Abstract
Neuro-oncology research has rediscovered a complexity of nervous system cancers through the incorporation of cellular heterogeneity into tumor models with cellular subsets displaying stem cell characteristics. Self-renewing cancer stem cells (CSCs) can propagate tumors and yield non-tumorigenic tumor bulk cells that display a more differentiated phenotype. The ability to prospectively isolate and interrogate CSCs is defining molecular mechanisms responsible for the tumor maintenance and growth. The clinical relevance of CSCs has been supported by their resistance to cytotoxic therapies and their promotion of tumor angiogenesis. Although the field of CSC biology is relatively young, continued elucidation of the features of these cells holds promise for the development of novel patient therapies.
Keywords: glioma, cancer stem cell, therapy resistance
Introduction
Fifteen years ago, at the time of the last printing of the Glia special issue on glioma, the prevailing model for the architecture of most solid tumors, including gliomas, was based on the stochastic clonal expansion model whereby any one cell having acquired enough mutations for transformation yielded a bulk tumor comprised of cells equal in their tumorigenic potential. A paradigm change occurred in 2003 when evidence for a more complex hierarchy within gliomas was described (Bao et al. 2006a; Galli et al. 2004; Hemmati et al. 2003; Ignatova et al. 2002; Singh et al. 2003; Singh et al. 2004; Wang et al. 2010b). This hierarchal organization within tumors is commonly termed the cancer stem cell (CSC) hypothesis and was first described in hematopoietic cancers (Bonnet and Dick 1997; Lapidot et al. 1994). At the apex of the hierarchy is the CSC and central to the CSC hypothesis is the ability of this population of cells to propagate tumors and promote tumor progression in an orthotopic xenograft transplantation model as compared to the non-tumorigenic cells within the tumor bulk. This ability to initiate secondary tumor formation upon transplantation has led to these cells also being termed tumor-initiating cells or tumor-propagating cells. However, it is crucial to clarify that the terms initiating and propagating are describing a function of the cells in the transplantation assay and do not refer to the cell-of-origin from which these cancers were derived. Although evidence exists for a contribution of the CSC population to tumor propagation following therapeutic intervention, and mouse models have yielded valuable insight into potential cells of origin for gliomas, there is currently no clear evidence that it is a cancer cell with stem-like characteristics responsible for the initiating events in the development of the disease in patients. In the context of this review, we will be using the term CSC to describe the population of self-renewing cells responsible for tumor formation in the transplantation assay. In solid tumors, a CSC population has been prospectively identified directly from surgical tumor specimens and interrogated in vivo for cancers of the breast (Al-Hajj et al. 2003), colon (O'Brien et al. 2007; Ricci-Vitiani et al. 2007), pancreas (Hermann et al. 2007; Li et al. 2007), head and neck (Prince et al. 2007), lung (Eramo et al. 2008) and skin (Boiko et al. 2010; Monzani et al. 2007; Schatton et al. 2008). Although the CSC hypothesis posits hierarchies in specific tissues akin to that described for stem cell populations in normal organs, and therefore is so named, it is important to limit comparisons. Both normal and neoplastic stem cell populations demonstrate self-renewal and differentiation capabilities, yet tight regulation of proliferation and differentiation to functionally integrating cell types only exists for the normal stem cell compartment.
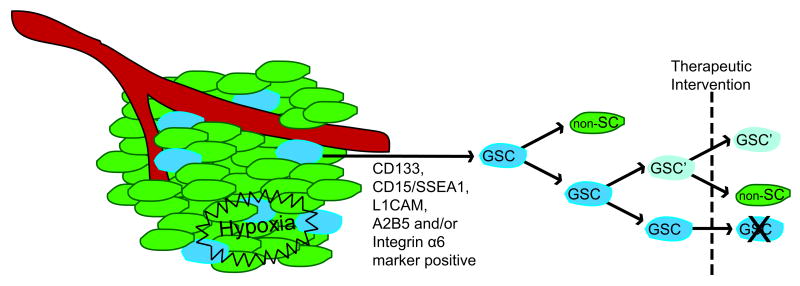
It is key to acknowledge that the hierarchy central to the CSC hypothesis may not be ubiquitous for all cancers or be represented in certain experimental cancer models (Kelly et al. 2007; Quintana et al. 2008). Furthermore, clonal expansion may still exist to some degree within the CSC compartment following acquisition of a genetic mutation favorable for survival, especially following therapeutic intervention (Fig. 1). A greater complexity may also exist whereby a non-CSC can evolve genetically and even acquire self-renewal potential that makes it phenotypically a CSC. Nonetheless, the demonstration of CSCs in human malignant glioma is strong evidence that the cellular heterogeneity of these tumors is associated with functional heterogeneity in terms of tumor initiation and propagation potential and warrants a discussion of how we identify and validate these cells and what their existence means for therapy and recurrence of the disease.
