Abstract
An early study performed in Bart Hoebel’s laboratory suggested that dopamine (DA) signaling in the nucleus accumbens was involved in learned flavor preferences produced by post-oral nutritive feedback. This paper summarizes our studies investigating the role of DA in flavor preferences conditioning using selective DA receptor antagonists. Food-restricted rats were trained to prefer a flavored saccharin solution (CS+) paired with intragastric (IG) sugar infusions over a flavored saccharin solution (CS−) paired with water infusions. Systemic injections of a D1-like receptor antagonist (SCH23390), but not a D2-like receptor antagonist (raclopride) during training blocked flavor preference learning. Neither drug prevented the expression of an already learned preference except at high doses that greatly suppressed total intakes. Central sites of action were examined by local microinjections of SCH23390 (12 nmol) during flavor training or testing. Drug infusions in the nucleus accumbens, amygdala, medial prefrontal cortex, or lateral hypothalamus during training blocked or attenuated CS+ flavor conditioning by IG glucose infusions. The same drug dose did not suppress the expression of a learned CS+ preference. The findings suggest that DA signaling within different components of a distributed brain network is involved in sugar-based flavor preferences. A possible role of DA in conditioned increases in flavor acceptance is discussed.
Keywords: Flavor conditioning, Sugar, Intragastric infusions
1. Introduction
There is considerable interest in the role of the dopamine (DA) reward system in normal and disordered feeding behavior as evidenced by publication of several recent reviews (Figlewicz et al., 2010;Fortuna, 2010;Kenny, 2011;Narayanan et al., 2010;Stice et al., 2010;Volkow et al., 2011;Vucetic et al., 2010). Bart Hoebel and his colleagues have published seminal studies on this topic during the last 25 years (Geiger et al., 2009;Hernandez et al., 1988a;Hernandez et al., 1988b;Hoebel, 1985;Rada et al., 2010). In this brief review, we describe how their early work greatly influenced our research program on learned food preferences.
In an early microdialysis study, Hernandez and Hoebel (1988a) reported feeding by hungry rats promoted DA release in the nucleus accumbens (NAc). They also observed NAc DA release in both hungry rats bar pressing for food pellets as well as in sated animals stimulated to eat by lateral hypothalamic electrical stimulation at sites that support self-stimulation behavior (Hernandez et al., 1988a;Hernandez et al., 1988b). Hernandez and Hoebel concluded that NAc DA was clearly related to feeding but its exact role was not clear. In a subsequent experiment, Mark, Blander and Hoebel (1991) investigated the relationship between NAc DA and the positive reward quality of the food taste. For this purpose, they examined whether the sweet taste of a noncaloric saccharin solution would stimulate DA release and, most importantly, if this response was altered in animals that developed a conditioned aversion to saccharin. Their results showed that intraoral infusions of saccharin stimulated NAc DA in naïve rats, but the same saccharin infusions significantly reduced DA release in rats previously conditioned to avoid the sweet solution by pairing it with LiCl injections. These findings indicated that the NAc DA response was related to the positive reward quality of the saccharin solution, not to its sweet taste per se, or alternatively, its arousing properties. The findings also provided early evidence that learning could modify the neurochemical response to food in mammals.
Mark et al. (1994) followed up on this learning effect by determining if a conditioned taste preference would increase NAc DA release, that is, have the opposite effect of a conditioned taste aversion. We had previously demonstrated that robust flavor (or taste) preferences were conditioned in rats by training them to drink a flavored solution paired with intragastric (IG) infusions of Polycose (a glucose polymer) (Elizalde et al., 1990;Sclafani et al., 1988;Sclafani, 1991). This conditioning effect was so strong that it reversed the rat's normal avoidance of a bitter taste to a strong preference (Sclafani, 1991). Using our IG conditioning technique, Mark et al. (1994) trained rats to prefer a bitter solution (sucrose octa acetate, the CS+) paired with IG Polycose infusions over a sour solution (citric acid, the CS−) paired with IG water infusions during 20 h/day training sessions. A control group was exposed to the same solutions but not paired with IG infusions. The rats were then fitted with a NAc microdialysis probe, water deprived overnight and offered the CS+ or CS− solution (without IG infusion) for 30 min during a dialysis session. The IG conditioned rats displayed a 28% increase in DA release during the CS+ drinking session, but no change during the CS− session. The control rats showed no DA response as they drank the bitter solution that served as the CS+ for the conditioned animals. Importantly, the conditioned and control groups consumed similar amounts of CS+ and CS− solutions during the microdialysis tests so that the differential DA response displayed by the conditioned animals was not related to amount of fluid consumed. Taken together, the results of Mark et al. (1991;1994) demonstrate that conditioned increases and decreases in the reward value of taste stimuli produced by positive and negative post-oral consequences, respectively, are associated with complementary changes in NAc DA release.
