Abstract
Largely on the basis of the first publication of findings of net harm with menopausal hormone treatment in the Women’s Health Initiative (WHI) hormone trials, current Food and Drug Administration recommendations limit menopausal hormone treatment to the “… shortest duration consistent with treatment goals …,” with goals generally taken to mean relief of menopausal symptoms and maximal duration as approximately 5 years. The WHI finding of net harm was due largely to the absence of beneficial effects on coronary heart disease incidence rates. Published analyses of WHI data by age or time since menopause find that excess coronary heart disease risk with menopausal hormone treatment is confined to more remotely menopausal or older women, with younger women showing nonsignificant trends toward benefit (the “timing hypothesis”). Moreover, a recently published reexamination of data from the WHI Estrogen plus Progestin trial suggests that reduced coronary heart disease risk may appear only after 5 to 6 years of treatment. Consistent with this finding, risk ratios for coronary heart disease were calculated as 1.08 (95% confidence interval, 0.86–1.36) in years 1 to 6 and as 0.46 (confidence interval, 0.28–0.78) in years 7 to 8+ in the WHI Estrogen Alone trial. Previous studies also support the beneficial effects of menopausal hormone treatment after prolonged exposure. Thus, current analyses do not support a generalized recommendation for short duration of menopausal hormone treatment. Rather, they suggest that current Food and Drug Administration practice guidelines should be reconsidered to allow individualized care based on risk:benefit considerations. New research is urgently needed evaluating influences of timing, duration, dose, route of administration, and agents on menopausal hormone treatment-related risks and benefits to better understand how to optimize recommendations for individual patients.
Keywords: Cardiovascular disease, Estrogen, Hormones, Menopause, Women’s health
Coronary heart disease remains the single greatest cause of death among women aged more than 50 years.1 Before 2002, it was widely believed that menopausal hormone treatment protected women against coronary heart disease, according to the results of several large-scale observational studies showing 40% to 50% lower coronary heart disease incidence in women receiving menopausal hormone treatment.2–9 In observational studies and randomized clinical trials, menopausal hormone treatment also has been found to significantly reduce the risks of osteoporosis-related fractures10–12 and colon cancer,13,14 while increasing breast cancer incidence by 20% to 30%.15–17 On the basis of the expectation of coronary heart disease protection, the benefit/risk ratio for menopausal hormone treatment has been calculated to be positive for most women.18 In 2002, primary results of the Women’s Health Initiative (WHI) Estrogen plus Progestin (E+P) trial14 showed the expected degrees of increase in breast cancer and thromboembolic disease without the expected coronary heart disease protection, leading to the conclusion that menopausal hormone treatment produces net harm. The results of the WHI Estrogen Alone (EA) trial, published in 2004,19 showed a trend toward decreased risk of breast cancer, increased risks of stroke and venous thromboembolic disease, and again no coronary heart disease benefit. Publication of these findings led tens of millions of symptomatic women in the United States alone to discontinue menopausal hormone treatment or to avoid starting it. Thus, it is crucial to understand the reasons for the apparent discrepancies in coronary heart disease risk outcomes between observational studies and WHI trials.
WOMEN’S HEALTH INITIATIVE FINDINGS AND THE “TIMING HYPOTHESIS”
One difference between the WHI hormone trials and the observational studies is that women enrolled in the WHI were an average of 63 years of age at menopausal hormone treatment initiation, approximately 12 years postmenopausal on average.14,19–21 In sharp contrast, enrollees in the observational studies tended to start menopausal hormone treatment at or near menopause, at an average age of 51 years.22 Thus, women in the WHI also were older and longer postmenopausal than is usual for initiation of menopausal hormone treatment in clinical practice.23 Because atherosclerotic lesions accumulate long before a first clinical event occurs,24,25 the older women in the WHI trials may have harbored significant subclinical coronary heart disease, and thus would not have been good candidates for a treatment such as menopausal hormone treatment, which seems to be more effective in primary rather than secondary prevention of atherosclerosis. The idea that differences in age or time since menopause when menopausal hormone treatment is initiated may account for differences in coronary heart disease outcomes, and even opposite effects of menopausal hormone treatment on coronary heart disease have become known as the “timing hypothesis.”24,26–28
