Abstract
The Human Papilloma Virus is the major cause of cervical cancer. Viral infection initiates cervical intraepithelial neoplasia which progresses through several stages to cervical cancer. The objective of this study is to identify the minimum effective dose of diindolylmethane that prevents the progression from cervical dysplasia to carcinoma in situ. We document cervical histology in K14-HPV16 mice receiving different doses of diindolylmethane. Urinary diindolylmethane concentrations are reported. Diindolylmethane could enhance the efficacy of human papilloma virus vaccines, creating a new therapeutic use for these vaccines in women already infected with the virus. Five doses (0 to 2500ppm) of diindolylmethane were incorporated into each mouse diet. The reproductive tract was serially sectioned and urine was obtained for analysis of urinary diindolylmethane. The results indicate that 62% of mice receiving 1,000ppm diindolylmethane, remained dysplasia free after 20 weeks compared to 16% of mice receiving no diindolylmethane and 18% receiving 500ppm. 1000ppm of 3,3′-diindolylmethane in the diet completely suppressed the development of cervical cancer. Urinary diindolylmethane levels increased significantly as diindolylmethane in food increased. These findings imply usefulness for diindolylmethane in the search to prevent cervical cancer when used in combination with prophylactic or therapeutic vaccines.
Keywords: cervical cancer; cervical intraepithelial neoplasia; 3,3′-diindolylmethane
Introduction
Although cervical cancer incidence and mortality rates have declined in the United States over the past three decades, this disease remains a serious national and international health threat. With new vaccines against the two most oncogenic human papillomavirus (HPV16 and HPV18), the further incidence of cervical cancer is likely to radically decrease. Even so, it will take decades for vaccination to take effect on cervical cancer rates (1).
Incidence rates for Hispanic women are higher than those for non-Hispanic women. Even though the mortality rate for African American women has declined more rapidly than the rate for White women, the African American mortality rate continues to be more than double that of Whites (2). Cervical cancer strikes nearly half a million women each year worldwide, claiming a quarter of a million lives (3).
The U.S. Food and Drug Administration (FDA) has approved two vaccines that are effective in preventing infection with types 16 and 18, two “high-risk” HPVs that cause most (70 percent) cervical cancers, and types 6 and 11, which cause most (90 percent) genital warts (4). Gardasil Quadrivalent Vaccine (Merck) and Cevarix (GlaxoSmithKline Biologicals) are highly effective in women with no prior exposure. Neither vaccine is effective in women already infected with the virus. There are also issues of patient compliance and duration of vaccine protection that need to be addressed.
Clinical trials have indicated that these vaccines have high efficacy in preventing persistent HPV infection, cervical cancer precursor lesions, vaginal and vulvar cancer precursor lesions, and genital warts caused by HPV types 6, 11, 16, or 18 among females who have not already been infected with the respective HPV type. No evidence exists for protection against disease caused in females infected with the HPV types at the time of vaccination. However, females infected with one or more vaccine HPV types before vaccination would be protected against disease caused by the other vaccine HPV types (5).
Gardasil and Cevarix are prophylactic or preventive vaccines. Neither provides protection against diseases from vaccine and non-vaccine HPV types to which a woman has previously been exposed. The vaccines do not block cervical epithelial changes and have no effect on cervical intraepithelial neoplasia (CIN) in those individuals already infected with HPV. For this reason therapeutic HPV vaccines are currently in development (6).
Data from the National Health and Nutrition Examination Survey (NHANES) published in the February 28, 2007 have indicated that a total of 26.8 percent of women aged 14 to 59 tested positive for one or more strains of HPV (7). The most troubling consequence of HPV infection is the potential to cause cervical cancer.
3,3′-diindolylmethane (DIM) effectively inhibits CIN lesions in women with cervical dysplasia (8–10). This has also been demonstrated in the K14-HPV16 mouse model used in this study (11–13). DIM is an acid condensation product of indole-3-carbinol (I3C). I3C is formed enzymatically from the indole glucobrassinin which is found in cruciferous vegetables and spices, When I3C contacts the acid environment of the stomach several acid condensation products are formed. DIM comprises at least 70% of these compounds. The conversion is also common in tissue culture studies. In studies that use I3C, the active biological agent is DIM. This has been reviewed previously (12).
