Abstract
The uterosacral ligaments (USLs) are key support structures of the uterus and upper vagina. Previously, we have shown that HOXA11 is necessary for the development of the USLs, is deficient in women with pelvic organ prolapse (POP) and regulates expression of extracellular matrix (ECM) proteins. Here we sought to determine if HOXA11 regulates cell proliferation in the USLs in women. Like others, we have found that, there is decreased cellularity in prolapsed USLs compared to USLs in women with normal pelvic support. We have also demonstrated that HOXA11 promotes cell proliferation in murine fibroblasts and primary human USL cells in vitro. These findings support a relationship between HOXA11 expression, rates of proliferation and phenotypic abnormalities in the USL. Based on these findings, we sought to determine if HOXA11 regulates p53, a tumor suppressor gene which controls progression through the cell cycle and regulates ECM genes. We have demonstrated that expression of HOXA11 represses expression of p53, suggesting a mechanism by which HOXA11 regulates of the morphology and integrity of the USLs. A better understanding of the influence of these genes on the homeostasis of the ECM and interactions with each other may prove beneficial in defining the underlying etiologies of the development of POP and aid in the development of new treatment options for women with this disorder.
Keywords: HOXA11, pelvic organ prolapse, uterosacral ligament, proliferation, p53 gene
INTRODUCTION
The uterosacral ligaments (USLs) are comprised of collagen, smooth muscle, elastin, and nerve bundles.1–3 They act as key support structures of the uterus and upper vagina.4 In pelvic organ prolapse (POP), the USLs are often attenuated, leading to descent of the pelvic organs.3 Although POP is a prevalent disorder, affecting an ever-increasing number of women, little is known about the molecular mechanisms involved in the development of POP.5–10
There are alterations in the smooth muscle content and extracellular matrix (ECM) metabolism as well as increased apoptosis and decreased cellularity in the USLs of women with POP.3,8–11 Biomechanical studies have also shown decreased strength of prolapsed USLs compared to normal controls.12
Homeobox genes (HOX) are evolutionarily conserved genes encoding transcription factors that regulate mammalian embryonic growth.13 The HOXA genes regulate the development of the urogenital tract.13 Previously, we have shown that HOXA11 is responsible for the development of the USLs and regulates the expression of collagen type III and matrix metalloproteinase 2 (MMP2).14 Additionally, we have shown that the expression of HOXA11 is dramatically decreased in the USLs of women with POP.
The primary aim of this study was to determine whether HOXA11 regulates cell proliferation in the USLs in women. Like others, we have found that there is decreased cellularity and less smooth muscle content in prolapsed USLs compared to USLs in women with normal pelvic support.11,15,16 Here, we have also shown that HOXA11 promotes cell proliferation, suggesting that decreased HOXA11 expression in the USLs in women with POP leads to decreased cell proliferation. Based on these findings, our secondary aim was to determine whether HOXA11 regulates p53, a tumor suppressor gene, which controls progression through the cell cycle. We have demonstrated that the expression of HOXA11 decreases the expression of p53, which may be a potential mechanism by which HOXA11 maintains the integrity of the USLs.
METHODS
Acquisition of Human Tissue
All experiments were performed with the approval of the Yale Human Investigation Committee. Specimens were collected from 22 women undergoing hysterectomy for benign indications. Prior to surgery, a pelvic examination was performed to evaluate for the presence of POP. Uter-ovaginal prolapse was graded according to the POP quantification system advocated by the International Continence Society.17 Women with stage II POP or greater were assigned to the POP group. Women with no evidence of POP were assigned to the control group. Data regarding age, menopausal status, and parity were recorded.
At the time of surgery, 3 to 5 mm samples of the USL were taken from the proximal ligament at its insertion into the cervix, where the ligament is consistently identifiable. Specimens were promptly placed in 10% formalin for immunohistochemistry. A portion from a specimen obtained from a patient with normal support was placed into a serum-free Dulbecco’s modified Eagle’s medium for digestion and propagation of a primary cell culture.
