Abstract
Manganese (Mn) exposure causes manganism, a neurological disorder similar to Parkinson’s disease. However, the cellular mechanism by which Mn impairs the dopaminergic neurotransmitter system remains unclear. We previously demonstrated that caspase-3-dependent proteolytic activation of protein kinase C delta (PKCδ) plays a key role in Mn-induced apoptotic cell death in dopaminergic neurons. Recently, we showed that PKCδ negatively regulates tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis, by enhancing protein phosphatase-2A activity in dopaminergic neurons. Here we report that Mn exposure can affect the enzymatic activity of TH, the rate-limiting enzyme in dopamine synthesis, by activating PKCδ - PP2A signaling pathway in a dopaminergic cell model. Low dose Mn (3–10 μM) exposure to differentiated mesencephalic dopaminergic neuronal cells for 3 h induced a significant increase in TH activity and phosphorylation of TH-Ser40. The PKCδ specific inhibitor rottlerin did not prevent Mn-induced TH activity or TH-Ser40 phosphorylation. On the contrary, chronic exposure to 0.1–1 μM Mn for 24 h induced a dose-dependent decrease in TH activity. Interestingly, chronic Mn treatment significantly increased PKCδ kinase activity and protein phosphatase 2A (PP2A) enzyme activity. Treatment with the PKCδ inhibitor rottlerin almost completely prevented chronic Mn-induced reduction in TH activity, as well as increased PP2A activity. Neither acute nor chronic Mn exposures induced any cytotoxic cell death or altered TH protein levels. Collectively, these results demonstrate that low dose Mn exposure impairs TH activity in dopaminergic cells through activation of PKCδ and PP2A activity.
Keywords: Manganese, Neurotoxicity, Dopamine synthesis, Protein Kinase C, phosphorylation, Parkinson’s disease
Introduction
Chronic exposure to manganese (Mn) has been shown to cause a Parkinson’s-like syndrome known as manganism. Increased incidences of manganism have been observed among miners and industrial welders, as well as farmers exposed to Mn-based pesticides such as the fungicides Maneb (Mn ethylene-bis-dithiocarbamate) and Mancozeb [2-[(dithiocarboxy)amino]ethyl]-carbamodithioato(2-)-κS,κS′]-zinc] (Roth and Garrick, 2003; Dobson et al., 2004). Adverse neurological effects of Mn also occurred in people who drank water containing high levels of Mn in many countries, and in abusers who used the Mn-containing compound Bazooka, a cocaine-based drug (Ensing, 1985). Exposures to infants and young children through infant food formulations have also been documented (Aschner and Aschner, 2005; Erikson et al., 2007). The recent introduction of the Mn-containing fuel additive methylcyclopentadienyl manganese tricarbonyl (MMT) to gasoline has raised concerns over potential chronic exposures to Mn.
Several lines of evidence suggest that exposure to Mn or Mn-containing compounds induces a variety of cellular changes including glutathione and dopamine depletion, increased oxidative stress, and impairment of energy metabolism and antioxidant systems (Kitazawa et al., 2002; Roth and Garrick, 2003; Dobson et al., 2004; Hirata et al., 2004; Olanow, 2004). Studies have also shown the role of Mn intoxication in astrocyte-derived injury to striatal-pallidal interneurons (Liu et al., 2006). The nigrostriatal system, including the globus pallidus and substantia nigra, is the primary target region of Mn neurotoxicity. Recently, we developed an in vitro model of dopaminergic neurotoxicity, namely N27 cells derived from the mesencephalon, a brain region directly affected by Parkinson’s disease. The N27 cells also represent a homogenous population of tyrosine hydroxylase-positive cells with functional characteristics similar to dopaminergic neurons, including dopamine synthesis and oxidative stress cellular signaling (Kaul et al., 2003a; Anantharam et al., 2004). We recently observed that caspase-3-mediated proteolytic activation of the proapoptotic kinase PKCδ plays a role in Mn-induced apoptosis in N27 mesencephalic dopaminergic cells (Latchoumycandane et al., 2005).
Depletion of dopamine in nigrostriatal neurons has been documented during Mn exposure (Goldstein and Lieberman, 1992; Aschner et al., 2006; Liu et al., 2006; Perl and Olanow, 2007; Erikson et al., 2008; Guilarte et al., 2008). Tyrosine hydroxylase (TH) is a rate-limiting enzyme in the biosynthesis of catecholamines and catalyzes the first step of a biochemical synthetic pathway in which L-tyrosine is converted to L-3,4-dihydroxyphenylalanine (L-dopa). Phosphorylation and dephosphorylation of TH represent important post-translational regulatory mechanisms of the enzymatic activity that in turn regulates the amount of catecholamine synthesized in a dopaminergic system (Goldstein and Lieberman, 1992; Dunkley et al., 2004; Fujisawa and Okuno, 2005). TH-Ser40 is a major residue that positively regulates the TH activity in vivo (Campbell et al., 1986; Wu et al., 1992). In addition to TH-Ser40 phosphorylation, TH activity can be regulated by phosphorylation of serine residues at positions 8, 19, 31 and 40, resulting in enhanced dopamine synthesis (Lee et al., 1989; Haycock, 1990; Haycock et al., 1992).
The phosphorylation state of TH can also be regulated by dephosphorylation reactions mediated by phosphatases. Protein phosphatase 2A (PP2A) has been demonstrated to be the major serine/threonine phosphatase that dephosphorylates TH, resulting in reduced TH activity (Haavik et al., 1989). Recently, we reported a novel functional interaction between PKCδ and TH, in which PKCδ negatively regulates TH activity and dopamine synthesis by enhancing PP2A activity in dopaminergic neurons (Zhang et al., 2007). Herein, we examined whether Mn exposure alters TH activity and dopamine synthesis by modulating PKCδ-PP2A signaling.
Methods
Chemicals
Manganese chloride (MnCl2, 99%), rottlerin, NSD-1015, dibutyryl cAMP, protease cocktail and anti-β-actin antibody were obtained from Sigma-Aldrich (St. Louis, MO); PhosphoTH-Ser40 antibody was purchased from Chemicon (Temecula, CA); Rabbit PKCδ antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The Bradford protein assay kit was purchased from Bio-Rad Laboratories (Hercules, CA). Anti-rabbit and anti-mouse secondary antibodies and the ECL chemiluminescence kit were purchased from Amersham Pharmacia Biotech (Piscataway, NJ). RPMI, fetal bovine serum, L-glutamine, penicillin, and streptomycin were purchased from Invitrogen (Gaithersburg, MD).