Evidence for the timing hypothesis comes from varied sources. In an experimental model with surgically menopausal monkeys that develop typical atherosclerosis when fed a high saturated fat diet, estrogen treatment reduces coronary atherosclerosis by 50% to 70% if begun immediately after ovariectomy.29–31 In contrast, estrogen treatment has no beneficial effect when delayed by 2 years, which is the equivalent of approximately 6 years delay in humans. 32 This finding is consistent with the lack of secondary coronary heart disease prevention by menopausal hormone treatment observed in trials in women with a clinical history of heart disease.33–35
In the Nurses’ Health Study, women initiating menopausal hormone treatment at or near menopause were observed to experience significant coronary heart disease protection (hazard ratio [HR] = 0.66, 95% confidence interval [CI], 0.54–0.80 for EA; HR = 0.72, 95% CI, 0.56–0.92 for E+P), whereas the few who started menopausal hormone treatment 10+ years after menopause were not (HR = 0.87, 95% CI, 0.69–1.10 for EA; HR = 0.90, 95% CI, 0.62–1.29 for E+P).36
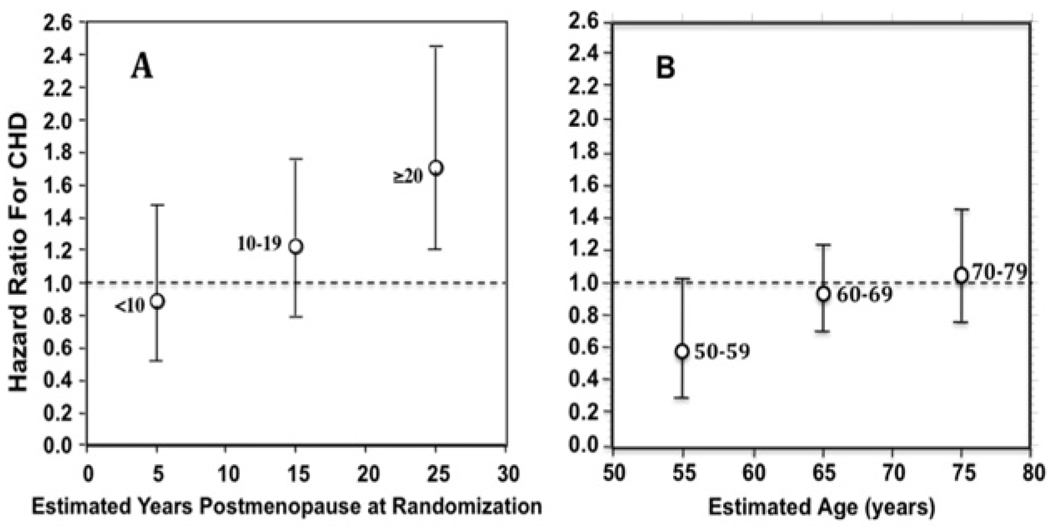
Subgroup analyses of WHI data also support the timing hypothesis. For example, as shown in Figure 1A, in the E+P trial a nonsignificant trend toward protection (HR = 0.89; 95% zCI, 0.5–1.5) was seen in women who were less than 10 years post-menopausal, whereas significant excess risk occurred in women more than 20 years postmenopausal (HR = 1.71; 95% CI, 1.1–2.5).20 Similarly, as shown in Figure 1B, in the EA trial19,37 there was a trend for coronary heart disease protection in women 50 to 59 years of age (HR = 0.63; 95% CI, 0.36–1.08), but a trend toward increased risk in women aged more than 70 years (HR = 1.11; 95% CI, 0.82–1.52). Similar trends were seen when data from both E+P and EA trials were pooled.38 Also, conjugated equine estrogen-treated women, who were 50 to 59 years old at randomization into the EA trial, showed a significantly lower mean coronary calcium burden compared with those treated with placebo when studied 8.7 (mean) years after randomization.39
Figure 1.
HRs and 95% CIs for coronary heart disease in WHI hormone trials. A, By time since menopause in the E+P study (redrawn from Manson et al20). B, By age group in EA study (redrawn from Anderson et al19 and Hsia et al37). CHD = coronary heart disease.
Finally, a large meta-analysis of randomized clinical trials comparing coronary heart disease outcomes after menopausal hormone treatment started in younger versus older women reported reduced coronary heart disease only in the younger women.40 In a complementary cost– benefit analysis, Salpeter et al41 found that quality adjusted life-years for menopausal hormone treatment are immediately positive in younger women, but, apropos of the duration effects discussed next, shift from negative to positive only after a significant delay in older women.
In contrast with those findings, a recent post hoc analysis that pooled observational data from WHI participants who initiated menopausal hormone treatment on their own when recently menopausal (but whose duration of treatment was unknown) with data from women randomized to study drug within 5 years of menopause failed to detect a timing effect. 42 This finding may reflect the fact that duration of treatment was not considered in the analysis.
The “timing hypothesis” has been discussed widely and is included in the conclusions of a consensus scientific statement recently published by the Endocrine Society, which states in part that “Subgroup analyses suggest that the lack of benefit or increase in coronary heart disease risk observed in the overall analysis of the WHI resulted from harmful effects of menopausal hormone treatment in older women starting therapy many years after onset of menopause.” 43 However, this hypothesis awaits rigorous testing. Two currently ongoing randomized clinical trials of menopausal hormone treatment address the timing hypothesis, the Kronos Early Estrogen Prevention Study,44 which compares 2 hormone regimens (oral conjugated equine estrogen vs transdermal estradiol, both with cyclic oral progesterone) with placebo in women less than 3 years postmenopausal, and the Early Versus Late Intervention Trial With Estradiol (Howard Hodis, Principal Investigator), which examines effects of oral estradiol in recently and more remotely menopausal women. Both trials monitor the development or progression of atherosclerosis as detected by noninvasive cardiovascular imaging. Neither is powered for clinical event outcomes.