Immunohistochemistry
Formalin-fixed specimens were embedded in paraffin and sectioned into 5-μm thickness and fixed to glass slides. Slides of each specimen were processed as previously described.13 In brief, the slides were incubated overnight at 4°C with antibodies specific for either HOXA11 (Abcam, Inc, Cambridge, MA) in a 1:250 concentration or α-smooth muscle actin (Santa Cruz Biotechnology, Santa Cruz, CA) in a 1:500 concentration. The slides were then washed and incubated in ABC Elite (Vector Laboratories, Burlingame, CA), followed by 3,3-diaminobenzidine (Vector Laboratories). Slides receiving ABC Elite with rabbit and mouse immunoglobulin G (IgG) as the primary antibody were used as negative controls.
Photographs of serial sections of the entire slide for each specimen were taken at a magnification of ×40 under the same lighting conditions. Images were digitally analyzed using the AxioVision Version 4.6 digital image processing software (Carl Zeiss MicroImaging GmBH, Germany). Using the digital analysis program, 22 slides were evaluated for cell count: 6 premenopausal control and POP specimens, 4 premenopausal control specimens and 6 postmenopausal POP specimens were evaluated. Areas of vascular smooth muscle were excluded from analysis. The nuclei were counted in each area staining positive for α-smooth muscle actin and separately in the connective tissue for each field. The nuclear counts were then averaged in both the smooth muscle and connective tissue compartments. HOXA11 expression was determined by counting the number of positively stained nuclei per 100 cells in each field.
Cell Culture
A primary cell culture was generated by digesting tissue and 2.5% collagenase and .1% DNAse I. The primary cultures and NIH/3T3 mouse fibroblast cells (ATCC, Manassas, VA) were cultured at 37°C in 90% Dulbecco’s modified Eagle’s medium with 4-mmol/L L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate and 4.5 g/L glucose and 10% fetal calf serum. Medium was changed every 3 to 4 days and cells were passaged at 70% confluence.
Bromodeoxyuridine Proliferation Assay
Both the NIH 3T3 cells and the primary USL cells were seeded in 6 well plates at a concentration of 1 × 105 cells and incubated overnight to approximately 70% confluence. They were then transfected with a pTriEX-4 vector with a cytomegalovirus promoter constitutively expressing HOXA11 (human cells) and Hoxa11 (mouse cell line) or with an empty vector using Mirus LT1 transit according to the manufacturers protocol (Novagen, North America). The vectors with the HOXA11 inserts were a generous gift from the Gunter Wagner Laboratory (Yale University). A total of 20 μL of bromodeoxyuridine (BrdU) was added to each well and incubated for 2 hours at 37°C according to the manufacturer’s protocol (BD Biosciences Pharminogen, San Diego, CA). Bromodeoxyuridine was not added to the wells that were used as negative controls. The medium was removed from the wells, and the wells were washed with phosphate-buffered saline (PBS). The cells were fixed and permeabilized by adding 100 μL of prewarmed fixation buffer to each well and incubating for 20 minutes at room temperature (RT). Cells were washed twice with 100 mL of Perm-Wash buffer then treated with 100 μL of CytoPerm Plus for 10 minutes at RT, followed by cytofix/cytoperm solution for 5 minutes at RT. The fetal bovine serum (FBS) was removed and the DNA in the cells was denatured by adding 50 μL of the sterile DNase I solution to each well and incubated for 1 hour at 37°C. The DNase I solution was removed from the wells and washed once with 100 μL of perm-wash buffer. The cells were washed twice with perm-wash buffer then blocked with 100 μL of 5% milk in PBS and incubated for 1 hour at RT. The cells were stained with 50 μL of the antibody solution and incubated for 1 hour at RT followed by the addition of BrdU conjugated to fluorescein isothiocyanate (FITC) for 1 hour at RT. The wells were washed twice in 100 μL of PBS. The nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI) solution. Slides were evaluated under ×60 magnification. Each well was divided into 4 quadrants. Cell counts were performed in each quadrant. The percentage of positive BrdU stained cells were counted per 100 cells in each area. Data were analyzed using the 2-proportion z test.
