Abstract
This review describes the results from X-ray absorption spectroscopy studies that have contributed to an understanding of the role of Ca in the photosynthetic water oxidation reaction. The results include the first Mn, Ca and Sr X-ray spectroscopy studies using Ca or Sr-substituted PS II samples that established the presence of a MnCa heteronuclear structure and its orientation, and the most recent Sr X-ray spectroscopy study using biosynthetically prepared Sr-containing PS II in the various S-states that provide important insights into the requirement for Ca in the mechanism of the Mn4Ca catalytic center.
Keywords: manganese, calcium, oxygen evolving complex, photosystem II, X-ray absorption spectroscopy
I. Introduction
Photosynthetic water oxidation is a fundamental chemical reaction that sustains the biosphere. It takes place at a catalytic Mn4Ca site within the oxygen-evolving complex (OEC) of photosystem II (PS II), which is embedded in the thylakoid membranes of green plants, cyanobacteria and algae [1]. Life-supporting oxygen, currently present at 20% in Earth's atmosphere, is a consequence of this process by oxygenic photosynthetic organisms that are able to use sunlight as the energy source and water as the primary reductant for the fixation of carbon. Single-electron photo-oxidations of a specialized chlorophyll molecules in the reaction center in PS II are coupled to the four-electron oxidation of water by the OEC in PS II. The OEC cycles through five states labeled S0-S4, where absorption of light in the reaction center drives transitions through the S0-S4 sequence, and S4 decays spontaneously to S0 with the release of dioxygen [2].
Calcium is an essential co-factor in oxygen evolution (recently reviewed in [3]). Most commonly, treatments with high NaCl concentrations (1.2 M) or with a low pH (3.0)/citrate incubation have been employed to deplete calcium from PS II. These treatments produce preparations in which oxygen evolution is inhibited, but which can be reactivated by the addition of Ca2+. Partial reactivation of oxygen evolution in inhibited preparations can also be achieved by addition of Sr2+ [4], and there is one study that reports that vanadyl ion (VO2+) can also activate oxygen evolution [5]. Although many other metal ions have been shown to compete with Ca2+ for binding sites in PS II (Na+, K+, Cd2+, various lanthanides), none of them results in reactivation of oxygen evolution activity [3]. A recent study has probed the requirement of Ca for all the S-state advances and reported that with the exception of the S0 to S1 transition, Ca is required for all the other S-state transitions [6]. Addition of Sr2+ to calcium-depleted preparations has been shown to reactivate the same number of centers as Ca2+, but with slower turnover in the S-state cycle, producing a lower overall rate of oxygen evolution at saturating light intensities [7].
Reactivation with Sr2+ produces a slightly altered manganese complex, as evidenced by changes in the S2-state multiline EPR signal [7]. The multiline signal produced in Sr2+-reactivated preparations has a narrower average line spacing (~71 G vs. ~88 G in untreated preparations) and a different pattern of intensities compared to the normal multiline signal from untreated PS II preparations. ESEEM (electron spin echo envelope modulation) spectroscopy using 87Sr has shown the proximity of Sr to the Mn cluster [8]. Furthermore, a 113Cd-NMR study showed that Ca2+ is close enough to the Mn4-cluster to be affected by its spin [9].
X-ray diffraction (XRD) studies at 2.9 - 3.8 Å resolution have located the Mn4Ca cluster in the electron-density map and confirmed the presence of Ca in the OEC cluster [10-14]. Prior to XRD studies the presence of Ca in the OEC was detected using Ca and Sr X-ray spectroscopy (XAS) studies [15-19]. Mn-, Ca- and Sr-EXAFS (extended X-ray absorption fine structure) studies of PS II frozen solutions have provided accurate distances (~0.02 Å) and information on the numbers of Mn-Mn, Mn-Ca and Mn/Ca-ligand vectors in the Mn4Ca cluster in all the S-states [20-23]. XAS experiments require a significantly lower X-ray dose than XRD measurements [24]. The onset of radiation damage can be precisely determined and controlled by monitoring the Mn K-edge position, thus allowing us to collect data from the intact Mn4Ca cluster of PS II.
X-ray Absorption Spectroscopy
X-ray Absorption Spectroscopy (XAS) is an excellent tool for examining the immediate structural environment of metal ions in proteins. XAS has the advantage of being element-specific and does not require a crystalline sample; thus, it has proved to be a useful technique for probing the structure of the Mn complex of PS II where many components (non-heme iron, cytochrome, etc.) normally present in active preparations can interfere with other spectroscopic techniques. X-ray absorption edges can be examined to determine the oxidation states and site symmetry of the Mn, and EXAFS gives information about the radial distribution of atoms around the Mn atoms of PS II. In addition to distances, EXAFS is sensitive to the numbers and atomic number of atoms around the absorbing atom (for reviews of XAS in biological systems and photosynthesis see [25-28]).
X-ray spectroscopy has been used to investigate the structure of the Mn4Ca complex and the mechanism of the water oxidation reaction (reviewed in [20,29,30]). Several different variations of XAS has been used to probe the role of Ca. Broadly they can be categorized as probing the Ca site using I) Mn XAS of PS II samples that contain Ca, Sr or depleted of Ca, II) Ca or Sr XAS on PS II samples that contain only the functional Ca or Sr (1Ca or 1Sr per 4Mn). These samples can be prepared either by replacing Ca by Sr, or biosynthetically grown using Sr instead of Ca, III) polarized XAS techniques at the Mn or Ca or Sr K-edges to deterimine the orientaion of the Ca/Sr-Mn vectors in relation to the membrane normal, and IV) the investigation of the structural changes in the Ca/Sr-Mn/O vectors as a function of the S-state changes that shows the involvement of Ca in the mechanism of photosynthetic water oxidation.
A comparison of the Mn EXAFS of Ca-containing, Sr-substituted and Ca-depleted PS II samples was the first study in 1995 that established the presence of a multinuclear MnCa complex in the OEC of PS II; such a mixed Ca-transition metal complex in biology had no precedence. This study [15,16], and the following Ca/Sr/Mn XAS studies, determined the structure [17,18] and orientation of the Ca [19,22] in the complex and the structural changes during the S-state transitions [23] from the perspective of Ca. The focus of this review is the role of Ca as determined by the use of X-ray spectroscopy and the emphasis is on the results from our laboratory.
II. Mn X-ray Absorption Spectroscopy
a) Mn XAS and Comparison of Ca and Sr-Substituted PS II
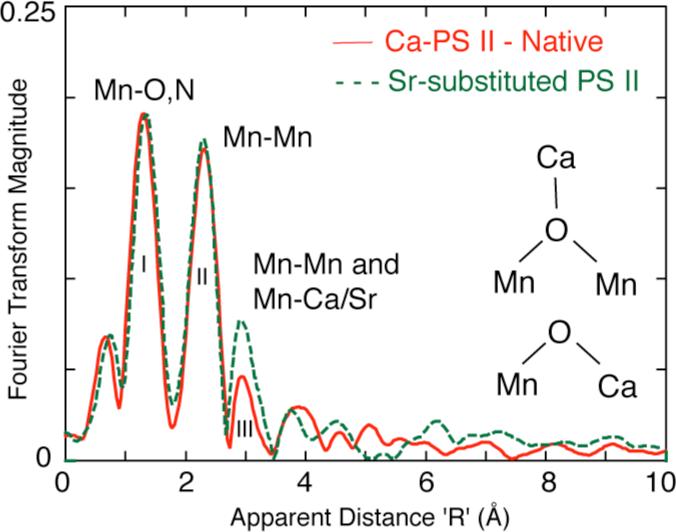
The first study that established the presence of a MnCa heteronuclear cluster involved removal of Ca and substitution of various metals into the Ca binding site, followed by EXAFS studies on the Mn cluster. One set of experiments from our laboratory, using Mn EXAFS on Sr-reactivated PS II membranes, was interpreted to indicate a 3.4-3.5 Å distance between the Ca (Sr) and the Mn cluster [15]. This conclusion was based on the observation of increased amplitude in Fourier peak III at 3.3 Å (Fig. 1) upon replacement of Ca with Sr, a heavier atom and better X-ray scatterer. Such a short Mn-Sr/Ca distance was interpreted as indicating a direct Mn-O-Ca bridged structure in the OEC. Analysis of EXAFS spectra from purified PS II membrane preparations indicated a Mn–Ca interaction at slightly longer distance (~3.6-3.7 Å) [31]. Ca depletion by NaCl-washing of PS II membranes removed the 16 and 23 kDa extrinsic proteins and led to a reduced amplitude for this 3.6 Å feature and because of the lower X-ray scattering ability of Na, this result was interpreted as possible Na+ substitution for Ca2+ at this distance [32]. Another Mn EXAFS study [33] did not detect any changes in the Fourier peak at 3.3 Å when Ca was replaced with Sr2+ or Dy3+ in PS II reaction center complexes lacking the 16 and 23 kDa extrinsic polypeptides; however, it was proposed that Ca might be linked via a hydrogen bond to a OH-/H2O ligand of the Mn cluster.