EFFECTS OF TREATMENT DURATION IN THE WOMEN’S HEALTH INITIATIVE ESTROGEN + PROGESTIN TRIAL
A second important issue, also apparently critical in the relationship between menopausal hormone treatment and atherosclerosis but less widely discussed to date, is that of treatment duration, that is, whether early and late menopausal hormone treatment effects on coronary heart disease risk may differ. The WHI findings led directly to a revised Food and Drug Administration “black box” warning for estrogens intended for menopausal hormone treatment, stating in part that “Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.”45 In practice, the “shortest duration” for menopausal hormone treatment is usually interpreted as no more than 5 years to reduce the risk for breast cancer, which may increase after 5 years of treatment.17 Ironically, observational studies addressing the issue of menopausal hormone treatment duration indicate that protective effects of menopausal hormone treatment against coronary heart disease may become apparent only after several years of treatment.46,47 This finding is supported by a recent post hoc analysis of data from the WHI E+P trial48 showing that, in adherent women initiating treatment at less than 10 years of menopause, the coronary heart disease event-free survival rate was slightly lower in the menopausal hormone treatment group than in the placebo group during the first 5 years of treatment, but at 6 years the placebo and treatment group curves crossed one another showing a nonsignificant, late trend toward greater event-free survival in the menopausal hormone treatment group (P = .44 for differences between these 2 survival curves). The survival curves for women of all ages and for women initiating treatment 10 or more years after menopause indicated a small advantage for the placebo-treated women at all time points, with P values of .057 and .011, respectively, consistent with the timing hypothesis. For continuous treatment of women initiating menopausal hormone treatment at less than 10 years since menopause, there were nonsignificant HRs indicating an increased risk for the first 2 years (HR = 1.29; 95% CI, 0.52–3.18), a decreased risk after 2 years (HR = 0.63; 95% CI, 0.27–1.52), and an overall 8-year decrease in risk (HR = 0.64; 95% CI, 0.21–1.99), the latter also nonsignificant. However, the difference between the ≤2-year and the > 2-year HRs was statistically significant (P = .038), suggesting a duration effect. The authors did not compare HRs for ≤5 years and > 5 years in all likelihood because so few women in the WHI E+P trial were treated beyond 5 years because the trial was stopped early for harm, seen primarily in the older women.
EFFECTS OF TREATMENT DURATION IN THE WOMEN’S HEALTH INITIATIVE ESTROGEN ALONE TRIAL
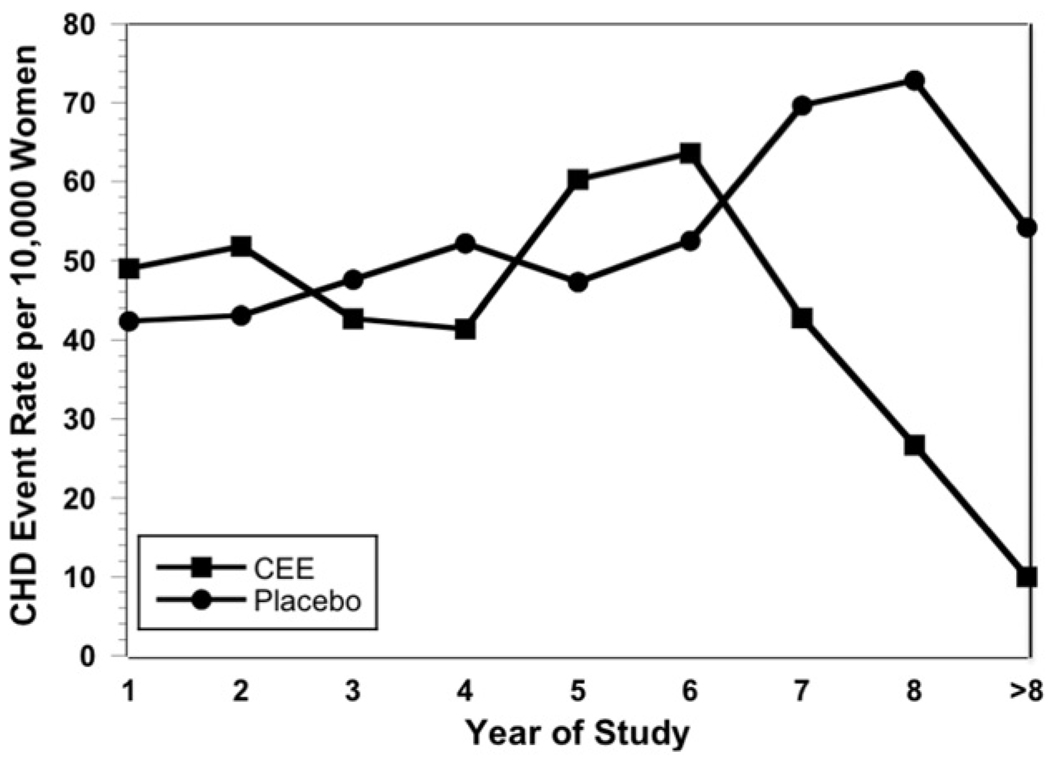
To investigate whether menopausal hormone treatment with EA might show a similar reduction in coronary heart disease risk with longer duration of use, as suggested by analyses of the E+P data, we evaluated published results of the WHI EA trial, which was continued for approximately 2 years longer than the E+P trial.19 On the basis of the findings of Toh et al48 in the WHI E+P study that survival curves crossed in the more recently menopausal women at 6 years, annual coronary heart disease event incidence rates for years 1 to 8+ and rate ratios and 95% CIs for coronary heart disease incidence rates pooled across 2 periods (years 1–6 and >6 years) were calculated using a Poisson model,49 accounting for person-years at risk in both groups. We approximated the person-years by summing the number of participants within the placebo and conjugated equine estrogen groups contributing follow-up during each of the years in the first and second pooled time periods.