2. Dopamine mediation of post-oral sugar conditioning: systemic studies
Based on the studies of Mark et al. (1994), we investigated the role of dopamine in flavor preference conditioning by sugars using DA receptor antagonist drugs. In early studies, we reported that systemic injection of the DA receptor antagonist pimozide reduced the rat's intake of a palatable, sweet solution (glucose + saccharin) more than that of plain water (Xenakis et al., 1981). Furthermore, the drug effect on licking patterns was similar to that produced by diluting the sweet solution (Xenakis et al., 1982). These results suggested that DA antagonism suppressed intake by reducing the reward value of sweet taste. A similar conclusion was reached by G.P. Smith and colleagues based on sham feeding studies which revealed that similar changes in sucrose licking and microstructure patterns were produced by pimozide and sugar dilution (Geary et al., 1985;Schneider et al., 1990). Because the sham-feeding procedure minimized post-oral effects, these findings demonstrated that DA receptor antagonism reduced the reward potency of sugar taste.
To determine if DA antagonism also interferes with the post-oral reward effect of sugars, we investigated drug effects on the flavor preferences conditioned by IG sugar infusions. Food-restricted rats were trained to drink flavored saccharin solutions (e.g., grape or cherry) during daily 30-min sessions. On alternate one-bottle training sessions, the intake of CS+ flavored solution was paired with an IG infusion of sugar (sucrose or glucose) and the intake of CS− flavored solution was paired with IG water infusion; infusion volumes were matched to CS volume intakes. In subsequent two-bottle tests the rats were given the choice between the CS+ vs. CS− solutions without IG infusions. Sugar conditioning was evaluated by analyzing percent CS+ intake (CS+ intake/total intake) with 100% indicating a total preference and 50% score no preference. Our initial study (Azzara et al., 2001) determined the effects of systemic injections of D1-like (SCH23390) and D2-like (raclopride) receptor antagonists on flavor conditioning. The effects of the drugs on the acquisition of learned preference were investigated by injecting separate groups of rats with drug or vehicle (saline) prior to the daily one-bottle training sessions. Flavor preferences were then evaluated in two-bottle choice tests without the drug. To control for the reduced training intakes produced by the DA antagonists, yoked vehicle-treated controls had their CS+ and CS− intakes limited to that of the drug groups. Other vehicle control groups were trained with unlimited access to the CS solutions. These control groups developed strong CS+ preferences and were used to evaluate the effects of DA antagonism on the expression of the learned preferences. This was accomplished by injecting the control rats with the DA antagonists just prior to the CS+ vs. CS− two-bottle tests.
Our first experiment revealed that treating rats with the D2 antagonist raclopride (200 or 400 nmol/kg) throughout training did not significantly alter flavor conditioning. The Raclopride group displayed a 77% preference for the CS+ flavor which was slightly greater than that displayed by the yoked control group (68%). A control group with unlimited training intakes displayed the strongest CS+ preference (85%). When treated with raclopride prior to two-bottle testing, the control rats displayed a selective decrease in their CS+ intake but they continued to drink more CS+ than CS− except at the highest dose tested (800 mg/kg) which reduced overall intakes to near zero. The dose-dependent reduction in the CS+ flavored saccharin solution intake is consistent with prior reports that D2 antagonists suppress sweet solution intakes (Geary et al., 1985;Schneider et al., 1986;Xenakis et al., 1981;Xenakis et al., 1982). Yet, our findings indicated that D2 receptors are not critically involved in the acquisition or expression of the flavor preference conditioned by IG sugar infusions.