Cell culture models
Differentiated N27 cells were cultured, as described in our earlier publications (Adams et al., 1996; Anantharam et al., 2004). Cells were grown in RPMI 1640 medium containing 10% fetal bovine serum, 2 mM L-glutamine, 50 units of penicillin, and 50 μg/ml streptomycin. Cells were maintained in a humidified atmosphere of 5% CO2 at 37°C. N27 cells were differentiated with 2 mM dibutyryl cAMP for 3–5 days and then used for experiments described below.
Treatment paradigm
Differentiated N27 dopaminergic cells were exposed to 0.1–10 μM MnCl2 for the duration of the experiment. For measurement of TH activity, cells were exposed to 2 mM NSD-1015 for 1 h prior to MnCl2 treatment. Untreated cells were used as control samples. In inhibitor studies, 3 μM rottlerin was used for PKCδ inhibition. We derived the concentrations of rottlerin used in this study from previously published literature. Rottlerin inhibits PKCδ activity with a Ki of 3–6 μM, whereas rottlerin inhibits PKCδ, β, γ, ε and λ with Ki values at least 5–10 times higher (Gschwendt, 1999; Kanthasamy et al., 2003; Reyland, 2007). In our previous study, we showed 3–10 μM rottlerin attenuated kinase activity to a greater extent (Anantharam et al., 2002). For this study, 3 μM rottlerin was used, which was lower than the Ki values of other PKC isoforms.
Cytotoxicity assay
Cytotoxicity measurements were performed using Sytox Green assay, as described previously (Anantharam et al., 2007). Membrane-impermeable DNA dye Sytox Green readily enters dying cells, resulting in increased fluorescence. The intensity of fluorescence is directly proportional to the amount of dead cells. After growing N27 cells in 24-well plates for 24 h, cells were immediately exposed to 300 μM MPP+ in the presence of NADPH oxidase inhibitors (100–1000 μM AEBSF, 100–1000 μM apocynin and 3–30 μM DPI) in a 1 μM Sytox-containing growth media. After 24 h, cytotoxic cell death was quantified by measuring DNA-bound Sytox Green using a Gemini fluorescence microplate reader (Ex 485 nm and Em 538 nm, Molecular Devices Corporation). Fluorescent images of Sytox-positive cells were taken after 24 h exposure with a NIKON TE2000 microscope, and pictures were captured with a SPOT digital camera.
Tyrosine hydroxylase activity
TH enzyme activity was measured by the modified method of Hayashi et al., in which DOPA levels are quantified as an index of TH activity after inhibition of DOPA decarboxylase with the decarboxylase inhibitor NSD-1015 (Hayashi et al., 1988). Briefly, cells were incubated with Krebs-HEPES buffer (pH 7.4) containing 2 mM NSD-1015 at 37°C for 1 h, and then subjected to the treatment paradigm, as described earlier. After treatment, cells were collected and resuspended in antioxidant solution, sonicated, and centrifuged. DOPA levels in the supernatants were measured by high-performance liquid chromatography with electrochemical detection (HPLC-EC). Samples were prepared as described previously (Kitazawa et al., 2001; Sun et al., 2006). Neurotransmitters were extracted from samples using 0.1 M perchloric acid containing 0.05% Na2 EDTA and 0.1% Na2S2O5. The extracts were filtered in 0.22 micron spin tubes and 20 μl of the samples were loaded for analysis. DOPA was separated isocratically by a reversed-phase column with a flow rate of 0.7 ml/min. An HPLC system (ESA Inc., Bedford, MA) with an ESA automatic sampler (model 542) was used for these experiments. The electrochemical detection system consisted of an ESA coulochem model 5100A with a microanalysis cell model 5014A and a guard cell model 5020 (ESA Inc., Bedford, MA). The peak areas of standard DOPA were compared with sample areas. The DOPA levels in the samples were measured and expressed as pg/μg protein, and retention times for DOPA were 2.5–4 min.
PP2A assay
To determine PP2A activity, the Serine/Threonine phosphatase assay kit was used (Promega, Madison, WI). For PP2A activity measurement, N27 cells were homogenized in lysis buffer (25 mM Tris-HCl, 10 mM β-mercaptoethanol, 2 mM EDTA, protease inhibitor) supplied with the kit. After centrifugation, the supernatants were used for the assay. PP2A activity was determined by the amount of free phosphate generated in a reaction and measured by the absorbance of a molybdate: malachite green: phosphate complex at 600 nm using a Spectramax plate reader (Molecular Devices, Sunnyvale, CA). The effective range for the detection of phosphate released in this assay is 100–4,000 pmol of phosphate.
Western blotting
Cell lysates containing equal amounts of protein were loaded in each lane and separated on a 10–12% SDS-PAGE gel, as described previously (Kaul et al., 2003b). After the separation, proteins were transferred to nitrocellulose membranes and nonspecific binding sites were blocked by treating with 5% nonfat dry milk powder. The membranes were then treated with primary antibodies directed against PKCδ (rabbit polyclonal or mouse monoclonal, 1:2000 dilution) and phospho TH-Ser40 (rabbit polyclonal, 1:1000). The primary antibody treatments were followed by treatment with secondary HRP-conjugated anti-rabbit IgG (1:2000) for 1 h at RT. Secondary antibody-bound proteins were detected using Amersham’s ECL chemiluminescence kit. To confirm equal protein loading, blots were reprobed with β-actin antibody (1:5000 dilution). Western blot images were captured with a Kodak 2000 MM imaging system and data were analyzed using 1D Kodak imaging analysis software.
Data analysis
Data analysis was performed using Prism 4.0 software (GraphPad Software, San Diego, CA). Data were analyzed using one-way ANOVA, and then Bonferroni’s post-test was performed to compare all treatment groups. Differences with p<0.05 were considered significant.
