Abstract
Study objective
To evaluate the effect of uterine leiomyomas on the endometrium using molecular markers of endometrial receptivity: HOXA10, HOXA11, LIF, and BTEB1.
Design
Case-control study
Setting
University medical center
Patients
Thirty reproductive-age women with submucosal, intramural, or no uterine myomas who underwent hysteroscopy or hysterectomy.
Interventions
Proliferative phase endometrial sampling was performed at the time of surgery. In uteri with a submucosal myoma, directed endometrial biopsies were obtained over the myoma and over normal myometrium.
Main outcome measures
Endometrial HOXA10 expression was evaluated as a primary end point using quantitative real time RT-PCR and immunohistochemistry. HOXA11, BTEB1, and LIF were evaluated using real time RT-PCR.
Results
Endometrial HOXA10 and HOXA11 mRNA expression were significantly decreased in uteri with submucosal myomas compared to controls and to uteri with intramural myomas. A similar trend was seen in BTEB1 mRNA expression, however no difference was found in LIF mRNA expression. Immunohistochemistry localized the decrease in endometrial HOXA10 protein expression to stroma. In the presence of a submucosal myoma, there were no regional differences in gene expression.
Conclusions
The molecular mechanism by which submucosal myomas adversely affect reproduction includes a global decrease in endometrial HOX gene expression, not simply a focal change over the myoma. This may explain the reproductive dysfunction observed with submucosal myomas.
Keywords: leiomyoma, fibroid, submucosal myoma, endometrium, endometrial receptivity, HOXA10
INTRODUCTION
Uterine leiomyomas are the most common benign tumor in women of reproductive age, affecting 20–50% of this population (1–5). Myomas are present in approximately 5–10% of women with infertility, and are the sole factor identified in 1–2.4% (1, 6, 7). Depending on location in the uterus, myomas have been implicated in both recurrent pregnancy loss and infertility (1, 2).
Submucosal myomas and intramural myomas that distort the endometrial cavity are associated with lower pregnancy, implantation, and delivery rates in women undergoing in vitro fertilization (IVF) compared to infertile women without myomas (6, 8, 9). Furthermore, there is an increased risk of infertility if the endometrial cavity is distorted by a submucous myoma (10, 11). Reproductive outcomes improve after myomectomy for a submucosal myoma, and the difference is more pronounced if the myoma was the only identifiable etiology of infertility (8, 10–13). Currently there are no molecular data to explain the mechanism behind these clinical observations. It is plausible that myomas adversely affect the overlying endometrium and hence impair endometrial receptivity, however little is known about the effect of myomas on known markers of endometrial receptivity.
HOXA10/Hoxa10 (human/mouse) is a homeobox-containing transcription factor that is essential for embryonic uterine development, and is necessary for proper adult endometrial development during each menstrual cycle (14–18). HOXA10 expression is necessary for endometrial receptivity (16, 18–21). Targeted mutation of Hoxa10 renders mice infertile due to implantation failure: they produce viable embryos, and these embryos implant and develop normally in a wild-type surrogate, however wild-type embryos fail to implant in Hoxa10 (−/−) mice (22). This phenotype is likely related to both the absence of Hoxa10 during embryonic uterine development, and lack of adult maternal Hoxa10 expression during cyclic endometrial development. Reduction of maternal Hoxa10 expression in mice using Hoxa10 antisense results in diminished implantation proportional to the level of Hoxa10 expression, indicating that altered levels of this protein regulate the degree of endometrial receptivity (20).
In the midluteal phase at the time of implantation, HOXA10 mRNA expression is up-regulated in both endometrial glandular and stromal cells in women (16, 23, 24). Endometrial HOXA10 expression in the stroma stays relatively constant throughout the menstrual cycle, while the glands are the location of the midsecretory increase in HOXA10 expression (23). Estrogen and progesterone each upregulate HOXA10 expression (16). HOXA10 has diverse effects on several aspects of adult endometrial development such as stromal decidualization, leukocyte infiltration, and pinopod development (19, 25). Furthermore HOXA10 regulates downstream target genes that are also involved in implantation such as β3 integrin, EMX2, and IGFBP-1, and BTEB1 (26–28). Defective endometrial HOXA10 expression has been described in association with endometriosis, polycystic ovary syndrome (PCOS), and hydrosalpinges, all conditions associated with abnormal implantation (17, 24, 29, 30). HOXA10 is a well-characterized marker of endometrial receptivity (15, 16).
Similar to HOXA10, the spatial and temporal pattern of HOXA11 expression in human endometrium suggests a role in endometrial development and implantation (31, 32). Moderate levels of HOXA11 are present throughout the menstrual cycle, and gene expression is markedly upregulated in the midsecretory phase at the time of implantation (31, 32). Estrogen and progesterone upregulate HOXA11 gene expression (31). Targeted disruption of Hoxa11 in mice results in uterine factor infertility, as well as a decrease in endometrial glandular development and LIF expression (33–35).