In contrast to these results, a second experiment revealed that rats treated with a D1-like antagonist (SCH23390, 200 nmol/kg) throughout training showed no preference for the CS+ flavor (50%) in the subsequent two-bottle test while the yoked control and control groups exhibited significant CS+ preferences of 72% and 80%, respectively. The control group continued to prefer the CS+ (by 76%) when injected with 200 nmol/kg SCH23390 prior to testing although their preference was reduced to 68% by a higher drug dose (400 nmol/kg) and was no longer significant. These findings demonstrate that the acquisition of a sugar-conditioned preference is dependent upon D1 receptor activity, but the expression of an already learned preference is much less affected by D1 receptor antagonism. The differential effects of the D1-like and D2-like receptor antagonists on flavor conditioning by IG sugar infusions are consistent with other flavor learning studies. In particular, D1-like, but not D2-like, receptor antagonists blocked the acquisition of LiCl-induced saccharin aversions in rats (Caulliez et al., 1996;Fenu et al., 2001). It should be noted that while the SCH23390 and raclopride drugs used in these studies are referred to as D1-like and D2-like antagonists, respectively, they act on multiple aspects of the DA receptor super-families in complex ways (Beaulieu et al., 2011) including activities at molecular, pharmacodynamic and genetic levels (Chen et al., 2009;Le Foll et al., 2009;Malo et al., 2010).
3. Dopamine mediation of post-oral sugar conditioning: central studies
In follow-up studies, we explored possible brain sites of action where D1-like signaling mediates flavor conditioning by IG sugar infusions. The microdialysis findings of Mark et al. (1994) pointed to the NAc as the first brain site to investigate. In addition, other investigators reported that DA receptor antagonism in the NAc impairs sugar reinforced learning in Pavlovian and operant tasks, and blocks sweet taste aversion learning produced by LiCl treatment (Fenu et al., 2001;Parkinson et al., 2002;Smith-Roe et al., 2000).
Rats were fitted with bilateral brain injection cannulae in the shell region of the NAc and with a gastric infusion catheter (Touzani et al., 2008). They were then trained in alternating one-bottle sessions to consume a CS+ flavored saccharin solution (e.g., grape) paired with IG infusion of 8% glucose and a CS− flavored saccharin solution (e.g., cherry) paired with IG water infusion. A series of two-bottle CS+ vs. CS− choice tests (with no IG infusions) was next conducted following bilateral microinjections of 0, 12, 24 or 48 nmol SCH23390 (doses are expressed as total nmol/brain). Only the D1-like antagonist was studied because systemic D2-like antagonism did not block flavor preference learning (Azzara et al., 2001). Following the 0 nmol (vehicle) microinjection the rats displayed a strong CS+ preference (92%). The SCH23390 microinjections produced dose-dependent reductions in CS+ intake, but the 12 and 24 nmol doses did not significantly suppress the CS+ preference (81, 80%) relative to 0 nmol treatment. Only the 48 nmol dose eliminated the expression of the preference, but it also reduced total CS intakes to near zero. We next determined the effects of D1 receptor antagonism in NAc shell or core on the acquisition of the CS+ preference (Touzani et al., 2008). Separate groups of rats were given bilateral injections of vehicle or 12 nmol SCH23390 prior to daily one-bottle training sessions with the CS+ and CS−. The training intakes of the vehicle control groups were limited to that of the drug groups and all rats were given fixed 8 ml infusions of glucose and water during CS+ and CS− sessions, respectively. In the post-training two-bottle choice tests, the rats given SCH23390 microinjections in the NAc shell or core did not display significant CS+ preferences (61%, 55%, respectively) in contrast to strong preferences displayed by the vehicle groups (83%, 89%). Thus, D1-like receptor signaling in the NAc is critical for the acquisition but not the expression of flavor preferences conditioned by IG sugar infusions (Touzani et al., 2008).
The NAc is one part of the mesocorticolimbic DA system implicated in reward processing. DA neurons originating in the ventral tegmental area (VTA) project not only to the NAc but to other areas including the amygdala (AMY) and medial prefortal cortex (mPFC) (Swanson, 1982). We previously observed that excitotoxic lesions of the AMY block flavor preference conditioning by IG Polycose infusions (Touzani et al., 2005), and other findings suggested that D1 receptors may be involved. In particular, D1 receptor antagonism blocked the acquisition of a sugar-reinforced operant response (Andrzejewski et al., 2005), and DA release in the AMY was stimulated by signals predictive of sugar, as well as by feeding and gastric nutrient infusion (Hajnal et al., 1997;Harmer et al., 1999;Heffner et al., 1980). We therefore next investigated the effects of D1-like receptor antagonism in the AMY on the expression and acquisition of sugar-conditioned flavor preferences (Touzani et al., 2009). Following IG glucose training, rats displayed a significant CS+ preference (89%) after vehicle injections in the AMY. Dose- related reductions in CS+ intake were produced by SCH23390, but the expression of the CS+ preference was preserved at the 12 nmol dose (81%) and nearly so at the 24 nmol dose (75%, p=0.087), but not at the 48 nmol dose that suppressed CS intake to near zero. In an acquisition experiment, rats treated with 12 nmol SCH23390 microinjections in the AMY throughout training did not develop a CS+ preference (55%), unlike the vehicle-treated rats (81%). Additional experiments revealed that SCH23390 microinjections specifically targeting the basolateral or central amygdalar nuclei attenuated (59%, 73%), but did not completely block the acquisition of CS+ preference conditioning. Consistent with these findings, whereas large AMY lesions blocked preference conditioning, selective basolateral lesions only attenuated the flavor learning (Touzani et al., 2005).