Effect of Expression of HOXA11 on Regulation of p53
NIH3T3 cells and USL cells were seeded at 1 × 105 cells in 6 well plates and transfected with the HOXA11 vector and the empty vector as described above. After 24 hours of transfection, total RNA was isolated with TRIzol reagent according to the manufacturer’s protocol (Invitrogen Corporation, Carlsbad, CA). The optical density (OD) of the RNA was measured using SmartSpex 300 (Bio Rad, Hercules, CA) at 260 nm to determine concentration. The purity of RNA specimens was assessed using the OD 260:280 ratios. One microgram of the total RNA was reverse transcribed using the Eppendorf Mastercycler (Eppendorf of North America) and the BioRad iScript complementary DNA (cDNA) synthesis kit. The reaction mix was incubated for 5 minutes at 25°C, 30 minutes at 42°C, and 5 minutes at 85°C according to the manufacturer’s protocol. The expression of p53 was evaluated using quantitative real-time polymerase chain reaction (PCR) with the following cycling conditions: preincubation at 95°C for 3 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds, annealing at 60°C for 20 seconds, and extension at 72°C for 25 seconds. The melting curve of each sample was routinely determined. All specimens were run in duplicate. The primer sequences for the p53 were as follows: forward: 5′-TGAAACGCCGACCTATCCTTA-3′ and reverse: 5′-GGCACAAACACGAACCTCAAA-3′. The primer sequences for the β-actin were as follows: forward 5′-AGAGGGAAATCGTGCGTGAC-3′ and reverse 5′-CAATAGTGATGACCTGGCCGT-3.′ The formula 2−ΔCt was used to compare relative values of RNA.
STATISTICS
Statistical analysis was performed with Excel software (Microsoft Office 2003). The results of the cell counts and relative messenger RNA (mRNA) expression of p53 are expressed as mean ± SEM. Data between the groups were compared using the unpaired Student t test. The HOXA11 expression and proliferation data using BrdU assays are reported as the percentage of cells expressing HOXA11 and percentage of proliferating cells incorporating intranuclear BrdU in each ×60 field evaluated. Data were analyzed using the 2-proportion z test. A P value less than .05 was considered statistically significant for all data analyzed.
RESULTS
Cell counts of all slides revealed an overall lower cellularity in the prolapsed USLs compared to control USLs in both the premenopausal (4.4 ± 0.93 vs. 9.7 ± 2.2 nuclei per 1000 μm2, P < .001) and postmenopausal groups (4.7 ± 2.2 vs. 10.8 ± 1.7 nuclei per 1000 μm2, P < .001). This difference persisted in both the smooth muscle (3.42 ± 0.84 vs.7.13 ± 2.14 nuclei per 1000 μm2 for the premenopausal group, P < .001; 3.8 ± 2.0 vs. 8.72 ± 2.0 nuclei per 1000 μm2 for the postmenopausal group) and connective tissue components (0.96 ± 0.32 vs. 2.25 ± 0.99 for the premenopausal group, P < .001; 1.0 ± 0.43 vs. 2.1 ± 0.2 nuclei per 1000 μm2 for the postmenopausal group, P < .001; Figure 1). The percent of proliferating cells expressing HOXA11 in each 60× field was significantly decreased in the USLs compared to controls (26.2% ± 6.2% vs. 60.2% ± 10.1%, z = 2.675, CI = 99.8%, P < .01) in the premenopausal group and (16.4% ± 6.5% vs. 54.6% ± 8.1%, (z = 3.139, CI = 99.8%, P = 0.01) in the postmenopausal group (Figure 2).
Figure 1.