The absolute numbers of coronary heart disease events and women at risk in the conjugated equine estrogen-treated and placebo-treated groups in each year of the WHI EA trial19 are shown in Table 1. The annual coronary heart disease event incidence rates appeared similar in conjugated equine estrogen- and placebo-treated women in years 1 to 6, but declined sharply in the conjugated equine estrogen-treated women thereafter (Figure 2). As shown in Table 2, the rate ratios for years 1 to 6 and 7 to 8+ identify a statistically significant (P = .003) reduction in coronary heart disease risk with more than 6 years of use of conjugated equine estrogen compared with placebo.
Table 1.
Numbers of Coronary Heart Disease Events and Women at Risk in Each Year of Women’s Health Initiative Estrogen Alone Trial
| Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | >8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Conjugated equine estrogen | Events | 26 | 27 | 22 | 21 | 30 | 31 | 13 | 6 | 1 |
| At risk | 5310 | 5210 | 5147 | 5067 | 4978 | 4874 | 3034 | 2248 | 999 | |
| Placebo | Events | 23 | 23 | 25 | 27 | 24 | 26 | 28 | 17 | 6 |
| At risk | 5429 | 5336 | 5254 | 5171 | 5072 | 4950 | 4015 | 2331 | 1106 |
Figure 2.
Annual incidence of coronary heart disease events per 10,000 women in the WHI EA trial. Data from Anderson et al19 and Hsia et al.37 CEE = conjugated equine estrogen; CHD = coronary heart disease.
Table 2.
Event Numbers and Rate Ratios for Coronary Heart Disease Events in Early and Late Periods of the Women’s Health Initiative Estrogen Alone Trial
| Years | Numbers | Placebo | Conjugated Equine Estrogen | Rate Ratio | Standard Error | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| 1–6 | Events | 148 | 157 | 1.08 | 0.124 | 0.86–1.36 |
| At risk | 31,212 | 30,586 | ||||
| 7–8+ | Events | 51 | 20 | 0.46 | 0.123 | 0.28–0.78 |
| At risk | 7452 | 6281 |
INTERPRETATION AND PERSPECTIVE
Our post hoc analysis of the WHI EA trial results corroborates recent findings in the E+P trial,48 demonstrating a trend for decrease in cardiovascular risk after 6 years of menopausal hormone treatment. The significantly reduced rate ratio after 6 years is particularly striking because it is not confined to the younger or more recently menopausal women, as was true for the E+P analysis48 but was calculated using data from all women enrolled in the WHI EA trial (ages 50–79 years). The finding of cardiovascular protection with treatment more than 6 years also is consistent with findings in the Nurses’ Health Study46,50 and a trend seen in older women with preexisting coronary heart disease in the Heart and Estrogen/Progestin Replacement Study.33 Furthermore, in a case-control study of women with an acute myocardial infarction versus age-matched community controls,47 menopausal hormone treatment was associated with a significant reduction in the likelihood for an acute myocardial infarction (HR = 0.42; 95% CI, 0.24–0.73) only when used for more than 60 months. Consistent with those findings, in postmenopausal women undergoing coronary angiography, strong inverse relationships were observed between years of menopausal hormone treatment exposure and both the degree of stenosis and the severity score.51
Although the timing of initiation of menopausal hormone treatment in relation to menopause onset has been a matter of debate, there has been little discourse and no working hypothesis regarding the likely effect of duration of menopausal hormone treatment on coronary heart disease risk. Because atherogenesis is a gradual process, progressing from fatty streaks to advanced plaques over a number of years, an intervention whose beneficial effects are confined to halting or slowing progression would not be expected to show benefit until sufficient time had elapsed to reduce the number of at-risk plaques. Menopausal hormone treatment reduces low-density lipoprotein cholesterol and lipoprotein(a), and increases high-density lipoprotein cholesterol,52–56 lowers fibrinogen levels,52 improves responsiveness of the arterial endothelium to vasodilatory stimuli,54,57 decreases expression of certain endothelial adhesion factors,58–60 reduces blood pressure,61–63 and acts as an antioxidant.64–68 All of these actions would be expected to inhibit initiation of atherosclerosis and slow progression of early atherosclerotic lesions.