Like the AMY, studies of the mPFC reported DA efflux was induced by food-related cues as well as eating, and D1-like receptor antagonism blocked sucrose-reinforced operant learning (Baldwin et al., 2002;Bassareo et al., 2002;Hernandez et al., 1990). In light of these results, we fitted rats with bilateral mPFC cannulae and trained them in our IG glucose conditioning paradigm (Touzani et al., 2010a). In two-bottle tests, expression of a strong CS+ preference was observed following vehicle microinjections in the mPFC (90%) as well as following microinjections of 12 and 24 nmol SCH23390 (93 and 87%) although the drug produced dose-related reductions in CS+ intake. (The 48 nmol dose was not included because of its near-total suppression of drinking observed in our prior studies). In contrast, microinjections of 12 nmol SCH23390 during training completely blocked the acquisition of the CS+ preference (50%).
A fourth brain site surveyed in our central studies is the lateral hypothalamus, which is innervated by DA neurons of the A13 cell group located in the zona incerta (Wagner et al., 1995). Prior excitotoxic lesion studies indicated LH involvement in both flavor preference and aversion learning (Touzani et al., 2001;Touzani et al., 2002) and other investigators reported that D1, but not D2-like receptor antagonism in the LH blocked flavor aversion learning (Caulliez et al., 1996). With our IG glucose conditioning protocol, we (Touzani et al., 2009b) found that LH microinjections of SCH23390 (12 nmol) during training attenuated the learning of a CS+ preference compared to vehicle treatment (61 vs. 87%). The same drug dose, however, had no effect on the expression of a previously conditioned CS+ preference (90% vs. 88% after vehicle) although it significantly reduced CS+ intake.
4. Discussion
Stimulated by the seminal studies performed in Bart Hoebel's laboratory, we investigated the involvement of brain DA systems in flavor preference conditioned by the post-oral actions of sugar. In our initial systemic study, antagonism of D1-like, but not D2-like receptors blocked flavor preference conditioning by IG sugar infusions. Subsequent central studies extended this finding and obtained highly consistent results: microinjections of SCH23390 in the NAc, AMY, mPFC, or LH blocked or significantly attenuated the acquisition of CS+ flavor preference learning produced by IG glucose infusions. Yet, the same drug dose (12 nmol) had no effect on the expression of a previously learned preference. As discussed elsewhere, these findings suggest the existence of an integrated DA network that is essential for post-oral flavor conditioning by sugars (Touzani et al., 2009;Touzani et al., 2010a;Touzani et al., 2010b). Direct evidence for the activation of this network by sugar is provided by the recent finding of Ren et al. (2010) that IG glucose infusions increase NAc DA release in mice. These authors hypothesized that glucose-based preference conditioning is due to the post-absorptive metabolic effects of the sugar, but findings from our laboratory implicate a pre-absorptive site of action. In particular, Ackroff et al. (2010) observed flavor preference conditioning by intestinal but not hepatic-portal glucose infusions in rats trained with a CS+ flavored saccharin solution. Much remains to be learned about the post-oral detection of glucose and how it activates central neural systems mediating flavor learning.
While D1-like receptor signaling is essential for rats to acquire a flavor preference based on the post-oral actions of sugar, our findings indicate that, once learned, the expression of the preference is relatively unaffected by D1-like receptor antagonism. Yet Mark et al. (1994) observed that following IG conditioning, the intake of the CS+ solution stimulated DA release in the NAc. It may be that this conditioned DA release, while not critical for the expression of the CS+ preference per se, influences the absolute intake of the CS+ solution. Several studies show that IG sugar infusions can substantially increase the intake of a CS+ solution during one-bottle sessions. This conditioned “acceptance” response, however, is not observed in all test situations and may be obscured when animals are highly motivated to drink because of their deprivation state or other factors. Thus, Mark et al. (1994) reported that, when water-restricted, their conditioned rats consumed similar amounts of the bitter and sour CS+ and CS− solutions during the dialysis sessions, although only the CS+ solution stimulated NAc DA release. In a subsequent study using the same CS solutions, we observed that food-restricted or non-deprived animals consumed significantly more of the CS+ than CS− (Pérez et al., 1998). The same study also reported that this conditioned increase in CS+ acceptance wanes with repeated extinction testing (i.e., without IG glucose infusions) unlike the conditioned CS+ preference which is rather resistant to extinction. It is possible that the conditioned increase in DA release observed by Mark et al. (1994) mediates the increased CS+ intake produced by IG sugar conditioning. Additional research is needed to explore DA involvement in the expression and extinction of conditioned flavor acceptance.