Results
Acute Mn exposure induces increases in TH activity
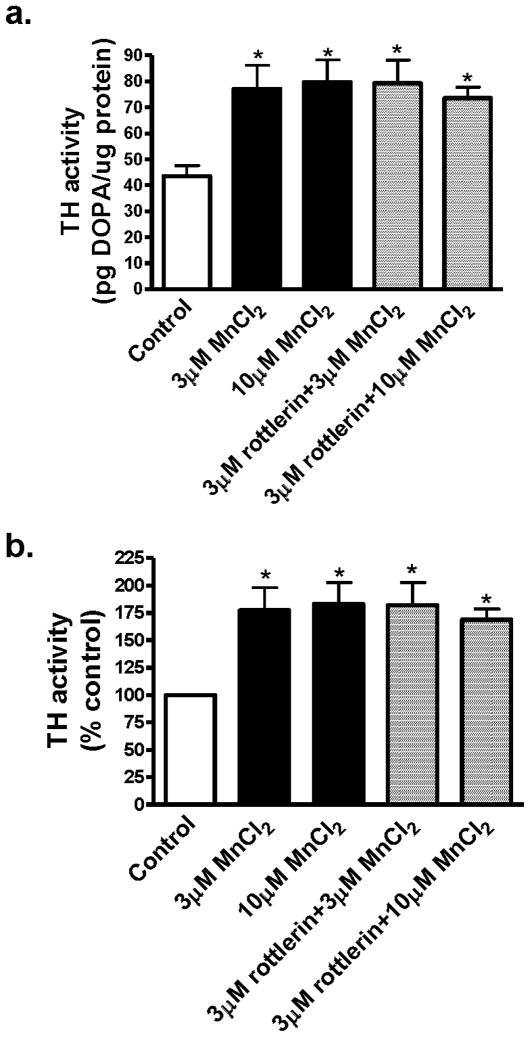
Recently we demonstrated that protein kinase C delta (PKCδ) negatively regulates tyrosine hydroxylase (TH) activity and dopamine synthesis by enhancing protein phosphatase 2A activity in dopaminergic neurons, both in cell culture and in animal brain (Zhang et al. 2007). PKCδ mediates apoptotic cell death in undifferentiated N27 dopaminergic neuronal cells following a high concentration of Mn (300 μM) for 24 h (Latchoumycandane et al. 2005). In this study, we examined whether Mn at non-toxic doses had any effect on TH activity. Differentiated N27 cells were exposed to 3 μM and 10 μM Mn, and after 3 h, cells were harvested, lysed, and measured for TH activity. As shown in Fig. 1A, both 3 μM and 10 μM Mn significantly increased TH activity as compared to untreated control cells. The increase was about 77% over control level (Fig 1B). To determine if PKCδ mediates Mn-induced increases in TH activity, N27 cells were pretreated with 3 μM rottlerin for 30 min prior to Mn treatment. The results show that Mn-induced increases in TH activity were unaffected by rottlerin treatment (Fig. 1A).
Fig. 1.
Effect of acute Mn treatment on TH activity in differentiated N27 cells. Differentiated N27 cells were incubated with 3 or 10 μM MnCl2 for 3 h with or without 3 μM rottlerin. For measurement of TH activity, cells were exposed to 2 mM NSD-1015 for 1 h prior to MnCl2 treatment. Cells were lysed after treatment and extracts were used for determining L-DOPA levels by HPLC. A, Data are presented as pg DOPA/μg protein; B, data are presented as percentage of control. The data represent a mean + SEM of six to eight individual measurements. Asterisks (*p<0.05) indicate significant differences between Mn treated cells and control cells.
Acute Mn exposure increases TH phosphorylation in dopaminergic cells
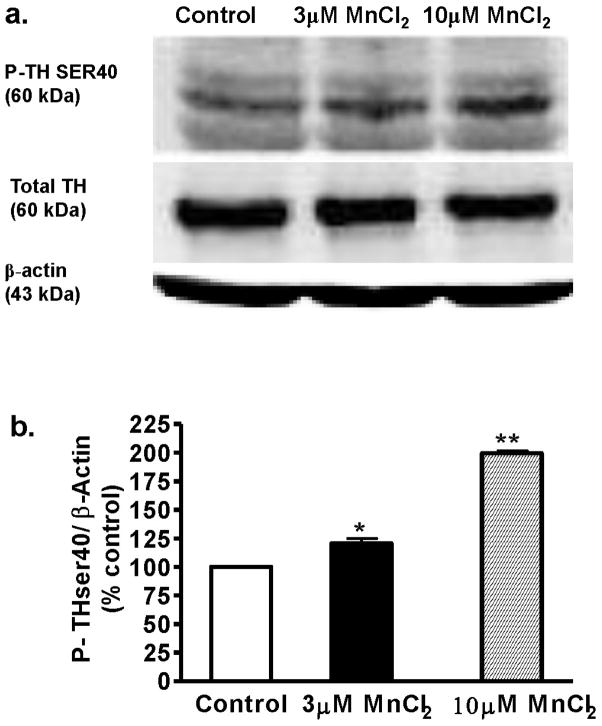
We previously demonstrated that PKCδ colocalizes with TH and also negatively regulates TH-Ser40 phosphorylation (Zhang et al. 2007). Since acute Mn exposure resulted in enhanced TH activity, we examined whether acute Mn treatment has any effect on the phosphorylation status of TH at Ser40. Differentiated N27 cells were exposed to 3 μM and 10 μM Mn for 3 h and the cell lysate was subjected to TH-Ser40 phospho-immunoblot analysis. As shown in Fig. 2, Mn treatment induced dose-dependent increases in the levels of TH-Ser40 phosphorylation. Densitometry analysis of the 60 kDa TH-Ser40 band in Fig. 2A revealed an almost twofold increase in phosphorylation in 10 μM Mn-treated cells compared to untreated control cells. A 43 kDa β-actin band was used for confirming equal protein loading of samples in each lane. These results demonstrate that acute Mn exposure results in enhanced TH phosphorylation.
Fig. 2.
Effect of acute Mn treatment on TH phosphorylation level in differentiated N27 cells. Differentiated N27 cells were exposed to 3 or 10 μM MnCl2 for 3 h. Cell extracts were prepared and separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. TH antibody (mouse, 1:1000) and phospho-specific antibodies directed against P-TH-Ser40 (rabbit, 1:1000) were used for immunoblotting. To confirm equal protein loading in each lane, the membranes were reprobed with β-actin antibody. The immunoblots were visualized using Amersham’s ECL detection agents. Densitometric analysis of 60 kDa P-TH-Ser40 bands represents the mean ± SEM from three separate experiments (*p < 0.05, **p < 0.01).