On the other hand, oral estrogens also can have adverse effects that are more rapidly manifest and that may help explain the observed trends for early increase in coronary heart disease risk in older or more remotely menopausal women, as observed in the WHI trials.14,19–21 Increases in thrombotic and decreases in thrombolytic factor syntheses by the liver induced by the first-pass effects of high concentrations of oral estrogen in the portal circulation69–74 may explain the 2- to 3-fold increase in risk of venous thromboembolic disease observed with oral but not transdermal estrogen,75,76 and could contribute to the risk of coronary thrombosis on existing plaque. Also, estrogens induce macrophage and vascular smooth muscle expression of matrix metalloproteinase in the fibrous caps of existing plaque,77–80 which may lead to plaque rupture and consequent vascular occlusion. These latter effects would tend to heighten the short-term risk of a coronary heart disease event in women with preexisting advanced plaques.
A potential weakness of our analysis is that the apparent late-onset coronary heart disease protection occurring in the WHI and other studies could be a “healthy survivor effect.” This might have occurred if estrogen-treated women at high risk because of the presence of advanced plaque had an increased incidence of early coronary heart disease events, thus, removing them from the study and leaving a residual group with better cardiovascular health for later follow-up. This explanation seems unlikely because during the first 6 years of the EA trial there was an excess of only 9 events (3.9/10,000 woman years) in the conjugated equine estrogen group, whereas in years 6 to 8+ there was an excess of 31 events (36.6/10,000 woman years) in the placebo group (Table 2). That said, it remains the case that our analysis of the WHI EA data could have been improved by access to original patient-level data allowing adjustment for compliance and other factors as carried out by Toh et al48 for the E+P study.
Both published clinical observations and considerations of mechanisms of estrogen actions influencing the pathogenesis of coronary heart disease make a plausible case that biphasic effects of menopausal hormone treatment on coronary heart disease risk depend on both timing of initiation and duration of treatment. The present analysis of the WHI EA trial, taken together with a recent analysis of E+P trial data,48 suggests that the longer the duration of menopausal hormone treatment, the more favorable the effects on coronary heart disease risk. Thus, treatment duration and timing of menopausal hormone treatment initiation seem to be distinct factors that may help explain the disparity in coronary heart disease outcomes between the observational studies and the WHI randomized clinical trials. Because younger, recently menopausal women have a relatively low prevalence of at-risk plaque and a correspondingly low incidence of coronary heart disease events, the “early harm” effect is likely to be clinically insignificant in this population. However, coronary heart disease risk in women increases steeply from age 55 to 70 years, just the time at which long-term menopausal hormone treatment initiated at menopause seems to confer coronary heart disease protection.
CONCLUSIONS
The current analysis and data reviewed do not support any specific limit on the duration of menopausal hormone treatment. Emerging data suggest that the decision to prescribe menopausal hormone treatment and how long to continue should be flexible, based on patient characteristics (eg, age and time since menopause) and the balance of benefits (symptom relief, coronary heart disease, and bone fractures) and risks (breast cancer, thromboembolic disease, and stroke). We believe that guidelines from the Food and Drug Administration and other official sources should be reconsidered and revised to reflect this personalized approach to the patient.81 New research is urgently needed to further evaluate influences of timing, duration, dose, route of administration, and choice of agents on menopausal hormone treatment risks and benefits to optimize recommendations for individual patients.
Acknowledgments
Funding: The Aurora Foundation, a private charitable foundation based in Phoenix, Arizona, via a grant to the Kronos Longevity Research Institute.
Footnotes
Conflict of Interest: None.
Authorship: All authors had access to the data and played a role in writing this manuscript.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Bush TL, Cowan LD, Barrett-Connor E, et al. Estrogen use and all-cause mortality. Preliminary results from the Lipid Research Clinics Program Follow-Up Study. JAMA. 1983;249:903–906. doi: 10.1001/jama.249.7.903. [DOI] [PubMed] [Google Scholar]
- 3.Bush TL, Barrett-Connor E, Cowan LD, et al. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PH, Madans JH, Finucane FF, Higgins M, Kleinman JC. Reduction of cardiovascular disease-related mortality among postmenopausal women who use hormones: evidence from a national cohort. Am J Obstet Gynecol. 1991;164:489–494. doi: 10.1016/s0002-9378(11)80006-2. [DOI] [PubMed] [Google Scholar]