The present paper has focused exclusively on post-oral sugar conditioning but flavor preferences are also conditioned by the sweet taste of sugars. As reviewed elsewhere (Touzani et al., 2010b), this flavor-taste learning also involves brain DA signaling although in this case, both D1-like and D2-like receptors are implicated. However, while SCH23390 (12 nmol) microinfusions in the NAc and AMY blocked the acquisition of post-oral sugar conditioned preferences, they did not prevent the acquisition of flavor preferences by the sweet taste of sugar although the preferences extinguished with repeated testing (Bernal et al., 2009;Bernal et al., 2008). Thus, D1-like receptor antagonism in the NAc and AMY had selective effects on flavor preferences conditioned by the sweet taste and post-oral consequences of sugar.
There is renewed interest in the DA reward system because of its possible role in the current obesity epidemic. According to one view, individuals with a hypersensitive DA reward system find food more rewarding and are therefore at risk for overeating and obesity (Davis et al., 2007; Dawe et al., 2004; Stice et al., 2008). There is also an opposing view that reduced food reward may, paradoxically, stimulate overeating in some individuals. This "reward deficiency" hypothesis emerged in part from clinical studies reporting decreased striatal D2 receptor expression and reduced striatal activation in response to food cues in obese compared to lean individuals (Stice et al., 2010a; Wang et al., 2009). Based on these and other findings, various investigators have hypothesized that "deficiency in DA pathways may lead to pathologic eating as a means to compensate for an understimulated reward system" (Davis et al., 2008a; Stice et al., 2010a; Stice et al., 2010b; Wang et al., 2009). These theories are not mutually exclusive and it is possible that the DA reward system changes over time from being hyper- to hyposensitive to food reward (Stice et al., 2011). Animal studies provide evidence for DA dysfunction in obesity although there are reports of both increased and decreased food reward in obese animal models (Geiger et al., 2008;Geiger et al., 2009;Hajnal et al., 2008; La Fleur et al., 2007; Thanos et al., 2008). Additional studies are required to explicate the involvement of the DA reward system in overeating and obesity. In view of our results demonstrating the importance of DA receptor activity in flavor preference learning in rats, it is possible that alterations in DA reward sensitivity in humans may disrupt food preference learning which may, in turn, influence food consumption.
Finally, flavor learning involves multiple neurotransmitter systems and published studies have investigated the effects of opioid, cannabinoid, benzodiazepine, and orexin receptor antagonists in this process (Azzara et al., 2000;Baker et al., 2004;Bernal et al., 2010;Bonacchi et al., 2010;Dwyer, 2009;Golden et al., 2007;Mediavilla et al., 2011;Yu et al., 1999). The interaction of these and other receptor systems in the acquisition and expression of conditioned food preferences awaits further study.