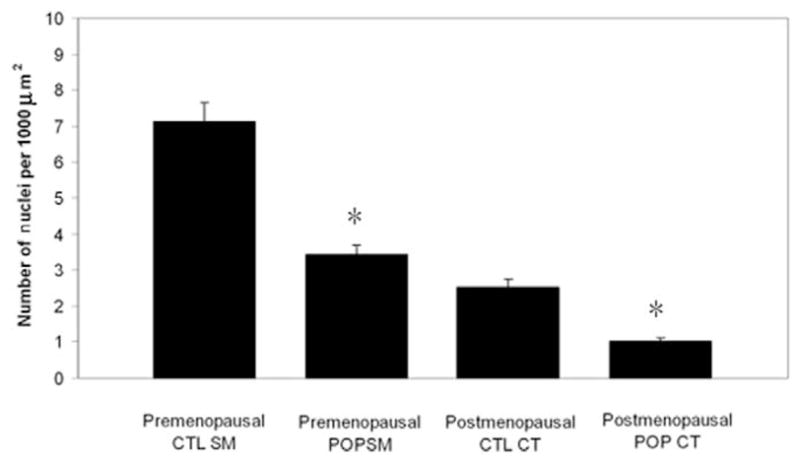
Comparison of the nuclear cell count/μm2 in the smooth muscle and connective tissue components in USLs of women with normal uterine support (controls) and women with pelvic organ prolapse. *P < .001. CTL indicates control; CT = connective tissue; POP = pelvic organ prolapse; SM = smooth muscle; USL = uterosacral ligament.
Figure 2.
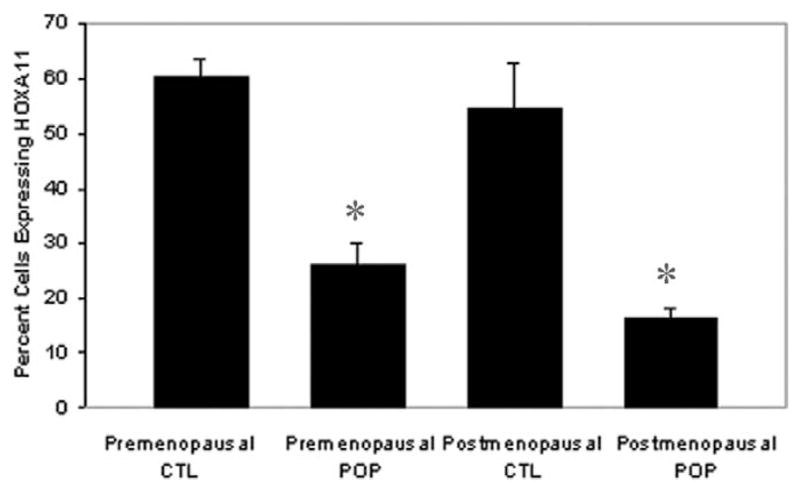
Comparison of HOXA11 expression between POP and healthy controls in both premenopausal and postmenopausal women. The y-axis represents number of cells expressing HOXA11 per 100 cells in each ×60 field. *P < .001. CTL indicates control; CT = connective tissue; POP = pelvic organ prolapse; SM = smooth muscle.