BTEB1 (basic transcriptional element binding protein 1) is an endometrial transcription factor which may play a role in regulation of endometrial cell growth by modulating gene transcription (36). This Krüppel-like family member gene directly interacts with the progesterone receptor (PR-B) to mediate progesterone-responsive gene expression in endometrial cells (37–39). Targeted mutation in BTEB1 has been shown to result in subfertility, uterine hypoplasia, and partial progesterone resistance (40). In human endometrial stromal and epithelial cell lines, HOXA10 downregulates BTEB1 (37). This regulation of BTEB1 by HOXA10 may lead to cyclic alterations in endometrial responsiveness to progesterone (37).
LIF (leukemia inhibitory factor) expression is essential for embryo-endometrium interaction and for blastocyst implantation in mice and humans (34, 41). In murine models, mice null for the Lif gene produce normal embryos which develop to blastocyst stage but do not implant; these Lif-null embryos successfully implant in wild-type mice (42, 43). Thus the implantation defect is maternal in origin, however the precise role of maternal LIF in implantation is unclear (34, 41). In humans, LIF mRNA and protein levels are low in the proliferative phase, and expression markedly increases in the secretory phase at the time of implantation (44–47).
We investigated the effect of uterine myomas on several markers of endometrial receptivity: HOXA10, HOXA11, BTEB1, and LIF. We studied the effect of submucosal and intramural myomas on endometrial receptivity, and determined the presence of either a focal or global endometrial abnormality.
MATERIALS AND METHODS
This case-control study included 30 subjects: 14 with submucosal myomas, 9 with intramural myomas and no distortion of the uterine cavity, and 7 without myomas. Subjects were identified prior to surgery, and all subjects underwent hysteroscopy (n=25) or hysterectomy (n=5). The 7 control subjects underwent hysteroscopy for assessment of the uterine cavity prior to fertility treatment. At the time of surgery, the following data were obtained: age, obstetric and gynecologic history, medical conditions, medications, surgical history, last menstrual period, preoperative and postoperative diagnoses, and operative findings. Subjects had not used hormonal medications for at least 3 months prior to surgery, and had regular menstrual periods. Subjects did not have any other condition previously demonstrated to affect endometrial receptivity such as endometriosis, PCOS, or hydrosalpinges.
All subjects underwent gynecologic procedures during the proliferative phase of the menstrual cycle; surgery was performed within 10 days of the onset of menses. When a submucosal leiomyoma was present, directed endometrial biopsies were obtained over the myoma and over normal myometrium in the same uterus. In subjects without a submucosal myoma, a sample of endometrium was obtained from the curettage specimen. The endometrial sample was then divided for pathology and laboratory evaluation. The study was approved by the Yale University School of Medicine Human Investigation Committee.
Real time reverse transcriptase-polymerase chain reaction
Endometrial expression of HOXA10, HOXA11, BTEB1, and LIF were evaluated using quantitative real time RT-PCR. Endometrial tissue was obtained at the time of surgery, and was immediately placed in RNAlater (Ambion, Austin, TX) and stored at − 80 C. To obtain total RNA, each sample was placed in 1 mL of TRIzol Reagent (Invitrogen, Carlsbad, CA) and homogenized. The cellular lysate was incubated, chloroform 0.2 mL was added, and the samples were centrifuged. The clear, aqueous phase was transferred to a fresh tube and RNA was precipitated twice with 75% ethanol. The RNA pellet was air-dried, then resuspended with RNase-free water. The samples were quantified at a 1:100 dilution, then stored at −20ºC until real time RT-PCR was performed.
Messenger RNA levels were analyzed by quantitative real-time RT-PCR using the Bio-Rad iCycler iQ system (Bio-Rad Laboratories, Hercules, CA). Messenger RNA was reverse transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories). Real-time RT-PCR was performed using the iQ SYBR Green Supermix kit (Bio-Rad Laboratories). Reaction conditions included cDNA template, each primer, water, and the iQ SYBR Green Supermix, for a final reaction volume of 25 μL. The sequences of all primers are identified in Table 1. The HOXA10 and BTEB1 real time RT-PCR reactions were performed for 1 cycle at 95 C for 3 minutes, then 40 cycles at 95 C for 15 seconds and 60 C for 20 seconds. HOXA11 amplification was performed for 40 cycles at 95 C for 15 seconds, 62 C for 20 seconds, and 72 C for 25 seconds. LIF amplification was performed for 1 cycle at 50 C for 2 minutes, 1 cycle at 95 C for 10 minutes, then 45 cycles at 95 C for 15 seconds and 60 C for 1 minute. Melting curve data were collected and analyzed. Each assay was run in duplicate with each set of primers, and samples without mRNA were included as negative controls. The mRNA level of each sample was normalized to that of the β-actin mRNA level. Relative mRNA level was presented as 2− ΔCt, and ΔCt for each sample refers to the difference between the average of the 2 study primer cycling times and the 2 β-actin cycling times.
Table 1.