- 5.Henderson BE, Paganini-Hill A, Ross RK. Decreased mortality in users of estrogen replacement therapy. Arch Intern Med. 1991;151:75–78. [PubMed] [Google Scholar]
- 6.Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses’ Health Study. N Engl J Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger B, Friedman GD, Bush T, Quesenberry CPJ. Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstet Gynecol. 1996;87:6–12. doi: 10.1016/0029-7844(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 8.Grodstein F, Stampfer MJ, Colditz GA, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769–1775. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 9.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 11.Randell KM, Honkanen RJ, Kroger H, Saarikoski S. Does hormone-replacement therapy prevent fractures in early postmenopausal women? J Bone Miner Res. 2002;17:528–533. doi: 10.1359/jbmr.2002.17.3.528. [DOI] [PubMed] [Google Scholar]
- 12.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 13.Folsom AR, Mink PJ, Sellers TA, Hong CP, Zheng W, Potter JD. Hormonal replacement therapy and morbidity and mortality in a prospective study of postmenopausal women. Am J Public Health. 1995;85:1128–1132. doi: 10.2105/ajph.85.8_pt_1.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Writing Group for the Women’s Health Initiative. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 15.Bergkvist L, Adami H-O, Persson I, Hoover R, Schairer C. The risk of breast cancer after estrogen and estrogen-progestin replacement. N Engl J Med. 1989;321:293–297. doi: 10.1056/NEJM198908033210505. [DOI] [PubMed] [Google Scholar]
- 16.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485–491. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 17.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 18.Col NF, Eckman MH, Karas RH, et al. Patient-specific decisions about hormone replacement therapy in postmenopausal women. JAMA. 1997;277:1140–1147. [PubMed] [Google Scholar]
- 19.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 20.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart Disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 21.Hsia J, Criqui MH, Rodabough RJ, et al. Estrogen plus progestin and the risk of peripheral arterial disease: the Women’s Health Initiative. Circulation. 2004;109:620–626. doi: 10.1161/01.CIR.0000115309.63979.92. [DOI] [PubMed] [Google Scholar]
- 22.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348:645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 23.Lemay A. The relevance of the women’s health initiative results on combined hormone replacement therapy in clinical practice. J Obstet Gynaecol Can. 2002;24:711–715. doi: 10.1016/s1701-2163(16)30326-7. [DOI] [PubMed] [Google Scholar]
- 24.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 25.Raggi P, Callister TQ, Cooil B, et al. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron- beam computed tomography. Circulation. 2000;101:850–855. doi: 10.1161/01.cir.101.8.850. [DOI] [PubMed] [Google Scholar]
- 26.Naftolin F, Taylor HS, Karas R, et al. The Women’s Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition. Fertil Steril. 2004;81:1498–1501. doi: 10.1016/j.fertnstert.2004.02.095. [DOI] [PubMed] [Google Scholar]
- 27.Brinton EA, Hodis HN, Merriam GR, Harman SM, Naftolin F. Can menopausal hormone therapy prevent coronary heart disease? Trends Endocrinol Metab. 2008;19:206–212. doi: 10.1016/j.tem.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Harman SM, Brinton EA. Biphasic effects of hormone treatment on risk of cardiovascular disease: resolving the paradox in postmenopausal women. Menopausal Med. 2009;17:S5–S8. [Google Scholar]
- 29.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 30.Clarkson TB, Anthony MS, Jerome CP. Lack of effect of raloxifene on coronary artery atherosclerosis of postmenopausal monkeys. J Clin Endocrinol Metab. 1998;83:721–726. doi: 10.1210/jcem.83.3.4617. [DOI] [PubMed] [Google Scholar]
- 31.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 32.Williams JK, Anthony MS, Honore EK, et al. Regression of atherosclerosis in female monkeys. Arterioscler Thromb Vasc Biol. 1995;15:827–836. doi: 10.1161/01.atv.15.7.827. [DOI] [PubMed] [Google Scholar]
- 33.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 34.Byington RP, Furberg CD, Herrington DM, et al. Effect of estrogen plus progestin on progression of carotid atherosclerosis in postmenopausal women with heart disease: HERS B-mode substudy. Arterioscler Thromb Vasc Biol. 2002;22:1692–1697. doi: 10.1161/01.atv.0000033514.79653.04. [DOI] [PubMed] [Google Scholar]
- 35.Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522–529. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 36.Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt) 2006;15:35–44. doi: 10.1089/jwh.2006.15.35. [DOI] [PubMed] [Google Scholar]
- 37.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 38.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 39.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 40.Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med. 2009;122:1016–1022. doi: 10.1016/j.amjmed.2009.05.021. e1. [DOI] [PubMed] [Google Scholar]