DIM alters the Phase I metabolism of estradiol and does not increase C-16 hydroxylation. C-16 hydroxylation produces 16α-hydroxyestrone, an endogenous carcinogen and a promoter of HPV 16 and 18 proliferation or lytic phase (14–17). DIM, by altering the directional pathway of estrogen metabolism, and through other non-estrogenic effects, suppresses viral oncogene expression. DIM also suppresses pathology in recurrent respiratory papillomatosis, an HPV caused disease state (14, 15). The change in estrogen metabolism is important since both primary cervical cells and foreskin cells transformed by HPV16 alter estrogen metabolism toward 16-hydroxylation (18).
Tiwari and co-workers (19) have demonstrated that the chemopreventive and antitumor effects of 13C (DIM) depend on the species and tissue type. They studied the mechanism of action of I3C in estrogen-responsive (MCF-7) and estrogen-nonresponsive (MDAMB-231) human breast cancer cell lines. I3C inhibited the growth of estrogen-responsive cell line MCF-7 but had little effect on estrogen-nonresponsive cell line MDA-MB-231. Specific C-2 hydroxylation of estrogen and induction of cytochrome P-4501A1 was enhanced in the MCF-7 but not in the MDA-MB-231 cells. Their conclusion was that the inhibitory effects of I3C may involve selective induction of estradiol metabolism and the related cytochrome P-450 system that may be limited to estrogen-sensitive cells.
DIM directly disrupts various stages in HPV proliferation in both the initiation and progression from cervical dysplasia to cervical cancer. It has been demonstrated that DIM specifically inhibits proliferation of viral transcripts E6 and E7 in CaSki cells (9,17). DIM can induce a G1 cell cycle arrest in MCF-7 cells that is accompanied by inhibition of cyclin-dependent kinase expression (20). DIM inhibits cell adhesion, spreading and invasion associated with the upregulation of PTEN (a tumor suppressor gene) and E-cadherin (a regulator of cell cell adhesion) in T-47-D human breast cancer cells (21). Additionally, DIM prevents PTEN loss in vivo in the K14HPV16 transgenic mouse (22). This mouse model is used in our research. DIM also elevates several key cytokines in vivo: Interferon-Gamma (IFN-γ), granulocyte colony stimulating factor (G-CSF), Interleukin-12 (IL-12), and Interleukin-6 (IL-6). IFN-γ is responsible for overall immune response system (23, 24).
We have shown a reduction in the severity of CIN and in some cases the complete reversal of cervical dysplasia in women taking DIM for 4 weeks. This may indicate that HPV16 viral oncogene expression is suppressed after continued DIM administration (8, 10). By utilizing the K14 HPV16 mouse model, we can determine how long DIM administration is necessary for viral oncogene inhibition.
The long range goal of this research is to test the theory that that 3,3′-diindolylmethane (DIM) acts to augment the efficacy of preventive and therapeutic HPV vaccines. An additional benefit may be that vaccine administration after DIM suppression of viral oncogenes will allow virus like particles (VLP) antibodies to form and thus prevent re-infection from new HPV exposure. Before the research using DIM with vaccines can begin, the most effective minimum dose of DIM needs to be determined. Here we present some of the results of a DIM dose response study. Histology of the cervical epithelium from each dose group is presented. Urinary DIM concentrations are assessed for the first time in this model.
Methods
This study employs a well known transgenic mouse model commonly used in studies involving cervical dysplasia and cervical cancer (11–13, 25, 26). Cervical neoplasia and cancer are linked to persistent infection of “high-risk” HPV viral types, where E6 and E7 oncogenes have enhanced affinity for cellular proteins controlling a collection of functions necessary for neoplastic progression or growth and spread of malignancies. The K14-HPV16 mouse model is transgenic for the entire HPV16 early region under control of the human keratin-14 promoter, and expresses HPV16 E6 and E7 oncogenes in basal squamous epithelial cells. Tumorigenesis in this model is hormone-dependent. Chronic estradiol treatment at 0.1–0.25 mg/60-day induces invasive squamous cancers in the vulva, vagina and outer cervix of K14-HPV16 mice. None of the tumors are metastatic. At low estradiol dose (0.05 mg/60-day) squamous cancers are almost exclusively localized to the transformation zone situated between the upper cervix and lower uterus. Epithelial dysplasia and metaplasia are observed after 4 months of estradiol treatment, leading to high-grade dysplasia and multifocal carcinomas by 6 months (26). We have found that high-grade dysplasia and multifocal carcinomas occur as early as 3 months. The mouse model and preliminary data that we have obtained are more fully described elsewhere (12, 13).