Real-time RT-PCR primer sequences
| Gene | Primer sequence | Amplicon length |
|---|---|---|
| HOXA10 | Sense 5’-AGGTGGACGCTGCGGCTAATCTCTA-3’ | 25bp |
| Anti-sense 5’-GCCCCTTCCGAGAGCAGCAAAG-3’ | 22bp | |
| β-actin | Sense 5’-CGTACCACTGGCATCGTGAT-3’ | 20bp |
| Anti-sense 5’-GTGTTGGCGTACAGGTCTTTG-3’ | 21bp | |
| HOXA11 | Sense 5’-GTACTTACTACGTCTCGGGTCCAG-3’ | 24bp |
| Anti-sense 3’-AGTCTCTGTGCACGAGCTCCT-3’ | 21bp | |
| LIF | Sense 5’-TGGTTCTGCACTGGAAACATG-3’ | 21bp |
| Anti-sense 5’-TGTAATAGAGAATAAAGAGGGCATTGG-3’ | 27bp | |
| BTEB | Sense 5’-ACAGTCGCTGTGGGAAAGTC-3’ | 20bp |
| Anti-sense 5’-AACTGCTTTTCCCCAGTGTG-3’ | 20bp |
Endometrial levels of HOXA10, HOXA11, BTEB1, and LIF mRNA expression were analyzed. Endometrial gene expression was compared using the Kruskal-Wallis One Way Analysis of Variance on Ranks with Dunn’s Method for pairwise multiple comparisons among the submucosal myoma, intramural myoma, and control groups, and the Wilcoxon Signed Rank Test for direct comparisons between two groups.
Immunohistochemistry
Endometrial HOXA10 protein expression was evaluated with immunohistochemistry. Endometrial tissue was fixed in formalin, embedded in paraffin, cut into 5-μm sections, and mounted onto slides. Endometrial dating was confirmed based on histology (48).
Slides were deparaffinized and dehydrated through a series of xylene and ethanol washes, followed by permeabilization in 95% cold ethanol. After a 5 minute rinse in distilled water, an antigen-presenting step was performed by steaming the slides in 0.01M sodium citrate buffer for 20 minutes, followed by cooling for 20 minutes. Slides were rinsed for 5 minutes in PBS with 0.1% Tween 20 (PBST), and sections were circumscribed with a hydrophobic pen. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 5 minutes followed by a 5 minute PBST wash. Nonspecific binding was blocked with 1.5% normal horse serum in PBST for 1 hour at room temperature. Slides were incubated in the primary antibody overnight at 4ºC. HOXA10 antibody (sc-17159) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Normal goat IgG (Santa Cruz Biotechnology) was used as a negative control for the HOXA10 antibody.
Biotinylated secondary antibodies were obtained from Vector Laboratories (Burlingame, CA). Horse α-goat secondary antibody was applied for 1 hour at room temperature. Slides were washed in PBST, incubated in ABC Elite (Vector Laboratories) for 15 minutes at room temperature, washed in PBST, and incubated for 5 minutes in diaminobenzidine (Vector Laboratories). A 15 second exposure to hematoxylin was used as a counterstain. Slides were rehydrated through 3 minute ethanol and xylene washes and mounted with Permount. All slides were processed simultaneously.
The HOXA10 immunohistochemistry results were quantified by two blinded evaluators. Each evaluator inspected 4 high-powered fields on each slide to determine the H-SCORE for the glandular cells and stromal cells. The H-SCORE was calculated with the following equation: H-SCORE = Σ Pi (i + 1). Intensity of HOXA10 nuclear staining is indicated by a value of 0, 1, 2, or 3 (none, weak, moderate, or strong respectively), and Pi is the percentage of stained nuclei for each intensity, ranging from 0 – 100% (49, 50). For each slide, the two H-SCORE results for glandular cells were averaged; the same process was employed for stromal cells. The glandular and stromal cell H-SCORES for the 3 study groups were compared using the Kruskal-Wallis One Way Analysis of Variance on Ranks with Dunn’s Method for pairwise multiple comparisons among the submucosal myoma, intramural myoma, and control groups, and the Mann-Whitney Rank Sum Test for direct comparisons between two groups.
RESULTS
The average subject age was 39.4 years (range 31 to 48 years). Of 23 subjects with myomas, 6 had a single myoma, 5 had myomas greater than 5 cm, and the average number of myomas was 3.2 (range 1-8). Among subjects with myomas, there were no significant differences in number of myomas or myoma size between the submucosal and intramural myoma groups. Between the 3 study groups, there were no differences in mean age, fertility history, or other medical conditions.
All samples underwent histologic evaluation, and normal proliferative endometrium was identified. Endometrium from subjects with submucosal myomas, with intramural myomas, and without myomas was evaluated for HOXA10 mRNA and protein expression, and mRNA expression of HOXA11, BTEB1, and LIF.