Figure 1.
Three major Fourier peaks are seen in the Mn EXAFS of Ca and Sr containing PS II samples [15]. The first peak is assigned to Mn-O,N ligands. The second FT peak is assigned to Mn-Mn distances. A third FT peak fits best to Mn-Mn and Mn-Ca/Sr distances The spectra are clearly different in the amplitude of the third peak, which isenhanced in the spectrum from the Sr-reactivated sample, as this peak consists of both Mn-Mn and Mn-Ca or Sr contributions. The insets shows the Ca oxo-bridging motifs to Mn that are compatible with the Mn-Ca and Mn-Sr distances of 3.3 and 3.4 Å respectively.
b) Mn XAS and Effect of Ca Depletion
The structural consequences of calcium depletion of PS II by treatment at pH 3.0 in the presence of citrate has been determined by Mn XAS [16]. X-ray absorption edge spectroscopy of Ca-depleted samples in the S1', S2', and S3' (S-states in Ca-depleted PS II; S3' was later determined to be S2YZ•) oxidation states reveals that there is Mn oxidation on the S1' to S2' transition, although no evidence of Mn oxidation was found for the S2' to S3' (i.e. S2YZ•) transition. This result is in keeping with the results from EPR studies where it has been found that the species oxidized to give the S3' (i.e. S2YZ•) broad radical signal found in Ca-depleted PS II is tyrosine Yz. The S2' state can be prepared by two methods; illumination followed by dark adaptation, and illumination in the presence of DCMU to limit to one turnover. Illumination followed by dark adaptation was found to yield a lower Mn K-edge inflection-point energy than illumination with DCMU, indicating vulnerability to reduction of the Mn complex even over the relatively short times used for dark adaptation (~15 minutes). EXAFS measurements of Ca-depleted samples in the three modified S-states (referred to as S'-states) reveals that the Fourier peak due to scatterers at ~3.3 Å from Mn, although strongly diminished retains some amplitude, consistent with assignments of a Ca-scattering contribution at this distance, and supporting conclusions from studies that the third peak (FT peak III) in native samples is comprised of both Mn and Ca scattering. The Mn-Mn contributions making up the second Fourier peak at ~2.7 Å are largely undisturbed by Ca-depletion.
Thus, while removal of the Ca2+ ion from the OEC does not lead to fundamental distortion or rearrangement of the Mn cluster indicating that the Ca2+ in the OEC is not essential for structural maintenance of the cluster, but fulfills a crucial function in the catalytic mechanism of the water oxidation reaction. This structural information provides important insights into the ligation schemes of the Ca to the Mn centers and mechanism for catalysis at the Mn4Ca cluster.
III. Ca or Sr X-ray Absorption Spectroscopy
a) Sr XAS
The most common approach, as described above [15,33], was to substitute other metals (such as Sr) for Ca and then use Mn EXAFS to detect changes in the cluster. Isolating the Mn-Ca or Sr component of the EXAFS spectrum from the combined EXAFS from all Mn-ligand and Mn-Mn interactions can be difficult. The reverse experiment where one probes for backscattering from Mn using Ca or Sr EXAFS (Ca/Sr cofactor point-of-view for nearby Mn) is an elegant alternative and is more definitive than Mn EXAFS results. Such studies on both isotropic and oriented PS II membranes have yielded unequivocal evidence for the proximity and mode of binding of Ca to the Mn cluster.
Several factors favor Sr as the better cofactor for XAS study. First, the X-ray energies involved (16 keV for the K-edge) are more penetrating and not attenuated by air. The higher X-ray absorption cross-section and fluorescence yield of Sr also make the experiment facile. The Sr EXAFS-based experiment requires PS II samples with Sr substituted for Ca while maintaining oxygen-evolving activity and a stoichiometry of 1 Sr per PS II, to focus on the functional cofactor binding site. Along with reactivated Sr-PSII, an inactivated sample can be prepared by treating with hydroxylamine (NH2OH) to disrupt the Mn cluster and suppress water oxidase activity [34].
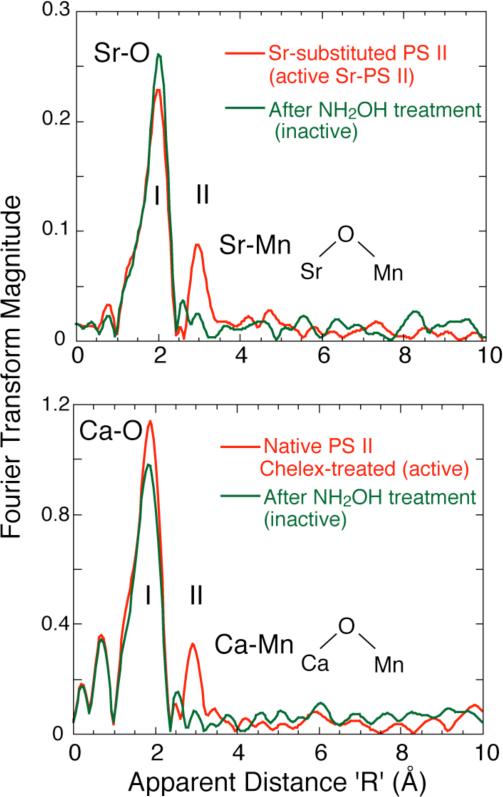
By using Sr EXAFS on isotropic Sr-reactivated PS II membranes, the proximity of Sr (and implicitly Ca) to within 3.5 Å of the Mn cluster [17] has been confirmed. The finding was based on the presence of a second Fourier peak (peak II, Fig. 2 Top) in the Sr EXAFS from functional samples, a peak that is absent from inactive, hydroxylamine-treated PS II. This Fourier peak was found to fit best to at least two Mn at ~3.5 Å rather than lighter atoms (C, O, P, S, Cl). Both types of samples share similar first coordination shells of oxygen (Peak I, Fig. 2 Top). The mode of binding of Sr to Mn must be via single-atom bridging O atoms in order for Sr to be within ~3.5 Å of Mn.
Figure 2.
Top. Fourier transforms of Sr EXAFS for intact and inactive Sr-substituted PS II samples (Chelex-treated). The dominant Fourier Peak I is due to ligating oxygens in the first coordination sphere and is common to both samples. The two Sr-PS II samples differ mainly at the R’ = 3.0 Å region, where the intact samples exhibit Fourier Peak II, which is from Sr-Mn vectors. Disruption of the Mn cluster in NH2OH-treated sample leads to the absence of Peak II. Bottom. Fourier Transform of Ca EXAFS from Chelex-treated, layered samples with 2 Ca/PS II with O2-evolving activity and an S2 EPR multiline signal. The FTs show the presence of a second Fourier peak in the 2Ca/PS II sample that fits to Ca-Mn that is absent in the control sample where the Mn complex has been disrupted with NH2OH.
b) Ca XAS
In a complementary and definitive experiment, Ca K-edge EXAFS studies have been used to probe the binding site of the native cofactor for any nearby Mn, within ~ 4 Å. The use of Ca EXAFS spectroscopy has produced results essentially congruent with those found by Sr EXAFS on Sr-reactivated PS II [17] and Mn EXAFS on similar samples [15], but it focuses on the native cofactor and avoids the treatments involving Ca depletion and Sr substitution. Like the earlier Sr EXAFS, the Ca EXAFS study has focused on the Ca cofactor of PS II (poised in the S1 state). This technique is a direct probe of the Ca binding site in PS II and the Ca EXAFS experiment probed the Ca cofactor in as close to a native system as possible.
The FT of the Ca EXAFS is shown in Fig. 2 (Bottom) and the spectra are remarkably similar to the Fourier transforms of the earlier Sr EXAFS study with Sr substituted for Ca. The first Fourier peak I corresponds to the coordinating oxygen atoms closest to Ca. In contrast to the control (NH2OH-treated) sample, the Chelex-treated PSII shows a second Fourier peak II. When Fourier peak II is isolated and simulated with possible scattering atoms, it corresponded best to Mn at 3.4 Å, rather than to light atom (C, O or Cl) neighbours. These results were consistent with the earlier Sr EXAFS studies.