The mice arrived at Hackensack University Medical Center’s Department of Biological Resources and were quarantined for as long as deemed necessary by the veterinarian based on serological testing and results. Once received from the NCI Mouse Repository (MMHCC, Frederick, MD), the mice were housed as breeder pairs. The cages have sterile Micro-isolator tops. Mice were fed irradiated AIN-93M diet (Purina TestDiets, Richmond, IN) and given sterile water via sterile water bottles. The animals were given daily health checks, which included a pain scale form.
During quarantine breeding of the animals began. The hemizygote K14-HPV16 was bred with the FVB wild type to produce the first litter of pups. At weaning the pups were genotyped (13). Those mice exhibiting the E6-E7 exon, (K14-HPV16 positive) were further bred with FVB wild type in order to generate progeny for the experimental protocols. All females (K14-HPV16 and FVB wild type) were trochar implanted with E2 pellets (0.250mg/90 day release, Innovative Research of America, Sarasota, Florida) under anesthesia. E2 pellets were replaced at 3 month intervals.
The animals were fed AIN-93M mouse diet until they were divided into respective groups. One group received AIN-93M mouse diet without DIM added and other groups received AIN-93M mouse diet enriched either 500,1000,1500, 2000 or 2500 ppm of DIM (LKT Laboratories, St. Paul, MN).
Histology
Mice were anesthetized and euthanized. Fresh tissue from the reproductive tract and surrounding soft tissue was acquired from each animal. The uterus and cervix were immediately dissected and frozen at −80C. Each specimen was serially sectioned at a 12 μm thickness using a Cryostat Leica CM 1850, and 10–15 sections were collected at 20 μm intervals for H&E staining. Examination of serial sections was done with a Leica CME binocular light microscope. The microscope has 4X, 10X and 40X objectives. Images were obtained using a Zeiss Axioplan microscope with a Sony color digital camera DXC-S500 attached. The calibration bar represents 0.1mm and was measured using a Reichert-Jung micrometer of 2mm divided into units of 0.01mm.
A histopathological grading system for this transgenic mouse model regarding cervical squamous carcinogenesis developed by Riley et al (16) was used to classify histological samples. The grading system is further described by Sepkovic et al (12, 13) and is based on the established criteria for classification of human cervical neoplasia or malignancy accounting for differences between the mouse model and patients.
Determination of DIM in Urine
The following protocol was used to extract DIM from urine. 500 μl of urine was combined with 1 ml of sodium acetate buffer (pH 4.8) and 20 μl of glucuronidase (110,200 units/ml; Sigma). The solution was incubated at 40°C for 24 h. The internal standard used previously was 4,4-dichlorodiindolylomethane (dichloro-DIM generously provided by Dr. Stephen Safe). This internal standard is no longer available to us so we modified the procedure by using [2,4,16α,16β-2H4] estradiol as an internal standard. Recovery data using the new internal standard in urine are presented in Table 1.
Table 1. Percent Recovery of DIM From Urine.
Known concentrations of DIM were aliquoted in triplicate to urine in each group. The internal standard was added and each sample was extracted and derivitized.
| ng DIM added | ng/ml | Percent Recovery | ||
|---|---|---|---|---|
| A1 | 0 | 0 | * | |
| A2 | 0 | 1 | * | |
| A3 | 0 | 1 | * | |
| mean | 1 | * | ||
| SD | 0 | * | ||
| B1 | 54 | 55 | 102 | |
| B2 | 54 | 58 | 107 | |
| B3 | 54 | 59 | 110 | |
| mean | 57 | 106 | ||
| SD | 2 | 4 | ||
| C1 | 270 | 270 | 100 | |
| C2 | 270 | 275 | 102 | |
| C3 | 270 | 274 | 102 | |
| mean | 273 | 101 | ||
| SD | 3 | 1 | ||
| D1 | 540 | 508 | 94 | |
| D2 | 540 | 509 | 94 | |
| D3 | 540 | 512 | 95 | |
| mean | 510 | 94 | ||
| SD | 2 | 0 | ||
| E1 | 1080 | 1069 | 99 | |
| E2 | 1080 | 1095 | 101 | |
| E3 | 1080 | 1066 | 99 | |
| mean | 1077 | 100 | ||
| SD | 16 | 1 |
The internal standard was added and the sample was extracted with chloroform. Twenty microliters of dry pyridine and 80 μl of N,O-bis(trimethylsilyl) trifluoroacetamide catalyzed with 1% trimethyl-chlorosilane (Pierce Chemical, Rockford, IL) were added, and the sample was heated to 100°C for 1h. The sample was injected without further treatment. Gas Chromatography-Mass Spectrometry conditions were the same as those described by Sepkovic et al (10). Creatinine determinations were performed colorimetrically using an ELISA kit provided by Assay Designs (Ann Arbor, MI).