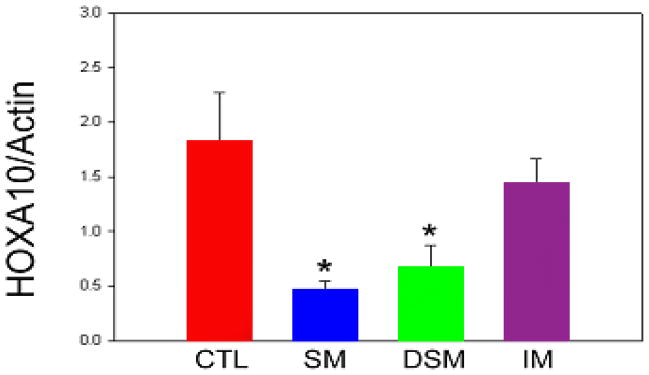
Compared to controls, endometrial HOXA10 mRNA expression (normalized to ß-Actin expression) was significantly decreased in biopsies from directly over the submucosal myoma (1.82 and 0.67 respectively, p<0.001) and from over normal myometrium remote from the submucosal myoma (0.47, p<0.001) (Figure 1). Furthermore, within a uterine cavity with a submucosal myoma, no differences in low levels of endometrial HOXA10 mRNA expression were identified between biopsies from over the myoma and remote from the myoma. Similarly, compared to endometrium from uteri with intramural myomas, significantly decreased endometrial HOXA10 mRNA expression was noted in biopsies from over the submucosal myoma (1.44 and 0.67 respectively, p<0.001) and from over normal myometrium remote from the submucosal myoma (0.47, p<0.001). Conversely, no difference in HOXA10 mRNA expression was noted in endometrium from normal uteri (controls) compared to endometrium from uteri with intramural myomas (Figure 1).
Figure 1.
Mean endometrial HOXA10 mRNA expression (normalized to β-actin). Endometrium was sampled from uteri without myomas (CTL), uteri with submucosal myomas (SM = biopsy from endometrium over normal myometrium, DSM = directed biopsy from endometrium overlying submucosal myoma), and uteri with intramural myomas (IM). * p < 0.05 compared to intramural myoma and control groups
Immunohistochemistry further evaluated HOXA10 expression in the endometrial glands and stroma, and confirmed and localized the real-time RT-PCR findings (Table 2). Representative slides are presented in Figure 2. There were no significant differences in glandular HOXA10 expression between the 3 groups. Compared to controls (H-SCORE 2.37), stromal HOXA10 expression was significantly decreased in biopsies from directly over a submucosal myoma (H-SCORE 1.90, p<0.05). Similarly, stromal HOXA10 expression in endometrium remote from a submucosal myoma was significantly decreased compared to controls (H-SCORE 1.58, p<0.05). Compared to the intramural myoma group (H-SCORE 2.20), stromal HOXA10 expression was significantly decreased in biopsies from over a submucosal myoma (H-SCORE 1.90, p<0.05). Furthermore, stromal HOXA10 expression in endometrium remote from a submucosal myoma was significantly decreased compared to the intramural myoma group (H-SCORE 1.58, p<0.05). There were no differences in stromal HOXA10 expression within a uterine cavity with a submucosal myoma. Additionally, there were no differences in stromal HOXA10 expression between the control and intramural myoma groups.
Table 2.
Mean H-SCORE results
| SM | IM | CTL | |
|---|---|---|---|
| n = 9 | n = 8 | n = 7 | |
| Glands | 0.32 | 0.20 | 0.47 |
| Stroma | 1.90* | 2.20 | 2.37 |
p < 0.05 compared to IM or CTL groups
Figure 2.
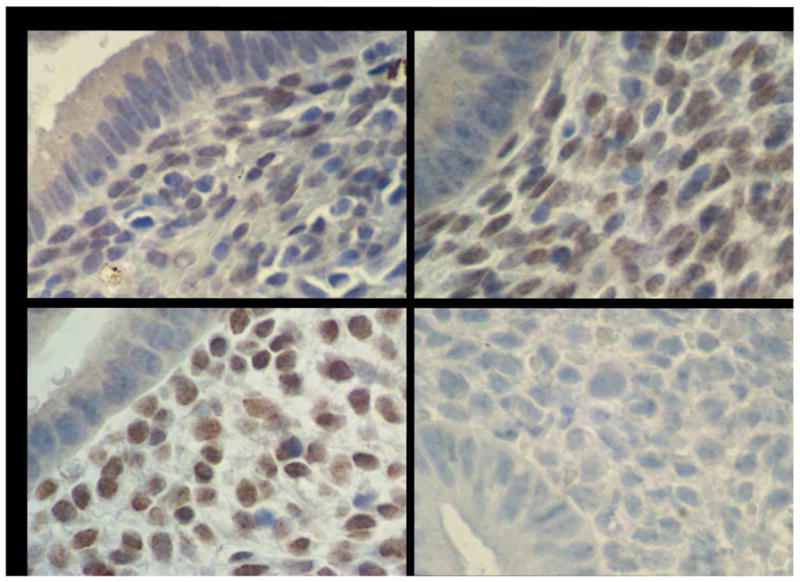
Endometrial HOXA10 Immunohistochemistry
Immunohistochemistry identified HOXA10 protein expression in endometrial glands and stroma. H-SCOREs were determined separately for the glands and stroma. Shown are representative photomicrographs demonstrating HOXA10 expression in endometrium in the setting of: (A) Submucosal myoma, (B) Intramural myoma, (C) No myomas, (D) HOXA10 negative control omitting primary antibody.
