel. Genomics. 1992;13:481–483. doi: 10.1016/0888-7543(92)90279-2. [DOI] [PubMed] [Google Scholar]
- Ujita M, Shinomura T, Kimata K. Molecular cloning of the mouse osteoglycin-encoding gene. Gene. 1995;158:237–240. doi: 10.1016/0378-1119(95)00070-m. [DOI] [PubMed] [Google Scholar]
- Vetter U, Vogel W, Just W, Young MF, Fisher LW. Human decorin gene: Intron-exon junctions and chromosomal location. Genomics. 1993;15:161–168. doi: 10.1006/geno.1993.1023. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Xu JH, Radhakrishnamurthy B, Srinivasan SR, Berenson GS. Primary structure of bovine aorta biglycan core protein deduced from cloned cDNA. Biochem Mol Biol Int. 1995;37:263–272. [PubMed] [Google Scholar]