Statistical Methods
Treatment of cervical histology data
The outcome of interest is the incidence rate of mice that were CIN free at each dose level at 20 weeks. Since normal histology (CIN free rate) at the end of the study period is desirable, we collapsed the ordinal response into CIN free (normal) and CIN to yield a binary response.
To examine evidence of a dose response relationship, a Cochran-Armitage test (27,28) was conducted. Further, the minimum effective dose was determined using pair wise Fisher exact tests for comparisons of treated mice and 0 DIM dose groups. The realized raw pair wise test p-values, were adjusted for family wise error rate (FWER) by the step-down Hochberg procedure (28–31) before identifying the smallest DIM dose with an effect on CIN freedom (29).
Treatment of urinary DIM data
DIM concentrations per mg creatinine were examined for evidence of DIM–dose dependent trend. A test of normality (Shapiro- Wilk) was conducted on each characteristic to ascertain the underlying distribution of the concentrations. If the data were found to be normally distributed, the mean (SD) was used to summarize the realized measurements, otherwise median (IQR: 25th – 75th percentiles) was utilized. Urinary DIM concentrations reported significant a Shapiro-Wilk test p-value of <0.05. Thus, nonparametric methods for summarizing the observed values and testing for trend in response along with comparisons with the control group (0ppm DIM), were sought. Trend was examined by performing a Jonckheere-Terpstra test, and using Dunn’s tests for pair wise comparisons to the concentration at the control group (0ppm DIM dose) to adjust for multiple testing using Dunn’s method as described in (30). To examine if each successive incremental DIM dose yielded a statistically different DIM concentration per mg creatinine, a modified Mann-Whitney described in (31) was utilized. This test compared the concentration at each level to the cumulative DIM concentrations observed in all DIM groups with less DIM Dose including the control group. This approach maintained the family wise error rate at 5% for all successive 5 DIM dose level tests. To provide a graphical presentation of the results obtained in the examination of urine DIM concentrations, we used boxplots. The box represents the middle 50% of the data set. The upper boundary (Q3) locates the 75th percentile of the data set while the lower boundary (Q1) indicates the 25th percentile. The line in the box that indicates the median. The vertical lines extending from the box, indicate the minimum and maximum values in the dataset. The diamond indicates the mean. The small circles indicate outliers.
All statistical testing was performed such that P-value<0.05% indicated statistical significance. All data analysis in this study was performed using SAS version 9.2 (SAS Institute Inc, Cary, NC) (30,31).
Results
Food consumption and body weights were measured weekly throughout the experimental period. Over the twenty week study, no significant differences in body weight were observed between wild type controls and transgenic mice in any DIM dose group. There were also no significant differences when the transgenic dose groups were compared. All groups had dietary food consumption levels that were consistent with animal feeding recommendations for mice on custom purified diet (3–6g/animal/day) (32). There were no significant differences in food consumption within or between groups.