Figure 1. The Cancer Stem Cell Hypothesis in Malignant Gliomas.
Within a tumor, there exists a heterogeneous mix of cell types. The cancer stem cell hypothesis holds that a fraction of cells within the tumor, the cancer stem cells, can initiate and maintain the tumor over the non-stem cell (non-SC) population. Such cells have been identified in malignant gliomas and have been termed glioma stem cells (GSCs). Although found throughout the tumor, these cells have a preferential location within perivascular and hypoxic regions, or niches, and can be isolated by surface marker expression (e.g.; CD133, CD15/SSEA-1, L1CAM, A2B5 and/or Integrin α6). Following therapeutic intervention, it is possible that some level of clonal expansion occurs within the GSC population, most likely through an existing genetic mutation favorable for resistance. These surviving GSCs could then contribute to tumor recurrence.
Current Enrichment Markers for Glioma Stem Cells
The field of CSC biology would not have evolved without the pioneering work performed in the hematopoietic field using fluorescent activated cell sorting (FACS) to lineage trace cells within the hematopoietic system based on cell surface markers and consequently identify a stem cell population with long-term reconstituting ability (Spangrude et al. 1988). Translation of this technique to leukemia allowed for the first prospective identification of a stem-like population of cells within a cancer that could alone reinitiate the disease in a transplantation model (Bonnet and Dick 1997; Lapidot et al. 1994). For gliomas, CD133, CD15/SSEA-1, L1CAM, A2B5 and, more recently, integrin α6, are some of the identified surface markers that enrich for stem cell populations within gliomas, termed glioma stem cells (GSCs) (Bao et al. 2008; Lathia et al. 2010; Ogden et al. 2008; Singh et al. 2004; Son et al. 2009). Some studies have used serum free conditions to generate tumor spheroids that are enriched in self-renewing tumorigenic stem cells then use serum or retinoic acid to induce a differentiated population from the sphere cultures. Although useful to enrich, not necessarily purify, populations of cells with stem cell properties and test their differentiation potential as a surrogate for hierarchy, these conditions do not replicate the original tumor phenotypes (Lee et al. 2006) and therefore do not appropriately model the cellular hierarchy as does marker enrichment. It must be emphasized that central to the CSC hypothesis is the ability to utilize these surface markers to prospectively isolate the putative stem population within the heterogeneous bulk tumor and functionally validate the stem cell phenotype of this population versus the non-stem population (Shackleton et al. 2009) (Fig. 1).
CD133, or Prominin-1, a cell membrane glycoprotein, is the most widely utilized antigen for enrichment of GSCs and has been repeatedly validated in freshly isolated patient specimens (Bao et al. 2006a; Singh et al. 2004; Wang et al. 2010b). Some criticism for CD133 exists based on reports that CD133 negative cells can form tumors. However, it is essential to note that the usefulness of markers is only reliably apparent when evaluating freshly isolated patient specimens, as many of the reports claiming that CD133 is not informative come from cells that have been cultured. Markers can become uninformative in culture as there is undoubtedly an ongoing selection process that negates the ability to make inferences about hierarchy or lack thereof. Therefore, these findings on markers from culture cannot be extended to the situation in primary tumors. This becomes more apparent when the microenvironment is taken into account. CD133 and other cell surface markers are molecular interactors that mediate signals between cells and the microenvironment. Therefore, their expression and usefulness in GSC isolation may be missed if evaluated in culture versus a freshly dissociated tumor. There have been reports where fresh specimens were used and CD133 negative cells were able to grow tumors (Wang et al. 2008). These findings highlight the complexity of the CSC hypothesis as well as the field's appreciation that no single marker will be adequate to identify all GSC populations.