Endometrial HOXA11 mRNA expression (normalized to ß-Actin expression) was significantly different between uteri with submucosal myomas and uteri with intramural myomas (0.17 and 0.52 respectively, p < 0.001), and between uteri with submucosal myomas and controls (0.17 and 0.41 respectively, p = 0.011) (Figure 3A). Analysis of endometrial BTEB1 mRNA expression demonstrated a similar trend between uteri with submucosal myomas and controls (0.36 and 0.08 respectively, p = 0.05) (Figure 3B). No significant difference was found in endometrial LIF mRNA expression between uteri with submucosal myomas and controls (0.33 versus 0.62, p > 0.05) (Figure 3C). There were no regional differences in endometrial HOXA11, BTEB1, or LIF expression within a uterine cavity with a submucosal myoma. Furthermore, there were no differences in endometrial HOXA11, BTEB1, or LIF expression between the control and intramural myoma groups.
Figure 3.
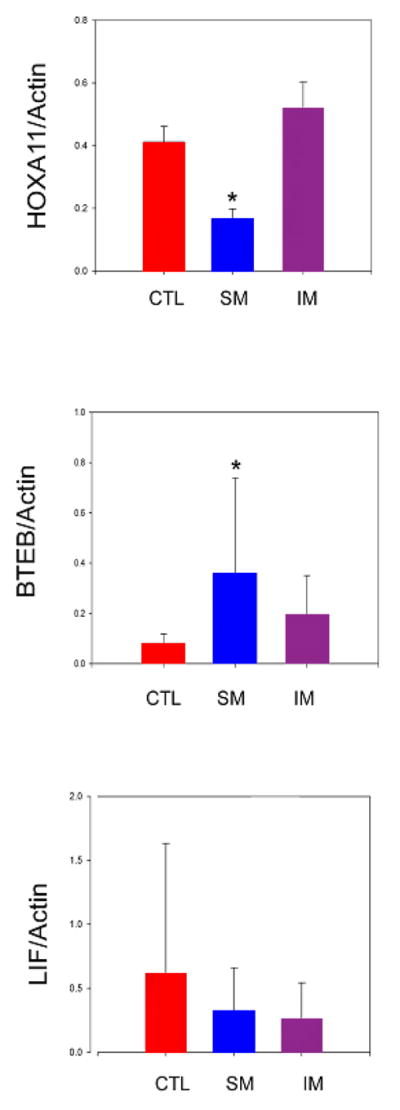
Mean endometrial HOXA11, BTEB1, and LIF mRNA expression (normalized to β-actin). Endometrium was sampled from uteri without myomas (CTL), uteri with submucosal myomas (SM), and uteri with intramural myomas (IM). * p < 0.05
DISCUSSION
Submucosal leiomyomas are associated with poor reproductive outcomes (6, 8). While defective implantation is likely due to an endometrial defect, no specific endometrial deficiency has been identified that would explain these clinical findings. In this study, histologic evaluation of endometrium from uteri with submucosal myomas revealed no consistent endometrial abnormality. This finding is consistent with recent reports demonstrating that endometrial assessment with histology is not able to reliably differentiate fertile and infertile women (51). Histology alone cannot effectively assess endometrial receptivity; molecular evaluation of the endometrium is a potential means of identifying defects in receptivity. Here we identify a molecular mechanism by which submucosal uterine myomas adversely affect reproduction. We identified a generalized alteration in key determinants of endometrial receptivity, extending throughout the entire cavity, rather than a focal effect over the submucosal myoma.
The effect of uterine myomas on the endometrium was evaluated using several established molecular markers of endometrial receptivity: HOXA10, HOXA11, and LIF, as well as BTEB1, a downstream target of HOXA10. Hoxa10, Hoxa11, and Lif have been demonstrated to be necessary for implantation in mice. Targeted disruption of any of these three genes results in sterility. The knock-out mice ovulate, but their embryos do not implant in their uterus. Wild-type embryos also do not implant in the knock-out mice, however knock-out mouse embryos implant in a wild-type uterus (22, 33, 35, 43, 52). Each targeted disruption results in an endometrial defect, not an embryo defect, in which implantation is severely altered.
Alterations in endometrial HOXA10 expression have been identified in several clinical conditions associated with impaired endometrial receptivity: endometriosis, PCOS, and hydrosalpinges. Women with endometriosis have lower implantation rates (53). In the presence of endometriosis, midsecretory endometrium demonstrates the absence of a dramatic rise in HOXA10 and HOXA11 (17). This reduction in endometrial HOXA10 expression is localized to the stroma (24). Women with PCOS also experience lower pregnancy rates and increased miscarriage rates (54, 55). In endometrial cells in vitro, testosterone suppresses HOXA10 expression and also reverses the stimulatory effect of estrogen and progesterone on HOXA10 (29). Furthermore, endometrial biopsies from ovulatory women with PCOS demonstrate decreased HOXA10 mRNA levels (29). Similarly, hydrosalpinges are associated with decreased implantation rates during in vitro fertilization, and implantation and pregnancy rates improve after salpingectomy is performed (56-58). When endometrial cells are cultured in hydrosalpinx fluid, HOXA10 mRNA levels are suppressed (30). Furthermore, endometrial HOXA10 expression has been shown to normalize after removal of the hydrosalpinges (59).