Histology
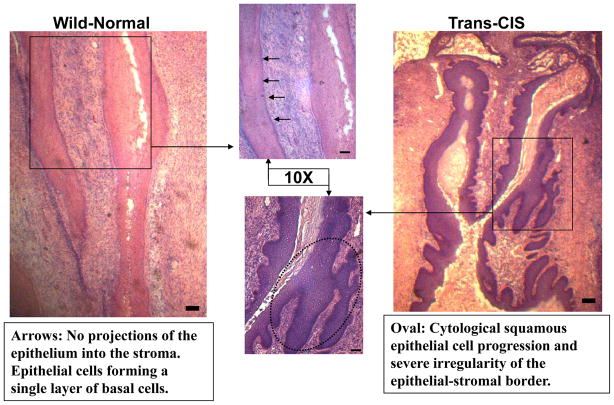
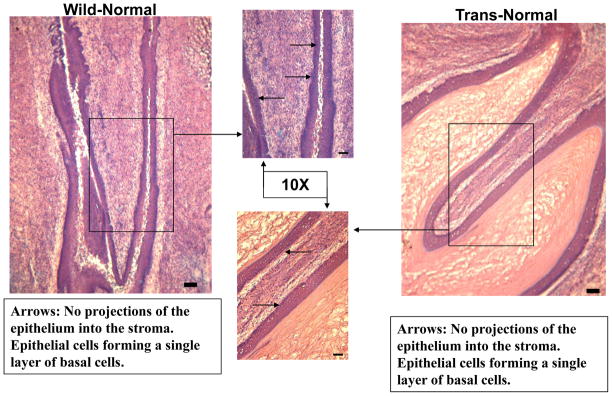
Each figure compares wild type and transgenic mouse cervical epithelia from the same dose group shown side by side. Figure 1 illustrates carcinoma in situ (NCIS) frequently found in transgenic mice that receive estradiol pellets without DIM administration (0ppm DIM transgenic). Commonly, cytological squamous cell progression and severe irregularity of the epithelial- stromal border are observed. In Figure 2, the cervical epithelium of a transgenic mouse receiving 1000ppm in the diet is normal.
Figure 1. Histo-Pathological Analysis No DIM-0ppm 5X.
Comparision of the cervical epithelium in wild type and transgenic mice receiving 0ppm DIM in the diet for 20 weeks. The wild type (left panel) shows a normal cervical epithelium. In the transgenic mouse (right panel) carcinoma in situ is illustrated.
Figure 2. Histo-Pathological Analysis DIM-1000ppm 5X.
Comparision of the cervical epithelium in wild type and transgenic mice receiving 1000ppm DIM in the diet for 20 weeks. The wild type (left panel) shows a normal cervical epithelium. In the transgenic mouse (right panel) normal cervical epithelium is shown.
Table 2 summarizes the histological comparison of the cervical transformation zone in each dose group. Results from the wild type mice that were assigned to the same dosing pattern as the transgenic mice are also presented. The endpoint in this analysis is the cervical histology examined at 20 weeks and the outcome of interest is the incidence rate of mice that were CIN free at each dose level.
Table 2. Histopathology of Wild Type and Transgenic Mice Receiving Different Doses of DIM for 20 Weeks.
Histopathological Response dichotomized as CIN free or not. For thebinary outcome, CIN freedom, Cochran-Armitage Test for trend, reported significant DIM Dose effect in Transgenic mice (P<0.0001) and not significant in Wild type (P=0.5327). Cochran –Armitage exact test with Hochberg procedure for multiple testing, identified 1000ppm as the minimum effective dose (MED) at (P<0.05)
| Cervical Epithelium | 0ppm DIM | 500ppm DIM | 1000ppm DIM | 1500ppm DIM | 2000ppm DIM | 2500ppm DIM |
|---|---|---|---|---|---|---|
| Transgenic | ||||||
| CIN-free | 3 | 4 | 13 | 14 | 13 | 14 |
| CIN1 | 1 | 3 | 2 | 0 | 0 | 1 |
| CIN2 | 5 | 9 | 4 | 5 | 9 | 5 |
| CIN3 | 6 | 5 | 2 | 3 | 1 | 1 |
| Carcinoma in situ (NCIS) | 2 | 0 | 0 | 0 | 0 | 0 |
| Squamous Cell carcinoma (SCC) | 2 | 1 | 0 | 0 | 0 | 0 |
| Total mice (Transgenic) | 19 | 22 | 21 | 22 | 23 | 21 |
| Percent CIN-free/Total | 16% | 18% | 62% | 64% | 57% | 67% |
| Wildtype | ||||||
| CIN-free | 26 | 27 | 21 | 18 | 19 | 20 |
| CIN1 | 5 | 5 | 0 | 5 | 2 | 4 |
| CIN2 | 3 | 0 | 0 | 2 | 4 | 2 |
| Wildtype Total | 34 | 32 | 21 | 25 | 25 | 26 |
| Percent CIN-free/Total | 76% | 84% | 100% | 72% | 76% | 77% |
To analyze the dose response, we first established whether or not there was a trend effect of the DIM dose on the binary response CIN free versus CIN. Based on the 128 transgenic mice that were randomly assigned to the control and five doses, this test indicated that there is significant positive linear trend (P<0.0001) such that as the DIM dose increased the proportion that were CIN free at 20 weeks also increased. With regard to the wild type mice the dose effect was not significant (P=0.5327).