Acute Mn exposure is not toxic to differentiated N27 cells
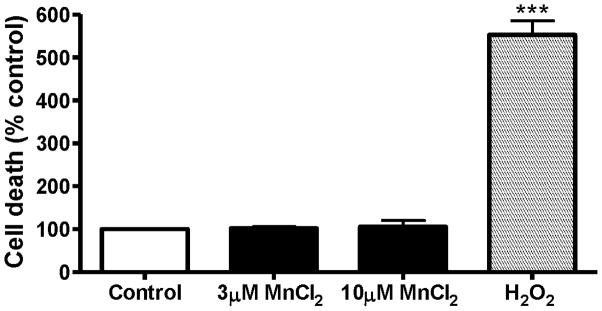
Next we determined if a 3 h exposure to 3–10 μM Mn induces any cytotoxicity in differentiated N27 cells. Cytotoxicity was measured using the Sytox Green fluorescence assay. An increase in the number of Sytox-positive green cells indicates an increase in cell death because the Sytox Green dye permeates compromised cell membranes to stain nuclear chromatin. Quantitative analysis of Sytox fluorescence using a fluorescence plate reader further confirmed that acute Mn exposure (3–10 μM) does not produce any significant cytotoxic response in differentiated N27 cells (Fig. 3). H2O2-treated N27 cells, used as a positive control, showed a fivefold increase in Sytox fluorescence (Fig. 3). These results suggest that the Mn concentration used in acute experiments is not toxic to the cells.
Fig. 3.
Cytotoxicity of acute MnCl2 treatment in differentiated N27 cells. Differentiated N27 cells were treated with 3 μM or 10 μM MnCl2 for 3 h. The effect of acute manganese treatment on cell death was quantified by Sytox Green fluorescence assay. The intensity of fluorescence was measured by fluorescence plate reader and shown as % of control. N27 cells exposed to 100 μM H2O2 for 3 h were used as a positive control. The data represent a mean + SEM of four individual measurements. Asterisks (***p<0.001) indicate significant differences between H2O2-treated cells and control cells.
Chronic Mn exposure decreases TH activity in N27 cells
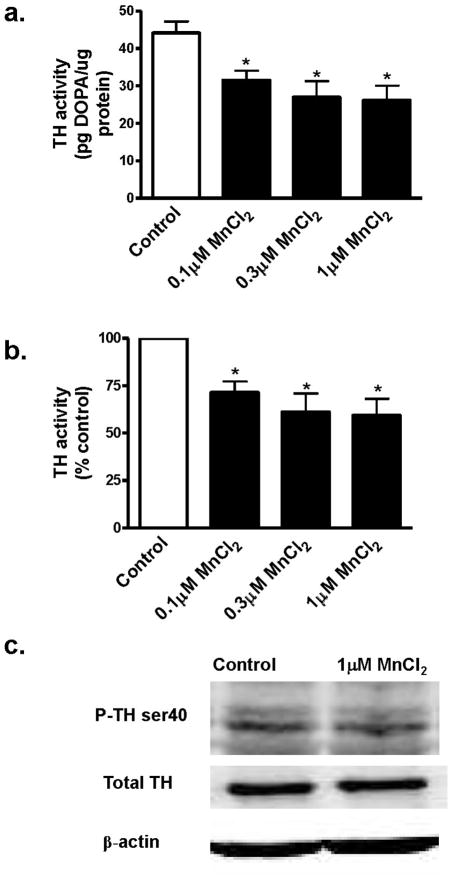
In the next set of experiments, we examined whether chronic Mn treatment also modulates TH activity. N27 cells were exposed to 0.1–1 μM Mn for 24 h, and TH activity was measured in the cell lysates. As shown in Fig. 4A, Mn induced a dose-dependent decrease in TH activity. Comparison with untreated controls revealed that TH activity was decreased to 70%, 61% and 59% in 0.1, 0.3 and 1 μM Mn-treated cells, respectively (Fig. 4B). Thus, unlike acute exposure, chronic Mn treatment produced a decrease in TH activity. Western blot analysis of TH-Ser40 phosphorylation revealed a marginal decrease in TH-Ser40 phosphorylation in 1 μM Mn-treated samples (Fig. 4C), however the decrease was not statistically significant. The lower concentrations of Mn did not significantly alter TH-Ser40 phosphorylation as compared to untreated samples (data not shown).
Fig. 4.
Effect of chronic MnCl2 on TH activity and phosphorylation levels in differentiated N27 cells. Differentiated N27 cells were incubated with 0.1 μM, 0.3 μM or 1 μM MnCl2 for 24 h. For measurement of TH activity, cells were exposed to 2 mM NSD-1015 for 1 h prior to MnCl2 treatment. Cells were lysed after treatment, and extracts were used to determine L-DOPA levels by HPLC. A, TH activity expressed as pg DOPA/μg protein; B, TH activity expressed as percentage of control. The data represent a mean + SEM of six to eight individual measurements. Asterisks (*p<0.05) indicate significant differences between MnCl2-treated cells and control cells. C, Western blot of P-TH-Ser40. Cell extracts from control and 1 μM MnCl2-treated N27 cells were prepared and separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. TH antibody and phospho-specific antibodies directed against P-TH-Ser40 were used for immunoblotting. To confirm equal protein loading in each lane, the membranes were reprobed with β-actin antibody. The immunoblots were visualized using Amersham’s ECL detection agents.
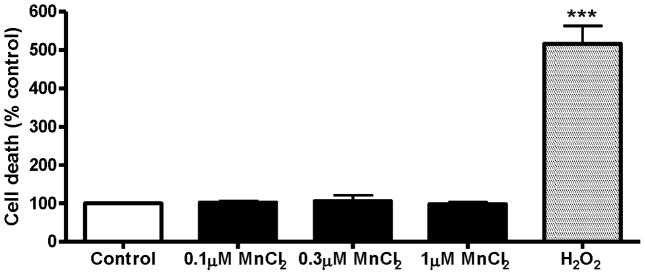
Chronic Mn exposure is not toxic to differentiated N27 cells
Next we examined if a 24 h exposure to 0.1–1 μM Mn induces cytotoxicity in differentiated N27 cells. Cytotoxicity was measured using the Sytox Green fluorescence assay. As shown in Fig. 5, quantitative analysis of Sytox fluorescence using a fluorescence plate reader revealed that differentiated N27 cells were unaffected by chronic Mn treatment (0.1–1 μM for 24 h). H2O2-treated N27 cells were again used as a positive control and a 100 μM H2O2 treatment for 3 h induced a greater than fivefold increase in Sytox fluorescence (Fig. 5). These results suggest that the Mn concentration used in chronic experiments is not toxic to the cells.