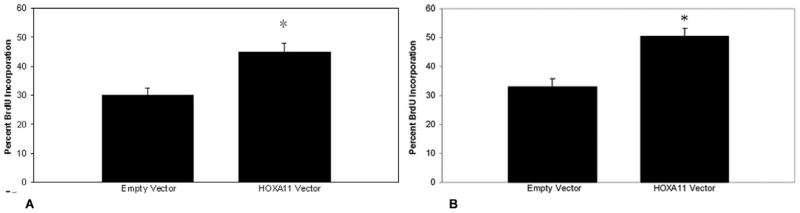
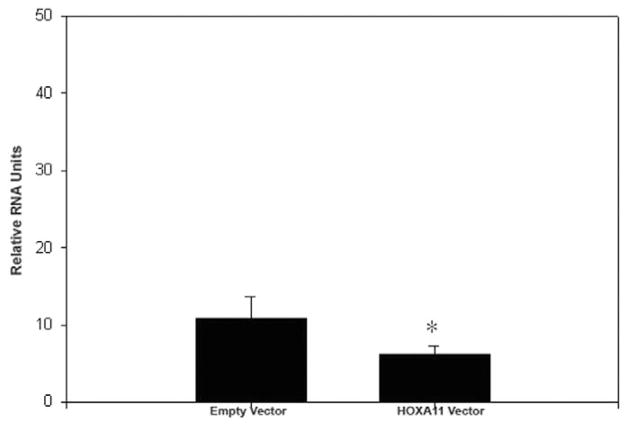
To determine whether decreased expression of HOXA11 is directly associated with decreased cellularity in the prolapse USLs, we sought to determine whether HOXA11 influences proliferation rates by constitutively expressing HOXA11 in NIH3T3 cells and primary USL cells. Bromodeoxyuridine assays showed that HOXA11 increases the proliferation rate in both the mouse and human cells by 15.4% (z = 1.687, CI = 90.8%, P < .05) and 21.2% (z = 1.88, CI = 94%, P = .03), respectively (Figures 3A, B and 4). To further explore potential mechanisms of this resultant increased cellularity, we evaluated the influence of HOXA11 on the expression of p53, which regulates the cell cycle and is intimately involved in cell proliferation and apoptosis. Constitutive expression of HOXA11 in the primary USL cells led to a decrease in the expression of p53 mRNA as determined by real-time polymerase chain reaction (PCR; P < .05; Figure 5).
Figure 3.
Comparison of proliferation rates in (A) NIH3T3 (*P < .05) and (B) USL cells (*P = .03) after constitutive expression of HOXA11. The y-axis represents the percentage of proliferating cells incorporating intranuclear BrdU in each ×60 field. BrdU indicates bromodeoxyuridine; USL = uterosacral ligament.
Figure 4.
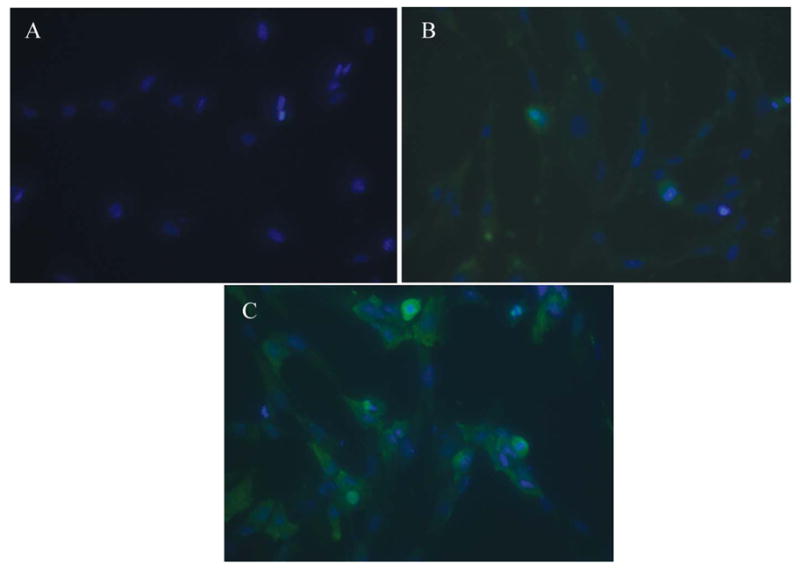
Immunohistochemistry demonstrating BrdU incorporation in USL cells after constitutive expression of HOXA11. A, Negative control (primary USL cells stained with DAPI that were not transfected with HOXA11 expression vector or empty vector). B, USL cells transfected with the empty vector, stained with DAPI and BrdU. C, USL cells transfected with the HOXA11 vector, stained with DAPI and BrdU. Blue immunofluorescence indicates DAPI staining of nuclei and green immunofluorescence indicates incorporation of intranuclear BrdU in proliferating cells. BrdU indicates bromodeoxyuridine; DAPI = 4,6-diamidino-2-phenylindole; USL = uterosacral ligament.
Figure 5.
Expression of p53 mRNA after constitutive expression of HOXA11 in USL cells. Data are means + standard error of the mean of the p53 gene expression adjusted to the internal control, β-actin. *P < .05. mRNA indicates messenger RNA; USL = uterosacral ligament.