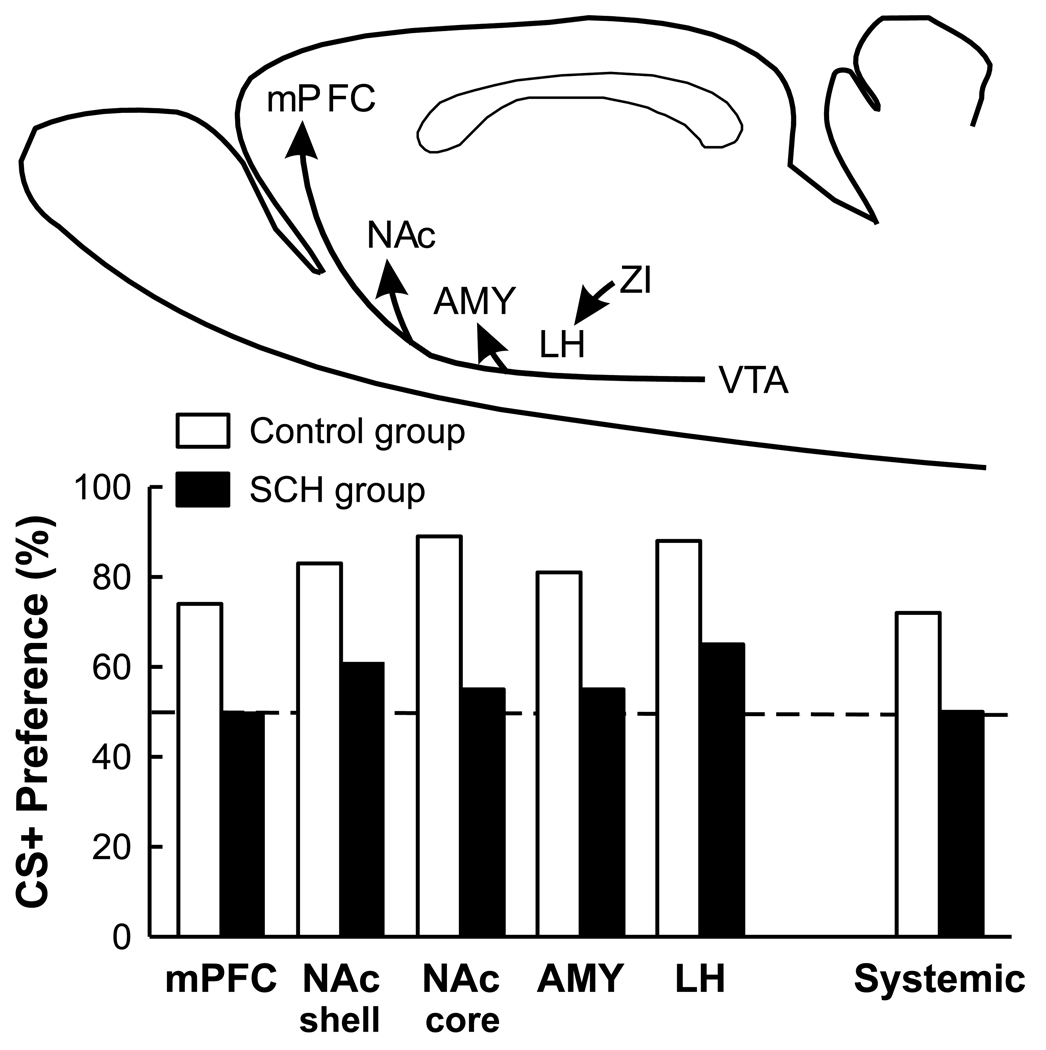
Figure 1.
Effects of D1-like receptor antagonism with SCH23390 on the acquisition of CS+ flavor preferences conditioned by IG sugar infusions. The bars represent percent CS+ intakes during four CS+ vs. CS- choice test (30 min/day) conducted after one-bottle training with the CS+ paired with IG sugar infusions and CS- paired with IG water infusions. The SCH group was treated with SCH23390 and Control group was treated with vehicle throughout training. Training CS+ and CS- intakes of the control rats were limited to the mean of the SCH rats. In the systemic study (Azzara et al., 2001), rats were given intraperitoneal injections of vehicle or SCH23390 (200 nmol/kg) 15 min prior to the training sessions. In the central studies, rats were given microinfusions of vehicle or SCH23390 (12 nmol/brain) in the NAc shell or core (Touzani et al., 2008), AMY (Touzani et al., 2009), mPFC (Touzani et al., 2010), or LH (Touzani et al., 2009) 10 min prior to the training sessions. No injections were given prior to the two-bottle choice tests. The arrows in the brain diagram are schematic representations of the DA projection pathways from the VTA to the NAc, AMY, and mPFC, and from the ZI to the LH. Abbreviations: VTA, ventral tegmental area; NAc, nucleus accumbens; AMY, amygdala, mPFC, medial prefrontal cortex; LH, lateral hypothalamus; ZI, zona incerta.
Acknowledgements
This paper is based on a presentation by the senior author at a Festschrift held in honor of Professor Bartley Hoebel at Princeton University, January 14, 2011. The senior author thanks Bart Hoebel for his inspired science and forty years of friendship.