This appreciation has been enhanced by the reports of alternative markers to isolate GSCs that can be overlapping as well as independent of CD133. The tumorigenicity of CD133-independent populations was validated for CD15/SSEA-1, A2B5 and integrin α6 (Lathia et al. 2010; Ogden et al. 2008; Son et al. 2009). Subpopulations of CSCs with unique biological properties were recently described for colorectal cancer (Pang et al. 2010). This finding underscores the need for such evaluation of GSCs isolated by different markers to identify potentially unique functional characteristics such as invasive potential and/or therapeutic resistance. For example, a functional role has been demonstrated for L1CAM and integrin α6 whereby knockdown or inhibition via a blocking antibody reduced tumor growth in a xenograft model (Bao et al. 2008; Lathia et al. 2010). A comprehensive analysis expanding and correlating marker expression with functional characteristics will hopefully strengthen our understanding of the GSC phenotype.
The Working Definition of a Glioma Stem Cell
Marker expression allows one to prospectively fractionate a bulk tumor into GSCs (marker positive) and non-GSCs (marker negative) populations, but it is through functional assessments that the CSC nature of one population over the other is verified. The key benchmark is the ability for the GSCs to reform a phenotypic copy of the original tumor in an orthotopic transplantation model, performed as a limiting dilution assay (Shackleton et al. 2009). Non-GSCs, by definition, lack this ability and fail in the transplant model. Additional criteria often evaluated include self-renewal/stem cell maintenance as measured by the neurosphere/tumorsphere assay and the ability to differentiate or express markers of downstream neural lineages in culture as a suggestion of hierarchal organization (e.g.; GFAP positive astrocytes or Tuj1 positive immature neurons). Many of these assays were adopted from the neural stem cell field and, in fact, the neurosphere/tumorsphere assay was one of the original methods used to identify a putative stem cell population within brain tumors (Galli et al. 2004; Hemmati et al. 2003; Ignatova et al. 2002; Singh et al. 2003). As touched on previously, culture undoubtedly confers some level of selection that makes it impossible to fully recapitulate the heterogeneity present in the primary patient sample. Nonetheless, culture is an informative and important surrogate. For example, the ability to culture a primary sphere, ideally from a single GSC, which can then be replated, as secondary spheres and so on, allows for the extrapolation of a self-renewal phenotype for the GSCs versus the non-GSCs. The ability of these serially passaged spheres to then remain tumorigenic in vivo supports the maintenance of the CSC hierarchy. More recently, it has been demonstrated that GSCs can be maintained in adherent conditions, with this approach highlighting the utility of culture systems for high-throughput screens (Pollard et al. 2009). However, the most important evaluation of a stem cell population is the orthotopic xenograft model performed as an in vivo limiting dilution assay. This assay evaluates GSC self-renewal through the ability to re-isolate GSCs from a primary xenograft and test their ability to form subsequent phenotypically heterogeneous tumors characteristic of glioma. Combined, our current methods allow for the isolation and interrogation of GSCs from the bulk tumor in an effort to better understand the disease.
Tumor Hierarchy in Mouse Models
Although mouse models of brain cancer are covered in detail elsewhere in this review issue, it is important to note that these genetically engineered systems have demonstrated support for the CSC hypothesis through the maintenance of a hierarchy for tumor initiation (Alcantara Llaguno et al. 2009; Bleau et al. 2009; Harris et al. 2008; Read et al. 2009; Tamase et al. 2009; Ward et al. 2009; Jacques et al 2010). These findings are important as they validate brain tumor initiating cells beyond xenograft models. Although caution must be exercised when comparing mouse models of cancer with the human disease, these systems nonetheless may offer preclinical models to test the impact of therapeutic interventions on tumor initiating cells (and less commonly cells that propagate secondary tumors) relative to the tumor bulk.
Glioma Stem Cell Influence on Tumor Vasculature and Therapeutic Resistance
Of key interest in CSC biology is determining which pathways the CSCs utilize to maintain their phenotype. These cells have been reported to promote tumor angiogenesis while being resistant to chemotherapy and radiotherapy (Bao et al. 2006a; Bao et al. 2006b; Liu et al. 2006; Singh et al. 2004). Identifying and targeting the pathways utilized by the GSCs to evade current modes of therapy, which predominantly includes fractionated radiation and treatment with the oral DNA alkylating/methylating agent temozolomide following surgical resection, offers promise for translation of therapies from bench to bedside.