In this study, the expression of HOX10, HOXA11, and BTEB1, several of the genes that regulate endometrial receptivity, were each significantly altered in the presence of a submucosal myoma. Moreover, endometrial mRNA expression was globally affected in the presence of a submucosal myoma, rather than focally changed in the endometrium over the myoma. Furthermore, immunohistochemisty localized the decrease in endometrial HOXA10 protein expression in the presence of a submucosal myoma to the endometrial stroma; HOXA10 expression in endometrial glands was not altered. Therefore, a submucosal myoma causes global changes in endometrial receptivity through an effect on the stromal compartment.
Although intramural myomas were not associated with a significant change in these markers of endometrial receptivity, a trend toward decreased endometrial HOXA10 mRNA and stromal protein expression was noted in the intramural myoma group compared to the control group. Similarly, endometrial BTEB1 mRNA expression in uteri with intramural myomas was increased compared to the control group. If submucosal myomas have a global effect on the endometrium that impairs endometrial receptivity, it is likely mediated by a diffusible signaling molecule that originates from the myoma. The same signaling pathway from intramural myomas to the endometrium may exist, however due to the greater distance and therefore low concentration, this signaling molecule causes a less pronounced effect on endometrial receptivity compared to that seen with submucosal myomas.
Endometrial LIF mRNA expression did not differ in the submucosal myoma, intramural myoma, or control groups. This marker of endometrial receptivity is minimally expressed in the proliferative phase, and has much higher level of expression in the secretory phase. Since this study sampled endometrium in the proliferative phase, the timing may have impaired the evaluation of LIF mRNA expression. Endometrial sampling was performed during the proliferative phase because hysteroscopic visualization is optimal at this time. Further studies in the secretory phase of the menstrual cycle may identify alterations in other markers of endometrial receptivity in the presence of a submucosal myoma.
The molecular mechanism by which submucosal uterine myomas adversely affect reproduction includes a global decrease in endometrial HOX gene expression. This generalized alteration in key determinants of endometrial receptivity, rather than a focal effect over the submucosal myoma, is seen in HOXA10 and HOXA11 expression, as well as BTEB1, a downstream HOX target gene. Therefore the effect of submucosal myomas on endometrial receptivity appears to be selective, specific to targeted signal transduction pathways including those involving HOX genes. These results also imply that the mechanism by which submucosal myomas impact endometrial receptivity is not simply a local mechanical effect over the myoma, but involves a signaling mechanism to the entire endometrium. Furthermore, this widespread endometrial effect does not simply correlate with the size of the submucosal myoma. These findings suggest that submucosal myomas affect endometrial receptivity through a specific and selective molecular mechanism of action with global endometrial consequences. These results may explain the reproductive dysfunction clinically observed in women with submucosal myomas. In addition to surgical resection, the identification of the signaling molecule may provide new therapeutic targets for treatment of infertility in women with myomas.
Acknowledgments
Support: National Institutes of Health HD36887
We would like to acknowledge contributions made to this study: Ivan Peña for assistance with data analysis; Hongling Du and Xiaolin Fei for assistance with laboratory procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buttram VC, Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–45. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 2.Manyonda I, Sinthamoney E, Belli AM. Controversies and challenges in the modern management of uterine fibroids. BJOG. 2004;111:95–102. doi: 10.1046/j.1471-0528.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 3.Olive DL, Lindheim SR, Pritts EA. Non-surgical management of leiomyoma: impact on fertility. Curr Opin Obstet Gynecol. 2004;16:239–43. doi: 10.1097/00001703-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104:393–406. doi: 10.1097/01.AOG.0000136079.62513.39. [DOI] [PubMed] [Google Scholar]
- 5.Myomas and reproductive function. Fertil Steril. 2006;86:S194–9. doi: 10.1016/j.fertnstert.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for a debate? Hum Reprod. 2002;17:1424–30. doi: 10.1093/humrep/17.6.1424. [DOI] [PubMed] [Google Scholar]
- 7.Verkauf BS. Myomectomy for fertility enhancement and preservation. Fertil Steril. 1992;58:1–15. doi: 10.1016/s0015-0282(16)55128-0. [DOI] [PubMed] [Google Scholar]