The results are summarized in a motif shown in Fig. 2 and this motif depicts the Ca/Sr linked to at least two Mn by single-O bridges, which can be supplied by protein residues or water. Only single-oxygen bridges (not bidentate bridges only, but which can be present in addition to the single-atom O bridges) can provide the required 3.4 Å distance indicated by the Ca EXAFS fitting. This single-atom O bridge may be derived from carboxylate ligands (aspartate or glutamate protein residues), phenoxy or alkoxy ligands (tyrosine or serine residues), protein backbone carboxyl, water, hydroxide or oxo groups.
It has been speculated that Ca controls substrate water binding to the catalytic Mn site [35] and recent mechanisms have suggested the crucial involvement of the cofactor [36-39]. The results of Mn EXAFS, Sr EXAFS and Ca EXAFS are mutually consistent and converge toward the conclusion that the Ca cofactor is intimately structurally linked with the Mn cluster in PS II and offer compelling evidence for the involvement of Ca in the mechanism of water oxidation.
IV. Orientation of Ca in the Mn4Ca Cluster
a) Sr XAS of Oriented PS II Samples
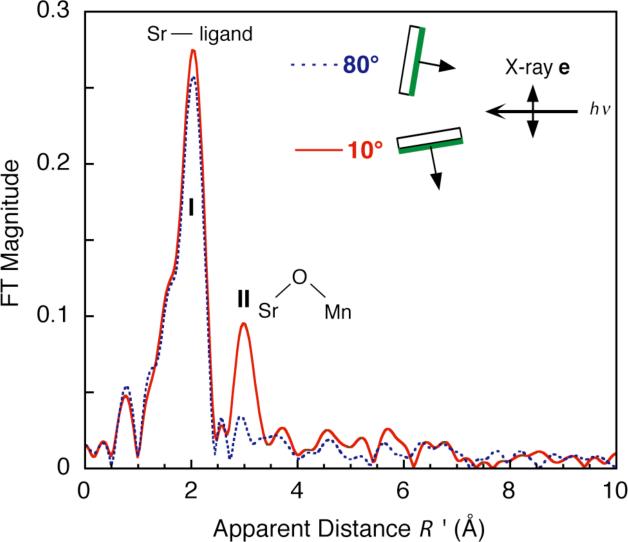
The technique of using Sr EXAFS has been extended to using polarized Sr EXAFS on layered Sr-substituted samples, to provide important angle information. Polarized EXAFS involves collecting spectra for different incident angles (θ) between the membrane normal of the layered sample and the X-ray electric field vector (Fig. 3, also see Fig. 4a). Dichroism in the EXAFS can occur, depending on how the particular absorber–backscatterer (A–B) vector is aligned with the electric field. Through analysis of the dichroism, the average orientation (φ) of this A–B vector relative to the membrane normal, and the average number of scatterers per absorbing atom (Niso) can be extracted. Constraints on the structural model are then imposed by these parameters (Fig. 4c).
Figure 3.
Fourier transform of Sr EXAFS from oriented Sr-PS II samples at two angles (θ). The dichroism is most readily apparent in Fourier peak II (R’ = 3.0 Å), where the amplitude is largest at 10° (solid line), and smallest at 80° (dashed line). The inset shows the layered PS II membranes and the underlying substrate in relation to the X-ray e-vector, which is perpendicular to the direction of propagation of X-rays. Fourier peak I is backscattering from O and peak II is backscattering from Mn.
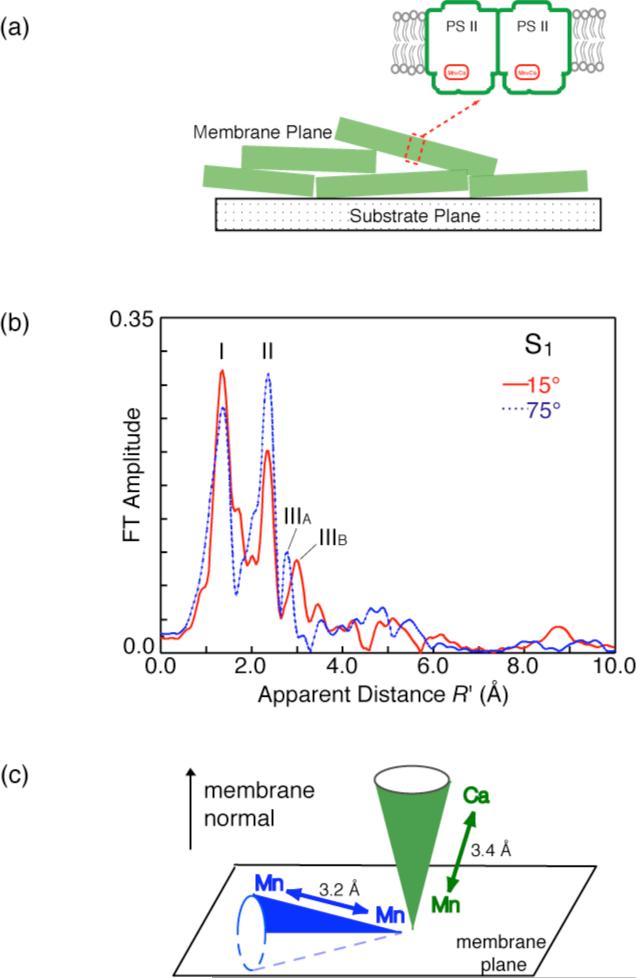
Figure 4.
a) Schematic of PS II membranes showing the orientation of the membrane plane.
b) Fourier Transform (FT) of Mn K-edge EXAFS spectra from oriented PS II membrane samples in the S1 state obtained with a high-resolution spectrometer (range-extended EXAFS). Angles indicate orientation of the membrane normal with respect to the X-ray e-field vector. FT peak IIIA and IIIB are from Mn-Mn and Mn-Ca vectors.
c) The orientation of the average Mn-Ca (~3.4 Å) and the Mn-Mn (~3.2 Å) vectors relative to the membrane normal.
Sr-substituted PS II samples made by a process of Ca depletion, Sr2+ reactivation, and Chelex treatment to remove excess Sr were layered onto flat Mylar films to produce oriented samples [40,41]. The Fourier transforms from the polarized Sr EXAFS showed extreme dichroism in Fourier peak II (Fig. 3). Nonlinear least-squares regression analysis translates to Sr–Mn vectors with an average angle of 0° to 23° between the Sr–Mn vectors and the membrane normal.
b) Mn Range-extended XAS of Oriented PS II Samples
EXAFS spectra of metallo-protein samples are normally collected as an excitation spectrum by electronically windowing the Kα emission (2p to 1s transition) from the metal atom [25-27]. The solid-state detectors used over the past decade have a resolution of 150-200 eV, making it impossible to discriminate the fluorescence of the absorbing atom (Mn in our case for PS II) from that of the following element (Fe in PS II) in the periodic table. Unfortunately, because of the presence of Fe in the PS II samples we have been limited to a k of about 12 Å-1, that places an inherent limit on the resolution of Mn distances to ~0.15 Å, because spatial resolution in EXAFS is inversely related to the spectral range. We have overcome this limitation by using a high resolution crystal monochromator [42], that has a resolution of 1 eV. Using the crystal monochromator, we were able to collect EXAFS spectra well beyond the Fe K-edge to k=16.5 Å-1, improving the distance resolution to ~0.1 Å. We have shown the feasibility of the range-extended EXAFS method and collected data without interference from Fe in the sample [21].
We can now fit two distances to the short Mn-Mn interactions in the S1 and S2 states (2 at ~2.7 Å and 1 at ~2.8 Å); while earlier solution EXAFS studies could discern only one distance of ~2.7 Å. We have also collected range-extended polarized EXAFS data using oriented PS II membranes. These studies also show the heterogeneity clearly; the Mn-Mn distance is ~2.7 Å or 2.8 Å depending on the angle the X-ray e-vector makes with the membrane normal. Interestingly, for the first time we were able to resolve the FT peak at ~3.3 Å into one Mn-Mn vector at ~3.2 Å and two Mn-Ca vectors at ~3.4 Å that are aligned at different angles to the membrane normal [43].
Fig. 4b shows the Fourier transforms (FTs) of the range-extended EXAFS for PS II in oriented membranes in the S1 state. Peaks, labeled I, II, III correspond to the shells of backscatterers from the Mn absorber. Peak I contains the Mn-O bridging and terminal interactions, peak II corresponds to the di-μ-oxo-bridged Mn-Mn moieties, and peak III has information about the mono-μ-oxo-bridged Mn-Mn and Mn-Ca interactions. Increased spectral resolution results in the detection of the orientation dependence of peaks II and III.