Following surgical resection, radiation is the mainstay of treatment for malignant gliomas. However, recurrence is common and highlights the potential presence of a resistant cellular population. Indeed, CD133 marker positive GSCs have been shown to be more resistant to radiation-induced apoptosis than non-GSCs (Bao et al. 2006a). Following exposure to radiation, the GSC population underwent dramatic expansion. Interestingly, GSCs demonstrated preferential activation of key components of the DNA damage response, likely leading to a corresponding increase in DNA repair. Notably, the fidelity of this repair is questionable due to an observed increase in mutation frequencies in recurrent malignant gliomas (The Cancer Genome Atlas Research Network 2008; Negrini et al. 2010) and may cause additional mutational events that evolve during the course of cancer therapy. Additional molecules linked to radioresistance of GSCs include Notch, SirT1, HSP90 and Bmi1 (Chang et al. 2009; Facchino et al. 2010; Sauvageot et al. 2009; Wang et al. 2010a), suggesting that there are multiple mechanisms that are coopted by CSCs to survive genotoxic stress. Targeting these molecules along with the identification of other key components involved in GSC radioresistance will offer multiple targeting strategies to increase GSC radiosensitization.
In addition to radiation, standard treatment of malignant gliomas includes administration of the DNA damaging alkylating agent, temozolomide. Temozolomide works by alkylation at the O6 position of guanine along with methylation at the N7 position, which, if left unrepaired, leads to cell death. Although one report claimed preferential targeting of GSCs by temozolomide, other studies have demonstrated a relative resistance of GSCs (Beier et al. 2008; Liu et al. 2006; Shervington and Lu 2008). Importantly, higher expression levels O6-methylguanine-DNA-methyltransferase (MGMT), the key repair enzyme for temozolomide-induced DNA adducts, have been reported in GSCs as compared to non-stem cells (Bleau et al. 2009; Liu et al. 2006) and these higher expression levels in GSCs have been shown to directly correlate to drug sensitivity (Beier et al. 2008). How this translates to the relative efficacy of temozolomid in the clinic has yet to be explored. Additional molecules upregulated in CSCs and linked to chemoresistance include the ABC (ATP-binding cassette) drug transporters (Hirschmann-Jax et al. 2004; Schatton et al. 2008). First identified in murine bone marrow, this class of membrane pumps has been associated with stem like cells termed “side-population” (SP) cells based on their ability to efflux the DNA dye Hoechst 33342 (Gussoni et al. 1999). SP cells were found to express high levels of the ABC drug transporter genes ABCG2/Bcrp1 and ABCA3 with later confirmation of increased expression of ABCG2/Bcrp1 in CD133 positive GSCs (Hirschmann-Jax et al. 2004; Liu et al. 2006). Additional support for a chemoresistance phenotype of SP cells has been demonstrated in a mouse model of glioma (Bleau et al. 2009). However, further evaluation of these pumps relative to stem cell marker expression is required as more recent reports have demonstrated that SP is not necessary or sufficient for GSC enrichment (Broadley et al. 2010). Taken together, the ability of GSCs to actively transport certain chemotherapeutic agents out of the cell coupled with improved DNA repair mechanisms results in a relatively drug-resistant population of cells that likely contribute to tumor recurrence. Such resistance to standard genotoxic therapies supports the need for alternative approaches to target these cells.