In transgenic mice, pair wise comparisons between the 5 dose levels and the 0 ppm DIM dose were performed to determine which doses were significantly from the control. There was no difference between the 0ppmDIM group and the 500ppm DIM group. At 1000ppm, there was a significant increase in normal mice (P=0.0123). This difference is reflected in the higher DIM dose groups.
The results show that DIM Dose of 500 ppm was not significantly different from the 0 ppm DIM (P=1.00) with respect to the rate of CIN free animals. Our analysis indicated that 1000 ppm of DIM was the smallest dose that reported a response significantly different from the control (P=0.0123), yielding the minimum effective dose for the study.
Notable was the fact that 21% of mice in the 0ppm DIM group and 5% on the 500ppm DIM groups had either NCIS or SCC. No cancers were reported at 1000ppm DIM or higher. With regard to the wild type mice the dose effect was not significant (P=0.5327), which means based on the 163 mice; there was no DIM dose effect on the CIN freedom at 20 weeks.
Urine DIM levels
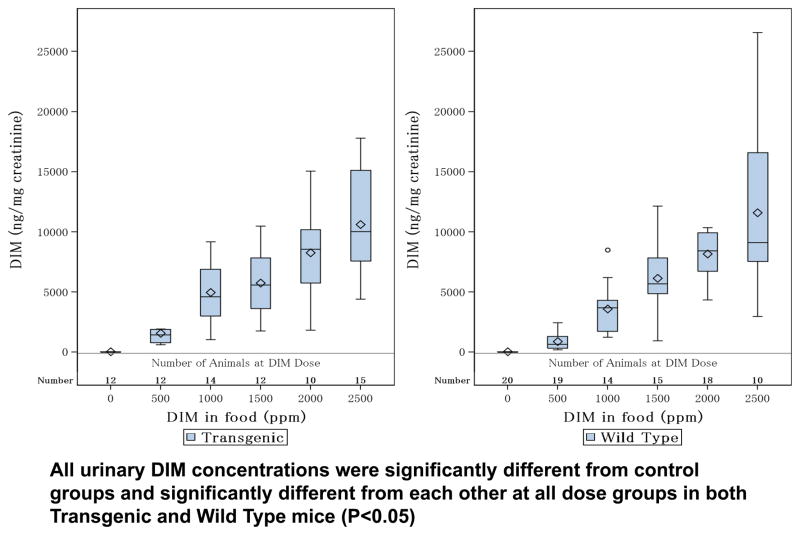
The DIM concentrations normalized by creatinine (ng/mg) were measured and summarized as median values as shown in Figure 3. There was a significant trend in this parameter (P<0.0001). Urine DIM concentrations increased significantly in a linear fashion with each DIM dose group in both transgenic and wild type mice (P<0.05).
Figure 3. Concentrations of DIM in Transgenic and Wild type Mice From Each Dose Groupg.
Box plots of DIM Levels at each DIM dose in transgenic and wild type mice. Jonckheere-Terpstra trend test result: both type reported significant trends (P<0.0001). Modified Mann-Whitney test was used to significant effect of each DIM dose cumulatively. Both transgenic and wild type reported significant dose effect (P<0.05) at consecutive DIM treated groups.
Discussion
In this study we determine the minimum effective dose of DIM that prevents cervical dysplasia using a transgenic mouse model. At 1000ppm (DIM in food) significant numbers of mice with normal cervical epithelia were obtained after 20 weeks. NCIS and SCC were completely eliminated in the 1000ppm DIM group compared to 21% of cancers in the 0ppm group and 5% in the 500ppm DIM group. This is a clear indication that DIM suppressed the oncogenic potential of the viral transgenes.
The goal of this research is to find the minimum effective dose of DIM that gives a normal cervical epithelium. At 1000ppm DIM continuously given in the diet several observations were apparent. Histology revealed a high percentage of mice with normal cervical epithelia. At this dose, the complete elimination of NCIS and SCC were observed. For these reasons 1000ppm was established as the minimum effective dose.
These findings imply usefulness for DIM in the quest to prevent cervical cancer. Vaccination using a preventive vaccine in combination with DIM in women after HPV infection could prevent re-activiation of oncogene expression. Additionally DIM administration could increase the effectiveness of therapeutic vaccines that currently are about 50% effective.