- 8.Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv. 2001;56:483–91. doi: 10.1097/00006254-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil Steril. 2001;75:405–10. doi: 10.1016/s0015-0282(00)01714-3. [DOI] [PubMed] [Google Scholar]
- 10.Garcia CR, Tureck RW. Submucosal leiomyomas and infertility. Fertil Steril. 1984;42:16–9. doi: 10.1016/s0015-0282(16)47951-3. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg M, Sivan E, Sharabi Z, Bider D, Rabinovici J, Seidman DS. Outcome of hysteroscopic resection of submucous myomas for infertility. Fertil Steril. 1995;64:714–6. doi: 10.1016/s0015-0282(16)57844-3. [DOI] [PubMed] [Google Scholar]
- 12.Vercellini P, Zaina B, Yaylayan L, Pisacreta A, De Giorgi O, Crosignani PG. Hysteroscopic myomectomy: long-term effects on menstrual pattern and fertility. Obstet Gynecol. 1999;94:341–7. doi: 10.1016/s0029-7844(99)00346-4. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez H, Sefrioui O, Virelizier C, Gervaise A, Gomel V, Frydman R. Hysteroscopic resection of submucosal myomas in patients with infertility. Hum Reprod. 2001;16:1489–92. doi: 10.1093/humrep/16.7.1489. [DOI] [PubMed] [Google Scholar]
- 14.Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. Faseb J. 2000;14:1101–8. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- 15.Taylor HS. The role of HOX genes in human implantation. Hum Reprod Update. 2000;6:75–9. doi: 10.1093/humupd/6.1.75. [DOI] [PubMed] [Google Scholar]
- 16.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101:1379–84. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–31. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- 18.Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57:1338–45. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- 19.Bagot CN, Kliman HJ, Taylor HS. Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev Dyn. 2001;222:538–44. doi: 10.1002/dvdy.1209. [DOI] [PubMed] [Google Scholar]
- 20.Bagot CN, Troy PJ, Taylor HS. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther. 2000;7:1378–84. doi: 10.1038/sj.gt.3301245. [DOI] [PubMed] [Google Scholar]
- 21.Taylor HS, Daftary GS, Selam B. Endometrial HOXA10 expression after controlled ovarian hyperstimulation with recombinant follicle-stimulating hormone. Fertil Steril. 2003;80 (Suppl 2):839–43. doi: 10.1016/s0015-0282(03)00985-3. [DOI] [PubMed] [Google Scholar]
- 22.Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–3. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- 23.Sarno JL, Kliman HJ, Taylor HS. HOXA10, Pbx2, and Meis1 protein expression in the human endometrium: formation of multimeric complexes on HOXA10 target genes. J Clin Endocrinol Metab. 2005;90:522–8. doi: 10.1210/jc.2004-0817. [DOI] [PubMed] [Google Scholar]
- 24.Gui Y, Zhang J, Yuan L, Lessey BA. Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol Hum Reprod. 1999;5:866–73. doi: 10.1093/molehr/5.9.866. [DOI] [PubMed] [Google Scholar]
- 25.Daftary GS, Taylor HS. Pleiotropic effects of Hoxa10 on the functional development of peri-implantation endometrium. Mol Reprod Dev. 2004;67:8–14. doi: 10.1002/mrd.20013. [DOI] [PubMed] [Google Scholar]
- 26.Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 2002;16:571–9. doi: 10.1210/mend.16.3.0792. [DOI] [PubMed] [Google Scholar]
- 27.Kim JJ, Buzzio OL, Li S, Lu Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod. 2005;73:833–9. doi: 10.1095/biolreprod.105.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troy PJ, Daftary GS, Bagot CN, Taylor HS. Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10. Mol Cell Biol. 2003;23:1–13. doi: 10.1128/MCB.23.1.1-13.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cermik D, Selam B, Taylor HS. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:238–43. doi: 10.1210/jc.2002-021072. [DOI] [PubMed] [Google Scholar]
- 30.Daftary GS, Taylor HS. Hydrosalpinx fluid diminishes endometrial cell HOXA10 expression. Fertil Steril. 2002;78:577–80. doi: 10.1016/s0015-0282(02)03306-x. [DOI] [PubMed] [Google Scholar]
- 31.Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999;84:1129–35. doi: 10.1210/jcem.84.3.5573. [DOI] [PubMed] [Google Scholar]
- 32.Wang LF, Luo HZ, Zhu ZM, Wang JD. Expression of HOXA11 gene in human endometrium. Am J Obstet Gynecol. 2004;191:767–72. doi: 10.1016/j.ajog.2004.02.069. [DOI] [PubMed] [Google Scholar]
- 33.Gendron RL, Paradis H, Hsieh-Li HM, Lee DW, Potter SS, Markoff E. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in Hoxa-11 null mice. Biol Reprod. 1997;56:1097–105. doi: 10.1095/biolreprod56.5.1097. [DOI] [PubMed] [Google Scholar]
- 34.Kimber SJ. Leukaemia inhibitory factor in implantation and uterine biology. Reproduction. 2005;130:131–45. doi: 10.1530/rep.1.00304. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K, et al. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995;121:1373–85. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XL, Simmen FA, Michel FJ, Simmen RC. Increased expression of the Zn-finger transcription factor BTEB1 in human endometrial cells is correlated with distinct cell phenotype, gene expression patterns, and proliferative responsiveness to serum and TGF-beta1. Mol Cell Endocrinol. 2001;181:81–96. doi: 10.1016/s0303-7207(01)00536-6. [DOI] [PubMed] [Google Scholar]