The range-extended EXAFS method allowed us to resolve the complex nature of the Peak III containing at least two peaks, IIIA and IIIB, having different distances of 3.2 Å and 3.4 Å. Previous Ca EXAFS studies of native PS II [18], Sr EXAFS of Sr-substituted PS II [17], and Mn EXAFS of Ca-depleted PS II [15,16] have shown the contribution of both Mn-Mn (~3.2 Å) and Mn-Ca (~3.4 Å) vectors to FT peak III. Peak IIIB can be assigned to the Mn-Ca vector and peak IIIA to the Mn-Mn vector; moreover, the dichroic behavior of the peak IIIB is strikingly similar to that reported for the Mn-Sr vector [19]. The dichroism of these peaks shows that the average Mn-Ca vector at ~3.4 Å is aligned at ~18° to the membrane normal.
c) Orientation of Ca in the Mn4 Ca Complex
The orientation data from the Sr EXAFS experiments can be combined with the dichroism data from Mn EXAFS data to calculate the orientation of the 3.3 Å Mn–Mn vector. The Fourier peak in the Mn EXAFS which contains the Mn–Mn (3.3 Å) and Mn–Ca (3.4 Å) contributions, is dichroic, with an average angle of 43 ± 10° with respect to the membrane normal [41]. By including the Mn-Ca vector at 23°, an angle of ~62° for the 3.3 Å Mn–Mn vector can be calculated. Previous polarized Mn EXAFS experiments on PS II have shown angles of 55° and 67° for the 2.7 Å Mn–Mn vectors [40,41]. Thus it follows that all Mn–Mn vectors lie at roughly the same angle (~61°) with respect to the membrane normal, but are not restricted to being collinear, because the PS II membranes are ordered in one dimension only. The electron density from the X-ray diffraction studies [10-14, 44] are in agreement with such an assignment; the plane containing the Mn electron density is at ~67° to the membrane normal, and the Mn-Ca vector is along the membrane normal.
V. Structural Changes in the Ca-Mn Distances and S-state Transitions
Biosynthetically exchanged Ca/Sr-PS II preparations and Sr and Mn XAS allowed us to monitor Mn-Mn and Ca(Sr)-Mn distances in the four intermediate S-states, S0 through S3, of the catalytic cycle [45]. The advantages over Ca/Sr exchange induced by biochemical procedures are: i) an enzyme fully competent in O2 evolution, ii) a 1:1 Sr to PS II ratio monitored by Sr quantitation, and iii) Sr K-edge EXAFS measurements (Fig. 5) that result in less X-ray damage and are preferred for experimental reasons to those at lower-energy Ca K-edge [17-19]. We have detected significant changes in the structure of the complex, especially in the Mn-Mn and Ca(Sr)-Mn distances, upon the S2 to S3 and S3 to S0 transitions [23]. These results implicate the involvement of at least one common bridging oxygen atom between the Mn-Mn and Mn-Ca(Sr) atoms in the S2 to S3 transition, and this oxo-bridge could possibly also be involved in O-O bond formation. Because PS II cannot advance beyond the S2 state in preparations that lack Ca(Sr), these results show that Ca(Sr) is one of the critical components in the mechanism of the enzyme. The results also show that Ca is not just a spectator atom involved in providing a structural framework but is actively involved in the mechanism of water oxidation and represents a rare example of a catalytically active Ca cofactor.
Figure 5.
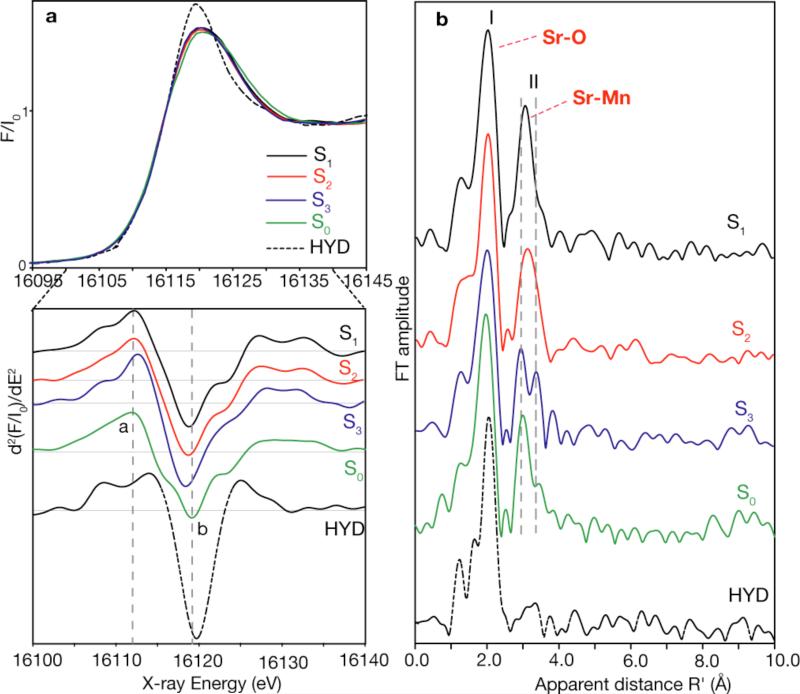
a) Sr K-edge XANES spectra of Sr-PS II from Thermosynechococcus elongatus (top), and the corresponding second derivatives of the XANES spectra (bottom) in the S1, S2, S3 and S0 states and an inactive control sample (HYD). The inflection point of the edges and the shape of the spectra are clearly different between the control and the intermediate S-states. There are small but distinct differences in the S-state spectra which are easier to see in the second derivatives (bottom). The two vertical dashed lines indicate the clear systematic differences in the Sr K-edge spectra between the S-states; the feature labeled a shifts to higher energy and feature b shifts to lower energy as we advance from S0 through S3 states. Other differences are also seen in the second derivatives between the S states, the most significant being that between the S2 and S3 and the S3 to S0 states. b) Fourier transforms of Sr EXAFS of Sr-PS II in the different S-states (S1, S2, S3, S0), and an inactive control sample prepared by hydroxylamine treatment of Sr-PS II. The dominant Fourier peak I is due to ligating oxygens in the first coordination sphere to Sr. Peak II is best fit by four Sr-Mn interactions; short distances at ~3.5 and longer Sr-Mn distances at ~4.0 Å. FT peak II from Sr-Mn is dependent on the particular S-state and shows that structure of cluster changes as we advance through the S-states, with a significant change occurring between the critical S2 to S3 transition suggesting that the cluster is flexible. The control sample shows only the FT peak from Sr-O backscattering, because the cluster is disrupted and the Sr-Mn interactions are lost.
a) Position of Sr in the OEC in the S1 State
Previously, Ca EXAFS of the native PS II [18] and Sr EXAFS of Sr-reactivated PS II membranes [17] indicated proximity of the Ca at 3.4 Å and Sr at 3.5 Å to the Mn-cluster in the S1 dark-stable state of the OEC. The study by Pushkar et al. [22] unambiguously demonstrates Sr to be proximate to the OEC in all S-states and that significant changes occur in the Sr(Ca)-Mn interactions as the enzyme proceeds through the catalytic cycle, as seen in the FTs in Fig. 5b. The Sr-Mn distances in the recently reported first synthetic heteronuclear Mn/Sr complex [46], where Mn and Sr are bridged by an oxo group, are similar to those observed in PS II. Therefore, it is likely that the Ca/Sr atom in PS II is bridged by μ-oxo groups to Mn atoms.
The best fits to the Sr EXAFS data (Fig. 5b) in the S0 through S3 states are obtained for Sr-Mn distances at ~3.5 Å (short) and ~ 4.0 Å (long). The fits for the S1 state are for 3:1, 2:2 and 2:1 short:long Sr-Mn ratio of vectors; however, the 3:1 fit is better than the others by 25-28%. The best fit for the S2 state is for a 3:1 or 2:2 short-to-long Sr-Mn ratio of vectors, with the fit for 2:2 being better by 21%. For the S3 and S0 states, a ratio of 2:2 provides the best fit. This information was used to model the position of Sr and the important changes in the short and long Sr-Mn distances during the S-state transitions.