There is a positive association between new sexual partners and re-infection and that true new infections with the same types harbored earlier in life are possible in older women (33). DIM, with subsequent vaccination, could prevent re-infection in this age group and prevent re-acquisition of HPV types in women who have been exposed to before and subsequently cleared.
Urinary DIM concentrations in the 1000ppm DIM group compare favorably to DIM measured in women taking either 200mg or 400mg of I3C (DIM) twice daily for 4 weeks (10). At 200mg of DIM, urinary DIM peak concentrations were around 12 μg per mg creatinine. Women receiving 400mg had urinary DIM values of about 16 μg/mg creatinine daily. In the mouse model, at 1000ppm DIM, urinary values were somewhat lower. Therefore, based on the results of this dose ranging study, 1000ppm DIM in the mouse model is the minimum effective dose and a viable dose for future human studies.
Acknowledgments
Grant Support: NIH Grant 5R01CA136847.
References
- 1.Sundström K, Eloranta S, Sparén P, Arnheim Dahlström L, Gunnell A, Lindgren A, et al. Prospective Study of Human Papillomavirus (HPV) Types, HPV Persistence, and Risk of Squamous Cell Carcinoma of the Cervix. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2469–78. doi: 10.1158/1055-9965.EPI-10-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services - National Cancer Institute, (NCI) Cancer Incidence – Surveillance, Epidemiology, and End Results (SEER) Registries Research Data. Bethesda, Maryland: National Cancer Institute, Cancer Statistics Branch; Apr 15, 2010. [Google Scholar]
- 3.Division of STD Prevention. Prevention of genital HPV infection and sequelae: Report of an external consultants’ meeting. Atlanta, GA: Centers for Disease Control and Prevention; 1999. [Google Scholar]
- 4.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347(21):1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 5.Villa L, Costa R, Petta C, Andrade R, Ault K, Giuliano A, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. The Lancet Oncology. 2005;6(5):271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 6.O’Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, Fabian CJ, et al. Treatment and Prevention of Intraepithelial Neoplasia: An Important Target for Accelerated New Agent Development Clin. Cancer Res. 2002;8:314–46. [PubMed] [Google Scholar]
- 7.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 8.Bell MC, Crowley-Norwick P, Bradlow HL, Sepkovic DW, Baugh S, Howell P, et al. Placebo controlled trial of indole-3-carbinol in the treatment of cervical dysplasia. Gynecologic Oncology. 2000;78:123–129. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 9.Yuan F, Chen D, Liu K, Sepkovic DW, Bradlow HL, Auborn K. Anti-estrogen effect of Indole-3-carbinol in cervical cancer cells: Implication for prevention of cervical cancer. Anticancer Res. 1999;19:1673–80. [PubMed] [Google Scholar]
- 10.Sepkovic DW, Bradlow HL, Bell M. Quantitative Determination Of 3,3′-Diindolylmethane In The Urine Of Individuals Receiving Indole-3-Carbinol. Nutrition and Cancer. 2002;41(1–2):57–63. doi: 10.1080/01635581.2001.9680612. [DOI] [PubMed] [Google Scholar]
- 11.Jin L, Qi M, Chen DZ, Anderson A, Yang GU, Arbiet JM, Auborn KJ. Indole-3-carbinol prevents cervical cancer in human papilloma virus type 16 transgenic mice. Cancer Research. 1999;59:3991–97. [PubMed] [Google Scholar]
- 12.Sepkovic DW, Stein J, Carlisle AD, Auborn K, Nyirenda T, Bradlow HL. Abst. ID: 156. Early findings from a dose response study using 3, 3′-diindolylmethane in the K14-HPV16 transgenic mouse model: Cervical histology. The 2010 Proceedings of ASCO-NCI-EORTC Annual Meeting on Molecular Markers in Cancer; Published by the American society of Clinical Oncology; 2010. p. 98. [Google Scholar]