DISCUSSION
Here, we have demonstrated decreased cellularity in the USLs of women with POP, a finding consistent with prior studies.15,16 Previously and here, we have also shown a decrease in the expression of HOXA11 in women with POP. Constitutive expression of HOXA11 led to increased proliferation of cells in vitro. These findings support a relationship between HOXA11 expression, rates of proliferation, and phenotypic abnormalities in the USL. Further exploration demonstrated that the expression of HOXA11 not only increased cell proliferation but also decreased p53 mRNA expression, suggesting a mechanism by which HOXA11 promotes cells to proliferate and may attenuate apoptosis.
Normal development and maintenance of tissues require a balance between complex processes of proliferation and cell death, both of which involve the participation of many genes.18 Hoxa11 is required for proper cellular proliferation and apoptotic responses in the developing neonatal uterus and is necessary for the development of the USLs.18,13 In both humans and mice, this gene has been shown to persist in the adult reproductive tract and is thought to play an important role in maintaining plasticity of the uterus during times of hormonal and structural changes that occur during the menstrual cycle and in pregnancy.13,19,20
The p53 gene encodes a transcription factor that regulates key cellular processes, including cell cycle arrest, DNA repair, apoptosis, and senescence in response to stress signals.21 The tightly regulated expression of p53 ensures genetic integrity of cells and prevents the proliferation of abnormal cells. p53 is maintained at low levels in the cell but becomes rapidly stabilized and activated in response to DNA damage, hypoxia, hyperproliferation, and other types of cellular stresses.21p53 is expressed during the late gap 1 (G1) phase and prevents cells from entering the synthesis (S) phase.22 The ability of cells to stop in the late G1 phase of the cell cycle is important for the maintenance of genome stability, as it prevents the replication of damaged DNA and propagation of aberrant cells.18,21,22
Recent studies suggest that the tumor suppressor p53 plays an important role in regulating ECM homeostasis.23 Expression of p53 in fibroblasts inhibits the formation of fibronectin fibrils, and inhibition of p53 expression increases fibronectin synthesis.24,25 Embryonic fibroblasts lacking p53 have increased type I collagen gene (COL1A2) promoter activity and collagen synthesis, indicating the physiological significance of cellular p53 in regulation of collagen gene expression.23 Wild-type p53 also stimulates the synthesis of MMP2.26–28 These results suggest a novel function for p53 in physiological regulation of connective tissue homeostasis. The significance and molecular mechanisms underlying p53 regulation of ECM gene expression are largely unknown.
Our results, like other authors, have shown decreased cellularity in the USLs of women with POP compared to controls, suggesting that decreased proliferation may be occurring in these deficient supportive ligaments.11,15,16 Our findings of increased proliferation rates and repression of p53 following acute upregulation of HOXA11 in murine fibroblasts and primary USL cells suggest that HOXA11 regulates a pathway involving inhibition of p53 to promote proliferation of healthy USL cells, perhaps following injury and subsequent DNA repair.
As mentioned above, p53 stimulates expression of MMP2.28 Consistent with this finding, we have previously shown that constitutive expression of HOXA11 results in decreased expression of MMP2. Whether this is a direct interaction of HOXA11 interacting at a binding site in the promoter of MMP2 directly or the indirect result by repressing p53 has yet to be determined. This may also be another mechanism by which HOXA11 promotes synthesis of structural ECM proteins in USLs.
Two studies have looked at the expression of p53 in the USL biopsy specimens and cultured primary cardinal ligament cells of women with and without POP. Bai et al demonstrated decreased expression of p53 in USL biopsy specimens from POP postmenopausal women compared to controls.29 Yamamoto et al showed that in cultured cardinal ligament cells, the growth rate during the logarithmic growth phase was not different between POP and control cells; however, the cell density at confluence (saturation density) was significantly higher in the POP cells and p53 protein and gene transcripts were decreased.30 These studies suggest that the expression of p53 is decreased in damaged prolapsed USLs with decreased cellularity in vivo as a potential compensatory mechanism and in cardinal ligament cells near confluence (which have surpassed the logarithmic growth phase) in vitro. Our findings suggest repression of p53 during acute stimulation of proliferation. Combined, these results reflect the critical importance of regulated p53 gene activity; the requirement for changes depends on the state of the cells and the need for a proliferative response during development or tissue repair.