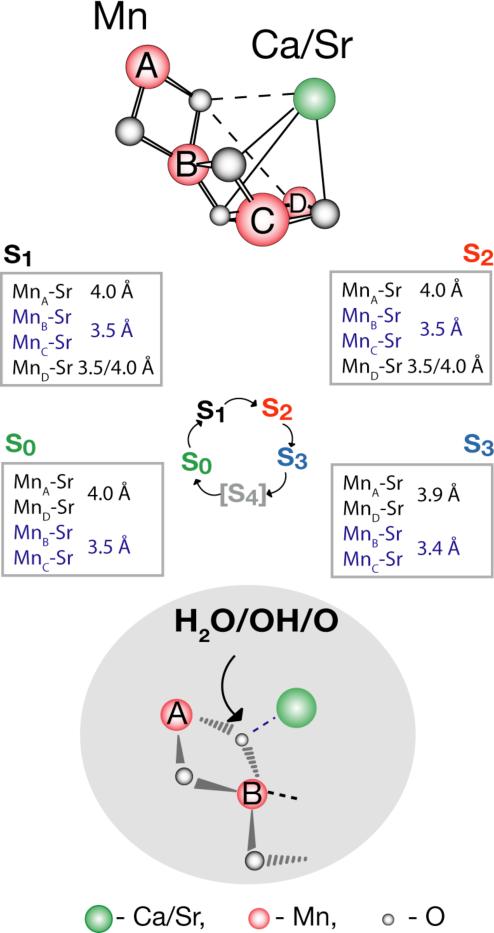
To model the position of the Sr atom in the S1 state we combined information from the EXAFS fits and from polarized EXAFS measurements of Ca-containing PS II single crystals [47], from which three Mn4Ca structural models (I, II, III) have been proposed. For the three best fits, there are 3 or 2 Sr-Mn vectors at ~3.5 Å and 1 or 2 Sr-Mn vectors at ~4.0 Å. For all three cases the Sr atom is displaced less than 1.2 Å from the position of Ca in the models, and is directly connected to the Mn-core through bridging oxygen atoms. The Ca- and Sr-PS II are slightly different in their kinetic and spectroscopic properties [45], which may reflect differences in the interaction of Ca and Sr with the Mn atoms. Previous results from Ca EXAFS of plant PS II have shown that there are only 2-3 Mn-Ca interactions [18] at <4 Å, compared to the four we have observed in Sr-PS II; it is possible that the longer interactions are at >4 Å or were not discernible at the S/N of the Ca EXAFS data [18]. There is also evidence that Ca protects two of the four Mn atoms from reductants, suggesting a closer interaction between Ca and two of the four Mn atoms in the cluster [48]. In Fig. 5 we use model III (from [47]) to illustrate the best possible position of the Sr atom (3:1 ratio) in the S1 state, requiring μ-oxo bridges between Sr and at least three of the Mn atoms to explain the presence of short distances of ~3.5/3.4 (Sr-Mn/Ca-Mn) Å.
b) Structural Changes in the OEC in all the S-states
Previous Mn-EXAFS experiments demonstrated the absence of major changes in Mn-Mn distances of the Mn4-core during the S1 to S2 transition. The Mn and Sr-EXAFS data from the Sr-PS II from biosynthetically grown cyanobacteria also show that there are no significant changes in the Mn-Mn and Sr-Mn distances during this transition. It is possible that the ratio of short:long Sr-Mn vectors changes from 3:1 (S1) to 2:2 (S2), on the basis of the quality of the fits, indicating that one Sr-Mn distance changes from ~3.5 to ~4.0 Å during this transition. However, we cannot rule out the presence of the 2:2 ratio in the S1 state or the 3:1 ratio in the S2 state, because of the uncertainties in EXAFS fitting procedures.
Fits of peak II in the Mn EXAFS of the S3 state show an elongation of the Mn-Mn distance as well as an increased Debye-Waller factor as compared to the S1 and S2 states (data not shown in this review) [23,49]. Introduction of the second Mn-Mn subshell improves the fit quality and resolves two distances (~2.8 Å and 2.9 Å) among the di-μ-oxo bridged Mn-Mn moieties [23].
The FT peak II in Sr EXAFS splits in the S3 state (Fig. 5b) suggesting changes in Sr-Mn distances. The best fit for the S3 state is for 2 short and 2 long Sr-Mn distances. The Sr-Mn interaction at ~3.5 Å shortens to ~3.4 Å, and the distance at ~4.0 Å decreases to ~3.9 Å during the S2 to S3 transition. The fact that Mn-Sr interactions change during the S2 to S3 transition is also in agreement with the different efficiency of Ca-depletion in the S3-state from that in other S-states [50]. Ca/Sr-depleted PS II cannot advance to the S3 state; instead, a state designated S2YZ• is formed, in which the Mn4-core structure is close to that of the S2 state and does not resemble the structure of the native S3 state [16,49]. We propose that a μ3-like-oxygen of the Mn4Ca structure is important for the formation of the S3 state and that its properties are significantly altered in the absence of a coordinated Ca/Sr atom. The oxo-bridge located between MnA and MnB is posssibly bridged to Ca and MnD in line with earlier suggestions by Kulik and coworkers [51] and Siegbahn [52].
Completing the catalytic cycle in the S3 to S0 transition, the di-μ-oxo bridged Mn-Mn interactions exhibit shortening in the Mn EXAFS (data not shown in this review) (Fig. 5b). From Sr EXAFS, we can see that the Sr-Mn distances at 3.42 Å and at 3.94 Å increase to 3.47 Å and 3.98 Å, respectively. During the S0 to S1 transition, the Sr-Mn distances increase further to ~3.5 Å and ~4.0 Å.
In addition, Fig. 5a shows the Sr XANES spectrum in all the S-states and in a sample where the Mn cluster was disrupted using NH2OH. There is a significant difference in the XANES spectrum between the intact samples and the NH2OH treated samples indicating that Sr is in a special environment in the PS II samples. Moreover, there are differences in the XANES between the S2 and S3 states, and S3 to S0 states (Fig. 5a) that complement the EXAFS results. These differences are more clear in the second derivatives of the XANES spectra in Fig. 5a.
The generally accepted proton release pattern during the S0 to S1, S1 to S2, S2 to S3 and S3 to S0 transitions is 1, 0, 1, 2, respectively [53]; although it has been reported that the proton release pattern is pH dependent [54]. EXAFS can provide only indirect information about protons, by the effect that protonations/deprotonations might have on the Mn-Mn/Sr(Ca) and Mn-O, N ligand atom distances. The shortening of one Mn-Mn distance observed during the S0 to S1 transition is compatible with the deprotonation of an hydroxo (OH-) bridge between Mn atoms to an oxo (O2-) bridge [55,56], and consistent with the release of one proton during. During the S1 to S2 transition there is no net release of protons and there are no significant changes in Mn-Mn/Ca distances. There is release of a proton during the S2 to S3 transition, and the absence of any shortening in Mn-Mn distances allows us to speculate that the proton release proceeds differently for this transition. There are no EXAFS studies on intermediate states between S3 and S0, when two protons are released. One speculation is that a proton is released from a hydroxo group that is a ligand of Ca/Mn (or from exogenous OH-/H2O) that is involved in the O-O bond formation, while another proton is released from a H2O ligand of Ca/Mn (or from exogenous H2O), which can be incorporated as a bridging OH- ligand in the S0 state. Ca which can accommodate 7 or 8 ligands is ideally suited for ferrying in H2O/OH- groups to the catalytic site (Hillier and Messinger in [1]).
Fig. 6 summarizes the structural changes accompanying the S0 to S3 catalytic cycle transition as deduced from Mn- and Sr-EXAFS. Upon the oxidation of one Mn atom, probably MnD [57,58], during the S1 to S2 transition, it is possible that the Sr atom moves away from one Mn (MnB, MnC or MnD) leading to the change from 3:1 short:long Sr-Mn distances to a 2:2 ratio in the S2 state. Alternatively, the 2:2 ratio can be present already in the S1 state, thus remaining unchanged in the S1 to S2 transition. During the S2 to S3 transition, although the ratio of short-to-long vectors remains 2:2, there is a significant decrease in the Sr-Mn distances. From the Mn EXAFS the di-μ-oxo bridged Mn-Mn distances increase during the S2 to S3 transition; from ~2.7-2.8 Å to ~2.8-2.9 Å. This result observed in Synechococcus PS II preparations is similar to that detected by Mn-EXAFS using PS II preparations from spinach [49]. Studies are in progress aimed at identifying which particular Mn-Mn moiety in the OEC model increases in distance.
Figure 6.