Fig. 5.
Cytotoxicity of chronic MnCl2 treatment in differentiated N27 cells. Differentiated N27 cells were treated with 0.1 μM, 0.3 μM or 1 μM MnCl2 for 24 h. The effect of chronic manganese treatment on cell death was quantified by Sytox Green fluorescence assay. The intensity of fluorescence was measured by fluorescence plate reader and shown as % of control. N27 cells exposed to 100 μM H2O2 for 3 h were used as a positive control. The data represent a mean + SEM of four individual measurements. Asterisks (***p<0.001) indicate significant differences between H2O2-treated cells and control cells.
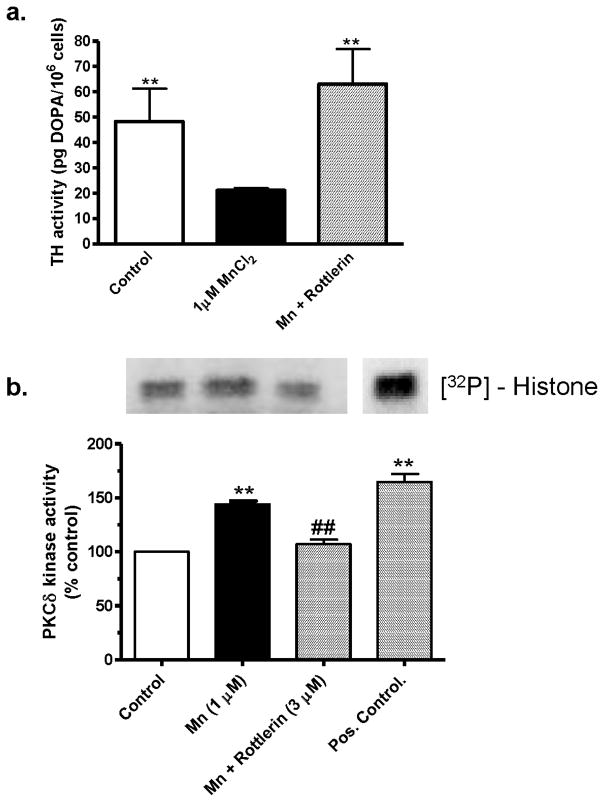
Rottlerin blocks Mn-induced changes in TH and PKCδ activities
To determine whether PKCδ plays any role in the chronic Mn-induced decreases in TH activity as shown in Fig. 4, differentiated N27 cells were cotreated with the PKCδ inhibitor rottlerin (3 μM). As shown in Fig. 6A, rottlerin cotreatment completely restored the TH activity in Mn-treated cells to the level of the untreated control cells. These data suggest that PKCδ plays a role in reduced TH activity during chronic Mn exposure in dopaminergic neuronal cells.
Fig. 6.
Effects of PKCδ inhibition on MnCl2-induced reduction of TH activity in differentiated N27 cells. Differentiated N27 cells were treated with 1 μM MnCl2 or cotreated with 3 μM rottlerin for 24 h. A, TH activity: Cells were pretreated with 2 mM NSD-1015 for 1 h before MnCl2 treatment and lysed. Extracts were used for determining L-DOPA levels by HPLC. The data represent a mean + SEM of six to eight individual measurements. Asterisks (**p<0.01) indicate significant differences between MnCl2-treated cells and control cells. B, PKCδ activity: cell extracts from the above treatment were used in the immunoprecipitation kinase assay. Recombinant PKCδ protein was used as a positive control. The bands were quantified by a PhosphoImager after scanning the dried gel and are expressed as a percentage of control. The data represent a mean + SEM of three individual measurements. Asterisks (**p<0.01) indicate significant differences between MnCl2 treated and control cells.
Since rottlerin treatment prevented Mn-induced decreases in TH activity, we examined if chronic Mn treatment has any effect on PKCδ activity. Differentiated N27 cells were exposed to 1 μM Mn in the presence or absence of 3 μM rottlerin and the cell lysates were used in the kinase assay. PKCδ was immunoprecipitated from the cell lysates and the kinase assay was performed by measuring PKCδ phosphorylation of histone H1 using [32P]-ATP, as described previously (Anantharam et al., 2002). As shown in Fig. 6B, a 24-h exposure to 1 μm Mn resulted in a 40% increase in histone phosphorylation compared to untreated control cells. Importantly, rottlerin cotreatment completely suppressed 1 μM Mn-induced increases in kinase activity. Pure recombinant PKCδ protein was used as a positive control. Together, these data imply that chronic exposure to low doses of Mn induces increases in PKCδ activity and that rottlerin at 3 μM effectively suppresses Mn-induced PKCδ activation.
PKCδ mediates Mn-induced increases in PP2A activity in dopaminergic cells
We previously demonstrated that PKCδ negatively regulates TH activity by enhancing protein phosphatase 2A (PP2A) activity in dopaminergic neurons (Zhang et al. 2007). Since chronic Mn treatment induced increases in PKCδ activity and decreased TH activity, we examined whether chronic Mn treatment also increases PP2A activity. As shown Fig. 7, PP2A enzyme activity increased by almost 50% in differentiated N27 cells exposed to 1 μM Mn for 24 h. Furthermore, 3 μM rottlerin significantly blocked Mn-induced increases in PP2A activity, suggesting that PKCδ may be activating PP2A to reduce TH activity.
Fig. 7.