CONCLUSION
Compared to women with normal uterine support, women with POP have USLs with decreased cellularity and decreased expression of HOXA11, which is necessary for the development and maintenance of these ligaments. Increased proliferation rates and decreased expression of p53 occur following acute upregulation of HOXA11 in murine fibroblasts and primary USL cells, suggesting that HOXA11 regulates a pathway involving inhibition of p53 allowing the proliferation of healthy USL cells following injury. A better understanding of the influence of these genes on the homeostasis of the ECM and interactions with each other may prove beneficial in defining the underlying etiologies of the development of POP and aid in the development of new treatment options for women with this disorder.
Acknowledgments
This work was supported by the NICHD Women’s Reproductive Health Research Career Development Program grant 5K12HD047018 to KAC and NIH HD 0368877, NIH ES10610 to HST.
Footnotes
For reprints and permissions queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav
References
- 1.Campbell RM. The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol. 1950;59(1):1–12. doi: 10.1016/0002-9378(50)90334-6. [DOI] [PubMed] [Google Scholar]
- 2.Cole EE, Leu PB, Gomelsky A, et al. Histopathological evaluation of the uterosacral ligament: is this a dependable structure for pelvic reconstruction? BJU Int. 2005;97(2):345–348. doi: 10.1111/j.1464-410X.2005.05903.x. [DOI] [PubMed] [Google Scholar]
- 3.Gabriel B, Denschlag D, Gobel H, et al. Uterosacral ligament in postmenopausal women with or without pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(6):475–479. doi: 10.1007/s00192-005-1294-5. [DOI] [PubMed] [Google Scholar]
- 4.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717–1724. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 5.Samuelsson EC, Victor FT, Tibblin G, Svardsudd K. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299–305. doi: 10.1016/s0002-9378(99)70203-6. [DOI] [PubMed] [Google Scholar]
- 6.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369(9566):1027–1038. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 7.Drutz H, Alarab M. Pelvic organ prolapse: demographics and future growth prospects. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S6–S9. doi: 10.1007/s00192-006-0102-1. [DOI] [PubMed] [Google Scholar]
- 8.Gabriel B, Watermann D, Hancke K, et al. Increased expression of metalloproteinase 2 in uterosacral ligaments associated with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(5):478–482. doi: 10.1007/s00192-005-0045-y. [DOI] [PubMed] [Google Scholar]
- 9.Phillips CH, Anthony F, Benyon C, Monga AK. Collagen metabolism in the uterosacral ligaments and vaginal skin of women with uterine prolapse. BJOG. 2006;113(1):39–46. doi: 10.1111/j.1471-0528.2005.00773.x. [DOI] [PubMed] [Google Scholar]
- 10.Suzme R, Yalcin O, Gurdol F, Gungor F, Bilir A. Connective tissue alterations in women with pelvic organ prolapse and urinary incontinence. Acta Obstetric Gynecol Scand. 2007;86(7):882–888. doi: 10.1080/00016340701444764. [DOI] [PubMed] [Google Scholar]
- 11.Takacs P, Nassiri M, Gualtieri M, Candiotti K, Medina C. Uterosacral ligament smooth muscle cell apoptosis is increased in women with uterine prolapse. Reprod Sci. 2008 doi: 10.1177/1933719108328611. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Reay Jones NHJ, Healy JC, King LJ, Saini S, Shousha S, Allen-Mersh TG. Pelvic connective tissue resilience decreases with vaginal delivery, menopause and uterine prolapse. Br J Surg. 2003;90(4):466–472. doi: 10.1002/bjs.4065. [DOI] [PubMed] [Google Scholar]