Schematic of the structural changes accompanying the S-state transitions in the Mn4Ca(Sr) cluster is placed within the context of the recent structural model III from single-crystal X-ray spectroscopy. The critical transition is the S2 to S3 advancement, when the Mn-Mn di-μ-oxo bridge distances of the Mn4-core become elongated from ~2.7-2.8 Å to ~2.8-2.9 Å. Simultaneously, Sr is drawn closer to the Mn core with the Sr-Mn interaction at ~3.5 Å shortening to ~3.4 Å and at ~4.0 Å distance decreasing to 3.94 Å. We propose that this change is triggered by the ligand-centered oxidation of the oxygen atom that bridges the Mn with the Ca atoms. The Ca(Sr)-Mn distances in all the S-states are indicated in the boxes next to the S-states. Changes in the Mn-Mn and Ca(Sr)-Mn distances may be triggered by changes at an oxo-bridge. The oxo bridge between MnA, MnB may also bridge to Ca and possiblly to MnD as shown by dotted lines. It may therefore fulfill the critical function in the O-O formation chemistry consistent with earlier proposals by Kulik and coworkers [51] and Siegbahn [52].
c) Mechanism of Photosynthetic Water Oxidation
It is difficult to rationalize the changes in the Mn-Mn and Mn-Sr distances without significant involvement of the bridging O atoms. One hypothesis is that during the S2 to S3 transition the oxidation occurs predominantly at a bridging oxygen ligand, triggering the structural changes in the OEC. An oxyl radical intermediate has been proposed on the basis of DFT calculations [59,60], including on a bridging position between two manganese atoms [38]. Preliminary RIXS data show that the charge density change during the S2 to S3 transition is much smaller than during the S1 to S2 transition, supporting the hypothesis that the oxidation is predominantly ligand-centered.
We think that the observed structural changes in the S2 to S3 transition are compatible with a primarily ligand-centered oxidation. The oxidized ligand in the S3 state can interconvert between a bridging and a terminal ligand O atom (OH or H2O) [61] resulting in an oxygen isotope exchange. Changes in Mn-O-Mn vibrational frequencies, the OEC EPR properties [45,62], rate of water exchange [63] upon Ca to Sr substitution and data presented here indicate that the critical oxygen atom is part of the Mn-O-Ca/Sr bridging structure [64].
Although, there are many proposed mechanisms for the photosynthetic water oxidation reaction that include variations on how the O-O bond is formed, there are two important favored mechanisms for water oxidation by the OEC: i) nucleophilic attack on Mn(V)=O or Mn(IV)-O• by a metal- (possibly Ca) bound water molecule or hydroxide [11,65]; or ii) reaction of a Mn-oxo unit with predominant radical character with an oxo/hydroxo/water ligand or an exogenous H2O [49]. Except for PS II there are few structurally defined catalysts competent to oxidize water at room temperature; Ru- and Mn-complexes provide examples [65-69]. A theoretical investigation of the Ru catalyst supported a RuIV-O• moiety to promote the water-splitting reaction [70]. Theoretical analysis of the OEC indicates that the formation of a low-lying ligand-oxygen radical precursor state may be required for forming the O-O bond [59]. To reach this state, a structural rearrangement is needed at the S2 to S3 transition. First, the changes in the Mn-Mn and Ca(Sr)-Mn distances require a model that involves a bridging oxygen atom (Fig. 6). This is probably the same oxygen where the oxidation occurs in the S3 state, which triggers the formation of the O-O bond. We note, however, that the oxidation of a different bridging oxygen cannot be excluded. Second, the decrease in the Ca(Sr)-Mn distance during the S2 to S3 transition favors the formation of the O-O bond between a Ca-bound water or hydroxide and a Mn-bound oxygen, leading to the decrease in the Mn-Ca distances. This shortening of the Mn-Ca distance may not by itself confirm the formation of the O-O bond in the S3 state between a Mn and a Ca atom, but it does show that this is a possibility during the S3 to S0 transition. These two observations lead us to propose that the Ca-bound water or hydroxide and a critical oxo-bridging atom with predominantly radical character are the oxygen atoms involved in the formation of the all-important O-O bond in the water oxidation reaction.
Acknowledgments
This work was supported by the NIH grant (GM 55302), and the DOE, Director, Office of Science, Office of Basic Energy Sciences (OBES), Chemical Sciences, Geosciences, and Biosciences Division, under Contract DE-AC02-05CH11231. Parts of this research were carried out at ALS, APS and SSRL funded by DOE, OBES. The SSRL SMB Program is supported by the DOE, OBER and by the NIH, NCRR. We are grateful to all the members of our group who have contributed to the work presented in this review, and we especially thank Drs. Matthew Latimer, Roehl Cinco, Yulia Pushkar, and our collaborator Dr. Alain Boussac.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wydrzynski T, Satoh S. Photosystem II: The Light-Driven Water:Plastoquinone Oxidoreductase. In: Govindjee, editor. Advances in Photosynthesis and Respiration. Springer; Dordrecht: 2005. [Google Scholar]
- 2.Kok B, Forbush B, McGloin M. Cooperation of charges in photosynthetic O2 evolution. Photochem. Photobiol. 1970;11:457–476. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- 3.Yocum CF. The calcium and chloride requirements of the O2 evolving complex. Coord. Chem. Rev. 2008;252:296–305. [Google Scholar]
- 4.Ghanotakis DF, Babcock GT, Yocum CF. Calcium reconstitutes high rates of oxygen evolution in polypeptide depleted Photosystem II preparations. FEBS Lett. 1984;167:127–130. [Google Scholar]
- 5.Lockett CJ, Demetriou C, Bowden SJ, Nugent JHA. Studies on calcium depletion of PS II by pH 8.3 treatment. Biochim. Biophys. Acta. 1990;1016:213–218. [Google Scholar]
- 6.Miqyass M, Marosvolgyi MA, Nagel Z, Yocum CF, van Gorkom HJ. S-state dependence of the calcium requirement and binding characteristics in the oxygen-evolving complex of photosystem II. Biochemistry. 2008;47:7915–7924. doi: 10.1021/bi8006059. [DOI] [PubMed] [Google Scholar]
- 7.Boussac A, Rutherford AW. Nature of the Inhibition of the Oxygen-Evolving Enzyme of Photosystem II Induced by NaCl Washing and Reversed by the Addition of Ca2+ or Sr2+ Biochemistry. 1988;27:3476–3483. [Google Scholar]
- 8.Kim SH, Gregor W, Peloquin JM, Brynda M, Britt RD. Investigation of the calcium-binding site of the oxygen evolving complex of photosystem II using 87Sr ESEEM spectroscopy. J. Am. Chem. Soc. 2004;126:7228–7237. doi: 10.1021/ja030614e. [DOI] [PubMed] [Google Scholar]
- 9.Matysik J, Alia, Gerda N, van Gorkom HJ, Hoff AJ, de Groot HJM. Exploring the Calcium-Binding Site in Photosystem II Membranes by Solid-State 113Cd NMR. Biochemistry. 2000;39:6751–6755. doi: 10.1021/bi0004145. [DOI] [PubMed] [Google Scholar]
- 10.Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Towards Complete Cofactor Arrangement in the 3.0 Å Resolution Structure of Photosystem II. Nature. 2005;438:1040–1044. doi: 10.1038/nature04224. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 12.Kamiya N, Shen JR. Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7 Å resolution. Proc. Natl. Acad. Sci. USA. 2003;100:98–103. doi: 10.1073/pnas.0135651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kargul J, Maghlaoul K, Murray JW, Deak Z, Boussac A, Rutherford AW, Vass I, Barber J. Purification, crystallization and X-ray diffraction analyses of the T-elongatus PSII core dimer with strontium replacing calcium in the oxygen-evolving complex. Biochim. Biophys. Acta-Bioenergetics. 2007;1767:404–413. doi: 10.1016/j.bbabio.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Guskov A, Kern J, Gabdulkhakov A, Broser M, Zouni A, Saenger W. Cyanobacterial photosystem II at 2.9-angstrom resolution and the role of quinones, lipids, channels and chloride. Nature Struct. & Mol. Biol. 2009;16:334–342. doi: 10.1038/nsmb.1559. [DOI] [PubMed] [Google Scholar]