The research conducted in the authors’ laboratories was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK071761. The authors thank Karen Ackroff for her helpful comments on this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav. 2010;99:402–411. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski ME, Spencer RC, Kelley AE. Instrumental learning, but not performance, requires dopamine D1-receptor activation in the amygdala. Neuroscience. 2005;135:335–345. doi: 10.1016/j.neuroscience.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. Naltrexone fails to block the acquisition or expression of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2000;67:545–557. doi: 10.1016/s0091-3057(00)00395-6. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D1 but not D2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2001;68:709–720. doi: 10.1016/s0091-3057(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Baker RW, Li Y, Lee M, Sclafani A, Bodnar RJ. Naltrexone does not prevent acquisition or expression of flavor preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2004;78:239–246. doi: 10.1016/j.pbb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Kelley AE. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J Neurosci. 2002;22:1063–1071. doi: 10.1523/JNEUROSCI.22-03-01063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bernal S, Miner P, Abayev Y, Kandova E, Gerges M, Touzani K, Sclafani A, Bodnar RJ. Role of amygdala dopamine D1 and D2 receptors in the acquisition and expression of fructose-conditioned flavor preferences in rats. Behav Brain Res 2009. 2009:183–190. doi: 10.1016/j.bbr.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A, Bodnar RJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behav Brain Res. 2008;190:59–66. doi: 10.1016/j.bbr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal SY, Touzani K, Gerges M, Abayev Y, Sclafani A, Bodnar RJ. Opioid receptor antagonism in the nucleus accumbens fails to block the expression of sugar-conditioned flavor preferences in rats. Pharmacol Biochem Behav. 2010;95:56–62. doi: 10.1016/j.pbb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacchi KB, Ackroff K, Touzani K, Bodnar RJ, Sclafani A. Opioid mediation of sugar and starch preference in the rat. Pharmacol Biochem Behav. 2010;96:507–514. doi: 10.1016/j.pbb.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulliez R, Meile M-J, Nicolaidis S. A lateral hypothalamic D1 dopaminergic mechanism in conditioned taste aversion. Brain Res. 1996;729:234–245. [PubMed] [Google Scholar]
- Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung Med J. 2009;32:148–154. [PubMed] [Google Scholar]
- Dwyer DM. The effects of midazolam on the acquisition and expression of fructose- and maltodextrin-based flavour preferences. Pharmacol Biochem Behav. 2009;91:503–510. doi: 10.1016/j.pbb.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric Polycose infusions: A detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47:63–77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- Fenu S, Bassareo V, Di Chiara G. A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J Neurosci. 2001;21:6897–6904. doi: 10.1523/JNEUROSCI.21-17-06897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Sipols AJ. Energy regulatory signals and food reward. Pharmacol Biochem Behav. 2010;97:15–24. doi: 10.1016/j.pbb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42:147–151. doi: 10.1080/02791072.2010.10400687. [DOI] [PubMed] [Google Scholar]
- Geary N, Smith GP. Pimozide decreases the positive reinforcing effects of sham fed sucrose in the rat. Pharmacol Biochem Behav. 1985;22:787–790. doi: 10.1016/0091-3057(85)90528-3. [DOI] [PubMed] [Google Scholar]
- Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden GJ, Houpt TA. NMDA receptor in conditioned flavor-taste preference learning: Blockade by MK-801 and enhancement by D-cycloserine. Pharmacol Biochem Behav. 2007;86:587–596. doi: 10.1016/j.pbb.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Lenard L. Feeding-related dopamine in the amygdala of freely moving rats. Neuroreport. 1997;8:2817–2820. doi: 10.1097/00001756-199708180-00033. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated D-amphetamine. Neuroscience. 1999;90:119–130. doi: 10.1016/s0306-4522(98)00464-3. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Hartman JA, Seiden LS. Feeding increases dopamine metabolism in the rat brain. Science. 1980;208:1168–1170. doi: 10.1126/science.7375926. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988a;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988b;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding can enhance dopamine turnover in the prefrontal cortex. Brain Res Bull. 1990;25:975–979. doi: 10.1016/0361-9230(90)90197-8. [DOI] [PubMed] [Google Scholar]