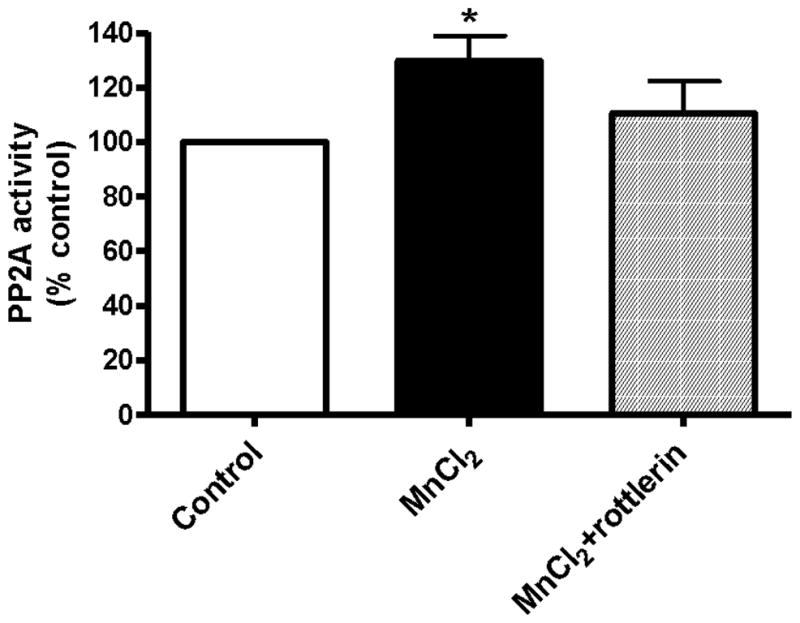
Effect of Mn on PP2A activity in dopaminergic cells. Differentiated N27 cells were treated with 1 μM MnCl2 or cotreated with 3 μM rottlerin for 24 h. Then cells were lysed and PP2A enzyme activity was measured using a serine/threonine phosphatase assay kit from Promega. The data represent a mean + SEM of four to six individual measurements. Asterisks (*p<0.05) indicate significant differences between MnCl2 treated and control cells.
Discussion
In the present study, we demonstrate that short-term and long-term low-dose Mn exposures have opposing effects on TH activity. While acute exposure to low μM Mn concentrations (1–10 μM for 3 h) increased TH activity, chronic exposure to 0.1–1 μM Mn over 24 h had an opposite effect (decreased TH activity) in differentiated dopaminergic neuronal cells. Chronic Mn exposure also caused PKCδ activation and increased PP2A activity, and these increases were blocked by the PKCδ inhibitor rottlerin. Importantly, inhibition of PKCδ reversed the chronic Mn-induced decrease in TH activity. The observed biochemical changes are independent of cytotoxicity because the doses of Mn used in the study did not induce any cytotoxicity. To our knowledge this is the first report showing that manganese regulates TH activity in dopaminergic neuronal cells via PKCδ and PP2A.
The nigrostriatal system, including the globus pallidus and substantia nigra, is the primary target of Mn (Baek et al., 2003). Accumulation of manganese in pallidal regions of the basal ganglia has been well described as ‘manganese hyperintensity signals’ by T1-weighted MRI imaging procedures (Krieger et al., 1995). Environmental Mn exposure range from moderate, as occurs via drinking water and food sources, to high, as found in occupational and industrial settings such as mining, welding, and steel manufacturing (Woolf et al., 2002; Roth and Garrick, 2003; Dobson et al., 2004; Olanow, 2004). The mean Mn level in the human brain is 0.261 μg/g, with wet weights of 6.31, 0.34, and 58.5 ng/mg in the putamen, substantia nigra, and neuromelanin, respectively (Zecca et al., 2004). Depending on the level of exposure, Mn levels can increase from 10 to 200-fold (Hauser et al., 1996; Lucchini et al., 1999; Woolf et al., 2002; McKinney et al., 2004). Higher concentrations (>100 μM) of Mn are generally required to observe neurotoxicity in cell cultures (Roth and Garrick, 2003; Hirata et al., 2004; Stredrick et al., 2004; Latchoumycandane et al., 2005), but we found that differentiated mesencephalic N27 dopaminergic cells are very sensitive to low doses of Mn, suggesting the utility of this model for studying neurochemical mechanisms underlying manganese neurotoxicity.
We previously showed that inorganic Mn (>100 μM) and an organic Mn compound, methylcyclopentadienyl manganese tricarbonyl (MMT, >300 μM), induce ROS generation, mitochondrial dysfunction and apoptotic cell death in two different dopaminergic cell lines: PC12 and undifferentiated N27 cells. (Anantharam et al., 2002; Latchoumycandane et al., 2005). We had also demonstrated that high-doses of Mn (100–300 μM) induced caspase-3-dependent proteolytic cleavage of PKCδ in undifferentiated N27 cells (Latchoumycandane et al. 2005). We recently showed that N27 cells express DMT1 and other metal transporter proteins that may play a role in Mn neurotoxicty. (Afeseh Ngwa et al., 2009)
TH is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to dihydroxyphenylalanine (DOPA), which is subsequently converted to dopamine, a key neurotransmitter that controls extrapyramidal motor function in the basal ganglia. TH activity can be regulated post-translationally by phosphorylation of serine residues at positions 8, 19, 31 and 40, resulting in enhanced dopamine synthesis (Lee et al., 1989; Haycock, 1990; Haycock et al., 1992). Recently, we demonstrated that PKCδ colocalizes and physically interacts with TH and PP2A, and that PKCδ phosphorylates PP2A to enhance the phosphatase activity (Zhang et al., 2007). Increased PP2A dephosphorylates TH-Ser40 and thereby reduces TH activity. In the present study, we observed that acute Mn exposure increases TH activity and TH-Ser40 phosphorylation. However, pretreatment with the PKCδ-specific inhibitor rottlerin did not block Mn-induced increases in TH activity, suggesting that PKCδ does not play a role in an acute-Mn treatment paradigm. A recent study showed increased TH activity without any increase in TH protein upregulation (Baek et al., 2007). During chronic Mn treatment, we noted decreased TH activity with no significant change in TH-Ser40 phosphorylation. Interestingly, the PKCδ inhibitor rottlerin restored the TH activity in Mn-treated cells to that of untreated control cells, suggesting that PKCδ may regulate TH activity. It is also possible that Mn-may mediate its effect by regulation the phosphorylation status of TH-associated proteins such as α-synuclein,14-3-3 and PP2A because PKCδ has been shown to interact with these proteins (Zhang et al., 2007; Wang et al., 2009). Recently, Xing et al. showed Mn is also required for PP2A activity in which PP2A-specific methylesterase directly binds to the active site of PP2A leading to its inactivation by evicting the manganese ions that are required for the phosphatase activity of PP2A (Xing et al., 2008).