- 15.Latimer MJ, DeRose VJ, Mukerji I, Yachandra VK, Sauer K, Klein MP. Evidence for the Proximity of Calcium to the Manganese Cluster of Photosystem II: Determination by X-Ray Absorption Spectroscopy. Biochemistry. 1995;34:10898–10909. doi: 10.1021/bi00034a024. [DOI] [PubMed] [Google Scholar]
- 16.Latimer MJ, DeRose VJ, Yachandra VK, Sauer K, Klein MP. Structural Effects of Calcium Depletion on the Manganese Cluster of Photosystem II: Determination by X-ray Absorption Spectroscopy. J. Phys. Chem. B. 1998;102:8257–8265. doi: 10.1021/jp981668r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cinco RM, Robblee JH, Rompel A, Fernandez C, Yachandra VK, Sauer K, Klein MP. Strontium EXAFS Reveals the Proximity of Calcium to the Manganese Cluster of Oxygen-Evolving Photosystem II. J. Phys. Chem. B. 1998;102:8248–8256. doi: 10.1021/jp981658q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cinco RM, Holman KLM, Robblee JH, Yano J, Pizarro SA, Bellacchio E, Sauer K, Yachandra VK. Calcium EXAFS establishes the Mn-Ca cluster in the oxygen-evolving complex of photosystem II. Biochemistry. 2002;41:12928–12933. doi: 10.1021/bi026569p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cinco RM, Robblee JH, Messinger J, Fernandez C, Holman KLM, Sauer K, Yachandra VK. Orientation of calcium in the Mn4Ca cluster of the oxygen-evolving complex determined using polarized strontium EXAFS of photosystem II membranes. Biochemistry. 2004;43:13271–13282. doi: 10.1021/bi036308v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yano J, Yachandra VK. Where Water is Oxidized to Dioxygen: Structure of the Photosynthetic Mn4Ca Cluster from X-ray Spectroscopy. Inorg. Chem. 2008;47:1711–1726. doi: 10.1021/ic7016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yano J, Pushkar Y, Glatzel P, Lewis A, Sauer K, Messinger J, Bergmann U, Yachandra VK. High-resolution Mn EXAFS of the Oxygen-evolving Complex in Photosystem II: Structural Implications for the Mn4Ca Cluster. J. Am. Chem. Soc. 2005;127:14974–14975. doi: 10.1021/ja054873a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pushkar Y, Yano J, Glatzel P, Messinger J, Lewis A, Sauer K, Bergmann U, Yachandra V. Structure and orientation of the Mn4Ca cluster in plant photosystem II membranes studied by polarized range-extended X-ray absorption spectroscopy. J. Biol. Chem. 2007;282:7198–7208. doi: 10.1074/jbc.M610505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pushkar YL, Yano J, Sauer K, Boussac A, Yachandra VK. Structural changes in the Mn4Ca cluster and the mechanism of photosynthetic water splitting. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1879–1884. doi: 10.1073/pnas.0707092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yano J, Kern J, Irrgang K-D, Latimer MJ, Bergmann U, Glatzel P, Pushkar Y, Biesiadka J, Loll B, Sauer K, Messinger J, Zouni A, Yachandra VK. X-ray Damage to the Mn4Ca Complex in Photosystem II Crystals: A Case Study for Metallo-Protein X-ray Crystallography. Proc. Natl. Acad. Sci. USA. 2005;102:12047–12052. doi: 10.1073/pnas.0505207102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott RA. X-ray absorption spectroscopy. In: Rousseau DL, editor. Structural and Resonance Techniques in Biological Research. Academic Press; Orlando: 1984. pp. 295–362. [Google Scholar]
- 26.Cramer SP. Biochemical Applications of X-ray Absorption Spectroscopy. In: Koningsberger DC, Prins R, editors. X-ray Absorption: Principles, Applications and Techniques of EXAFS, SEXAFS, and XANES. Wiley-Interscience; New York: 1988. pp. 257–326. [Google Scholar]
- 27.Yachandra VK. X-ray absorption spectroscopy and applications in structural biology. Methods Enzymol. 1995;246:638–675. doi: 10.1016/0076-6879(95)46028-4. [DOI] [PubMed] [Google Scholar]
- 28.Yano J, Yachandra VK. X-ray absorption spectroscopy. Photosynth. Res. 2009;102:241–254. doi: 10.1007/s11120-009-9473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yano J, Yachandra VK. Oxidation State Changes of the Mn4Ca Cluster in Photosystem II. Photosynth. Res. 2007;92:289–303. doi: 10.1007/s11120-007-9153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yachandra VK, Sauer K, Klein MP. Manganese Cluster in Photosynthesis: Where Plants Oxidize Water to Dioxygen. Chem. Rev. 1996;96:2927–2950. doi: 10.1021/cr950052k. [DOI] [PubMed] [Google Scholar]
- 31.MacLachlan DJ, Hallahan BJ, Ruffle SV, Nugent JHA, Evans MCW, Strange RW, Hasnain SS. An EXAFS study of the manganese oxygen-evolving complex in purified photosystem II membrane fractions. The S1 and S2 states. Biochem. J. 1992;285:569–576. doi: 10.1042/bj2850569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLachlan DJ, Nugent JHA, Bratt PJ, Evans MCW. The effects of calcium depletion on the O2-evolving complex in spinach PS II: the S1*, S2* and S3* states and the role of the 17 kDa and 23 kDa extrinsic polypeptides. Biochim. Biophys. Acta. 1994;1186:186–200. [Google Scholar]
- 33.Riggs-Gelasco PJ, Mei R, Ghanotakis DF, Yocum CF, PennerHahn JE. X-ray absorption spectroscopy of calcium-substituted derivatives of the oxygen-evolving complex of phostosystem II. J. Am. Chem. Soc. 1996;118:2400–2410. [Google Scholar]
- 34.Tamura N, Cheniae G. Effects of Photosystem-II Extrinsic Proteins On Microstructure of the Oxygen-Evolving Complex and Its Reactivity to Water Analogs. Biochim. Biophys. Acta. 1985;809:245–259. [Google Scholar]
- 35.Chen C, Kazimir J, Cheniae GM. Calcium Modulates the Photoassembly of Photosystem II (Mn)4-Clusters by Preventing Ligation of Nonfunctional High-Valency States of Manganese. Biochemistry. 1995;34:13511–13526. doi: 10.1021/bi00041a031. [DOI] [PubMed] [Google Scholar]
- 36.Limburg J, Szalai VA, Brudvig GW. A mechanistic and structural model for the formation and reactivity of a MnV=O species in photosynthetic water oxidation. J. Chem. Soc., Dalton Trans. 1999:1353–1361. [Google Scholar]
- 37.Renger G. Mechanistic and structural aspects of photosynthetic water oxidation. Physiol. Plant. 1997;100:828–841. [Google Scholar]
- 38.Siegbahn PEM. Theoretical Models for the Oxygen Radical Mechanism of Water Oxidation and of the Water Oxidizing Complex of Photosytem II. Inorg. Chem. 2000;39:2923–2935. doi: 10.1021/ic9911872. [DOI] [PubMed] [Google Scholar]
- 39.Ananyev GM, Dismukes GC. Calcium Induces Binding and Formation of a Spin-Coupled Dimanganese (II, II) Center in the Apo-Water Oxidation Complex of Photosystem II as Precursor to the Functional Tetra-Mn/Ca Cluster. Biochemistry. 1997;36:11342–11350. doi: 10.1021/bi970626a. [DOI] [PubMed] [Google Scholar]
- 40.Dau H, Andrews JC, Roelofs TA, Latimer MJ, Liang W, Yachandra VK, Sauer K, Klein MP. Structural Consequences of Ammonia Binding to the Manganese Cluster of the Photosynthetic Oxygen-Evolving Complex: An X-ray Absorption Study of Isotropic and Oriented Photosystem II Particles. Biochemistry. 1995;34:5274–5287. doi: 10.1021/bi00015a043. [DOI] [PubMed] [Google Scholar]
- 41.Mukerji I, Andrews JC, DeRose VJ, Latimer MJ, Yachandra VK, Sauer K, Klein MP. Orientation of the oxygen-evolving manganese complex in a photosystem II membrane preparation: an X-ray absorption spectroscopy study. Biochemistry. 1994;33:9712–9721. doi: 10.1021/bi00198a042. [DOI] [PubMed] [Google Scholar]