- 13.Sepkovic DW, Stein J, Carlisle AD, Ksieski HB, Auborn K, Bradlow HL. Diindolylmethane Inhibits Cervical Dysplasia, Alters Estrogen Metabolism, and Enhances Immune Response in the K14-HPV16 Transgenic Mouse Model. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2957–64. doi: 10.1158/1055-9965.EPI-09-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auborn K, Abramson A, Bradlow HL, Sepkovic DW, Mullolly V. Estrogen Metabolism and Respiratory Papillomatosis: A Pilot Study on Dietary Prevention. Anticancer Res. 1998;18:4569–74. [PubMed] [Google Scholar]
- 15.Coll DA, Rosen CA, Auborn K, Potsic WP, Bradlow HL. Treatment of recurrent respiratory papillomatosis with Indole-3-Carbinol. Am J Otolaryngol. 1997;18:283–85. doi: 10.1016/s0196-0709(97)90012-0. [DOI] [PubMed] [Google Scholar]
- 16.Riley RR, Duensing S, Brake T, Münger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;15:63(16):4862–71. [PubMed] [Google Scholar]
- 17.Carter TH, Liu K, Ralph W, Jr, Chen D, Qi M, Fan S, et al. Diindolylmethane alters gene expression in human keratinocytes in vitro. J Nutr. 2002;132(11):3314–24. doi: 10.1093/jn/132.11.3314. [DOI] [PubMed] [Google Scholar]
- 18.Auborn KJ, Woodworth C, DiPaolo JA, Bradlow HL. The interaction between HPV infection and estrogen metabolism in cervical carcinogenesis. Int J Cancer. 1991;2;49(6):867–9. doi: 10.1002/ijc.2910490611. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari RK, Guo L, Bradlow HL, Telang NT, Osborne MP. Selective responsiveness of human breast cancer cells to indole-3-carbinol, a chemopreventave agent. J Natl Cancer Inst. 1994;86:126–131. doi: 10.1093/jnci/86.2.126. [DOI] [PubMed] [Google Scholar]
- 20.Cram EJ, Liu BD, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter. J Biol Chem. 2001;276(25):22332–40. doi: 10.1074/jbc.M010539200. [DOI] [PubMed] [Google Scholar]
- 21.Meng Q, Goldberg ID, Rosen EM, Fan S. Inhibitory effects of Indole-3-carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res Treat. 2000;63(2):147–52. doi: 10.1023/a:1006495824158. [DOI] [PubMed] [Google Scholar]
- 22.Mei Q, Anderson AE, Chen DZ, Shishinn S, Auborn KJ. Indole-3-carbinol prevents PTEN loss in cervical cancer in vivo. Mol Med. 2005;11(1–12):59–63. doi: 10.2119/2006-00007.Auborn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riby JE, Xue L, Chatterji U, Bjeldanes EL, Firestone GL, Bjeldanes LF. Activation and Potentiation of Interferon-(gamma) Signaling by 3,3′-Diindolylmethane in MCF-7 Breast Cancer Cells. Molecular Pharmacology. 2006;69(2):430–9. doi: 10.1124/mol.105.017053. [DOI] [PubMed] [Google Scholar]
- 24.Xue L, Pestka J, Maoxiang L, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane stimulates murine immune function in vitro and in vivo. Journal of Nutritional Biochemistry. 2008;19(5):336–44. doi: 10.1016/j.jnutbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arbeit JM, Munger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol. 1994;68:4358–68. doi: 10.1128/jvi.68.7.4358-4368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. PNAS. 1996;93:2930–35. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armitage P. Test for linear trends in proportions and frequencies. Biometrics. 1955;11:375–85. [Google Scholar]
- 28.Cochran WG. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10:417–51. [Google Scholar]
- 29.Dmitrienko A, Tamhane AC, Bretz F. Multiple Testing Problems in Pharmaceutical Statistics. CRC Press; Boca Raton, FL: 2010. [Google Scholar]
- 30.Dmitrienko A, Chuang-Stein C, D’Agostino R. Pharmaceutical Statistics Using SAS: A Practical Guide. Cary, NC: SAS Institute Inc; 2007. [Google Scholar]
- 31.Chen YI. Biometrics. Vol. 55. A Practical Guide, SAS Institute Inc; Cary, NC: 1999. Nonparametric Identification of Minimum Effective Dose; pp. 1236–1240. 2007. [DOI] [PubMed] [Google Scholar]
- 32.Institute of Laboratory Animal Resources. Commission on Life Sciences. National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C: 1996. [Google Scholar]
- 33.Munoz N, Mendez F, Posso H, et al. Incidence duration and determinants of cervical human papillomavirus infection in a cohort of Columbian women with normal cytological results. J Infect Dis. 2004;190:2077–87. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]