Furthermore, chronic Mn treatment increased PKCδ activity and PP2A activity, consistent with our previous finding of synergistic interaction between PKCδ activity and PP2A. A recent study by Bevilaqua et al. demonstrated 1.5 mM Mn can mediate dephosphorylation of TH-Ser40 by activating PP2A in in vitro experiments (Bevilaqua et al., 2003). Of note, the Mn concentration used in the Bevilaqua study was 1000-fold higher than the concentration used in this study. While our manuscript was under review, Posser et al. demonstrated that Mn induced a sustained Ser40 phosphorylation of TH and increased TH activity in pheochromocytoma cells (PC12 cells) (Posser et al., 2009). The sustained Ser40 phosphorylation may be due to the 10–100-fold higher concentrations of Mn used in PC12 cell model. Together with the observation that PKCδ inhibition can restore both TH activity and PP2A activity to levels present in control cells, our study suggests that Mn-induced reduction in TH activity may in part be mediated by the PKCδ-PP2A signaling pathway.
The functional consequences of biphasic alterations of TH activity during Mn exposure, an increased TH activity during acute Mn-treatment and decreased TH activity during chronic Mn treatment, observed in this study need to be examined in future studies in animal models. Previous studies in animal models have shown some significant changes in the nigrostriatal dopaminergic system. A recent study by Liu et al. showed that chronic administration of large doses of Mn (100 mg/kg) for 8 weeks via oral gavage in mice depletes striatal dopamine level by 50% without inducing injury to dopaminergic neurons in nigra (Liu et al., 2006). Guilarte et al. showed that Mn-exposed non-human primates exhibited subtle motor function deficits while apparently retaining an intact but dysfunctional nigrostriatal DA system (Guilarte et al., 2006). Very recently, Guilarte et al. reported that chronic Mn exposure in primates significantly reduces amphetamine-induced dopamine release, as measured by the PET imaging procedure (Guilarte et al., 2008). The authors also found reduced dopamine and 3,4-dihydroxyphenylacetic acid in the caudate putamen of Mn-treated monkeys without any significant change in the nigral dopaminergic neurons. Inhibition of TH activity observed in the present study might explain the reduced tissue dopamine levels and decreased stimulated-dopamine release observed in animal models of Mn toxicity. The differential alterations in TH activity observed in our study also may be modeling the toxicity occurring in human manganism cases resulting in neurological symptoms that are distinct in three stages (initial, intermediate and late).
In conclusion, we provide novel evidence that acute and chronic Mn exposures induce differential alterations in TH enzyme activity. Acute Mn exposure increased TH activity, whereas chronic Mn treatment decreased TH activity. Chronic Mn treatment also induced activation of PKCδ, which in turn activated PP2A, resulting in decreased TH activity due to dephosphorylation of TH-Ser40. Our results suggest that Mn-induced decreases in TH activity mediated through PKCδ and PP2A may explain the dopaminergic deficits observed during chronic Mn exposure.
Acknowledgments
This work is supported in NIH grants ES10586 and NS 386344. The support from W. Eugene and Linda Lloyd Endowment to AGK is also acknowledged. We would like to acknowledge Keri Henderson and Mary Ann deVries for their assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams FS, La Rosa FG, Kumar S, Edwards-Prasad J, Kentroti S, Vernadakis A, Freed CR, Prasad KN. Characterization and transplantation of two neuronal cell lines with dopaminergic properties. Neurochem Res. 1996;21:619–627. doi: 10.1007/BF02527762. [DOI] [PubMed] [Google Scholar]
- Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, Kanthasamy AG. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson’s disease. Toxicol Appl Pharmacol. 2009;240:273–285. doi: 10.1016/j.taap.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharam V, Kaul S, Song C, Kanthasamy A, Kanthasamy AG. Pharmacological inhibition of neuronal NADPH oxidase protects against 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress and apoptosis in mesencephalic dopaminergic neuronal cells. Neurotoxicology. 2007;28:988–997. doi: 10.1016/j.neuro.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Blockade of PKCdelta proteolytic activation by loss of function mutants rescues mesencephalic dopaminergic neurons from methylcyclopentadienyl manganese tricarbonyl (MMT)-induced apoptotic cell death. Ann N Y Acad Sci. 2004;1035:271–289. doi: 10.1196/annals.1332.017. [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Lukey B, Tremblay A. The Manganese Health Research Program (MHRP): status report and future research needs and directions. Neurotoxicology. 2006;27:733–736. doi: 10.1016/j.neuro.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Baek SY, Kim YH, Oh SO, Lee CR, Yoo CI, Lee JH, Lee H, Sim CS, Park J, Kim JW, Yoon CS, Kim Y. Manganese does not alter the severe neurotoxicity of MPTP. Hum Exp Toxicol. 2007;26:203–211. doi: 10.1177/0960327107070567. [DOI] [PubMed] [Google Scholar]
- Baek SY, Lee MJ, Jung HS, Kim HJ, Lee CR, Yoo C, Lee JH, Lee H, Yoon CS, Kim YH, Park J, Kim JW, Jeon BS, Kim Y. Effect of manganese exposure on MPTP neurotoxicities. Neurotoxicology. 2003;24:657–665. doi: 10.1016/S0161-813X(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Bevilaqua LR, Cammarota M, Dickson PW, Sim AT, Dunkley PR. Role of protein phosphatase 2C from bovine adrenal chromaffin cells in the dephosphorylation of phospho-serine 40 tyrosine hydroxylase. J Neurochem. 2003;85:1368–1373. doi: 10.1046/j.1471-4159.2003.01792.x. [DOI] [PubMed] [Google Scholar]
- Campbell DG, Hardie DG, Vulliet PR. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem. 1986;261:10489–10492. [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- Ensing JG. Bazooka: cocaine-base and manganese carbonate. J Anal Toxicol. 1985;9:45–46. doi: 10.1093/jat/9.1.45. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dorman DC, Lash LH, Aschner M. Duration of airborne-manganese exposure in rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Neurotoxicology. 2008;29:377–385. doi: 10.1016/j.neuro.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007;113:369–377. doi: 10.1016/j.pharmthera.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H, Okuno S. Regulatory mechanism of tyrosine hydroxylase activity. Biochem Biophys Res Commun. 2005;338:271–276. doi: 10.1016/j.bbrc.2005.07.183. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Lieberman A. The role of the regulatory enzymes of catecholamine synthesis in Parkinson’s disease. Neurology. 1992;42:8–12. discussion 41-18. [PubMed] [Google Scholar]