- 42.Bergmann U, Cramer SP. SPIE Conference on Crystal and Multilayer Optics. SPIE; San Diego, CA: 1998. A high-resolution large-acceptance analyzer for x-ray fluorescence and Raman spectroscopy; pp. 198–209. [Google Scholar]
- 43.Pushkar Y, Yano J, Glatzel P, Messinger J, Lewis A, Sauer K, Bergmann U, Yachandra VK. Structure and orientation of the Mn4Ca cluster in plant photosystem II membranes studied by polarized range-extended X-ray absorption spectroscopy. J. Biol. Chem. 2007;282:7198–7208. doi: 10.1074/jbc.M610505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zouni A, Witt HT, Kern J, Fromme P, Krauss N, Saenger W, Orth P. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature. 2001;409:739–743. doi: 10.1038/35055589. [DOI] [PubMed] [Google Scholar]
- 45.Boussac A, Rappaport F, Carrier P, Verbavatz JM, Gobin R, Kirilovsky D, Rutherford AW, Sugiura M. Biosynthetic Ca2+/Sr2+ exchange in the photosystem II oxygen-evolving enzyme of Thermosynechococcus elongatus. J. Biol. Chem. 2004;279:22809–22819. doi: 10.1074/jbc.M401677200. [DOI] [PubMed] [Google Scholar]
- 46.Mishra A, Yano J, Pushkar Y, Yachandra VK, Abboud KA, Christou G. Heteronuclear MnCa/Sr Model Complexes, and Ca/Sr EXAFS Spectral Comparisons with the Oxygen-Evolving Complex of Photosystem II. Chem. Comm. 2007:1538–1540. doi: 10.1039/b701355h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yano J, Kern J, Sauer K, Latimer M, Pushkar Y, Biesiadka J, Loll B, Saenger W, Messinger J, Zouni A, Yachandra VK. Where Water is Oxidized to Dioxygen: Structure of the Photosynthetic Mn4Ca Cluster. Science. 2006;314:821–825. doi: 10.1126/science.1128186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuntzleman T, McCarrick M, Penner-Hahn J, Yocum C. Probing reactive sites within the Photosystem II manganese cluster: Evidence for separate populations of manganese that differ in redox potential. Phys. Chem. Chem. Phys. 2004;6:4897–4904. [Google Scholar]
- 49.Liang W, Roelofs TA, Cinco RM, Rompel A, Latimer MJ, Yu WO, Sauer K, Klein MP, Yachandra VK. Structural Change of the Mn Cluster during the S2 → S3 State Transition of the Oxygen-Evolving Complex of Photosystem II. Does It Reflect the Onset of Water/Substrate Oxidation? Determination by Mn X-ray Absorption Spectroscopy. J. Am. Chem. Soc. 2000;122:3399–3412. doi: 10.1021/ja992501u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boussac A, Rutherford AW. Ca2+ binding to the oxygen evolving enzyme varies with the redox state of the Mn cluster. FEBS Lett. 1988;236:432–436. [Google Scholar]
- 51.Kulik LV, Epel B, Lubitz W, Messinger J. Electronic structure of the Mn4OxCa cluster in the S0 and S2 states of the oxygen-evolving complex of photosystem II based on pulse 55Mn-ENDOR and EPR Spectroscopy. J. Am. Chem. Soc. 2007;129:13421–13435. doi: 10.1021/ja071487f. [DOI] [PubMed] [Google Scholar]
- 52.Siegbahn PEM. Structures and Energetics for O2 Formation in Photosystem II. Acc. Chem. Res. 2009;42:1871–1880. doi: 10.1021/ar900117k. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki H, Sugiura M, Noguchi T. Monitoring Proton Release during Photosynthetic Water Oxidation in Photosystem II by Means of Isotope-Edited Infrared Spectroscopy. J. Am. Chem. Soc. 2009;131:7849–7857. doi: 10.1021/ja901696m. [DOI] [PubMed] [Google Scholar]
- 54.Rappaport F, Lavergne J. Coupling of electron and proton transfer in the photosynthetic water oxidase. Bichim. Biophys. Acta. 2001;1503:246–259. doi: 10.1016/s0005-2728(00)00228-0. [DOI] [PubMed] [Google Scholar]
- 55.Baldwin MJ, Stemmler TL, Riggs-Gelasco PJ, Kirk ML, Penner-Hahn JE, Pecoraro VL. Structural and Magnetic Effects of Successive Protonations of Oxo Bridges in High-Valent Manganese Dimers. J. Am. Chem. Soc. 1994;116:11349–11356. [Google Scholar]
- 56.Robblee JH, Messinger J, Cinco RM, McFarlane KL, Fernandez C, Pizarro SA, Sauer K, Yachandra VK. The Mn Cluster in the S0 State of the Oxygen-Evolving Complex of Photosystem II Studied by EXAFS Spectroscopy: Are There Three Di-μ-oxo-bridged Mn2 Moieties in the Tetranuclear Mn Complex? J. Am. Chem. Soc. 2002;124:7459–7471. doi: 10.1021/ja011621a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chu HA, Hillier W, Debus RJ. Evidence that the C-terminus of the D1 polypeptide of photosystem II is ligated to the manganese ion that undergoes oxidation during the S1 to S2 transition: An isotope-edited FTIR study. Biochemistry. 2004;43:3152–3166. doi: 10.1021/bi035915f. [DOI] [PubMed] [Google Scholar]
- 58.Kimura Y, Mizusawa N, Yamanari T, Ishii A, Ono T. Structural changes of D1 C-terminal alpha-carboxylate during S-state cycling in photosynthetic oxygen evolution. J. Biol. Chem. 2005;280:2078–2083. doi: 10.1074/jbc.M410627200. [DOI] [PubMed] [Google Scholar]
- 59.Siegbahn PEM, Crabtree RH. Manganese oxyl radical intermediates and O-O bond formation in photosynthetic oxygen evolution and a proposed role for the calcium cofactor in photosystem II. J. Amer. Chem. Soc. 1999;121:117–127. [Google Scholar]
- 60.Lundberg M, Blomberg MRA, Siegbahn PEM. Oxyl radical required for O-O bond formation in synthetic Mn-catalyst. Inorg. Chem. 2004;43:264–274. doi: 10.1021/ic0348188. [DOI] [PubMed] [Google Scholar]
- 61.McEvoy JP, Brudvig GW. Water-Splitting Chemistry of Photosystem II. Chem. Rev. 2006;106:4455–4483. doi: 10.1021/cr0204294. [DOI] [PubMed] [Google Scholar]
- 62.Chu H-A, Hillier W, Law NA, Babcock GT. Vibrational spectroscopy of the oxygen-evolving complex and of manganese model compounds. Biochim. Biophys. Acta. 2001;1503:69–82. doi: 10.1016/s0005-2728(00)00216-4. [DOI] [PubMed] [Google Scholar]
- 63.Hendry G, Wydrzynski T. 18O Isotope Exchange Measurements Reveal that Calcium is Involved in the Binding of one Substrate-water Molecule to the Oxygen-Evolving Complex in Photosystem II. Biochemistry. 2003;42:6209–6217. doi: 10.1021/bi034279i. [DOI] [PubMed] [Google Scholar]
- 64.Su JH, Messinger J. Is Mn-Bound Substrate Water Protonated in the S2 State of Photosystem II? Appl. Mag. Res. 37:123–136. doi: 10.1007/s00723-009-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vrettos JS, Limburg J, Brudvig GW. Mechanism of photosynthetic water oxidation: combining biophysical studies of photosystem II with inorganic model chemistry. Biochim. Biophys. Acta. 2001;1503:229–245. doi: 10.1016/s0005-2728(00)00214-0. [DOI] [PubMed] [Google Scholar]
- 66.Yamada H, Siems WF, Koike T, Hurst JK. Mechanism of Water Oxidation Catalyzed by the cis,cis-[(bpy)2Ru(OH2)]2O4+ ion. J. Am. Chem. Soc. 2004;126:9786–9795. doi: 10.1021/ja030594g. [DOI] [PubMed] [Google Scholar]
- 67.Romain S, Bozoglian F, Sala X, Llobet A. Oxygen-Oxygen Bond Formation by the Ru-Hbpp Water Oxidation Catalyst Occurs Solely via an Intramolecular Reaction Pathway. J. Am. Chem. Soc. 2009;131:2768–2769. doi: 10.1021/ja808166d. [DOI] [PubMed] [Google Scholar]
- 68.Concepcion JJ, Jurss JW, Brennaman MK, Hoertz PG, Patrocinio AOT, Iha NYM, Templeton JL, Meyer TJ. Making Oxygen with Ruthenium Complexes. Acc. Chem. Res. 2009;42:1954–1965. doi: 10.1021/ar9001526. [DOI] [PubMed] [Google Scholar]
- 69.Wasylenko DJ, Ganesamoorthy C, Henderson MA, Koivisto BD, Osthoff HD, Berlinguette CP. Electronic Modification of the [RuII(tpy)(bpy)(OH2)]2+ Scaffold: Effects on Catalytic Water Oxidation. J. Am. Chem. Soc. 2010;132:16094–16106. doi: 10.1021/ja106108y. [DOI] [PubMed] [Google Scholar]
- 70.Yang X, Baik M-H. cis,cis-[(bpy)2Ru(V)O]2O4+ Catalyzes Water Oxidation Formally via in Situ Generation of Radicaloid RuIV-O. J. Am. Chem. Soc. 2006;128:7476–7485. doi: 10.1021/ja053710j. [DOI] [PubMed] [Google Scholar]