- 41.Salpeter SR, Buckley NS, Liu H, Salpeter EE. The cost-effectiveness of hormone therapy in younger and older postmenopausal women. Am J Med. 2009;122:42–52. doi: 10.1016/j.amjmed.2008.07.026. e2. [DOI] [PubMed] [Google Scholar]
- 42.Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170:12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society Scientific Statement. J Clin Endocrinol Metab. 2010;95(7) Suppl 1:s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller VM, Black DM, Brinton EA, et al. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) J Cardiovasc Translat Res. 2009;2:228–239. doi: 10.1007/s12265-009-9104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.USFDA: PREMARIN®. (conjugated estrogens tablets, USP). 2010. [Accessed April 8, 2010];Wyeth Pharmaceuticals. 2002 Available at: http://www.wyeth.com/products/prescription?product=/wyeth_html/home/products/prescription/PREMARIN%C2%AE%20%28conjugated%20estrogens%20tablets,%20USP%29/PREMARIN%C2%AE%20%28conjugated%20estrogens%20tablets,%20USP%29_overview.html. [Google Scholar]
- 46.Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the Nurses’ Health Study. A prospective, observational study. Ann Intern Med. 2001;135:1–8. doi: 10.7326/0003-4819-135-1-200107030-00003. [DOI] [PubMed] [Google Scholar]
- 47.Chilvers CE, Knibb RC, Armstrong SJ, Woods KL, Logan RF. Post menopausal hormone replacement therapy and risk of acute myocardial infarction—a case control study of women in the East Midlands, UK. Eur Heart J. 2003;24:2197–2205. doi: 10.1016/j.ehj.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Toh S, Hernandez-Diaz S, Logan R, Rossouw JE, Hernan MA. Coronary heart disease in postmenopausal recipients of estrogen plus progestin therapy: does the increased risk ever disappear? A randomized trial. Ann Intern Med. 2010;152:211–217. doi: 10.1059/0003-4819-152-4-201002160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frome EL. The analysis of rates using Poisson regression models. Biometrics. 1983;39:665–674. [PubMed] [Google Scholar]
- 50.Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bairey Merz CN, Johnson BD, Berga SL, et al. Total estrogen time and obstructive coronary disease in women: insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) J Womens Health (Larchmt) 2009;18:1315–1322. doi: 10.1089/jwh.2008.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Writing Group for the PEPI Trial. Effects of estrogen or estrogen/ progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 53.Darling GM, Johns JA, McCloud PI, Davis SR. Estrogen and progestin compared with simvastatin for hypercholesterolemia in postmenopausal women. N Engl J Med. 1997;337:595–601. doi: 10.1056/NEJM199708283370903. [DOI] [PubMed] [Google Scholar]
- 54.Herrington DM, Werbel BL, Riley WA, Pusser BE, Morgan TM. Individual and combined effects of estrogen/progestin therapy and lovastatin on lipids and flow-mediated vasodilation in postmenopausal women with coronary artery disease. J Am Coll Cardiol. 1999;33:2030–2037. doi: 10.1016/s0735-1097(99)00128-x. [DOI] [PubMed] [Google Scholar]
- 55.Spencer C, Crook D, Ross D, Cooper A, Whitehead M, Stevenson J. A randomised comparison of the effects of oral versus transdermal 17beta-oestradiol, each combined with sequential oral norethisterone acetate, on serum lipoprotein levels. Br J Obstet Gynaecol. 1999;106:948–953. doi: 10.1111/j.1471-0528.1999.tb08435.x. [DOI] [PubMed] [Google Scholar]
- 56.Futterman LG, Lemberg L. Lp(a) lipoprotein—an independent risk factor for coronary heart disease after menopause. Am J Crit Care. 2001;10:63–67. [PubMed] [Google Scholar]
- 57.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on endothelium-dependent vasodilation in postmenopausal women receiving estrogen. Circulation. 2001;104:1773–1778. doi: 10.1161/hc4001.097035. [DOI] [PubMed] [Google Scholar]
- 58.Stork S, von Schacky C, Angerer P. The effect of 17beta-estradiol on endothelial and inflammatory markers in postmenopausal women: a randomized, controlled trial. Atherosclerosis. 2002;165:301–307. doi: 10.1016/s0021-9150(02)00242-3. [DOI] [PubMed] [Google Scholar]
- 59.Guzic-Salobir B, Keber I, Seljeflot I, Arnesen H, Vrabic L. Combined hormone replacement therapy improves endothelial function in healthy postmenopausal women. J Intern Med. 2001;250:508–515. doi: 10.1046/j.1365-2796.2001.00910.x. [DOI] [PubMed] [Google Scholar]
- 60.Seljeflot I, Arnesen H, Hofstad AE, Os I. Reduced expression of endothelial cell markers after long-term transdermal hormone replacement therapy in women with coronary artery disease. Thromb Haemost. 2000;83:944–948. [PubMed] [Google Scholar]
- 61.McCubbin JA, Helfer SG, Switzer FS, 3rd, Price TM. Blood pressure control and hormone replacement therapy in postmenopausal women at risk for coronary heart disease. Am Heart J. 2002;143:711–717. doi: 10.1067/mhj.2002.121262. [DOI] [PubMed] [Google Scholar]