- 13.Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57(6):1338–1345. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- 14.Connell KA, Guess MK, Chen H, Andikyan V, Bercik R, Taylor HS. HOXA11 is critical for development and maintenance of uterosacral ligaments and deficient in pelvic prolapse. J Clin Invest. 2008;118(3):1050–1055. doi: 10.1172/JCI34193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kökçü A, Yanik F, Çetinkaya M, Alper T, Kandemir B, Malatyalioglu E. Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch Gynecol Obstet. 2002;266(2):75–78. doi: 10.1007/s004040100194. [DOI] [PubMed] [Google Scholar]
- 16.Cole EE, Leu P, Gomelsky A, et al. Histopathological evaluation of the uterosacral ligament: is this a dependable structure for pelvic reconstruction? BJU Int. 2005;97(2):345–348. doi: 10.1111/j.1464-410X.2005.05903.x. [DOI] [PubMed] [Google Scholar]
- 17.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 18.Jänicke RU, Sohn D, Schulze-Osthoff K. The dark side of a tumor suppressor: anti-apoptotic p53. Cell Death Differ. 2008;15(6):959–976. doi: 10.1038/cdd.2008.33. [DOI] [PubMed] [Google Scholar]
- 19.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101(7):1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999;84(3):1129–1135. doi: 10.1210/jcem.84.3.5573. [DOI] [PubMed] [Google Scholar]
- 21.Chumakov PM. Versatile functions of p53 protein in multicellular organisms. Biochemistry (Mosc) 2007;72(13):1399–1421. doi: 10.1134/s0006297907130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kastan MB, Zhan Q, El-Deiry WS, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh AK, Bhattacharrya S, Varga J. The tumor suppressor p53 abrogates smad-dependent collagen gene induction in mesenchymal cells. J Biol Chem. 2004;279(46):47455–47463. doi: 10.1074/jbc.M403477200. [DOI] [PubMed] [Google Scholar]
- 24.Alexandrova A, Ivanov A, Chumakov P, Kopnin B, Vasiliev J. Changes in p53 mouse fibroblasts and extracellular matrix organization. Oncogene. 2000;19(50):5826–5830. doi: 10.1038/sj.onc.1203944. [DOI] [PubMed] [Google Scholar]
- 25.Iotsova V, Stehelin D. Downregulation of fibronectin gene expression by the p53 tumor suppressor protein. Cell Growth Differ. 1996;7(5):629–634. [PubMed] [Google Scholar]
- 26.Bian J, Sun YI. Transcriptional activation by the p53 of the human type IV collagenase (gelatinase A or matrix metalloproteinase 2) promoter. Mol Cell Biol. 1997;17:6330–6338. doi: 10.1128/mcb.17.11.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Cheung JM, Martel-Pelletier J, et al. Wild type and mutant p53 differentially regulate the gene expression of human collagenase-3 (hMMP-13) J Biol Chem. 2000;275(15):11327–11332. doi: 10.1074/jbc.275.15.11327. [DOI] [PubMed] [Google Scholar]
- 28.Ala-aho R, Grenman R, Seth P, Kahari V-M. Adenoviral delivery of p53 gene suppresses expression of collagenase-3 (MMP13) in squamous carcinoma cells. Oncogene. 2002;21:1187–1195. doi: 10.1038/sj.onc.1205198. [DOI] [PubMed] [Google Scholar]
- 29.Bai SW, Chung DJ, Yoon JM, Shin JS, Kim SK, Park KH. Roles of estrogen receptor, progesterone receptor, p53 and p21 in pathogenesis of pelvic organ prolapse. Int Urogynecol J. 2005;16:492–496. doi: 10.1007/s00192-005-1310-9. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M, Aoyagi M, Akazawa K, Tajima S, Yamamoto K. Decrease in p53 protein in cultured cardinal ligament fibroblasts from patients with prolapsus uteri. Cell Bio Int. 1998;22(1):31–40. doi: 10.1006/cbir.1997.0207. [DOI] [PubMed] [Google Scholar]