- 37.Du H, Sarno JL, Taylor HS. Basic transcription element binding protein (BTEB1) is regulated by HOXA10 in endometrial cells. J Soc Gynecol Investig. 2006;13:71A–2A. [Google Scholar]
- 38.Zhang D, Zhang XL, Michel FJ, Blum JL, Simmen FA, Simmen RC. Direct interaction of the Kruppel-like family (KLF) member, BTEB1, and PR mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology. 2002;143:62–73. doi: 10.1210/endo.143.1.8590. [DOI] [PubMed] [Google Scholar]
- 39.Simmen RC, Chung TE, Imataka H, Michel FJ, Badinga L, Simmen FA. Trans-activation functions of the Sp-related nuclear factor, basic transcription element-binding protein, and progesterone receptor in endometrial epithelial cells. Endocrinology. 1999;140:2517–25. doi: 10.1210/endo.140.6.6625. [DOI] [PubMed] [Google Scholar]
- 40.Simmen RC, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman L, Jr, et al. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem. 2004;279:29286–94. doi: 10.1074/jbc.M403139200. [DOI] [PubMed] [Google Scholar]
- 41.Cavagna M, Mantese JC. Biomarkers of endometrial receptivity--a review. Placenta. 2003;24 (Suppl B):S39–47. doi: 10.1016/s0143-4004(03)00184-x. [DOI] [PubMed] [Google Scholar]
- 42.Cheng JG, Rodriguez CI, Stewart CL. Control of uterine receptivity and embryo implantation by steroid hormone regulation of LIF production and LIF receptor activity: towards a molecular understanding of "the window of implantation". Rev Endocr Metab Disord. 2002;3:119–26. doi: 10.1023/a:1015402811650. [DOI] [PubMed] [Google Scholar]
- 43.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–9. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 44.Arici A, Engin O, Attar E, Olive DL. Modulation of leukemia inhibitory factor gene expression and protein biosynthesis in human endometrium. J Clin Endocrinol Metab. 1995;80:1908–15. doi: 10.1210/jcem.80.6.7775640. [DOI] [PubMed] [Google Scholar]
- 45.Charnock-Jones DS, Sharkey AM, Fenwick P, Smith SK. Leukaemia inhibitory factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at this time. J Reprod Fertil. 1994;101:421–6. doi: 10.1530/jrf.0.1010421. [DOI] [PubMed] [Google Scholar]
- 46.Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci U S A. 1996;93:3115–20. doi: 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senturk LM, Arici A. Leukemia inhibitory factor in human reproduction. Am J Reprod Immunol. 1998;39:144–51. doi: 10.1111/j.1600-0897.1998.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 48.Ferenczy A. Anatomy and histology of the uterine corpus. In: Kurman RJ, editor. Blaustein's pathology of the female genital tract. New York: Springer-Verlag; 1994. pp. 327–66. [Google Scholar]
- 49.Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643–9. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 50.Sharpe-Timms KL, Ricke EA, Piva M, Horowitz GM. Differential expression and localization of de-novo synthesized endometriotic haptoglobin in endometrium and endometriotic lesions. Hum Reprod. 2000;15:2180–5. doi: 10.1093/humrep/15.10.2180. [DOI] [PubMed] [Google Scholar]
- 51.Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–72. doi: 10.1016/j.fertnstert.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 52.Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–96. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 53.Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–55. doi: 10.1016/s0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- 54.Balen AH, Tan SL, MacDougall J, Jacobs HS. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum Reprod. 1993;8:959–64. doi: 10.1093/oxfordjournals.humrep.a138174. [DOI] [PubMed] [Google Scholar]
- 55.Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of chances to conceive in ovulatory patients during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab. 1999;84:1617–22. doi: 10.1210/jcem.84.5.5705. [DOI] [PubMed] [Google Scholar]
- 56.Strandell A, Waldenstrom U, Nilsson L, Hamberger L. Hydrosalpinx reduces in-vitro fertilization/embryo transfer pregnancy rates. Hum Reprod. 1994;9:861–3. doi: 10.1093/oxfordjournals.humrep.a138606. [DOI] [PubMed] [Google Scholar]
- 57.Zeyneloglu HB, Arici A, Olive DL. Adverse effects of hydrosalpinx on pregnancy rates after in vitro fertilization-embryo transfer. Fertil Steril. 1998;70:492–9. doi: 10.1016/s0015-0282(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 58.Strandell A, Lindhard A. Why does hydrosalpinx reduce fertility? The importance of hydrosalpinx fluid. Hum Reprod. 2002;17:1141–5. doi: 10.1093/humrep/17.5.1141. [DOI] [PubMed] [Google Scholar]
- 59.Daftary GS, Kayisli U, Seli E, Bukulmez O, Arici A, Taylor HS. Salpingectomy increases peri-implantation endometrial HOXA10 expression in women with hydrosalpinx. Fertil Steril. 2007;87:367–72. doi: 10.1016/j.fertnstert.2006.06.041. [DOI] [PubMed] [Google Scholar]