- Hoebel BG. Brain neurotransmitters in food and drug reward. Am J Clin Nutr. 1985;42:1133–1150. doi: 10.1093/ajcn/42.5.1133. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Malo M, Brive L, Luthman K, Svensson P. Selective pharmacophore models of dopamine D(1) and D(2) full agonists based on extended pharmacophore features. ChemMedChem. 2010;5:232–246. doi: 10.1002/cmdc.200900398. [DOI] [PubMed] [Google Scholar]
- Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res. 1991;551:308–310. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- Mark GP, Smith SE, Rada PV, Hoebel BG. An appetitively conditioned taste elicits a preferential increase in mesolimbic dopamine release. Pharmacol Biochem Behav. 1994;48:651–660. doi: 10.1016/0091-3057(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Mediavilla C, Cabello V, Risco S. SB-334867-A, a selective orexin-1 receptor antagonist, enhances taste aversion learning blocks taste preference learning in rats. Pharmacol Biochem Behav. 2011;98:385–391. doi: 10.1016/j.pbb.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol. 2010;31:104–112. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Pérez C, Lucas F, Sclafani A. Increased flavor preference and acceptance conditioned by the postingestive actions of glucose. Physiol Behav. 1998;64:483–492. doi: 10.1016/s0031-9384(98)00104-8. [DOI] [PubMed] [Google Scholar]
- Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol Behav. 2010 doi: 10.1016/j.physbeh.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, De Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LH, Davis JD, Watson CA, Smith GP. Similar effect of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding. Eur J Pharmacol. 1990;186:61–70. doi: 10.1016/0014-2999(90)94060-b. [DOI] [PubMed] [Google Scholar]
- Schneider LH, Gibbs J, Smith GP. D-2 selective receptor antagonists suppress sucrose sham feeding in the rat. Brain Res Bull. 1986;17:605–611. doi: 10.1016/0361-9230(86)90231-5. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Conditioned food preferences. Bull Psychon Soc. 1991;29:256–260. [Google Scholar]
- Sclafani A, Nissenbaum JW. Robust conditioned flavor preference produced by intragastric starch infusions in rats. Am J Physiol. 1988;255:R672–R675. doi: 10.1152/ajpregu.1988.255.4.R672. [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Dagher A. Genetic variation in dopaminergic reward in humans. Forum Nutr. 2010;63:176–185. doi: 10.1159/000264405. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Activation of dopamine D1 receptors in the nucleus accumbens is critical for the acquisition, but not the expression, of flavor preference conditioned by intragastric glucose in rats. Eur J Neurosci. 2008;27:1525–1533. doi: 10.1111/j.1460-9568.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Dopamine D1-like receptor antagonism in amygdala impairs the acquisition of glucose-conditioned flavor preference in rats. Eur J Neurosci. 2009;30:289–298. doi: 10.1111/j.1460-9568.2009.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Acquisition of glucose-conditioned flavor preference requires the activation of dopamine D1-like receptors within the medial prefrontal cortex in rats. Neurobiol Learn Mem. 2010a;94:214–219. doi: 10.1016/j.nlm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Neuropharmacology of learned flavor preferences. Pharmacol Biochem Behav. 2010b;97:53–62. doi: 10.1016/j.pbb.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Sclafani A. Conditioned flavor preference and aversion: Role of the lateral hypothalamus. Behav Neurosci. 2001;115:84–93. doi: 10.1037/0735-7044.115.1.84. [DOI] [PubMed] [Google Scholar]
- Touzani K, Sclafani A. Lateral hypothalamic lesions impair flavor-nutrient and flavor-toxin trace learning in rats. Eur J Neurosci. 2002;16:2425–2433. doi: 10.1046/j.1460-9568.2002.02404.x. [DOI] [PubMed] [Google Scholar]
- Touzani K, Sclafani A. Critical role of amygdala in flavor but not taste preference learning in rats. Eur J Neurosci. 2005;22:1767–1774. doi: 10.1111/j.1460-9568.2005.04360.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med. 2010;2:577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Eaton MJ, Moore KE, Lookingland KJ. Efferent projections from the region of the medial zona incerta containing A13 dopaminergic neurons: A PHA-L anterograde tract-tracing study in the rat. Brain Res. 1995;677:229–237. doi: 10.1016/0006-8993(95)00128-d. [DOI] [PubMed] [Google Scholar]
- Xenakis S, Sclafani A. The effects of pimozide on the consumption of a palatable saccharin-glucose solution in the rat. Pharmacol Biochem Behav. 1981;15:435–442. doi: 10.1016/0091-3057(81)90274-4. [DOI] [PubMed] [Google Scholar]
- Xenakis S, Sclafani A. The dopaminergic mediation of a sweet reward in normal and VMH hyperphagic rats. Pharmacol Biochem Behav. 1982;16:293–302. doi: 10.1016/0091-3057(82)90163-0. [DOI] [PubMed] [Google Scholar]
- Yu W-Z, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: Effects of naltrexone. Pharmacol Biochem Behav. 1999;64:573–584. doi: 10.1016/s0091-3057(99)00124-0. [DOI] [PubMed] [Google Scholar]