- 62.Angerer P, Stork S, von Schacky C. Influence of 17beta-oestradiol on blood pressure of postmenopausal women at high vascular risk. J Hypertens. 2001;19:2135–2142. doi: 10.1097/00004872-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Scuteri A, Bos AJ, Brant LJ, Talbot L, Lakatta EG, Fleg JL. Hormone replacement therapy and longitudinal changes in blood pressure in postmenopausal women. Ann Intern Med. 2001;135:229–238. doi: 10.7326/0003-4819-135-4-200108210-00007. [DOI] [PubMed] [Google Scholar]
- 64.Telci A, Cakatay U, Akhan SE, Bilgin ME, Turfanda A, Sivas A. Postmenopausal hormone replacement therapy use decreases oxidative protein damage. Gynecol Obstet Invest. 2002;54:88–93. doi: 10.1159/000067718. [DOI] [PubMed] [Google Scholar]
- 65.Yen CH, Hsieh CC, Chou SY, Lau YT. 17Beta-estradiol inhibits oxidized low density lipoprotein-induced generation of reactive oxygen species in endothelial cells. Life Sci. 2001;70:403–413. doi: 10.1016/s0024-3205(01)01486-2. [DOI] [PubMed] [Google Scholar]
- 66.Bhavnani BR, Cecutti A, Gerulath A, Woolever AC, Berco M. Comparison of the antioxidant effects of equine estrogens, red wine components, vitamin E, and probucol on low-density lipoprotein oxidation in postmenopausal women. Menopause. 2001;8:408–419. doi: 10.1097/00042192-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Arteaga E, Rojas A, Villaseca P, Bianchi M. The effect of 17beta-estradiol and alpha-tocopherol on the oxidation of LDL cholesterol from postmenopausal women and the minor effect of gamma-tocopherol and melatonin. Menopause. 2000;7:112–116. [PubMed] [Google Scholar]
- 68.Hermenegildo C, Garcia-Martinez MC, Tarin JJ, Cano A. Estradiol reduces F2alpha-isoprostane production in cultured human endothelial cells. Am J Physiol Heart Circ Physiol. 2002;283:H2644–H2649. doi: 10.1152/ajpheart.00369.2002. [DOI] [PubMed] [Google Scholar]
- 69.Luyer MD, Khosla S, Owen WG, Miller VM. Prospective randomized study of effects of unopposed estrogen replacement therapy on markers of coagulation and inflammation in postmenopausal women. J Clin Endocrinol Metab. 2001;86:3629–3634. doi: 10.1210/jcem.86.8.7768. [DOI] [PubMed] [Google Scholar]
- 70.Brussaard HE, Leuven JA, Krans HM, Kluft C. The effect of 17 beta-oestradiol on variables of coagulation and fibrinolysis in postmenopausal women with type 2 diabetes mellitus. Vascul Pharmacol. 2002;39:141–147. doi: 10.1016/s1537-1891(02)00303-8. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, Howard BV, Cowan LD, et al. Associations of postmenopausal hormone therapy with markers of hemostasis and inflammation and lipid profiles in diabetic and nondiabetic American Indian women: The Strong Heart Study. J Womens Health (Larchmt) 2004;13:155–163. doi: 10.1089/154099904322966137. [DOI] [PubMed] [Google Scholar]
- 72.Vehkavaara S, Silveira A, Hakala-Ala-Pietila T, et al. Effects of oral and transdermal estrogen replacement therapy on markers of coagulation, fibrinolysis, inflammation and serum lipids and lipoproteins in postmenopausal women. Thromb Haemost. 2001;85:619–625. [PubMed] [Google Scholar]
- 73.Collins P, Flather M, Lees B, Mister R, Proudler AJ, Stevenson JC. Randomized trial of effects of continuous combined HRT on markers of lipids and coagulation in women with acute coronary syndromes: WHISP Pilot Study. Eur Heart J. 2006;27:2046–2053. doi: 10.1093/eurheartj/ehl183. [DOI] [PubMed] [Google Scholar]
- 74.Hoibraaten E, Qvigstad E, Andersen TO, Mowinckel MC, Sandset PM. The effects of hormone replacement therapy (HRT) on hemostatic variables in women with previous venous thromboembolism—results from a randomized, double-blind, clinical trial. Thromb Haemost. 2001;85:775–781. [PubMed] [Google Scholar]
- 75.Scarabin PY, Oger E, Plu-Bureau G. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428–432. doi: 10.1016/S0140-6736(03)14066-4. [DOI] [PubMed] [Google Scholar]
- 76.Canonico M, Oger E, Plu-Bureau G, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens, The ESTHER study. Circulation. 2007;115:840–845. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 77.Hu P, Greendale GA, Palla SL, et al. The effects of hormone therapy on the markers of inflammation and endothelial function and plasma matrix metalloproteinase-9 level in postmenopausal women: the postmenopausal estrogen progestin intervention (PEPI) trial. Atherosclerosis. 2006;185:347–352. doi: 10.1016/j.atherosclerosis.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 78.Lewandowski KC, Komorowski J, Mikhalidis DP, et al. Effects of hormone replacement therapy type & route of administration on plasma matrix metalloproteinases and their tissue inhibitors in postmenopausal women. J Clin Endocrinol Metab. 2006;91:3123–3130. doi: 10.1210/jc.2005-2789. [DOI] [PubMed] [Google Scholar]
- 79.Suzuki T, Sullivan DA. Estrogen stimulation of proinflammatory cytokine and matrix metalloproteinase gene expression in human corneal epithelial cells. Cornea. 2005;24:1004–1009. doi: 10.1097/01.ico.0000160973.04072.a5. [DOI] [PubMed] [Google Scholar]
- 80.Wingrove CS, Garr E, Godsland IF, Stevenson JC. 17beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophys Acta. 1998;1406:169–174. doi: 10.1016/s0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 81.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. No abstract available. Erratum in: N Engl J Med. 2010;363:1092. [DOI] [PubMed] [Google Scholar]