Another mechanism by which GSCs facilitate tumor growth is by promoting tumor vasculature. Specifically, GSCs have been shown to influence angiogenesis and vasculogenesis through increased expression of VEGF and SDF-1, respectively (Bao et al. 2006b; Folkins et al. 2009). Recently, the complexity of GSC influence on tumor vasculature has expanded based on studies demonstrating direct differentiation of GSCs to tumor endothelium and subsequent reduced tumor burden when GSC-derived endothelial cells are directly targeted (Ricci-Vitiani et al. 2010; Wang et al. 2010b). It has also been shown that therapeutic targeting of GSC-expressed VEGF with the humanized neutralizing anti-VEGF antibody bevacizumab (Avastin) results in tumor inhibition in xenograft models (Bao et al. 2006b; Calabrese et al. 2007). Indeed, the demonstrated effectiveness of bevacizumab in recurrent GBM led to its recent FDA approval and it is currently in clinical trials in combination with other standard and investigational agents in newly diagnosed and recurrent GBM patients (Chamberlain 2010). More recently, it has been proposed that inhibition of vasculogenesis represents a novel mode of treatment to prevent recurrence (Kioi et al. 2010). While angiogenesis relies on the formation of new capillaries from pre-existing vessels, tumor vasculogenesis involves the recruitment of endothelial precursor cells or bone marrow-derived hematopoietic cells to initiate de-novo capillary formation. It has been reported that following radiation induced damage to tumor vasculature, the tumor becomes more dependent on vasculogenesis to reinitiate tumor growth. Koio et al. (2010) explored this hypothesis utilizing a murine intracranial GBM xenograft model and demonstrated that following radiation treatment there was a cascade of events that resulted in increased vasculogenesis. This cascade was initiated by increased hypoxia in the tumor, resulting in increased HIF-1 expression that led to increased levels of SDF-1. The chemokine receptor, CXCR4, is then activated by SDF-1 to promote recruitment of CD11b+ myelomonocytes to the tumor to form new blood vessels. Importantly, this process was inhibited using the FDA-approved drug, AMD3100, which blocks the interaction of SDF-1 with CXCR4 (Broxmeyer et al. 2005). Given the reported increased expression levels of SDF-1 in GSCs and their inherent radioresistance phenotype, it would be of significant therapeutic value to further define the role of GSCs in post-irradiation vasculogenesis.
The Glioma Stem Cell Niche
It is becoming increasingly clear that an integral component of GSC-related therapy resistance and tumor initiation is the specified location, or niche, where GSCs reside within the tumor. The niche for normal adult stem cells controls the balance between stem cell quiescence and tissue regeneration through the transmission of pro- or anti-proliferative signaling. While a direct parallel for niche-dependence in CSCs may not be apparent, the vascular endothelial cells have been shown to be an important factor for regulating stem cell maintenance in what is referred to as the perivascular niche (Calabrese et al. 2007; Tavazoie et al. 2008). Furthermore, in medulloblastoma, the perivascular niche has been reported to offer a protective advantage to CSCs following radiation treatment (Hambardzumyan et al. 2008). As discussed previously, disruption of the vasculature, and hence the perivascular niche, depletes the CSC population and halts tumor growth (Calabrese et al. 2007). More recent studies have focused on defining components of the perivascular niche that help maintain the CSC phenotype. Using a PDGF-induced glioma mouse model, it has been demonstrated that nitric oxide produced by endothelial cells contributes to tumor growth through activation of the Notch pathway (Charles et al. 2010). Interestingly, inhibition of the Notch pathway using gamma-secretase inhibitors has been shown to decrease the radioresistance phenotype of GSCs, highlighting a potential mechanism for targeting GSCs via disruption of niche signaling (Wang et al. 2010a). This approach is further supported by a recent report using a three-dimensional GBM explant system from patient derived specimens whereby the combination of Notch inhibition, shown in this study to target both the stem cells and endothelial cells, combined with radiation reduced stem cell expansion (Hovinga et al. 2010). Additionally, a population of GSCs reported to be CD44high/Id1high demonstrated a preferential localization in the perivascular niche and regulation by TGF-β (Anido et al. 2010). Importantly, the therapeutic potential of targeting these cells through anti-TGF-β treatment was validated in mouse models as well as in a patient sample obtained from a phase I-II trial. These in vivo results are intriguing, but caution must be exercised when evaluating CD44 expressing cells under current GSC culture conditions as FGF, a major component in the culturing media, has been reported to induce CD44 expression (Pollard et al. 2008).
Although seemingly counterintuitive to the aforementioned location within the presumed nutrient rich perivascular niche, GSCs have also been described to reside in a hypoxic niche. It is likely that GSCs reside within a hypoxic microenvironment transiently, prior to the induction of angiogenesis and/or vasculogenesis by the GSCs. Indeed, the increased GSC-mediated VEGF expression driving angiogenesis is likely a direct result of hypoxic induced stabilization of the HIF transcription factors, namely HIF2α, as depletion of HIF2α in GSCs resulted in depletion of VEGF (Li et al. 2009). Furthermore, HIF2α has been shown to regulate the transcription of stem cell genes crucial to maintaining the GSC phenotype and is required for self-renewal as measured by tumorsphere formation and tumor initiation in a xenograft model (Heddleston et al. 2009; Jogi et al. 2002; Li et al. 2009; McCord et al. 2009; Seidel et al. 2010). Not only does hypoxia have diverse effects on GSCs but it can drive non-stem cells into a more stem-like state (Bar et al. 2010; Heddleston et al. 2009; Soeda et al. 2009). Thus, the potential plasticity of the non-stem population should be taken into consideration when designing novel therapeutic strategies. Approaches aimed at targeting just the GSCs, and not the perivascular or hypoxic niches, may offer a selective advantage for the non-stem population to repopulate the tumor.