- Gschwendt M. Protein kinase C delta. Eur J Biochem. 1999;259:555–564. doi: 10.1046/j.1432-1327.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, McGlothan JL, Verina T, Zhou Y, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, Schneider JS. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): Implications to manganese-induced parkinsonism. J Neurochem. 2008;107:1236–1247. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Haavik J, Schelling DL, Campbell DG, Andersson KK, Flatmark T, Cohen P. Identification of protein phosphatase 2A as the major tyrosine hydroxylase phosphatase in adrenal medulla and corpus striatum: evidence from the effects of okadaic acid. FEBS Lett. 1989;251:36–42. doi: 10.1016/0014-5793(89)81424-3. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Zesiewicz TA, Martinez C, Rosemurgy AS, Olanow CW. Blood manganese correlates with brain magnetic resonance imaging changes in patients with liver disease. Can J Neurol Sci. 1996;23:95–98. doi: 10.1017/s0317167100038786. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Miwa S, Lee K, Koshimura K, Kamel A, Hamahata K, Fujiwara M. A nonisotopic method for determination of the in vivo activities of tyrosine hydroxylase in the rat adrenal gland. Anal Biochem. 1988;168:176–183. doi: 10.1016/0003-2697(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Haycock JW. Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J Biol Chem. 1990;265:11682–11691. [PubMed] [Google Scholar]
- Haycock JW, Ahn NG, Cobb MH, Krebs EG. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc Natl Acad Sci U S A. 1992;89:2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Furuta K, Miyazaki S, Suzuki M, Kiuchi K. Anti-apoptotic and pro-apoptotic effect of NEPP11 on manganese-induced apoptosis and JNK pathway activation in PC12 cells. Brain Res. 2004;1021:241–247. doi: 10.1016/j.brainres.2004.06.064. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Role of proteolytic activation of protein kinase Cdelta in oxidative stress-induced apoptosis. Antioxid Redox Signal. 2003;5:609–620. doi: 10.1089/152308603770310275. [DOI] [PubMed] [Google Scholar]
- Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003a;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003b;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Wagner JR, Kirby ML, Anantharam V, Kanthasamy AG. Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganese tricarbonyl. J Pharmacol Exp Ther. 2002;302:26–35. doi: 10.1124/jpet.302.1.26. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Lee KY, Lew JY, Tang D, Schlesinger DH, Deutch AY, Goldstein M. Antibodies to a synthetic peptide corresponding to a Ser-40-containing segment of tyrosine hydroxylase: activation and immunohistochemical localization of tyrosine hydroxylase. J Neurochem. 1989;53:1238–1244. doi: 10.1111/j.1471-4159.1989.tb07420.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol Sci. 2006;91:521–531. doi: 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, Mergler D, Sassine MP, Palmi S, Alessio L. Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20:287–297. [PubMed] [Google Scholar]
- McKinney AM, Filice RW, Teksam M, Casey S, Truwit C, Clark HB, Woon C, Liu HY. Diffusion abnormalities of the globi pallidi in manganese neurotoxicity. Neuroradiology. 2004;46:291–295. doi: 10.1007/s00234-004-1179-1. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson’s disease. Ann N Y Acad Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J Neuropathol Exp Neurol. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Posser T, Franco JL, Bobrovskaya L, Leal RB, Dickson PW, Dunkley PR. Manganese induces sustained Ser40 phosphorylation and activation of tyrosine hydroxylase in PC12 cells. J Neurochem. 2009;110:848–856. doi: 10.1111/j.1471-4159.2009.06185.x. [DOI] [PubMed] [Google Scholar]
- Reyland ME. Protein kinase Cdelta and apoptosis. Biochem Soc Trans. 2007;35:1001–1004. doi: 10.1042/BST0351001. [DOI] [PubMed] [Google Scholar]
- Roth JA, Garrick MD. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol. 2003;66:1–13. doi: 10.1016/s0006-2952(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Stredrick DL, Stokes AH, Worst TJ, Freeman WM, Johnson EA, Lash LH, Aschner M, Vrana KE. Manganese-induced cytotoxicity in dopamine-producing cells. Neurotoxicology. 2004;25:543–553. doi: 10.1016/j.neuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Sun F, Anantharam V, Zhang D, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Proteasome inhibitor MG-132 induces dopaminergic degeneration in cell culture and animal models. Neurotoxicology. 2006;27:807–815. doi: 10.1016/j.neuro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Wang J, Lou H, Pedersen CJ, Smith AD, Perez RG. 14-3-3zeta contributes to tyrosine hydroxylase activity in MN9D cells: localization of dopamine regulatory proteins to mitochondria. J Biol Chem. 2009;284:14011–14019. doi: 10.1074/jbc.M901310200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf A, Wright R, Amarasiriwardena C, Bellinger D. A child with chronic manganese exposure from drinking water. Environ Health Perspect. 2002;110:613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Filer D, Friedhoff AJ, Goldstein M. Site-directed mutagenesis of tyrosine hydroxylase. Role of serine 40 in catalysis. J Biol Chem. 1992;267:25754–25758. [PubMed] [Google Scholar]
- Xing Y, Li Z, Chen Y, Stock JB, Jeffrey PD, Shi Y. Structural mechanism of demethylation and inactivation of protein phosphatase 2A. Cell. 2008;133:154–163. doi: 10.1016/j.cell.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Zecca L, Stroppolo A, GATT A, Tampellini D, Toscani M, Gallorini M, Giaveri G, Arosio P, Santambrogio P, Fariello RG, Karatekin E, Kleinman MH, Turro N, Hornykiewicz O, Zucca FA. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc Natl Acad Sci U S A. 2004;101:9843–9848. doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Yang Y, Anantharam V. Protein kinase C delta negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J Neurosci. 2007;27:5349–5362. doi: 10.1523/JNEUROSCI.4107-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]