Conclusions and Future Directions
The complexity of malignant gliomas can be further appreciated with the identification of a hierarchal stem cell population. As we move forward in translating our understanding of the heterogeneous populations within the tumor to the design of more cell-specific and effective therapies, it is imperative to maintain a stringent definition of a CSC while ensuring careful interpretation of studies evaluating this phenotype. Of particular caution is the interpretation of differential responses between marker positive and negative cells in culture. CSCs were first defined from the direct sorting of fresh tissue based on functional differences in populations segregated by markers and not in culture. If cells are cultured, the markers may be less informative, and one cannot necessarily infer differences in positive and negative populations anymore. Additionally, it must be determined how CSCs contribute to recurrence following therapeutic intervention in order to incorporate new strategies that can eradicate these cells following primary presentation of the disease. Finally, correlating the cell of origin for glioma (covered elsewhere in this review issue) with the hierarchal CSC model will yield valuable insights to the development of the disease. That is, does the initiating tumor cell already demonstrate CSC properties or is there an evolution in the hierarchy? It is an exciting time in glioma biology whereby application of the conceptual and methodological framework of stem cell biology is leading to fresh insights into this terrible disease and hopefully, in the not to distant future, leading to improved patient care.
Acknowledgments
We thank members of the Rich lab for their constructive criticism. Work in the Rich lab is supported by the National Brain Tumor Society, Goldhirsh Foundation, and NIH grants NS054276 (JNR), CA129958 (JNR), CA116659 (JNR), CA154130 (JNR), and National Research Service Awards NS058042 (MV). JNR is a Damon Runyon-Lilly Clinical Investigator supported by the Damon Runyon Cancer Research Foundation. MV is supported by an American Brain Tumor Association Basic Research Fellowship. Work in the Dirks laboratory is funded by the Canadian Cancer Society Research Institute, the Canadian Institutes for Health Research, the Ontario Institute for Cancer Research, the Terry Fox Foundation, the Hospital for Sick Children Foundation, and Jessica's Footprint Foundation. Work in the Fine laboratory is funded by National Cancer Institute, National Institutes of Neurological Disorders and Stroke/National Institutes of Health.
References
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15(1):45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anido J, Saez-Borderias A, Gonzalez-Junca A, Rodon L, Folch G, Carmona MA, Prieto-Sanchez RM, Barba I, Martinez-Saez E, Prudkin L, et al. TGF-beta Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18(6):655–68. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, McLendon RE, Hjelmeland AB, Rich JN. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68(15):6043–8. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006a;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006b;66(16):7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. 2010;177(3):1491–502. doi: 10.2353/ajpath.2010.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier D, Rohrl S, Pillai DR, Schwarz S, Kunz-Schughart LA, Leukel P, Proescholdt M, Brawanski A, Bogdahn U, Trampe-Kieslich A, et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68(14):5706–15. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4(3):226–35. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466(7302):133–7. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Broadley KW, Hunn MK, Farrand KJ, Price KM, Grasso C, Miller RJ, Hermans IF, McConnell MJ. Side Population is not Necessary or Sufficient for a Cancer Stem Cell Phenotype in Glioblastoma Multiforme. Stem Cells. 2010 doi: 10.1002/stem.582. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Chamberlain MC. Emerging clinical principles on the use of bevacizumab for the treatment of malignant gliomas. Cancer. 2010;116(17):3988–99. doi: 10.1002/cncr.25256. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Hsu CC, Yung MC, Chen KY, Tzao C, Wu WF, Chou HY, Lee YY, Lu KH, Chiou SH, et al. Enhanced radiosensitivity and radiation-induced apoptosis in glioma CD133-positive cells by knockdown of SirT1 expression. Biochem Biophys Res Commun. 2009;380(2):236–42. doi: 10.1016/j.bbrc.2009.01.040. [DOI] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6(2):141–52. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15(3):504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- Facchino S, Abdouh M, Chatoo W, Bernier G. BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. J Neurosci. 2010;30(30):10096–111. doi: 10.1523/JNEUROSCI.1634-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69(18):7243–51. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401(6751):390–4. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22(4):436–48. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MA, Yang H, Low BE, Mukherjee J, Guha A, Bronson RT, Shultz LD, Israel MA, Yun K. Cancer stem cells are enriched in the side population cells in a mouse model of glioma. Cancer Res. 2008;68(24):10051–9. doi: 10.1158/0008-5472.CAN-08-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8(20):3274–84. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100(25):15178–83. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101(39):14228–33. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovinga KE, Shimizu F, Wang R, Panagiotakos G, Van Der Heijden M, Moayedpardazi H, Correia AS, Soulet D, Major T, Menon J, et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010;28(6):1019–29. doi: 10.1002/stem.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- Jacques TS, Swales A, Brzozowski MJ, Henriquez NV, Linehan JM, Mirzadeh Z, O'Malley C, Naumann H, Alvarez-Buylla A, Brandner S. Combinations of genetic mutations in the adult neural stem cell compartments determine brain tumour phenotypes. EMBO J. 2010;29(1):222–35. doi: 10.1038/emboj.2009.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Pahlman S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci U S A. 2002;99(10):7021–6. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317(5836):337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120(3):694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6(5):421–32. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15(6):501–13. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord AM, Jamal M, Shankavaram UT, Lang FF, Camphausen K, Tofilon PJ. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Mol Cancer Res. 2009;7(4):489–97. doi: 10.1158/1541-7786.MCR-08-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43(5):935–46. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11(3):220–8. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, et al. Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery. 2008;62(2):505–14. doi: 10.1227/01.neu.0000316019.28421.95. discussion 514-5. [DOI] [PubMed] [Google Scholar]
- Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6(6):603–15. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Pollard SM, Wallbank R, Tomlinson S, Grotewold L, Smith A. Fibroblast growth factor induces a neural stem cell phenotype in foetal forebrain progenitors and during embryonic stem cell differentiation. Mol Cell Neurosci. 2008;38(3):393–403. doi: 10.1016/j.mcn.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4(6):568–80. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456(7222):593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read TA, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, Febbo PG, Wechsler-Reya RJ. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15(2):135–47. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- Sauvageot CM, Weatherbee JL, Kesari S, Winters SE, Barnes J, Dellagatta J, Ramakrishna NR, Stiles CD, Kung AL, Kieran MW, et al. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro Oncol. 2009;11(2):109–21. doi: 10.1215/15228517-2008-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, et al. Identification of cells initiating human melanomas. Nature. 2008;451(7176):345–9. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel S, Garvalov BK, Wirta V, von Stechow L, Schanzer A, Meletis K, Wolter M, Sommerlad D, Henze AT, Nister M, et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain. 2010;133(Pt 4):983–95. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138(5):822–9. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Shervington A, Lu C. Expression of multidrug resistance genes in normal and cancer stem cells. Cancer Invest. 2008;26(5):535–42. doi: 10.1080/07357900801904140. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–8. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T, Kassam AB, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28(45):3949–59. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4(5):440–52. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Tamase A, Muraguchi T, Naka K, Tanaka S, Kinoshita M, Hoshii T, Ohmura M, Shugo H, Ooshio T, Nakada M, et al. Identification of tumor-initiating cells in a highly aggressive brain tumor using promoter activity of nucleostemin. Proc Natl Acad Sci U S A. 2009;106(40):17163–8. doi: 10.1073/pnas.0905016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122(4):761–8. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, Rich JN, Sullenger BA. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010a;28(1):17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010b;468(7325):829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Lee L, Graham K, Satkunendran T, Yoshikawa K, Ling E, Harper L, Austin R, Nieuwenhuis E, Clarke ID, et al. Multipotent CD15+ cancer stem cells in patched-1-deficient mouse medulloblastoma. Cancer Res. 2009;69(11):4682–90. doi: 10.1158/0008-5472.CAN-09-0342. [DOI] [PubMed] [Google Scholar]